Introduction
Angiomyolipomas (AMLs) are benign mixed mesenchymal
neoplasms that arise predominantly in the kidneys (1). Extrarenal AMLs are rare and, in
particular, AMLs of the duodenum are uncommon. To the best of our
knowledge, only two cases have been reported based on a search of
the PubMed and Medline databases (2,3). Among the
extrarenal sites, the liver is the most frequently involved
(4).
AMLs have been originally described as hamartomas or
choristomas, formed of smooth muscle, abnormal blood vessels and
fat tissue (5). However, evidence has
indicated that AMLs belong to the family of perivascular
epithelioid cell tumors (PEComas) (6). The first-line treatment of AMLs is
surgical excision. Even though AMLs are considered to be benign
tumors, they may be invasive and recurrent (5,6).
The majority (~80%) of AMLs are sporadic, whereas
the remainder are associated with tuberous sclerosis complex (TSC)
(6,7).
Most patients with TSC have renal manifestations, whereas
extrarenal AMLs are usually not associated with TSC (8). The etiology is not completely
understood. In the present case report, a rare AML case arising in
the duodenum and presenting with multiple systemic vascular
malformations and aneurysms is described.
Case report
A 22-year-old female patient, suffering from early
satiety, intermittent upper abdominal pain and vomiting for 2
years, was admitted into the Zhongnan Hospital of Wuhan University
(Hubei, China) in February 2013, with upper abdominal pain and
symptoms of hematemesis. The patient had no previous history of
TSC. Physical examination revealed moderate pallor and mild
tenderness of the middle upper abdominal quadrant; however, the
patient's vital signs were stable. No abnormalities in laboratory
examinations, including blood biochemical tests, urinalysis and
serum tumor markers; other than a low hemoglobin concentration (82
g/l, compared with normal values of 120–160 g/l), were
observed.
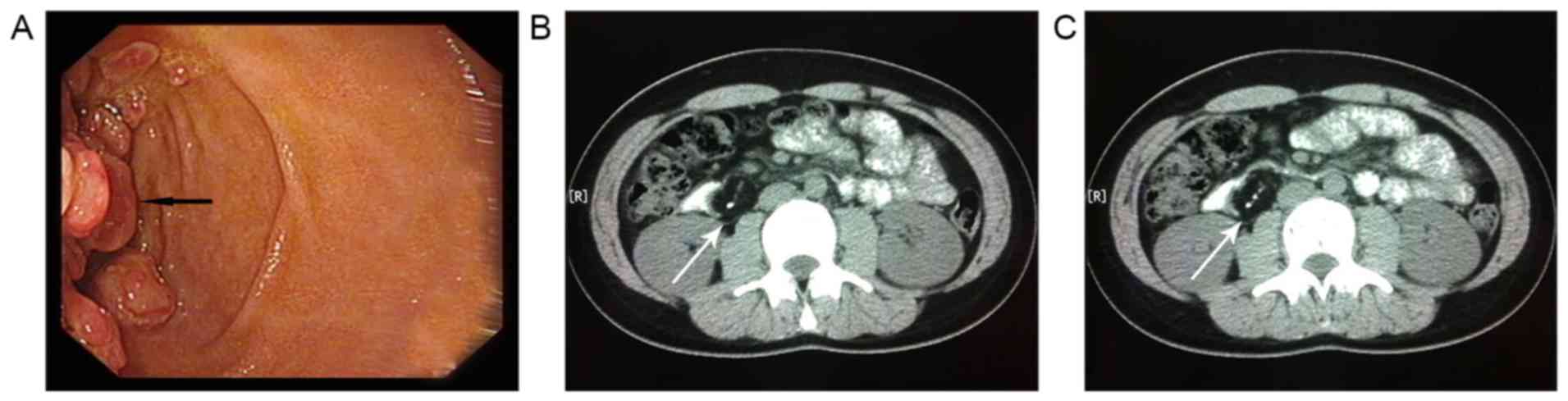
Esophagogastroduodenoscopy (upper gastrointestinal
endoscopy) revealed multiple duodenal mucosa polypoid masses in the
bowel wall of the descending duodenum (Fig. 1A). Biopsy from the growth indicated a
benign lesion. However, it was not possible to perform an
immunohistochemical examination, as the biopsy material was
insufficient. Computed tomography (CT) of the abdomen revealed a
6.5×3.3 cm well-circumscribed mass in the second part of the
duodenum, formed predominantly of fat tissue and presenting a
banded calcified center (Fig. 1B and
C). Laparotomy was performed through an abdominal midline
incision. During surgery, multiple mobile pedunculated polypoid
masses with maximum diameter 3.5 cm were observed in the second
part of the duodenum. These masses protruded into the duodenal
lumen. Owing to the large volume of the lesion, the patient
underwent duodenopancreatectomy and the tumor was completely
resected.
