Introduction
Leukemia stem cells (LSCs) are similar to normal
hematopoietic stem cells (HSCs) but with self-renewal capacity and
differentiation potential (1).
Although the number of LSCs is limited, they have a critical role
in leukemia resistance, relapse and prognosis. Recent reports
indicate that leukemia can be considered as a rare and abnormal
form of hematopoiesis induced by cancer stem cells or LSCs.
Moreover, stem cells can maintain or obtain unlimited proliferative
capacity through the accumulation of mutations and/or changes in
epigenetic regulation of genes (2).
Increasing evidence shows that LSCs have an important role in
resistance to chemotherapy drug-induced cell death and in leukemia
relapse (3). LSCs exhibit a number of
resistance mechanisms observed in HSCs, which allow the transport
of chemotherapeutic agent out of the cells via transporters to
avoid cytotoxic effects (4).
Furthermore, it has been shown that 95% of LSCs are in the
G0 phase, where cells rarely proliferate (5), therefore avoiding the cytotoxic effects
of the chemotherapeutic agents. Additionally, some other rare
subpopulations of LSCs (such as CD34 + CD38-phenotype) can enter
the cell cycle, differentiate and proliferate into new leukemia
cells following appropriate stimulation, therefore inducing the
incidence of refractory or relapsed leukemia (6). Phosphatase and tensin homolog (PTEN) can
inhibit mechanistic target of rapamycin (mTOR) activity via
negative regulation of the phosphatidylinositol 3-kinase (PI3K)/Akt
signaling pathway, therefore inhibiting proliferation of cancer
cells, promoting apoptosis and reversing multidrug resistance
(7). DNA topoisomerase II (Topo II)
is the target of anthracycline chemotherapeutic drugs
(topoisomerase inhibitors), which inhibit cell replication by
inhibiting DNA annealing following binding with Topo II (8).
The KG1a leukemia cell line expresses high levels of
cluster of differentiation (CD)34, therefore exhibiting the main
biological characteristic of LSCs. Yi-qi-yang-yin-tang (YQYYT) is
the decoction of Chinese medicine, according to the theory of TCM
syndrome differentiation, in which the combination of drugs have
activating, excitement and promotion effects in the theory of
Traditional Chinese Medicine. Therefore, it was speculated that
YQYYT is able to activate LSCs, upregulate expression of negative
regulatory genes, increase the sensitivity of chemotherapy drug
targets, reverse multidrug resistance and clear residual LSCs in
patients. YQYYT may be a new strategy for the clinical treatment of
leukemia.
In the present study, the KG1a cell line was used to
investigate the role of YQYYT-containing serum on LSC proliferation
and cell cycle progression, and on the levels of mRNA and protein
expression of PTEN, Topo II and mTOR.
Materials and methods
Experimental animals
A total of 20 adult male New Zealand white rabbits
(5 weeks old; weight, 2.2–2.5 kg) were purchased from Beijing
Huafukang Biotechnology Ltd., (Beijing, China), and were housed in
an air-conditioned room at 25°C with a 12-h dark/light cycle
(specific-pathogen-free conditions) at the Institute of Radiation
Medicine, Chinese Academy of Medical Sciences (Tianjin, China). The
rabbits received humane care with unlimited access to food and
water during the present study. Ethical approval was obtained from
Tianjin University of Traditional Chinese Medicine (Tianjin,
China).
YQYYT preparation
A water decoction of YQYYT was prepared at the
Laboratory of Traditional Chinese Medicine Preparation, the First
Affiliated Hospital of Tianjin University of Traditional Chinese
Medicine (Tianjin, China). The water decoction of YQYYT (1 g/ml)
was prepared from the following combination of Chinese medicines:
30 g astragalus (Leguminosa, Astragalus L.), 10 g ginseng
(Araliaceae, PanaxLinn L.), 15 g Ligustrum lucidum
(Oleaceae, Ligustrum L.), 15 g Eclipta (Composite, Eclipta
L.), 10 g Angelica (Umbelliferae, Angelica L.), 10 g
Atractylodes (Composite, Atractylodes L.), 10 g poria
(Polyporaceae, Wolfiporia L.) and 10 g licorice
(Papilionoideae, Glycyrrhiza L.). All the herbs were
provided by The First Affiliated Hospital of Tianjin University of
Traditional Chinese Medicine (Tianjin, China). The solution was
placed into an infusion bottle (250 ml), and sterilized at 105°C
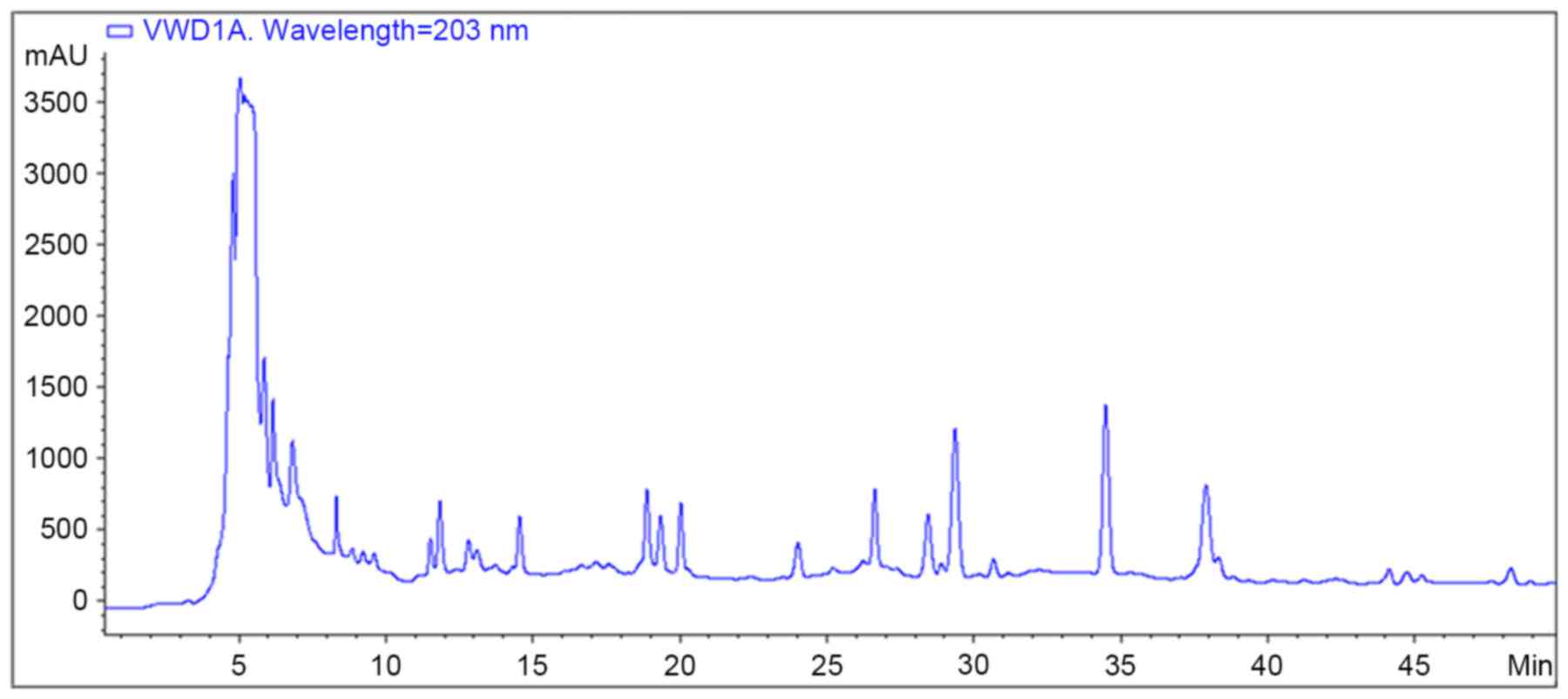
under circulation steam. High performance liquid chromatography
(HPLC) fingerprint analysis was performed to quantify the
components in the water decoction of YQYYT (Fig. 1).
