Introduction
The phyllodes tumors are rare breast tumors that
account for <1% of breast neoplasms worldwide (1). They are fibroepithelial tumors
resembling fibroadenomas but have a predominant connective tissue
component. They are divided into three categories: Benign,
borderline and malignant (2). Wide
excision with clear margins is typically performed and prognosis
depends on the histological characteristics of the connective
tissue component (3). Multivariate
analysis indicated benign histology, negative tumor margins and
absence of residual disease following initial treatment and
radiation therapy as favorable independent prognostic factors
(4). However, the role of neoadjuvant
treatment in phyllodes tumors remains unclear and requires
elucidation on a case-by-case basis (5).
Case report
A 77-year-old female patient from Changhua City
underwent simple mastectomy on 25 February 2015 in Changhua
Christian Hospital (Changhua, Taiwan) for a malignant phyllodes
tumor in the left breast. Pathological examination revealed a soft
tumor mass, 13×12 cm in size, extended in the whole breast. A
rapidly progressing >5 cm mass, with bleeding and a central
necrosis area, was observed on 25 April 2015. A recurrent phyllodes
tumor was suspected and the patient underwent a second surgery on
28 April 2015. Pathological examination revealed a protruding
tumor, 10×4.5×4.8 cm in size, associated with skin ulceration.
Multiple nodules were observed in the operated wound area 10 days
after the second surgery and the patient was admitted to the Breast
Surgery Outpatient Department (Changhua Christian Hospital). During
initial physical examination, the patient was afebrile with normal
