Introduction
Worldwide, in terms of incidence, colorectal cancer
(CRC) is the third most commonly occurring cancer in males (after
lung and prostate cancer) and the second most commonly occurring in
females (after breast cancer) (1).
Annually, ~1.36 million new cases of CRC are estimated to occur
worldwide, accounting for 9.7% of all cancers (1). Approximately 694,000 CRC-associated
mortalities occur worldwide annually, accounting for 8.5% of all
cancer-associated mortalities and making CRC the third most common
cause of cancer-associated mortality (1). In total, 75–80% of patients with colon
cancer present with localized diseases (2,3). However,
despite curative surgery, patients still have a significant
probability of disease relapse and cancer-associated mortality
(4). Adjuvant therapy is administered
immediately following surgery to target any residual tumor cells
and reduce the risk of recurrence (5). Much interest has been generated in the
last few decades regarding adjuvant treatment (5–7).
A series of large randomized studies performed by
the National Surgical Adjuvant Breast and Bowel Project and the
National Cancer Institute sponsored co-operative groups has defined
the role of adjuvant chemotherapy in patients with stage III colon
cancer (8–12). Encouraged by the initial results from
the North Central Cancer Treatment Group, 5-fluorouracil
(5-FU)/leucovorin (LV) was shown to increase disease-free survival
(DFS) and overall survival (OS) in the confirmatory US
Intergroup-0035 study, with mature results showing a 40% reduction
in recurrence and a 33% reduction in mortality (9). This led to the recommendations from the
National Institutes of Health consensus conference for this drug
combination to be administered as an adjuvant therapy in patients
with stage III colon cancer (12).
5-FU/LV became the standard of care in the US and formed the
control arm in numerous studies conducted in the 1990s (13,14).
In Taiwan, CRC is one of the most common of all
malignancies and the third leading cause of cancer-associated
mortality (15). The incidence of CRC
in Taiwan was 40/100,000 in 2011, and this rate has gradually been
approaching those of Western nations in recent decades (15). Stage III colon cancer denotes lymph
node involvement (16). Radical
surgical resection and anastomosis are the only way to cure stage
III colon cancer (17–20), and adjuvant chemotherapy has
previously been demonstrated to improve clinical outcomes (8).
Post-operative adjuvant chemotherapy for colon
cancer is one of the most important clinical advances in oncology
to have been introduced in recent years (21–23). Prior
to 2000, 5-FU was the only useful cytotoxic agent in the adjuvant
setting for patients with stage III colon cancer (24). Subsequently, treatment with levamisole
plus 5-FU, rather than with 5-FU alone, was found to reduce the
risk of cancer recurrence among patients with stage III colon
cancer by 41% (P<0.0001), while the overall mortality rate was
reduced by 33% (P=0.006) (5).
Subsequent to that, a multicenter international study of LV, 5-FU
and oxaliplatin (FOLFOX4) in the adjuvant treatment of colon cancer
[MOSAIC (NCT00275210)] was completed, wherein the toxic effects and
efficacy of 6 months of FOLFOX4 treatment were compared with those
of 6 months of a 5-FU/LV regimen without oxaliplatin among 2,246
patients with resected stage II or stage III colon cancer (25). According to the results of this MOSAIC
trial, adjuvant FOLFOX4 was demonstrated to prolong OS time for
patients with stage III colon cancer compared with patients
receiving 5-FU/LV without oxaliplatin (25). Therefore, adding oxaliplatin to a
regimen of 5-FU and LV improved the adjuvant treatment of colon
cancer (25). Another study
demonstrated that the 6-year OS rate in patients with stage III
colon cancer was 72.9% among patients receiving FOLFOX4 and 68.7%
among patients receiving 5-FU/LV [hazard ratio (HR), 0.80; 95%
confidence interval (CI), 0.65–0.97; P=0.023] (26). Since then, FOLFOX4 has become the gold
standard adjuvant therapy for patients with stage III colon cancer
(26).
In an experimental model, oral uracil-tegafur (UFUR;
which consists of tegafur and uracil at a molar ratio of 1:4) plus
cisplatin prolonged the survival of murine intraperitoneal
implanting colon carcinoma in mice and maintained these mice in a
relatively improved condition compared with continuous infusion of
5-FU plus cisplatin (27). In 2000, a
randomized comparison of the relative efficacies of 5-FU plus LV
and UFUR plus LV in 1,530 evaluable patients indicated that the two
regimens have similar toxicity profiles (28). Evidence that UFUR plus oral LV is
associated with significant antitumor activity and has a
well-tolerated toxicity makes this a logical formulation for the
adjuvant treatment of colon cancer (29–31). Since
UFUR can be taken orally, patients receiving oral UFUR therapy are
not required to stay in hospital for long periods (27). Additionally, an improved quality of
life and prolonged survival also highlight the potential clinical
usefulness of the UFUR therapy in patients with metastatic colon
cancer (32).