Serum preparation
Male Adult New Zealand white rabbits (n=20) were
randomly divided into treatment and control groups, with 10 rabbits
in each group. Rabbits in the treatment group received the water
decoction of YQYYT (10 ml twice each day) by gavage for 3 days,
while rabbits in the control group received saline. At 2 h
following the last drug administration, 3% sodium pentobarbital was
given to anesthetize the rabbits, and cardiac blood samples were
collected. The serum was separated and filtered for distribution
and stored at −20°C. Gavage method was performed as follows. The
animals were fixed and the opening device was fixed between the
upper and lower incisors. The tube was then inserted into the mouth
of the animal from the mouth of the mouthpiece and into the
esophagus along the posterior wall of the pharynx. After insertion,
the gastric canal should be checked for insertion into the
esophagus. The outer opening of the gastric tube can be placed in
the beaker of the water, and if no bubbles are produced, it
indicates that the gastric tube is inserted into the stomach
properly and that the trachea has not strayed into the trachea. The
syringe was then connected to the tube and injected into the
solution.
Cell culture
KG1a cells (obtained from the Institute of
Hematology, Chinese Academy of Medical Sciences, Tianjin, China)
were cultured in RPMI-1640 medium (HyClone; GE Healthcare, Chicago,
IL, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
U/ml streptomycin (HyClone; GE Healthcare, Chicago, IL, USA) at
37°C under 5% CO2 and saturated humidity. The cells were
passaged into fresh culture medium every 2–3 days. The cells in the
logarithmic growth phase were used in experiments.
Cell Counting Kit-8 (CCK-8) assay
KG1a cells in the logarithmic growth phase were
adjusted to a density of 1×105 /ml, seeded in 96-well
culture plates (100 µl/well), and then divided into experimental
and control groups. Different volumes of YQYYT-containing serum (0,
5, 10, 20 and 40, 20, 10, 5 and 0 µl) were added to the
experimental group, whereas the corresponding volume of rabbit
serum was administered to the control group. RPMI-1640 medium was
added so that the final volume of each well is 200 µl. Each group
was tested at least in triplicate. The cells were cultured at 37°C
under 5% CO2 and saturated humidity for 24, 48 and 72 h.
Subsequently, 10 µl CCK-8 reagent (Tianjin Solomon Biotechnology
Co., Ltd., Tianjin, China) was added into each well, and the
samples were incubated for a further 2 h at 37°C. The absorbance
(OD) value was measured at room temperature using multi-function
enzyme-labeling measuring instrument (Thermo Fisher Scientific,
Inc.) at 450 nm. The proliferation rate was calculated as follows:
Proliferation rate (%)=(1-experimental group OD/control group OD) ×
100%.
The cells were cultured as aforementioned. The cells
were divided into blank group, daunorubicin (DNR), rabbit serum
control and serum containing YQYYT groups (5, 10, 20 and 40 µl;
made up to 100 µl with medium). RPMI-1640 medium (100 µl) was added
to the blank and DNR groups. The final volume in each well was 200
µl. Each group was set up at least in triplicate. After 24 h, 10 µl
DNR (5 µg/ml; Pfizer, Inc., New York, NY, USA) was added into the
experimental, rabbit serum control and DNR groups, while 10 µl
RPMI-1640 medium was added to the blank group. Following a further
24 or 48 h, the OD value in each well was measured using a
multi-function enzyme-labeling at 450 nm.
Cell cycle analysis
KG1a cells in the logarithmic growth phase were
adjusted to a density of 5×105 /ml and seeded in a
6-well culture plate (1 ml/well). The cells were divided into the
control group, high (200 µl) and low (100 µl) dose of YQYYT. After
culturing for 24, 48 and 72 h, the cells were harvested by
centrifugation (400 × g; 5 min). Following the removal of the
supernatant, the cells were washed twice with pre-cooled PBS and
then stored at −20°C overnight. Each sample was incubated with 0.5
ml propidium iodide (Shanghai Megiddo Biological Pharmaceutical
Co., Shanghai, China) in the dark for 30 min. Cell cycle analysis
was performed by flow cytometry (BD Accuri C6; BD Biosciences,
Franklin Lakes, NJ, USA).
Cell sorting
KG1a cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum, 100 U/ml penicillin and 100 U/ml
streptomycin at 37°C under 5% CO2 and saturated
humidity. The cells were passaged every 2–3 days. The cells in the
logarithmic growth phase were harvested.