vital signs. Examination of the left breast revealed multiple
lobulated masses with irritated overlying skin. The right breast
was normal. Positron emission tomography-computed tomography
(PET-CT) and magnetic resonance imaging (MRI) were performed for
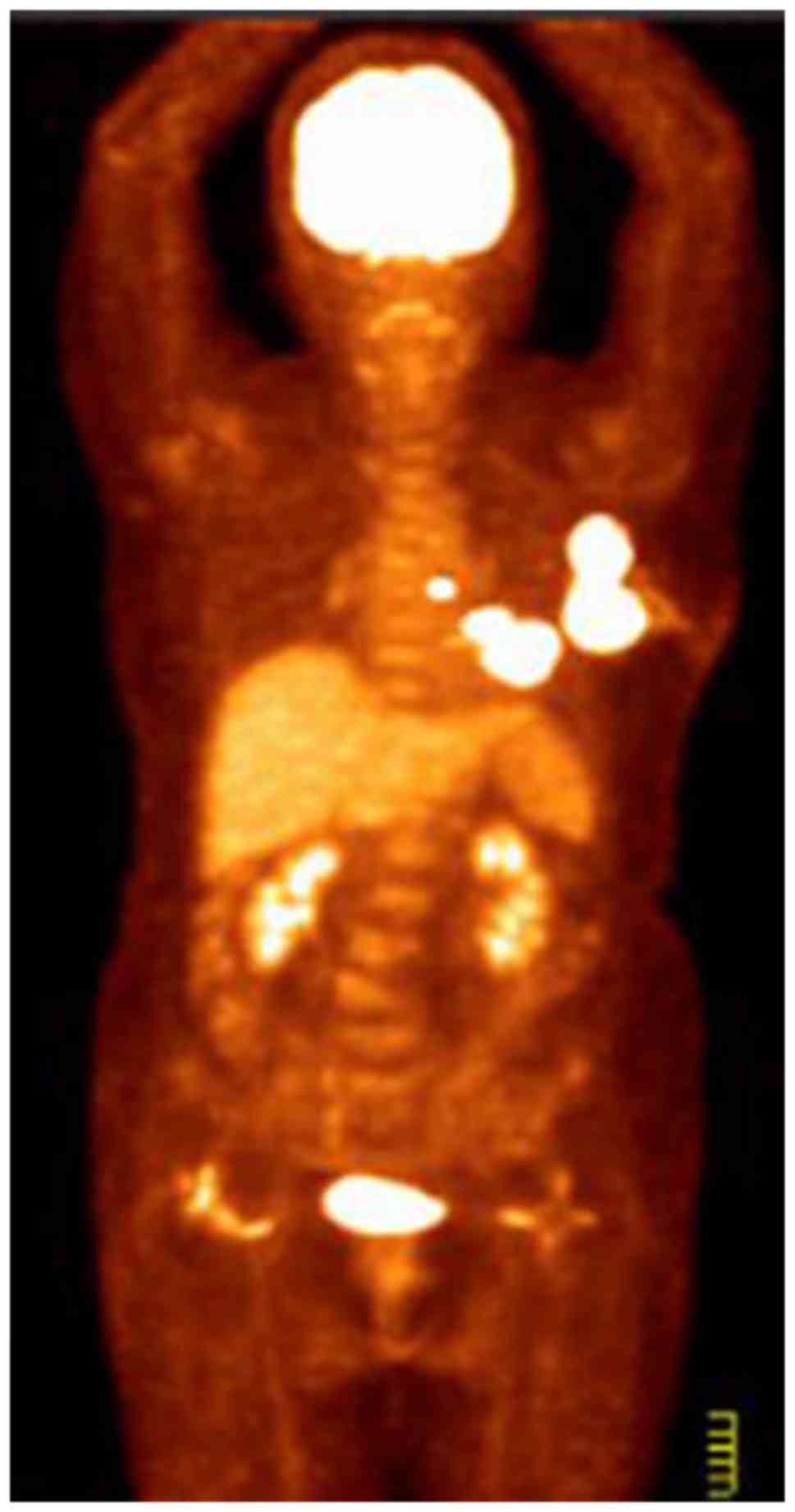
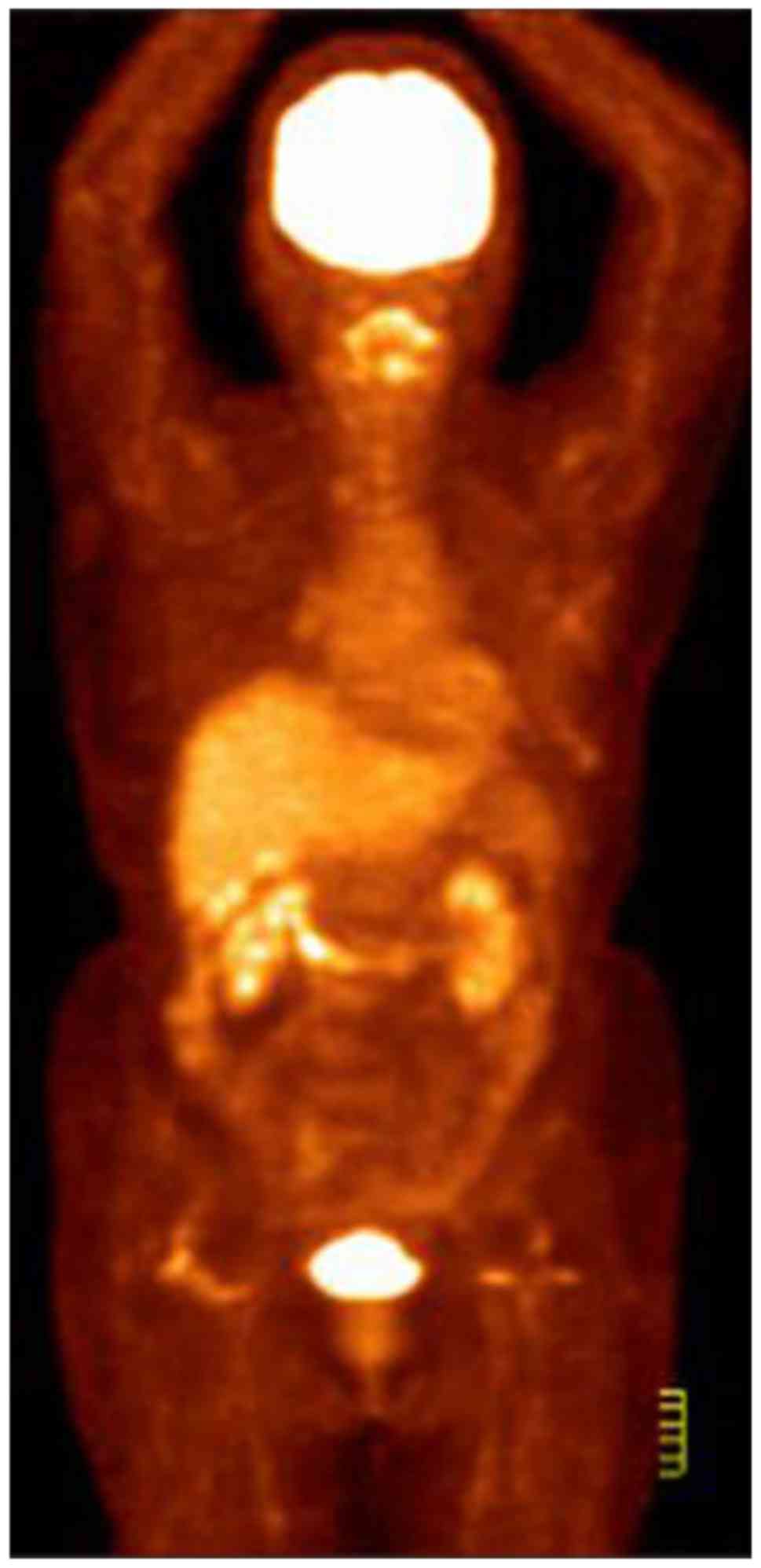
further evaluation. CT and [18F] fluorodeoxyglucose
(FDG)-PET scans revealed multiple areas with markedly increased FDG
uptake in the left breast, the adjacent left third rib and the left
internal mammary region. These areas demonstrated increased
standardized uptake values in the subsequent analysis, suggesting
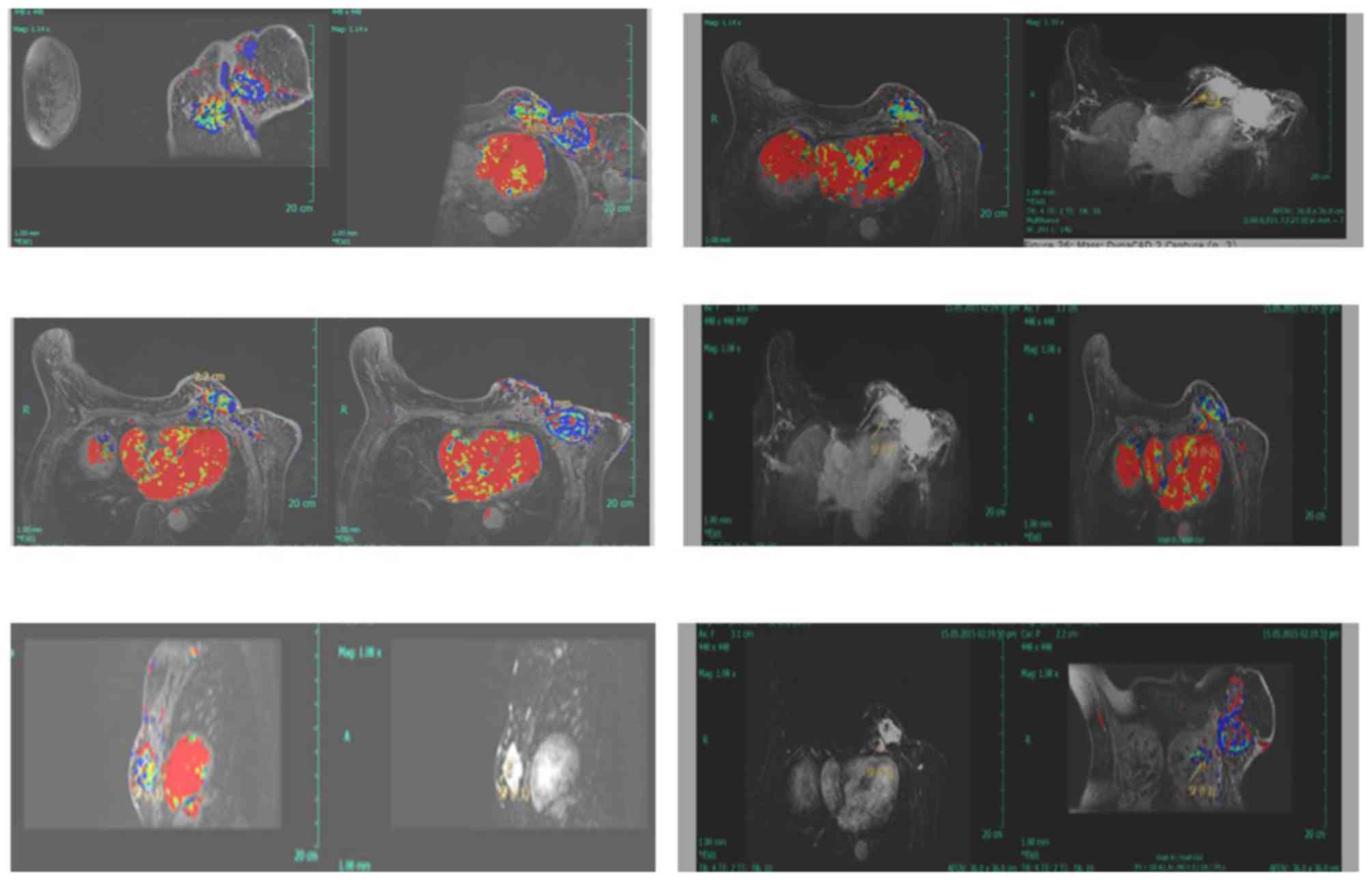
malignancy (Fig. 1). MRI examination
revealed an ovoid heterogeneous enhancement mass 5.0×4.3×3.3 cm in
the left breast (lower-outer quadrant), along with a 3.7×3.2×2.3 cm
mass within the pectoralis muscle layer (Fig. 2).
Malignant phyllodes tumors are rare and aggressive
fibroepithelial neoplasms. An accurate diagnosis of metastasis
should be based on clinicopathological examinations. To achieve
successful management of these tumors, early detection and complete
resection prior to dissemination are of marked importance (6). The present case report describes a
46-year-old female patient with a metastatic phyllodes tumor in the
anterior chest wall. Even though it was not possible to treat the
lesion by surgery or chemotherapy, the tumor size was markedly
decreased following radiation therapy, one of the palliative
therapies (7). The present case
report suggests that radiation therapy should be recognized as a
treatment of choice for palliative medicine. Hashimoto et al
(8) reported a case of preoperative
chemoembolization of a large malignant phyllodes tumor in which