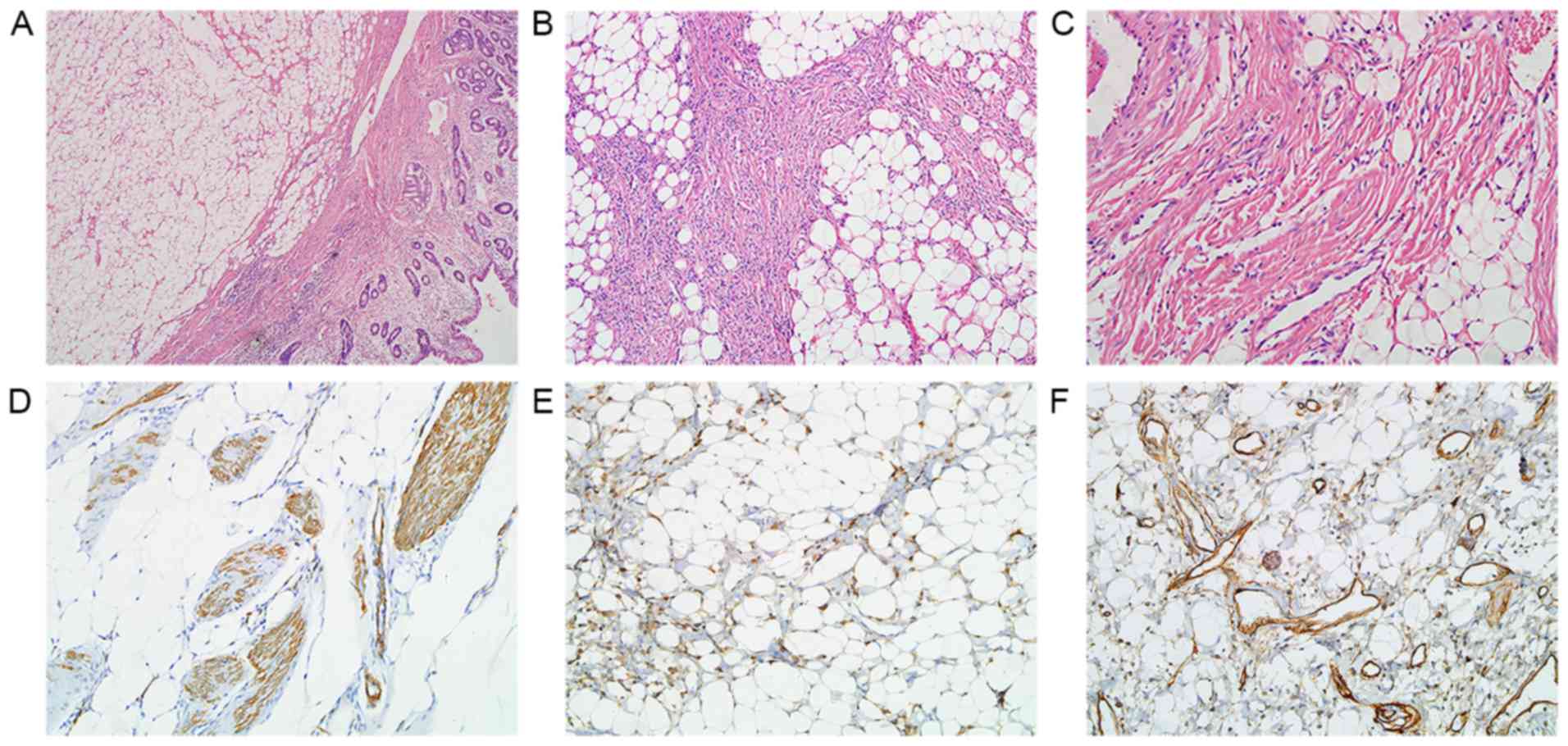
Multiple well-circumscribed polypoid submucosal
masses in the descending duodenum near the ampulla of Vater, with
dimensions 6.5×3.5×2.2 cm, were observed in gross pathological
examination (Fig. 2A).