CD34+CD38− cells were sorted, and the purity
of the resulting populations was determined by flow cytometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The sorted CD34+CD38− KG1
cells were grouped and treated using the protocols as described
previously. Total RNA was isolated using the RNA isolation kit
(Kangwei Shiji, Biotechnology Co., Ltd., Beijing, China), and cDNA
was prepared using the SuperRT cDNA Synthesis kit (Kangwei Shiji,
Biotechnology Co., Ltd.). The following primers (Sangon
Biotechnology, Shanghai, China) were used: β-actin forward,
5′-CCAAGGCCAACCGCGAGAAGATGAC-3′ and reverse,
5′-AGGGTACATGGTGGTGCCGCCAGAC-3′; PTEN forward,
5′-ACCAGGACCAGAGGAAACCT-3′ and reverse, 5′-GCTAGCCTCTGGATTTGACG-3′;
Topo IIα forward, 5′-GTGCGTGAAGTTGTGAATA-3′ and reverse,
5′-GAGAGACACCAGAATTCAA-3′; mTOR forward, 5′-TAACGAGCTGGTCCGAATCA-3′
and reverse, 5′-AGGGTGGACTTAGCTGGACT-3′. RT-qPCR was performed on
the qPCR instrument (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with the SYBR-Green PCR kit (Beijing BioTake Biotechnology,
Beijing, China). The optimized parameters for PCR were: 95°C for 2
min, 95°C for 15 sec, 60°C for 30 sec and 72°C for 40 sec (40
cycles). The mean relative expression of PTEN, TopoII and mTOR in
triplicate samples was calculated according to the
2−ΔΔCq method (9).
Western blot analysis
The sorted CD34+CD38− KG1
cells were grouped and treated using the protocols as
aforementioned. Proteins were extracted using RIPA lysis buffer
(Beijing BioTake Corporation, Beijing, China), and protein
concentration was determined using the Bradford method. Proteins
(20 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes using a semi-dry transfer method.
The membranes were blocked with 5% non-fat milk, and incubated with
primary antibodies (1:3,000) overnight at 4°C. After washing with
TBST three times, the membranes were then incubated with secondary
antibodies (1:1,000) for 1 h at room temperature. Proteins were
detected using enhanced chemiluminescence kit and visualized under
ultraviolet light. Primary antibodies: Anti-PTEN antibody (catalog
no. ab32199; Abcam, Cambridge, UK), anti-topoisomerase IIα antibody
(catalog no. ab52934; Abcam), anti-mTOR antibody (catalog no.
ab2732; Abcam). Secondary antibodies: Goat anti-rabbit IgG,
horseradish peroxidase-conjugated (catalog no. CW0103S; Kangwei
Shiji, Biotechnology Co., Ltd.).
Statistical analysis
Each experiment was repeated more than three times,
and statistical analysis of data was performed using SPSS software
(version 13.0; SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. Comparisons between two groups were
analyzed using Student's t-test, and comparisons between different
groups were analyzed by one-way analysis of variance test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
YQYYT-containing serum does not
inhibit proliferation of KG1a cells in vitro
As indicated in Table
I, YQYYT-containing serum did not inhibit proliferation KG1a
cells at any of the doses tested at 24, 48 and 72 h.
 | Table I.Effect of YQYYT-containing serum at
various concentrations on proliferation of KG1a cells. |
Table I.
Effect of YQYYT-containing serum at
various concentrations on proliferation of KG1a cells.
|
|
| Control serum | YQYYT-containing
serum |
|---|
|
|
|
|
|
|---|
| Volume of
YQYYT-containing serum (µl) | Time (h) | OD value, mean ±
SD | Inhibition rate
(%) | OD value, mean ±
SD | Inhibition rate
(%) |
|---|
| 0 | 24 | 0.52±0.02 | – | 0.51±0.01 | – |
|
| 48 | 0.62±0.01 | – | 0.64±0.01 | – |
|
| 72 | 0.76±0.01 | – | 0.81±0.02 | – |
| 5 | 24 | 0.47±0.03 | 8.3 | 0.47±0.02 |
8.1 |
|
| 48 | 0.48±0.03 | 12.7 | 0.57±0.01 |
9.6 |
|
| 72 | 0.70±0.02 | 7.6 | 0.73±0.01 | 10.2 |
| 10 | 24 | 0.43±0.05 | 16.1 | 0.45±0.03 | 12.4 |
|
| 48 | 0.41±0.01 | 14.8 | 0.56±0.04 | 11.6 |
|
| 72 | 0.68±0.01 | 9.3 | 0.69±0.01 | 14.5 |
| 20 | 24 | 0.41±0.04 | 20.3 | 0.42±0.03 | 19.0 |
|
| 48 | 0.45±0.01 | 24.8 | 0.53±0.02 | 19.7 |
|
| 72 | 0.67±0.02 | 11.7 | 0.68±0.03 | 17.7 |
| 40 | 24 | 0.40±0.01 | 20.9 | 0.41±0.01 |
19.9a |
|
| 48 | 0.45±0.01 | 25.5 | 0.49±0.03 |
23.5a |
|
| 72 | 0.62±0.02 | 18.8 | 0.61±0.01 |
24.3a |
Combination of YQYYT-containing serum
and DNR inhibits proliferation of KG1a cells in vitro
To determine whether the YQYYT-containing serum
increases the sensitivity of KG1a cells to DNR, the IC50
value of DNR for KG1a cells was determined using a previously
described method (10). The
IC50 value was indicated to be 5 µg/ml (data not
shown).
The effects of the YQYYT-containing serum at
different concentrations on the sensitivity of KG1a cells to DNR
were subsequently examined using the IC50 value. At 24
h, treatment with YQYYT-containing serum (5, 10, 20 and 40 µl in a
total volume of 100 µl) and DNR did not inhibit the proliferation
of KG1a cells compared with the group treated with DNR alone.