marked tumor shrinkage was achieved prior to surgery.
Intra-arterial Epirubicin infusion and subsequent embolization with
Embosphere microspheres were administered three times over the
course of 6 weeks. The patient underwent surgery without skin
grafting, and no local recurrence or distant metastasis was
observed within 6 months of surgery.
Pathological examination was performed in samples
obtained from the first and second surgery. Samples were fixed in
10% neutralized formalin at room temperature for 24 h. Paraffin
embedded tumor tissue sections (4 µm) were placed on coated slides
and washed with xylene to remove the paraffin, then rehydrated
using serial dilutions of alcohol (70 and 90%, respectively).
Sections were washed with PBS (pH 7.2) and incubated with
anti-cluster of differentiation 34 (CD34) antibody (1:50 dilution;
Thermo Fisher Scientific, Inc., Waltham, MA, USA; clone QBEend/10;
ab81289) for 60 min at room temperature. Subsequently, slides were
washed 3 times with PBS and the conventional streptavidin
peroxidase method (LSAB kit K675; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) was performed for signal development.
Incubation with ready-to-use secondary antibodies from the kit was
performed at room temperature for 10–30 mins. PBS, instead of
primary antibodies, was used as a negative control. Stromal
endothelial cells were used as a positive control. Samples were
viewed under a light microscope (magnification, ×40).
Paraffin-embedded tumor tissue sections (4 µm) were
processed with standard hematoxylin and eosin staining using
Tissue-Tek DRS (Sakura Finetek Europe B.V., Flemingweg, The
Netherlands), according to the manufacturer's protocol.