Microscopically, they were primarily formed of mature adipose
tissue, thick- or thin-walled blood vessels and interspersed areas
of spindle-shaped smooth muscle fibers (Fig. 2B and C). Immunohistochemical staining
was performed using an automated stainer (Benchmark, XT; Ventana
Medical Systems, Inc., Tucson, AZ, USA) with 4-µm thick sections
from formalin-fixed and paraffin-embedded tissues. Tissue sections
were deparaffinized in xylene and rehydrated in a graded alcohol
series. Following retrieval by the autoclave retrieval technique
(0.01 M citrate buffer (pH 6.0); 10–20 min) and inhibition of
endogenous peroxidase activity with 0.3% H2O2
for 5–10 min at 37°C, the slides were incubated with primary
antibodies for 15–24 min at 37°C. The primary antibodies used were
α-smooth muscle actin (α-SMA) monoclonal mouse (dilution, 1:200;
catalog no., ab7817; Abcam, Cambridge, MA, USA), human melanoma
black-45 (HMB-45) monoclonal mouse (dilution, 1:100; catalog no.,
sc-59305; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and
cluster of differentiation 34 (CD34) monoclonal mouse (dilution,
1:100; catalog no., sc-74499; Santa Cruz Biotechnology, Inc.).
Negative controls were performed by omitting primary antibodies.
After washing with Tris buffer, the slides were incubated for 30
min with a corresponding HRP Multimer polyclonal secondary antibody
(ready-to-use; catalog no., G05739; Ventana Medical Systems, Inc.).
The antigen-antibody reaction was visualized with
3,3′-diaminobenzidine (DAB) (Dako; Agilent Technologies GmbH,
Waldbronn, Germany). The sections were counterstained with
hematoxylin for 2–3 min at room temperature, dehydrated and
mounted. Results were interpreted using a light microscope at a
magnification of ×200. Immunohistochemically, the proliferative
smooth muscle tissue was positive for α-smooth muscle actin (α-SMA)
(Fig. 2D). Focal positivity with
HMB-45 was also observed (Fig. 2E).
The vascular components were immunoreactive for the hematopoietic
progenitor cell antigen cluster of differentiation 34 (CD34)