However, when the volume of serum containing with YQYYT was
increased to 40 µl, there was a significant inhibitory effect
(60.4%) compared with the DNR group on the growth of KG1a cells
(P<0.05; Table II). Following the
treatment of DNR and YQYYT-containing serum (volume, 20 and 40 µl)
for 48 h, an inhibitory effect on KG1a cells was observed (58.9 and
72.5%, respectively) compared with treatment with DNR alone
(Table II). This inhibitory effect
on proliferation was significant when the cells were treated with
40 µl YQYYT-containing serum (P<0.01; Table II). The rate of inhibition following
the treatment with YQYYT-containing serum for 48 h (72.5%) was
significantly higher compared with treatment for 24 h (60.4%,
P<0.01; Table II). Taken
together, these results indicated that the YQYYT-containing serum
was able to promote the inhibitory effect of DNR on KG1a cells in a
time- and dose-dependent manner.
 | Table II.Effect of treatment with a
combination of YQYYT-containing serum and DNR on proliferation of
KG1a cells. |
Table II.
Effect of treatment with a
combination of YQYYT-containing serum and DNR on proliferation of
KG1a cells.
|
|
| Control serum | YQYYT-containing
serum |
|---|
|
|
|
|
|
|---|
| Groups | Time
(h)a | OD value, mean ±
SD | Inhibition rate
(%) | OD value, mean ±
SD | Inhibition rate
(%) |
|---|
| Blank | 24+24 | 0.97±0.06 | – | 1.01±0.05 | – |
|
| 24+48 | 1.35±0.01 | – | 0.31±0.02 | – |
| DNR | 24+24 | 0.52±0.01 | 49.2 | 0.52±0.01 |
48.6 |
|
| 24+48 | 0.71±0.06 | 46.8 | 0.67±0.04 | 49 |
| 5 µl serum
+DNR | 24+24 | 0.49±0.01 | 46.7 | 0.50±0.01 |
50.1 |
|
| 24+48 | 0.74±0.04 | 45.1 | 0.67±0.03 |
48.5 |
| 10 µl serum
+DNR | 24+24 | 0.49±0.02 | 48.8 | 0.48±0.02 |
52.1 |
|
| 24+48 | 0.76±0.05 | 47.3 | 0.70±0.05 |
56.5 |
| 20 µl serum
+DNR | 24+24 | 0.48±0.03 | 49.6 | 0.45±0.01 |
53.9 |
|
| 24+48 | 0.78±0.04 | 43.3 | 0.54±0.02 |
58.9a |
| 40 µl serum
+DNR | 24+24 | 0.51±0.02 | 50.6 | 0.40±0.01 |
60.4a |
|
| 24+48 | 0.69±0.02 | 48.8 | 0.37±0.03 |
72.5b |
YQYYT-containing serum promotes cell
cycle progression of KG1a cells from the G0 phase
As shown in Tables
III–V, YQYYT-containing serum was
able to promote cell cycle progression of KG1a cells from the
G0 phase. The G0/G1 ratio was
decreased and the percentage of S and G2M phase cells
was increased in a dose-dependent manner. However, there was no
significant difference between the effects of treatment with 200
and 100 µl YQYYT-containing serum.
 | Table III.Effect of YQYYT-containing serum on
cell cycle of KG1a cells following 24 h treatment. |
Table III.
Effect of YQYYT-containing serum on
cell cycle of KG1a cells following 24 h treatment.
| Group |
G0/G1 (%) | S (%) | G2/M
(%) |
|---|
| Blank | 84.07±1.63 | 8.96±2.01 | 7.14±3.39 |
| Control |
|
|
|
| 100
µl | 82.52±2.31 | 12.10±1.37 | 9.17±4.41 |
| 200
µl | 81.15±2.45 | 12.57±3.11 | 9.48±2.27 |
| Treatment |
|
|
|
| 100
µl | 77.81±2.05 | 10.68±3.01 | 11.24±1.24 |
| 200
µl |
76.50±2.33a |
14.41±1.64a | 9.19±2.15 |
 | Table V.Effect of YQYYT-containing serum on
cell cycle of KG1a cells following 72 h treatment. |
Table V.
Effect of YQYYT-containing serum on
cell cycle of KG1a cells following 72 h treatment.
| Group |
G0/G1 (%) | S (%) | G2/M
(%) |
|---|
| Blank | 77.20±1.87 | 12.27±2.61 | 8.73±3.12 |
| Control |
|
|
|
| 100
µl | 74.15±1.95 | 16.16±1.73 | 9.68±2.56 |
| 200
µl | 72.81±2.72 | 18.27±3.28 | 6.92±2.11 |
| Treatment |
|
|
|
| 100
µl |
62.94±1.49a | 25.23±1.80 | 11.83±1.79 |
| 200
µl |
59.33±1.81b |
27.77±1.35b | 12.90±2.93 |
The effect of YQYYT-containing serum on the
G0/G1 ratio of the KG1a cells was
time-dependent, with a decreased proportion of cells in the
G0/G1 phase following treatment for 72 h
compared with the ratios following treatment for 24 and 48 h. There
were no significant differences in the proportion of
G2/M cells between the control (24, 48 and 72 h) and
blank groups, or among the treatment groups at different
time-points (24, 48 and 72 h). (Tables
III–V).