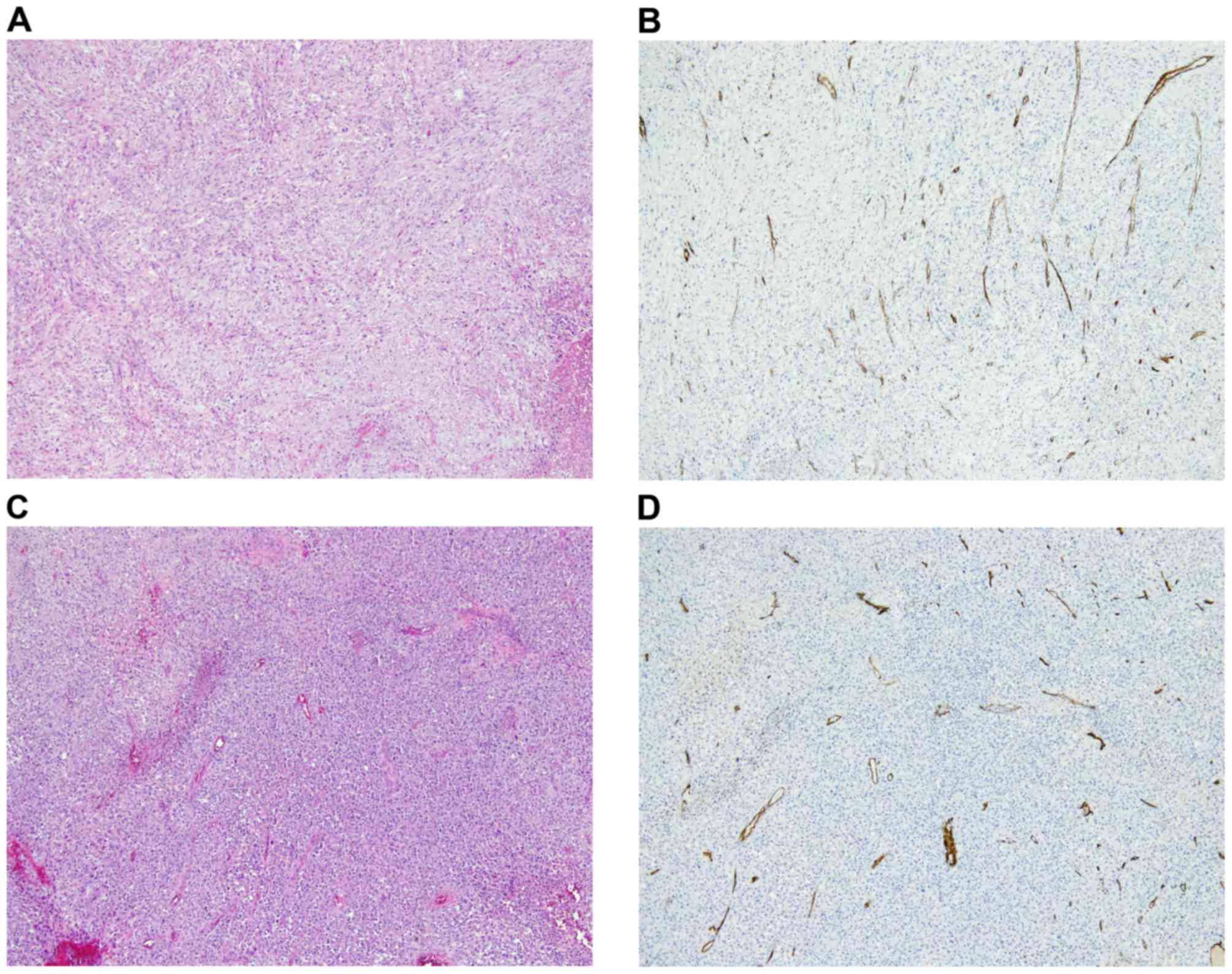
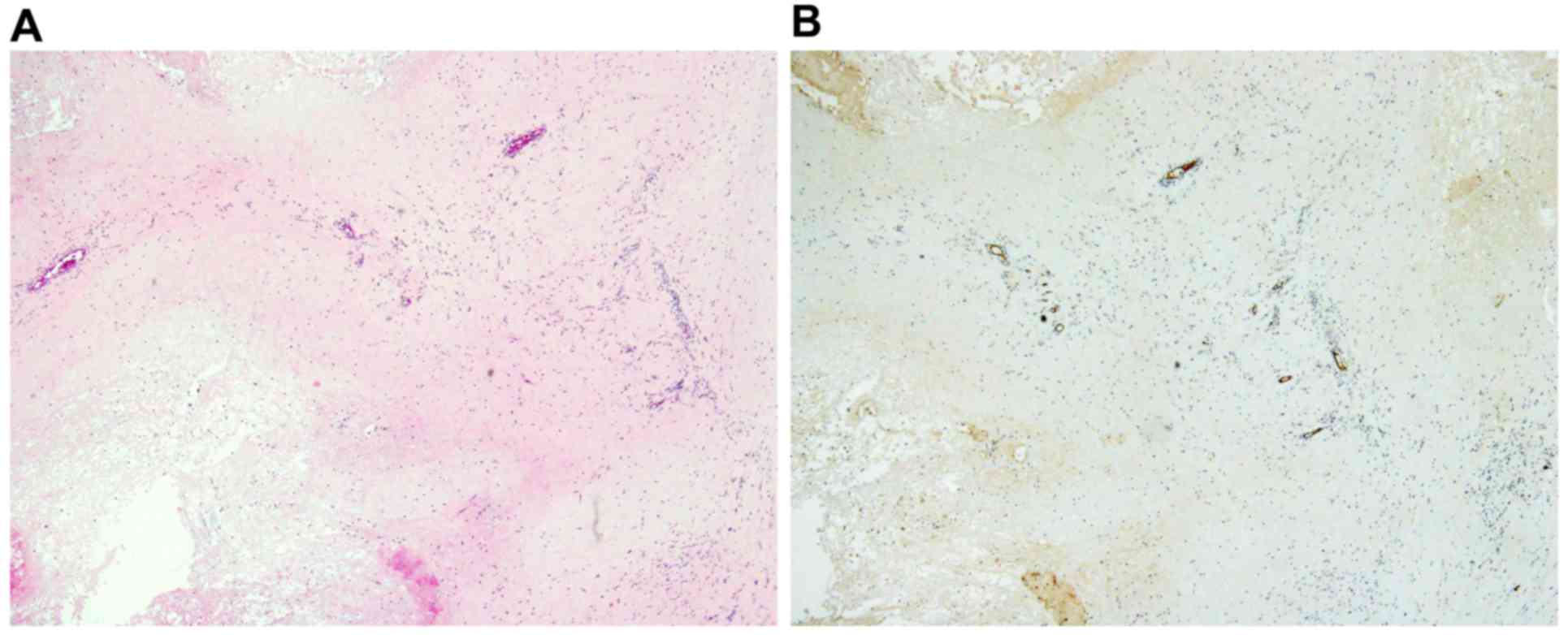
Hypercellular areas with malignant tumor cells (Fig. 3A) and abundant intratumoral vessels
(Fig. 3B), immunoreactive for the
hematopoietic progenitor cell antigen CD34 were observed in the
primary tumor prior to chemotherapy. Similarly, hypercellular areas
with atypical tumor cells (Fig. 3C)
and abundant intratumoral vessels immunoreactive for CD34 (Fig. 3D) were observed in the recurrent
malignant phyllodes tumor prior to chemotherapy. Owing to the
patient's advanced age and the multiple nodules, bevacizumab (5
mg/m2) on the first day in combination with Lipodox (30
mg/m2) on the second day was administered for 3 cycles
every 2 weeks, commencing on 22 May 2015. The patient's clinical
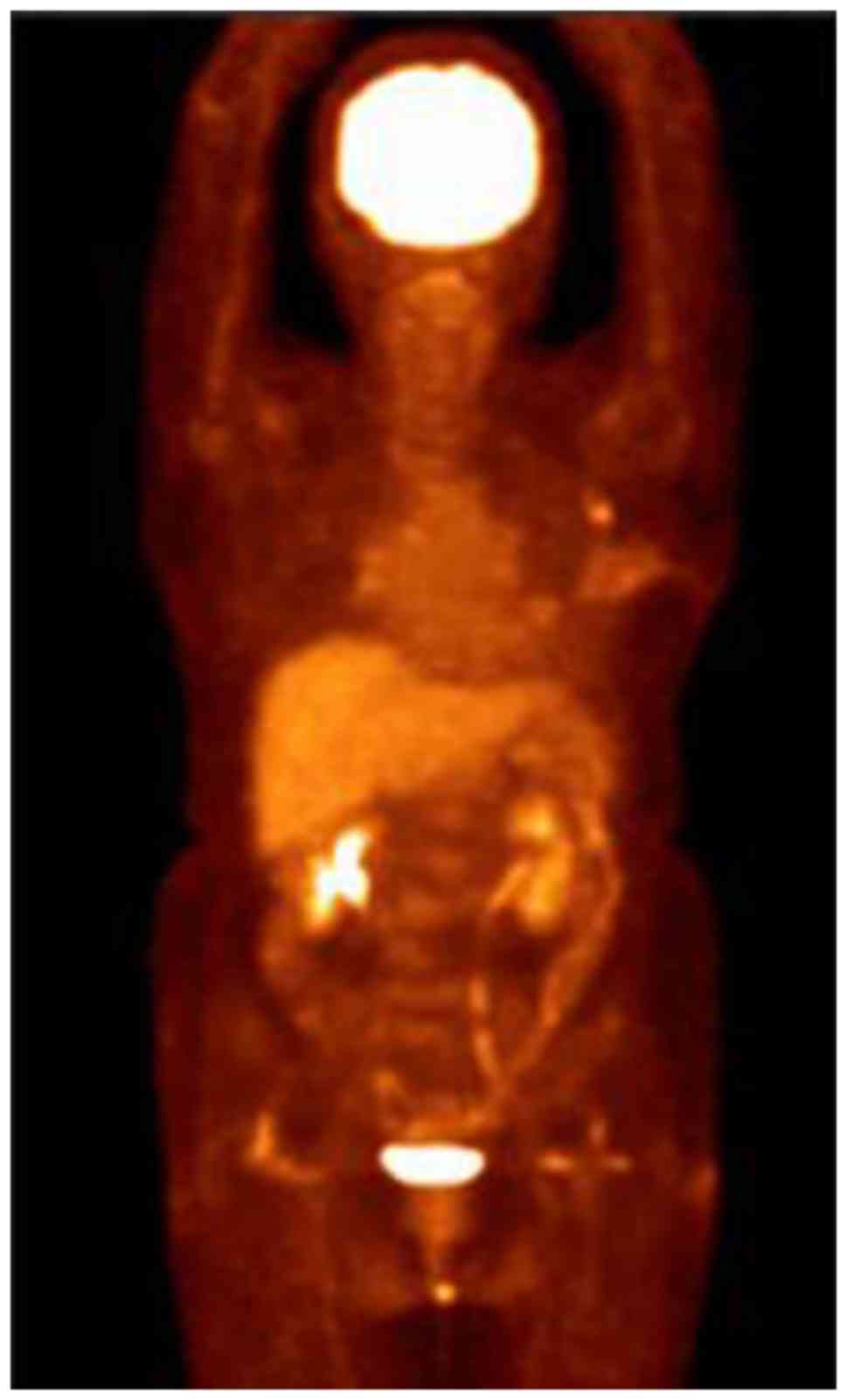