(Fig. 2F). The pathological diagnosis
was AML.
No significant postoperative complications were
observed. However, on the eighth postoperative day, hematemesis
recurred (~60 ml). Further inquiry revealed a history of aneurysm
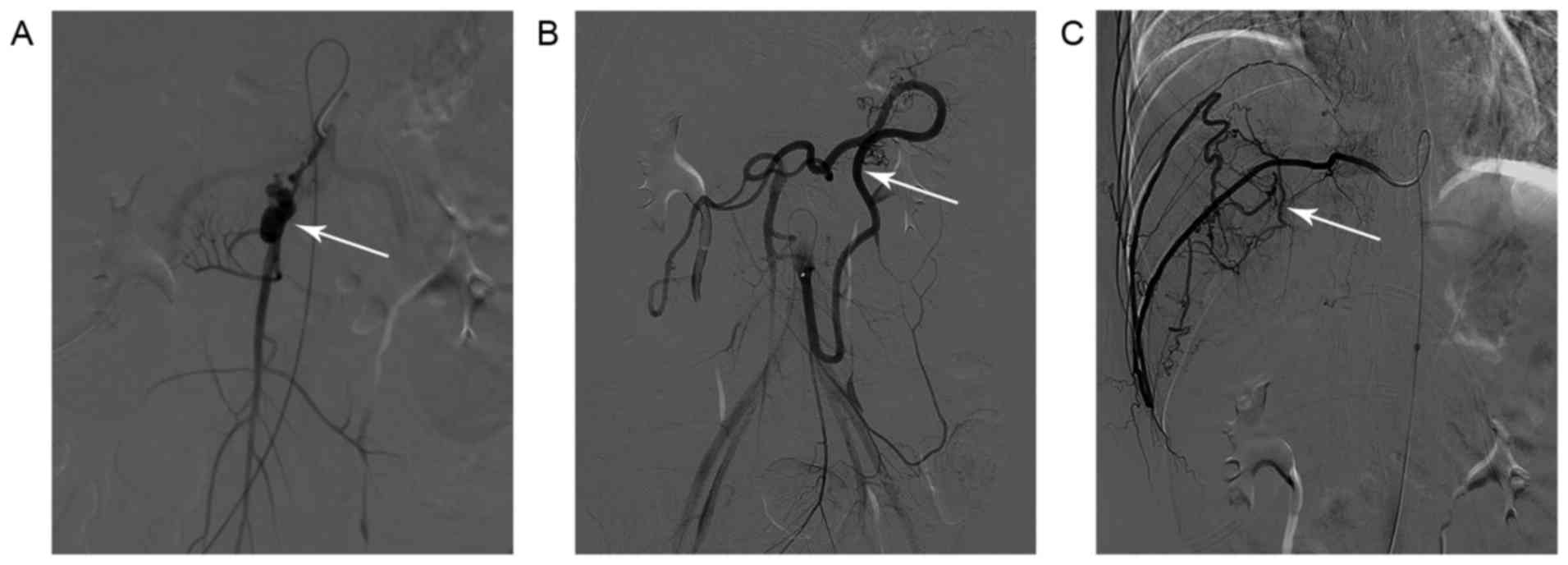
rupture of the right subclavian artery. An emergency digital
subtraction angiography (DSA) did not detect any evident bleeding
point in the gastrointestinal tract. However, a narrow segment of
the superior mesenteric artery with beaded appearance, relatively
narrow distal vascular wall and possible aneurysmal dilatation was
observed (Fig. 3A). In addition,
marked dilatation and tortuosity of the inferior mesenteric artery
was observed (Fig. 3B). Furthermore,
severe tortuosity and variation of the right intercostal artery
were also observed (Fig. 3C).
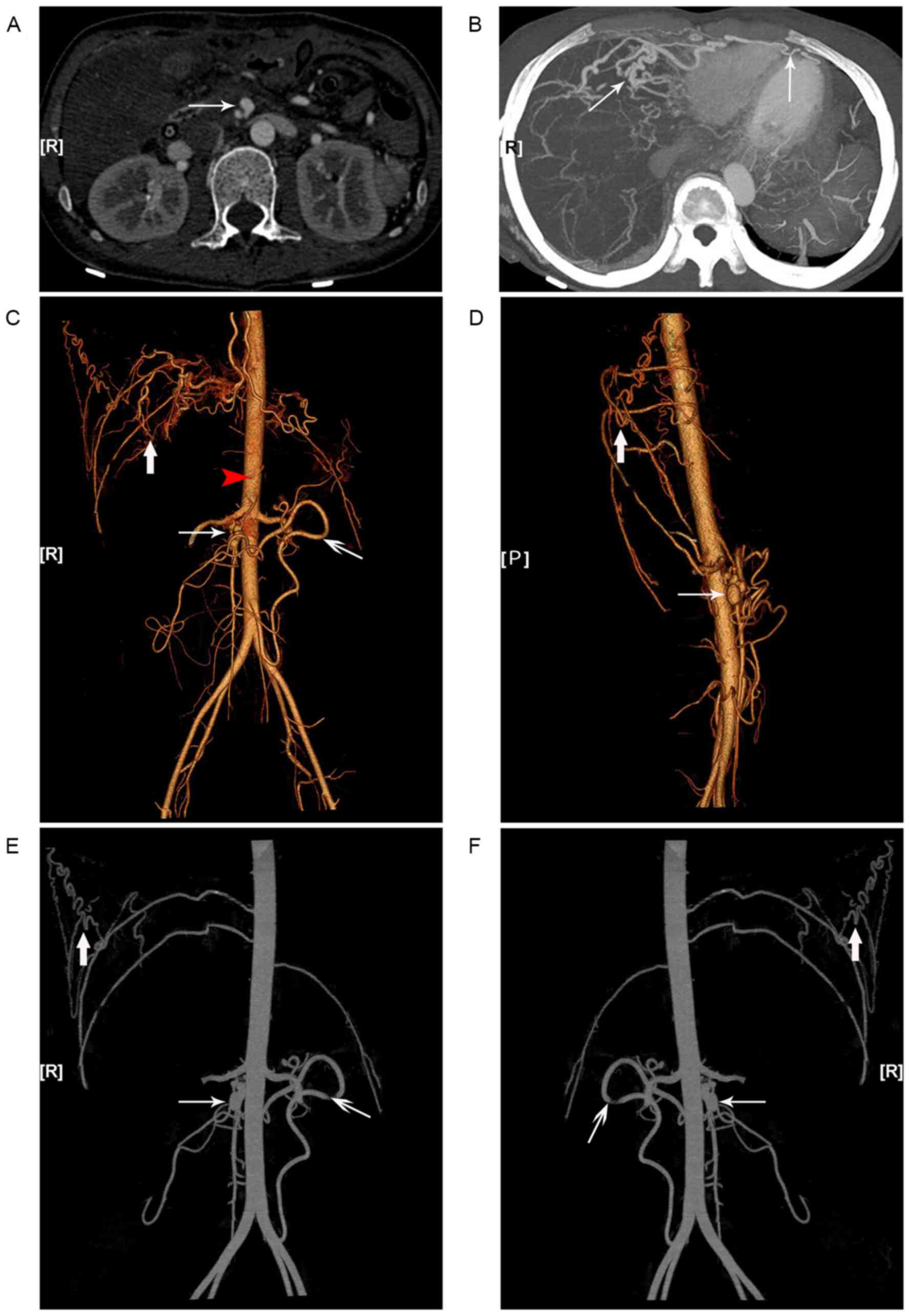
Subsequent CT angiography (CTA) and
three-dimensional analyses further confirmed the existence of
multiple systemic vascular alterations and aneurysms. Additionally,
severe tortuosity and malformations of the right intercostal and
the bilateral internal thoracic arteries, responsible for the blood
supply to the liver and the spleen in the absence of the celiac
trunk, were observed (Fig. 4).
Following treatment, the patient was stable and
discharged from the hospital 20 days after surgery. Follow-up
visits to the interventional center of the Zhongnan Hospital of
Wuhan University for further evaluation and treatment were
recommended. Endovascular aneurysm repair was suggested as a
treatment strategy for the abdominal vascular aneurysms. However,
the patient did not elect to have treatment. The patient was in
good health and no signs of recurrence or progression of the
patient's vascular lesions were detected in the first 24 follow-up
months. Written informed consent was obtained from the patient
prior to publication of the present study.
Discussion
AMLs are histologically defined as benign
mesenchymal hamartomas that contain at least two mesenchymal tissue