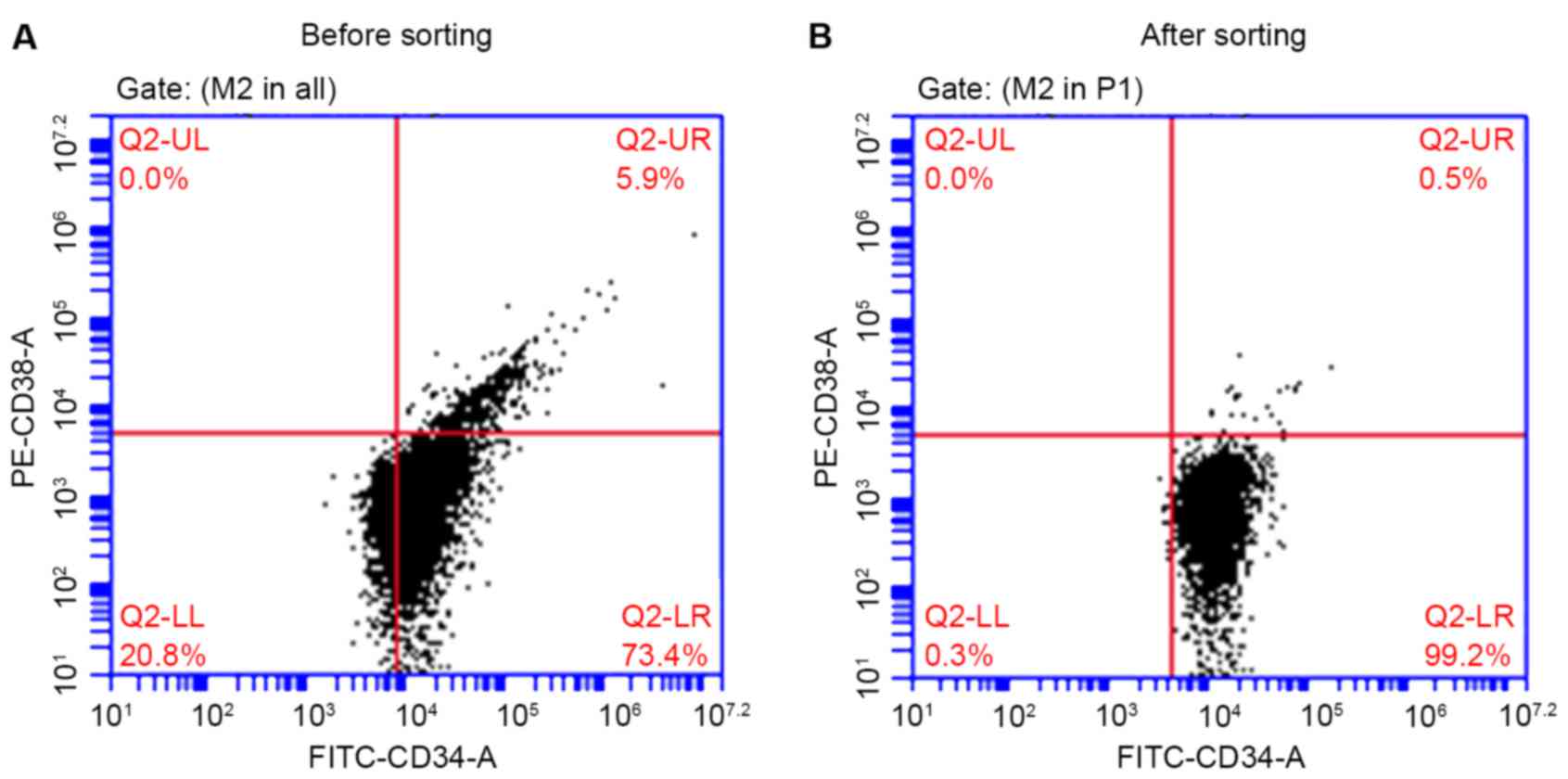
CD34+CD38− KG1a
cell subpopulation was isolated using an immunomagnetic cell
sorting system
Following magnetic separation, the percentage of
CD34+CD38− KG1a cells was 99.2%, which was
significantly higher (P<0.01) compared with the percentage prior
to sorting (73.4%, Fig. 2). This
phenotype was consistent with the biological characteristics of
stem cells.
Effects of YQYYT-containing serum on
the mRNA expression of PTEN, Topo II and mTOR in KG1a cells
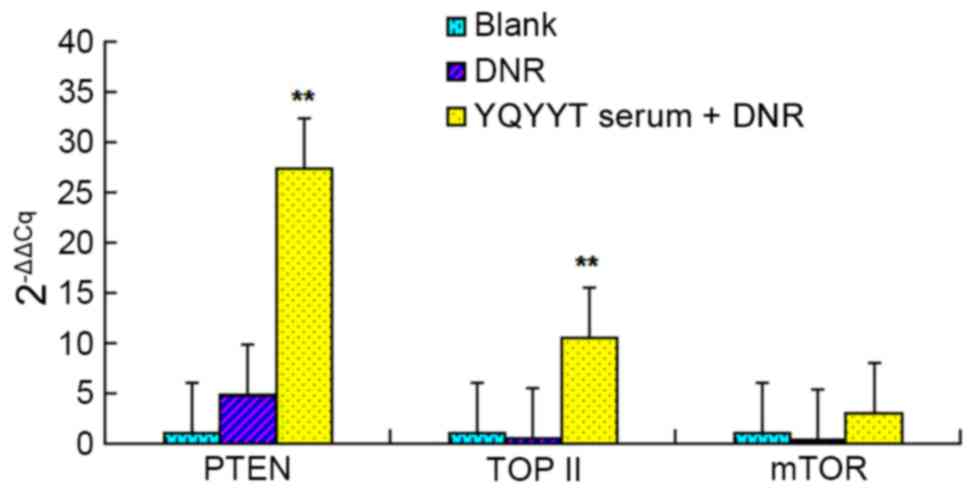
As shown in Fig. 3,
PTEN gene expression was upregulated in KG1a cells when treated
with DNR alone and in combination with YQYYT-containing serum
compared with the blank group. The level of upregulation was
significantly higher in KG1a cells treated with a combination of
DNR and serum containing YQYYT compared with the blank group
(P<0.01).
The mRNA expression of Topo II was decreased in the
DNR group, but was significantly increased in the group treated
with the combination of DNR and YQYYT-containing serum (P<0.01),
when compared with the blank group.
The mRNA expression of mTOR was decreased in the DNR
group, but was increased in the group treated with the combination
of DNR and serum containing YQYYT when compared with the blank
group (Fig. 3).