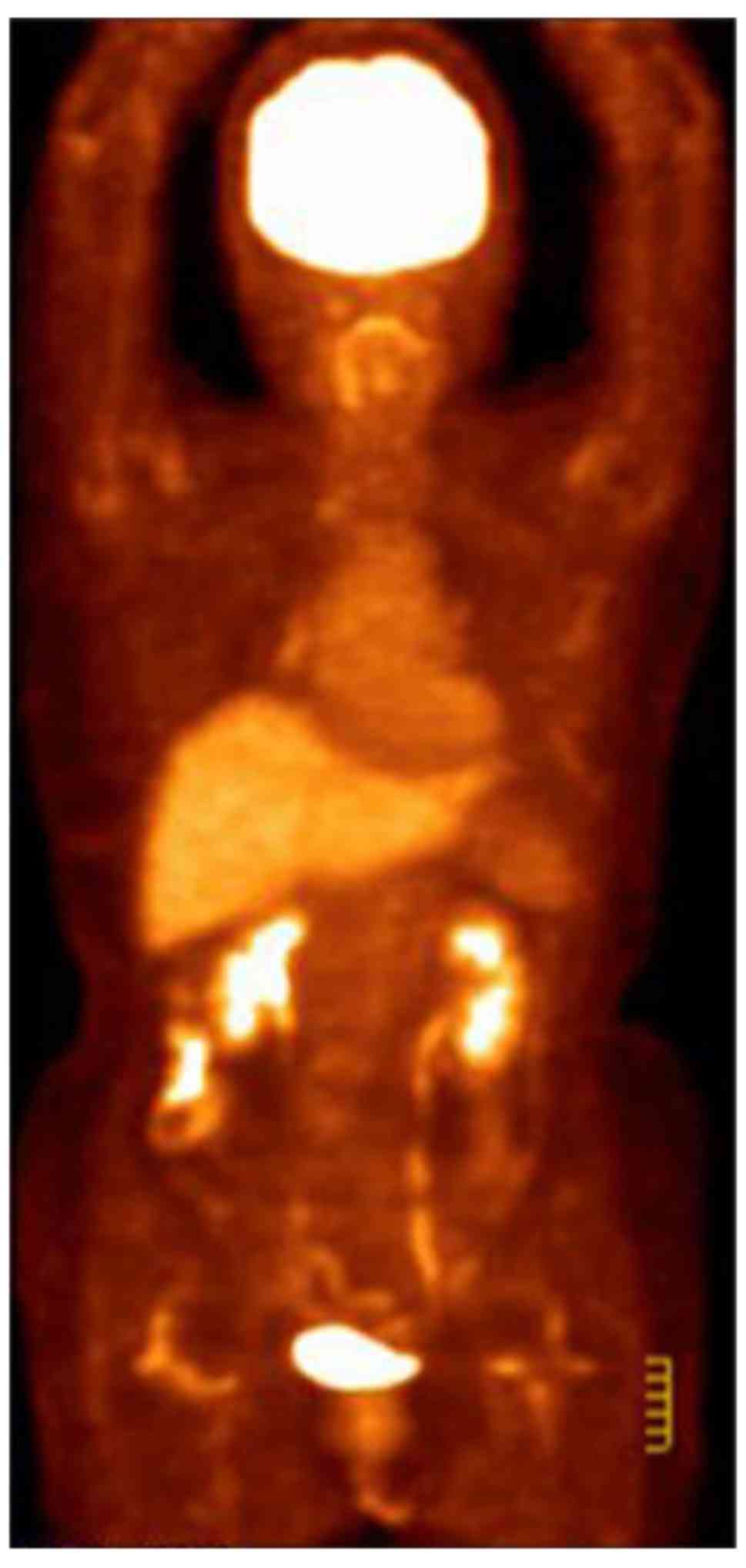
condition improved significantly. A PET-CT follow-up scan was
performed on 13 July 2015 and revealed a postoperative alteration
in the left breast and a small metastatic lymph node in the left
axillary region (Fig. 4). A recurrent
left breast phyllodes tumor was suspected and wide surgical
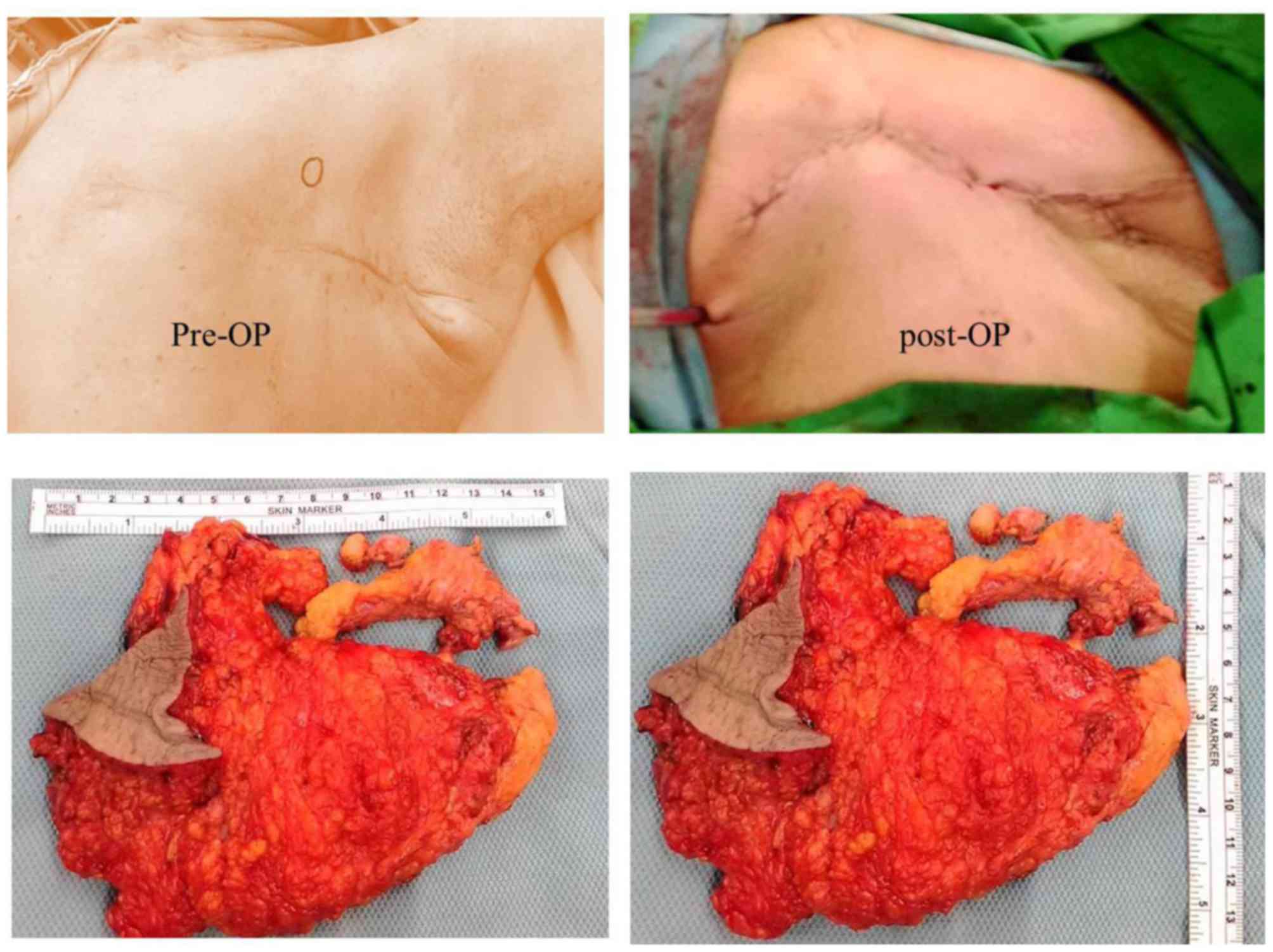
excision was conducted on 27 July 2015 (Fig. 5A). The sample obtained was 16×11×3 cm
in size and consisted of breast tissue, pectoral fascia and muscle
fibers in fresh state (Fig. 5B). The
breast sample was covered by skin with dimensions 7×5.5 cm.
Surgical scars were observed on the skin. However, there was no
evidence of edema or inflammation. The areola and nipple were not
observed. Following tissue sectioning, two nodules measuring