components. They were first described in the kidneys in 1951 by
Morgan et al (7). The vast
majority of AMLs primarily involve one or both kidneys. However,
extrarenal AMLs have also been reported in several tissues,
including the mediastinum, the retroperitoneum, the abdominal wall,
the heart, the lungs, the stomach, the spleen, the bones, the
transverse colon, the vagina, the upper lip, the nasal cavity, the
spinal cord, the parotid glands and the skin (5,9–13). Duodenal AMLs are markedly rare and
present clinically with satiety, melena, anemia, generalized
weakness, vomiting and abdominal pain (2,3).
Endoscopic diagnosis of extrarenal AMLs is
challenging. Usually, CT and magnetic resonance imaging are
sufficient to diagnose AMLs which are depicted as fat-containing
lesions with abnormal vascularity (14). Radiological diagnosis and endoscopic
ultrasound are the methods of choice for the evaluation of these
tumors, but they do not have diagnostic value. Surgical excision is
the first-line treatment for tumors with diameter larger than 4 cm.
However, inadequate resection may result in rapid local relapse
(15). Minja et al (16) reported that selective arterial
embolization has been used effectively to control the hemorrhagic
lesions of patients with extrarenal AMLs.
Histologically, the classic AML contains various
proportions of vascular tissue, smooth muscle and adipose tissue
(17). However, in certain cases, one
of the components may predominate or be virtually absent. AMLs with
a large component of epithelioid cells resemble PEComas, a group of
tumors originating from perivascular epithelioid cells (6). Even though AMLs are considered to be
benign lesions, malignant behavior has been observed in epithelioid
angiomyolipomas (18). However,
malignant features in AMLs arising in the gastrointestinal tract
have not been reported to date. Positive immunoreactivity for α-SMA
and HMB-45 is typical of AMLs and may be used to distinguish AMLs
from other similar lesions including angiolipomas, angioleiomyomas,
liposarcomas and leiomyosarcomas (13,15).