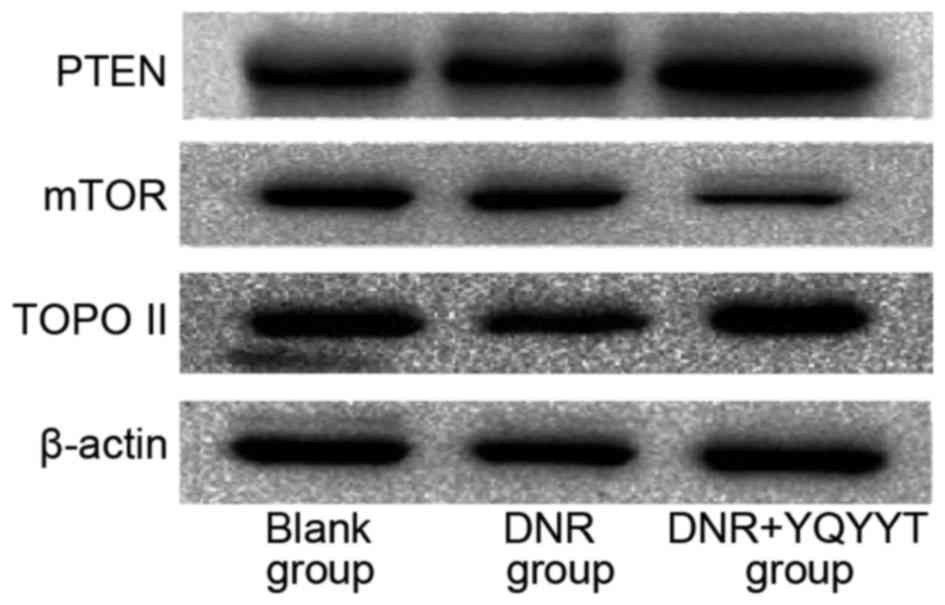
Effects of YQYYT-containing serum on
the protein expression of PTEN, Topo II and mTOR in KG1a cells
The blank group was used as the standard group
(relative quantitative value of 1), and the relative quantitative
values of each group were calculated using Image Lab 4.0 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Compared with the
blank group, the expression of PTEN protein in the DNR group and
the group treated with a combination of DNR and serum-containing
YQYYT increased, the mTOR protein levels in the group treated with
a combination of DNR and serum containing YQYYT decreased
significantly (Table VI; Fig. 4). In the DNR group, the level of Topo
II protein was downregulated compared with the blank group, while
the level of Topo II was upregulated in the group treated with a
combination of DNR and YQYYT-containing serum (Table VI; Fig.
4).
 | Table VI.Image Lab 4 software analysis of the
relative quantitative values of western blot analysis of PTEN, Topo
II and mTOR. |
Table VI.
Image Lab 4 software analysis of the
relative quantitative values of western blot analysis of PTEN, Topo
II and mTOR.
| Group | PTEN | mTOR | Topo II |
|---|
| Blank | 1 | 1 | 1 |
| Control |
1.62±0.23a | 0.93±0.12 |
0.62±0.19a |
| Treatment |
2.17±0.34a |
0.33±0.11a |
1.74±0.18a |
Discussion
Acute leukemia is a type of malignant cancer for
which long-term disease-free survival can be potentially achieved
through chemotherapy (11). Although
the current induction therapy achieves complete remission (CR) in
80% of patients, >60% of patients become resistant to
chemotherapy and even relapse (12).
Although LSCs, which express high levels of CD34+,
CD38− and CD123+, represent only a small
subpopulation of cells in patients with acute myeloid leukemia
(AML) (13), these cells appear to be
the cause of drug resistance and relapse (14,15).
Due to untargeted toxicity, chemotherapy drugs not
only kill leukemia cells, but also induce damage of normal tissues
and cells to various degrees. This result in impaired
proliferation, differentiation and maturation of HSCs or progenitor
cell, and induces potentially life-threatening effects, including
bone marrow suppression, pancytopenia, low immunity and increased
susceptibility to infection (16).
Recent studies showed that many novel markers, including C-type
lectin-like molecule-1 (CLL-1), CD96, T cell immunoglobulin mucin
3, CD47, CD32 and CD25, are expressed by CD34+
CD38− LSCs, but not by HSCs. Therefore, these markers
have been used to confirm the presence of LSCs in patients in
clinical studies (17,18). Tettamanti et al (19) indicated that the combination of
anti-CD123 antibody and chimeric antigen receptor-integrated
cytokines induced cytotoxic T cells that efficiently eliminated
LSCs. Additional reports demonstrated that the incidence of
biphenotypic acute leukemia (BAL) is between 1.3–8%, and BAL cells
are derived from multipotent progenitor cells (20). During the development of leukemia,
multipotent progenitor cells can differentiate into myeloid and
lymphoid leukemia cells, and these patients with BAL are associated
with poor prognosis. Furthermore, the expression of CD34 is
negatively correlated with therapeutic efficacy in BAL (21). It was observed that the rate of
expression of the LSC immunological markers, CD123 and CD47, as
well as CLL-1 and CD96, reached 100% and >60%, respectively
(22). These markers can be used to
improve the identification and elimination of LSCs. Song et
al (23) identified CD44 as an
important target for LSC therapy, and CD44 monoclonal antibodies
can induce the differentiation and inhibit proliferation of primary
leukemia cells, as well as effectively promoting programmed cell
death of leukemia cells.
The capacity of LSCs for self-renewal is closely
associated with the regulation of the cell cycle, which is also
closely linked with DNA damage and repair. These processes are
important for survival of LSCs when treated with DNA-damaging
agents (24). It has been
demonstrated that >95% of LSCs are in the G0 phase
(25), where they do not proliferate
and have a low ability for replication. In the G0 phase,
the cells have an infinite ability for self-renewal and are not
sensitive to chemotherapy. Therefore, the cells in the
G0 phase can easily evade the destructive effects of
chemotherapeutic agents (26). Drugs
which specifically target the cell cycle, particularly
5-fluorouracil, methotrexate and cytarabine antimetabolite, are not
effective against LSCs (27). Saito
et al (28) reported that
G0 phase AML-LSC in the endosteum can be induced to
enter the cell cycle by granulocyte colony-stimulating factor, with
apoptosis and elimination of LSCs being enhanced by combination
therapy with cell cycle-dependent chemotherapeutic drugs (28). Song et al (29) investigated the effects of Zuigui pill,
Yougui pill and their disassembled prescriptions on the cell cycle
progression of bone marrow mesenchymal stem cells (BMSCs) which are
involved in osteogenic differentiation, and reported that Zuigui
pill and YQYYT were able to significantly promote proliferation of
BMSCs, and alter the cell cycle and apoptosis of BMSCs (29). In the present study, it was detected
that treatment with YQYYT-containing serum promoted cell cycle
progression of KG1a cells, therefore indicating that treatment with
YQYYT increases the sensitivity of leukemia cells to
chemotherapeutic agents and enhances the chemotherapeutic response.
In this way, not only can drug resistance be inhibited, residual
LSCs can also be eliminated, therefore greatly reducing the
possibility of disease recurrence.