4.2×2.5×2.3 cm and 2.2×2×1.8 cm in size, respectively, were
revealed. The tumors presented yellowish with necrotic features and
the distance between them was 0.5 cm. They were located 0.3 and 1.5
cm underneath the skin, respectively, and <0.1 cm above the
pectoral fascia and muscle. The lower peripheral margin measured
0.2 cm. No clear evidence of invasion in the overlying skin was
observed. The parenchyma in the remaining breast was not marked.
Microscopically, sections of the breast exhibited extensive
necrotic areas and hemorrhagic regions surrounded by granulation
tissue with chronic inflammation, hyalinization and foreign body
reaction with multinucleated giant cells. Hemosiderin deposition
was also observed. The presentation was consistent with
post-treatment appearance. Following chemotherapy, the tumor
exhibited marked necrosis. Scant and residual tumor cells (Fig. 6A) along with decreased intratumoral
vessels, as demonstrated by CD34 immunostaining, were observed
(Fig. 6B). Lipodox (40
mg/m2) was administered for 3 cycles every 3 weeks and
surgical wide excision was conducted 4 weeks later. PET-CT was
performed on 16 October 2015. Postoperative alterations along with
post-treatment reaction were observed in the left lateral chest
wall. No evident metastatic lesion was observed (Fig. 7). A follow-up PET-CT scan was
performed on 15 January 2016 and no evidence of residual
hypermetabolic malignancy was detected. Compared with the previous
scan (on 16 October 2015), persistent complete metabolic response
was indicated (Fig. 8). Written
informed consent was obtained from the patient prior to publication
of the present study.