However, certain cases of gastrointestinal AML demonstrated weak or
no immunoreactivity for HMB-45 in spindle cells (3,15). The
patient described in the present case report was positive for α-SMA
and only focal positive for HMB45, whereas the vascular component
was positive for CD34. These results are consistent with those of
previously reported cases of duodenal AML (2,3).
Toye and Czarnecki (2)
reported the first case of duodenal AML in a 60-year-old female
presenting with anemia, satiety and a 36×36 mm well-circumscribed
duodenal mass. De Padua et al (3) reported another case in a 66-year-old
male presenting with generalized weakness, severe anemia
(hemoglobin concentration 6 mg/dl), melena and a 40×40 mm
pedunculated polyp duodenal mass. The two lesions demonstrated a
single polypoid pattern, whereas in the patient described in the
present case report, multiple polypoid neoplasms were observed.
None of the three cases was associated with tuberous sclerosis. The
three patients underwent surgical excision and no recurrence was
observed.
The majority of AMLs are sporadic (80%) and a total
of 20% are associated with TSC or lymphangioleiomyomatosis
(7,18). TSC, a hereditary syndrome with
autosomal dominant inheritance, is associated with benign tumors in
a number of organs, including the skin, the brain, the kidneys, the
heart and the lungs (19). AMLs occur
in between 55 and 80% of patients and are the most common cause of
TSC-associated mortality in adults (8,19).
Abnormal vasculature is a typical feature of AMLs. Aneurysmal
dilatations of intratumoral vessels and micro- or macro-aneurysms,
associated with an increased risk of hemorrhage, are frequently
observed (19,20). It is worthy of mention that patients
with Ehlers-Danlos syndrome (EDS) type IV display similar severe
arterial complications, which cannot be differentially diagnosed.
However, the diagnosis of type IV EDS is confirmed by a mutation in
the type III procollagen gene (21).
Certain limitations to the treatment protocol
followed in the present case report are discussed below. First,
detailed medical history of this patient was not initially
obtained. Further inquiry revealed a history of aneurysm rupture in
the right subclavian artery. Thus, a preoperative digital angiogram
on the duodenal AML was not performed. Secondly, due to a
subjective diagnosis of benign polyps, no postoperative macroscopic
sample was obtained. The postoperative DSA and CTA revealed
multiple systemic vascular malformations and aneurysms. However,
there is no solid evidence that these multiple vascular lesions are
certainly associated with TSC. Currently, the diagnosis of TSC is
based on clinical observations. Molecular genetic sequencing is not
recommended as a diagnostic tool for TSC, due to the complexity and
variability of genetic mutations (22). In the patient described in the present
case report, no evidence of other lesions associated with TSC was
obtained. Examination of genetic mutations is required to confirm
the final diagnosis. However, the patient was unwilling to accept
it.
Duodenal AMLs are markedly rare benign tumors. Their
differential diagnosis is complicated and challenging, particularly
in cases arising in the duodenum, presenting with multiple vascular
lesions. Surgical excision is considered the most effective
treatment. Even though molecular genetic sequencing was not
performed, the available data suggest that this case may not be
associated with TSC. However, further research is required to
elucidate the genetic mutations associated with AML presenting with
multiple systemic vascular malformations and aneurysms.
Acknowledgements
The authors would like to thank Dr Chao Qin
(Department of Urology, The First Affiliated Hospital of Nanjing
Medical University, Jiangsu, China) for his assistance with the
language editing of the present paper before submission.
Glossary
Abbreviations
Abbreviations:
|
AML
|
angiomyolipoma
|
|
BITA
|
bilateral internal thoracic artery
|
|
CT
|
computed tomography
|
|
CTA
|
CT angiogram
|
|
DSA
|
digital subtraction angiography
|
|
EDS
|
Ehlers-Danlos syndrome
|
|
SMA
|
superior mesenteric artery
|
|
TSC
|
tuberous sclerosis complex
|
References
|
1
|
Tong YC, Chieng PU, Tsai TC and Lin SN:
Renal angiomyolipoma: Report of 24 cases. Br J Urol. 66:585–589.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toye LR and Czarnecki LA: CT of a duodenal
angiomyolipoma [corrected]. AJR Am J Roentgenol. 178:922002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Padua M, Gupta N, Broor SL and Govil D:
Duodenal angiomyolipoma: A case report. Indian J Pathol Microbiol.