PTEN is a highly conserved tumor suppressor gene,
which can inhibit cancer cell proliferation, induce apoptosis and
cell cycle arrest, simultaneously regulate a variety of molecules,
and reduce tumor invasiveness (30)
It is currently accepted that PTEN inhibits cancer cell growth,
infiltration, invasiveness and metastasis via inhibition of
multiple signal transduction pathways (31). However, although PTEN gene deficiency
leads to the generation and proliferation of LSCs, it does not
affect differentiation and survival of HSCs (32). Therefore, this indicates that
targeting PTEN may be a potential strategy for LSC-targeted
therapy. In addition, the PTEN gene can inhibit mTOR activity by
negatively regulating the PI3K/Akt signaling pathway, therefore
inhibiting cancer cell proliferation, promoting apoptosis and
reversing multidrug resistance (7).
Furthermore, it has been previously demonstrated that
overexpression of PTEN can slow disease progression and prolong the
survival of leukemic mice (33).
Using RT-PCR techniques, Xu et al (34) reported that YQYYT is able to decrease
the mRNA expression of Fms related tyrosine kinase 3 and N-ras in
bone marrow mononuclear cells in patients with AML, inhibit
proliferation of leukemic cells in patients with AML and exhibits a
therapeutic effect on AML (34). In
the present study, it was observed that treatment of KG1a cells
with YQYYT-containing serum was able to increase PTEN expression of
RNA or protein.
Topoisomerase has an important role in DNA
replication, transcription, repair and recombination (35). Topo II is an enzyme that acts on the
topology of DNA and regulates changes in the conformation of DNA
(36). Changes in the quality and
quantity of Topo II would directly affect binding between
chemotherapeutic drugs and DNA, which would lead to a decrease in
drug-induced cleavage complex formation and result in the induction
of drug resistance. The majority of LSCs express low levels of Topo
II, and the upregulation of Topo II expression and activity
increases sensitivity to anthracycline (37). Shi et al (38) reported that Six Spirits pill, a
Chinese traditional medicine, was able to reverse multidrug
resistance in leukemia cells by reducing the expression of p170 and
increasing the expression of Topo IIβ (38). Kim et al (39) observed that ginsenoside Rg3 and
verapamil were able to increase the sensitivity of multidrug
resistant leukemia cells to chemotherapeutic drugs, and a limited
number of side-effects were detected (39). In the present study, it was observed
that treatment of KG1a cells with YQYYT-containing serum was able
to increase Topo II expression of RNA or protein.
Chinese herbal formulae are flexible, with a variety
of regulatory mechanisms and multiple targets for anti-cancer
effects that may significantly reduce the damage induced by
chemotherapeutic drugs. Therefore, Chinese herbal agents may have
an important role in increasing the efficacy of chemotherapy in the
clinical treatment of leukemia, as well as reducing toxicity and
reversing drug resistance (40,41). Yan
and Shi (42) reported that the
clinical efficacy of combination chemotherapy with YQYYT and
refractory acute leukemia (RAL) is higher compared with the
efficacy achieved with conventional chemotherapy alone, with
limited side effects. This effect on efficacy may be associated
with the promotion of cell cycle progression of leukemia cells by
YQYYT (42).
The results showing the effects of YQYYT-containing
serum on cell cycle progression and the expression of
resistance-associated genes in KG1a cells are preliminary and
require further investigation in in vivo studies.
Furthermore, changes in cell cycle of tumor cells are regulated by
multiple proteins and cytokines, and further studies are necessary
to reveal the detailed mechanisms underlying these processes.
However, these results indicate the potential of YQYYT in enhancing
the sensitivity of LSC to DNR chemotherapy.
References
|
1
|
Hu Y and Li S: Survival regulation of
leukemia stem cells. Cell Mol Life Sci. 73:1039–1050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Passegué E and Weisman IL: Leukemic stem
cells: Where do they come from? Stem Cell Rev. 1:181–188. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Styczynski J and Drewa T: Leukemic stem
cells: From metabolic pathways and signaling to a new concept of
drug resistance targeting. Acta Biochim Pol. 54:717–726.
2007.PubMed/NCBI
|
|
4
|
Guzman ML, Neering SJ, Upchurch D, Grimes
B, Howard DS, Rizzieri DA, Luger SM and Jordan CT: Nuclear
factor-kappaB is constitutively activated in primitive human acute
myelogenous leukemia cells. Blood. 98:2301–2307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Won EJ, Kim HR, Park RY, Choi SY, Shin JH,
Suh SP, Ryang DW, Szardenings M and Shin MG: Direct confirmation of
quiescence of CD34+CD38-leukemia stem cell populations using single
cell culture, their molecular signature and clinicopathological
implications. BMC Cancer. 15:2172015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Irons RD and Stillman WS: Cell
proliferation and differentiation in chemical leukemogenesis. Stem
Cells. 11:235–242. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng C, Chen Y, Li D and Li S: Role of
Pten in leukemia stem cells. Oncotarget. 1:156–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald TL, Labroli MA and Tepe JJ: 7.16
- DNA Topoisomerase InhibitorsComprehensive Natural Products
Chemistry. 7. Elsevier Ltd.; Philadelphia, PA: pp. 593–614. 1999,
View Article : Google Scholar
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He K, Yu P, Xing HY, Li Y, Tian Z, Wang M,
Tang KJ and Rao Q: Resistance of leukemia KG1a cells with positive
N-cadherin in phase G(0) against killing activity of VP16. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 19:1102–1106. 2011.(In Chinese).
PubMed/NCBI
|
|
11
|
Colado E, Paino T, Maiso P, Ocio EM, Chen
X, Alvarez-Fernández S, Gutiérrez NC, Martín-Sánchez J,
Flores-Montero J, San Segundo L, et al: Zalypsis has in vitro
activity in acute myeloid blasts and leukemic progenitor cells
through the induction of a DNA damage response. Haematologica.