Discussion
Malignant breast phyllodes tumors frequently recur
following breast-conserving surgery (9). The high rate of local recurrence renders
the research for therapeutic improvement highly important. It is
well-documented that adjuvant radiation therapy decreases the local
recurrence rate of borderline and malignant phyllodes tumors in
patients undergoing breast conserving surgery. However, to the best
of our knowledge, an effect on overall or disease-free survival has
not been reported (10,11). Surgical wide excision with a clear
margin is considered the treatment of choice. Patients with tumors
with infiltrating margins, stromal overgrowth and hypercellularity
are at a high risk of metastasis (12). Several predictive factors of
recurrence and metastasis, including positive surgical margins,
increased mitotic activity, stromal atypia, stromal
hypercellularity and overgrowth have been described. However, the
role of adjuvant therapies (radiotherapy and chemotherapy) remains
unclear (13). Lipodox is used as a
monotherapy for the treatment of metastatic breast cancer, where
there is increased cardiac risk (www.drugs.com/pro/lipodox.html). Several agents
targeting the vascular endothelial growth factor pathway have been
used in combinatorial strategies and improved the efficacy of other
anticancer drugs for the treatment of lung, stomach, colorectal,
ovarian and breast carcinomas (14).
A meta-analysis study of randomized controlled trials demonstrated
that in the neoadjuvant setting, bevacizumab in combination with
chemotherapy compared with chemotherapy alone increased the
percentage of patients with non-metastatic breast cancer that
achieved a pathological complete response. Bevacizumab was
particularly effective in patients with human epidermal growth
factor receptor 2-negative and hormone receptor-negative breast
tumors (15). Malignant phyllodes
tumors do not respond to chemotherapy or radiotherapy. The novel
formulation of bevacizumab (5 mg/m2, first day) in
combination with Lipodox (30 mg/m2, second day), twice a
week, achieved sufficient tumor shrinkage as neoadjuvant
chemotherapy. To the best of our knowledge, effective neoadjuvant
treatment of malignant phyllodes tumors resulting in clear margins
following resection has not been reported previously.
References
|
1
|
Nathan Roberts* and Dianne M: Runk:
Aggressive malignant phyllodes tumor. Int J Surg Case Rep.
8:161–165. 2015. View Article : Google Scholar
|
|
2
|
Norat F, Dreant N, Riah Y and Lebreton E:
Extraordinary case of malignant phylloid tumor of the breast:
Surgical reconstruction treatment. Ann Ital Chir. 80:475–478.
2009.(In Italian). PubMed/NCBI
|
|
3
|
Matar N, Soumani A, Noun M, Chraibi T,
Himmi A, el Mansouri A, Aderdour M and Bekkay M: Phyllodes tumors
of the breast. Forty one cases. J Gynecol Obstet Biol Reprod
(Paris). 26:32–36. 1997.PubMed/NCBI
|
|
4
|
Belkacémi Y, Bousquet G, Marsiglia H,
Ray-Coquard I, Magné N, Malard Y, Lacroix M, Gutierrez C, Senkus E,
Christie D, et al: Phyllodes tumor of the breast. Int J Radiat
Oncol Biol Phys. 70:492–500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guillot E, Couturaud B, Reyal F, Curnier
A, Ravinet J, Laé M, Bollet M, Pierga JY, Salmon R and Fitoussi A:
Breast Cancer Study Group of the Institut Curie: Management of
phyllodes breast tumors. Breast J. 17:129–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
EI Ochi MR, Toreis M, Benchekroun M,
Benkerroum Z, Allaoui M, Ichou M, El Khannoussi B, Albouzidi A and
Oukabli M: Bone metastasis from malignant phyllodes breast tumor:
Report of two cases. BMC Clin Pathol. 16:42016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sazuka T, Matsuzaki H, Kanada Y, Tohnosu
N, Yoshiwara C, Aruga T, Iwata K, Sasahara N, Kobayashi H, Yokoyama
M, et al: A case of malignant phyllodes tumor effectively treated
by radiation therapy as a palliative medicine. Gan To Kagaku Ryoho.
42:1698–1699. 2015.(In Japanese). PubMed/NCBI
|
|
8
|
Hashimoto K, Mimura H, Arai Y, Doi M,
Kojima Y, Tsugawa K and Nakajima Y: Successful preoperative
chemoembolization in the treatment of a giant malignant phyllodes
tumor. Cardiovasc Intervent Radiol. 39:1070–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barth RJ Jr: Histologic features predict
local recurrence after breast conserving therapy of phyllodes
tumors. Breast Cancer Res Treat. 57:291–295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng S, Zhang X, Yang D, Wang X and Ren G:
Effects of adjuvant radiotherapy on borderline and malignant
phyllodes tumors: A systematic review and meta-analysis. Mol Clin
Oncol. 3:663–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gnerlich JL, Williams RT, Yao K, Jaskowiak
N and Kulkarni SA: Utilization of radiotherapy for malignant
phyllodes tumors: Analysis of the National Cancer Data Base,
1998–2009. Ann Surg Oncol. 21:1222–1230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WH, Cheng SP, Tzen CY, Yang TL, Jeng
KS, Liu CL and Liu TP: Surgical treatment of phyllodes tumors of
the breast: Retrospective review of 172 cases. J Surg Oncol.
91:185–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khosravi-Shahi P: Management of non
metastatic phyllodes tumors of the breast: Review of the
literature. Surg Oncol. 20:e143–e148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arjaans M, Schröder CP, Oosting SF, Dafni
U, Kleibeuker JE and de Vries EG: VEGF pathway targeting agents,
vessel normalization and tumor drug uptake: From bench to bedside.
Oncotarget. 7:21247–21258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao L, Yao GY, Liu MF, Chen LJ, Hu XL and
Ye CS: Neoadjuvant bevacizumab plus chemotherapy versus
chemotherapy alone to treat non-metastatic breast cancer: A
meta-analysis of randomised controlled trials. PLoS One.
10:e01454422015. View Article : Google Scholar : PubMed/NCBI
|