50:568–569. 2007.PubMed/NCBI
|
|
4
|
Petrolla AA and Xin W: Hepatic
angiomyolipoma. Arch Pathol Lab Med. 132:1679–1682. 2008.PubMed/NCBI
|
|
5
|
Candas F, Berber U, Yildizhan A, Yiyit N,
Görür R and Isitmangil T: Anterior mediastinal angiomyolipoma. Ann
Thorac Surg. 95:1431–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lienert AR and Nicol D: Renal
angiomyolipoma. BJU Int. 110 Suppl 4:S25–S27. 2012. View Article : Google Scholar
|
|
7
|
Morgan GS, Straumfjord JV and Hall EJ:
Angiomyolipoma of the kidney. J Urol. 65:525–527. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Curatolo P, Bombardieri R and Jozwiak S:
Tuberous sclerosis. Lancet. 372:657–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramírez Daniel L, García Sabela L, Rey
Jorge R and Antonio O Calvo: Retroperitoneal angiomyolipoma: Review
of literature and report of a new case. Actas Urol Esp. 34:815–817.
2010.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdulkader M, Abercrombie J, McCulloch TA
and Kaye PV: Colonic angiomyolipoma with monotypic expression and a
predominant epitheloid component. J Clin Pathol. 58:1107–1109.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Helwig K, Talabiska D, Cera P and Komar M:
Gastric angiomyolipoma: A previously undescribed cause of upper GI
hemorrhage. Am J Gastroenterol. 93:1004–1005. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosado P, Villalain L, De Vicente JC,
Vivanco B and Torre A: Angiomyolipoma of the parotid gland: Report
of a case and review of the literature. J Oral Maxillofac Surg.
68:2609–2612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Silva AA, Carlos R, Contreras E, de
Almeida OP, Lopes MA and Vargas PA: Angiomyolipoma of the upper
lip: Case report and review of the literature. Med Oral Patol Oral
Cir Bucal. 12:E101–E104. 2007.PubMed/NCBI
|
|
14
|
Israel GM, Bosniak MA, Slywotzky CM and
Rosen RJ: CT differentiation of large exophytic renal
angiomyolipomas and perirenal liposarcomas. AJR Am J Roentgenol.
179:769–773. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CH, Kim JH, Yang DH, Hwang Y, Kang MJ,
Kim YK and Lee MR: Ileal angiomyolipoma manifested by small
intestinal intussusception. World J Gastroenterol. 15:1398–1400.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minja EJ, Pellerin M, Saviano N and
Chamberlain RS: Retroperitoneal extrarenal angiomyolipomas: An
evidence-based approach to a rare clinical entity. Case Rep
Nephrol. 2012:3741072012.PubMed/NCBI
|
|
17
|
Boorjian SA, Frank I, Inman B, Lohse CM,
Cheville JC, Leibovich BC and Blute ML: The role of partial
nephrectomy for the management of sporadic renal angiomyolipoma.
Urology. 70:1064–1068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassan M, El-Hefnawy AS, Elshal AM, Mosbah
A, El-Baz M and Shaaban A: Renal epithelioid angiomyolipoma: A rare
variant with unusual behavior. Int Urol Nephrol. 46:317–322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Waele L, Lagae L and Mekahli D:
Tuberous sclerosis complex: The past and the future. Pediatr
Nephrol. 30:1771–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guridi J, Tuñón M, Caballero C, Gallo-Ruiz
A, Vázquez A and Zazpe I: Intracranial hemorrhage from an
arteriovenous malformation (AVM) in a tuberous sclerosis patient.
Neurologia. 16:281–284. 2001.(In Spanish). PubMed/NCBI
|
|
21
|
Germain DP: Ehlers-Danlos syndrome type
IV. Orphanet J Rare Dis. 2:322007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sancak O, Nellist M, Goedbloed M,
Elfferich P, Wouters C, Maat-Kievit A, Zonnenberg B, Verhoef S,
Halley D and van den Ouweland A: Mutational analysis of the TSC1
and TSC2 genes in a diagnostic setting: Genotype-phenotype
correlations and comparison of diagnostic DNA techniques in
Tuberous Sclerosis Complex. Eur J Hum Genet. 13:731–741. 2005.
View Article : Google Scholar : PubMed/NCBI
|