96:687–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vergez F, Green AS, Tamburini J, Sarry JE,
Gaillard B, Cornillet-Lefebvre P, Pannetier M, Neyret A, Chapuis N,
Ifrah N, et al: High levels of CD34+CD38low/−CD123+ blasts are
predictive of an adverse outcome in acute myeloid leukemia: A
Groupe Ouest-Est Des Leucemies Aigues et maladies du sang (GOELAMS)
study. Haematologica. 96:1792–1798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
She M, Niu X, Chen X, Li J, Zhou M, He Y,
Le Y and Guo K: Resistance of leukemic stem-like cells in AML cell
line KG1a to natural killer cell-mediated cytotoxicity. Cancer
Lett. 318:173–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Long J, Liu S, Li K, Zhou X, Zhang P and
Zou L: High proportion of CD34+/CD38-cells is positively correlated
with poor prognosis in newly diagnosed childhood acute
lymphoblastic leukemia. Leuk Lymphoma. 55:611–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sohl SJ, Schnur JB and Montgomery GH: A
meta-analysis of the relationship between response expectancies and
cancer treatment-related side effects. J Pain Symptom Manage.
38:775–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horton SJ and Huntly BJ: Recent advances
in acute myeloid leukemia stem cell biology. Haematologica.
97:966–974. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chávez-González A, Dorantes-Acosta E,
Moreno-Lorenzana D, Alvarado-Moreno A, Arriaga-Pizano L and Mayani
H: Expression of CD90, CD96, CD117, and CD123 on different
hematopoietic cell populations from pediatric patients with acute
myeloid leukemia. Arch Med Res. 45:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tettamanti S, Biondi A, Biagi E and Bonnet
D: CD123 AML targeting by chimeric antigen receptors: A novel magic
bullet for AML therapeutics? Oncoimmunology. 3:e288352014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu L, Reader JC, Chen C, Zhao XF, Ha JS,
Lee C, York T, Gojo I, Baer MR and Ning Y: Activation of a novel
palmitoyltransferase ZDHHC14 in acute biphenotypic leukemia and
subsets of acute myeloid leukemia. Leukemia. 25:367–371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zulfikar O, Koc B and Kebudi R: The oue
center experience. J Clin Oncol. 31:136–139. 2013.
|
|
22
|
Majeti R: Monoclonal antibody therapy
directed against human acute myeloid leukemia stem cells. Oncogene.
30:1009–1019. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song QX, Wu Y and Chen YZ: The effect of
CD44 antibody-HI44a on proliferation and differentiation of HL-60
cells. Chine Pharmacol Bulletin. 3:222011.
|
|
24
|
Misaghian N, Ligresti G, Steelman LS,
Bertrand FE, Bäsecke J, Libra M, Nicoletti F, Stivala F, Milella M,
Tafuri A, et al: Targeting the leukemic stem cell: The holy grail
of leukemia therapy. Leukemia. 23:25–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hope KJ, Jin L and Dick JE: Human acute
myeloid leukemia stem cells. Arch Med Res. 34:507–514. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pollyea DA, Gutman JA, Gore L, Smith CA
and Jordan CT: Targeting acute myeloid leukemia stem cells: A
review and principles for the development of clinical trials.
Haematologica. 99:1277–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C and Chen Y: The study of molecular
mechanism of leukemia stem cell resistance. J Mol Diagno Ther.
3:203–206. 2009.
|
|
28
|
Saito Y, Uchida N, Tanaka S, Suzuki N,
Tomizawa-Murasawa M, Sone A, Najima Y, Takagi S, Aoki Y, Wake A, et
al: Induction of cell cycle entry eliminates human leukemia stem
cells in a mouse model of AML. Nat Biotechnol. 28:275–280.
2010.PubMed/NCBI
|
|
29
|
Song N, He WZ, Wang ZM and Ren YL: Effects
of serum containing Zuigui pill, Yougui pill and their disassembled
prescriptions on the cell cycle and cell apoptosis of bone marrow
mesenchymal stem cells in osteogenic differentiation. Chin J Trad
Chin Med Pharm. 5:772013.
|
|
30
|
Wu H, Goel V and Haluska FG: PTEN
signaling pathways in melanoma. Oncogene. 22:3113–3122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morotti A, Panuzzo C, Crivellaro S, Carrà
G, Torti D, Guerrasio A and Saglio G: The Role of PTEN in myeloid
malignancies. Hematol Rep. 7:58442015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheung AM and Mak TW: PTEN in the
haematopoietic system and its therapeutic indications. Trends Mol
Med. 12:503–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng C, Chen Y, Yang Z, Zhang H, Osterby
L, Rosmarin AG and Li S: PTEN is a tumor suppressor in CML stem
cells and BCR-ABL-induced leukemias in mice. Blood. 115:626–635.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu R, Wang X and Wang J: Study on the
expression of Flt3 and N-ras genes human leukemia stem cells using
RT-PCT. Zhe Int Trad Chin West Med. 5:278–280. 2009.
|
|
35
|
Cowell IG and Austin CA: Mechanism of
generation of therapy related leukemia in response to
anti-topoisomerase II agents. Int J Environ Res Public Health.
9:2075–2091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JC and Kirkegaard K: DNA
topoisomerases. Gene Amplif Anal. 2:455–473. 1981.PubMed/NCBI
|
|
37
|
Liu MY, Wang WZ, Liao FF, Wu QQ, Lin XH,
Chen YH, Cheng L, Jin XB and Zhu JY: Selective and effective
targeting of chronic myeloid leukemia stem cells by topoisomerase
II inhibitor etoposide in combination with imatinib mesylate in
vitro. Cell Biol Int. 41:16–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi Z and Yang W: The effect and
underlying mechanism of liushenpill in reversing multidrug
resistance in leukemia cells. J Tian Uni Trad Chin Med. 1:11–13.
2007.
|
|
39
|
Kim SS, Seong S and Kim SY: Synergistic
effect of ginsenoside Rg3 with verapamil on the modulation of
multidrug resistance in human acute myeloid leukemia cells. Oncol
Lett. 7:1265–1269. 2014.PubMed/NCBI
|
|
40
|
Li J and Liu XB: Advances in medicine of
toxicity and synergistic effects in cancer treatment. J Pharmaceut
Res. 4:229–231. 2015.
|
|
41
|
Long X, Geng Y and Guo Q: Recent progress
in anti-tumor medicine and the role of the active ingredients in
recent years. Chin Arch Tradl Chin Med. 4:862–864. 2015.
|
|
42
|
Yan L and Shi Z: Clinical study on
reversal of multidrug resistance in refractory acute leukemia by
Supplementing qi and nourishing yin. J Beij Uni Trad Chin Med.
1:68–72. 2015.
|