In 1998, O'Connell et al (10) reported that there was no significant
improvement in patient survival when chemotherapy (either
intensive-course 5-FU and LV combined with levamisole, or a
standard regimen of 5-FU plus levamisole) was administered for 12
months compared with 6 months. In 2005, Haller et al
(23) assessed the three chemotherapy
regimens: Low-dose leucovorin plus 5-FU regimen; high-dose LV plus
5-FU regimen; and low-dose LV plus levamisole plus 5-FU regimen,
each administered for 30–32 weeks. The control arm was levamisole
plus 5-FU for 1 year. Among the four arms, none were statistically
superior in DFS time or OS time (23). The duration of adjuvant chemotherapy
is usually 6 months; however, the results of studies were all
limited to 5-FU plus LV and levamisole.
Although FOLFOX4 has become established as a
standard chemotherapeutic regimen, no relevant information
regarding the sequential administration of oral UFUR/LV following
FOLFOX4 as an auxiliary subsequent adjuvant treatment for patients
with stage III colon cancer has been reported thus far. The goal of
the present study was to evaluate the efficacy and safety of oral
UFUR/LV following FOLFOX4 chemotherapy as adjuvant therapy for
patients with stage III colon cancer.
Materials and methods
Study population
The present study retrospectively analyzed data
between January 2007 and October 2012 for 143 resected patients
(median, 64 years; age range, 20–84 years; 81 male and 62 female
patients) with stage III colon cancer treated with FOLFOX4 adjuvant
chemotherapy plus subsequent oral UFUR/LV (FOLFOXU) or FOLFOX4
adjuvant chemotherapy alone at Kaohsiung Medical University
Hospital (Kaohsiung, Taiwan). To exclude cases of FOLFOX regimen
failure, inclusion criteria required that patients to show no
evidence of recurrence (local recurrence or distant metastasis)
within the FOLFOX4 treatment period, as well as an Eastern
Cooperative Oncology Group performance status of 0–2 (33). Patients with other malignant diseases
in their medical history were also excluded. The clinical
characteristics of patients are listed in Table I. The present study was approved by
the Institutional Review Board of the Kaohsiung Medical University
Hospital.
 | Table I.The clinicopathological features of
the FOLFOX4 group and the FOLFOXU group patients. |
Table I.
The clinicopathological features of
the FOLFOX4 group and the FOLFOXU group patients.
| Feature | Overall, n | FOLFOX4, n (%) | FOLFOXU, n (%) | P-value |
|---|
| Total | 143 | 62 | 81 |
|
| Sex |
|
|
|
|
| Male | 81 | 36 (58.1) | 45 (55.6) | 0.764 |
|
Female | 62 | 26 (41.9) | 36 (44.4) |
|
| Age |
|
|
|
|
| <60
years | 58 | 25 (40.3) | 33 (40.7) | 0.960 |
| ≥60
years | 85 | 37 (59.7) | 48 (59.3) |
|
| Tumor size |
|
|
|
|
| <5
cm | 75 | 29 (46.8) | 46 (56.8) | 0.235 |
| ≥5
cm | 68 | 33 (53.2) | 35 (43.2) |
|
| Histology |
|
|
|
|
|
WD+MD | 122 | 54 (87.1) | 68 (84.0) | 0.598 |
| PD | 21 | 8 (12.9) | 13 (16.0) |
|
| T status |
|
|
|
|
|
T1+T2 | 20 | 6 (9.7) | 14 (17.3) | 0.194 |
|
T3+T4 | 123 | 56 (60.3) | 67 (82.7) |
|
| N status |
|
|
|
|
| N1 | 98 | 40 (64.5) | 58 (71.6) | 0.366 |
| N2 | 45 | 22 (35.5) | 23 (28.4) |
|
| Vascular
invasion |
|
|
|
|
| No | 93 | 39 (62.9) | 54 (66.7) | 0.640 |
|
Yes | 50 | 23 (37.1) | 27 (33.3) |
|
| Perineural
invasion |
|
|
|
|
| No | 100 | 42 (67.7) | 58 (71.6) | 0.618 |
|
Yes | 43 | 20 (32.3) | 23 (28.4) |
|
| Pre-op CEA,
ng/ml |
|
|
|
|
|
<5 | 84 | 35 (56.5) | 49 (60.5) | 0.627 |
| ≥5 | 59 | 27 (43.5) | 32 (39.5) |
|
| Post-op CEA,
ng/ml |
|
|
|
|
|
<5 | 119 | 53 (85.5) | 66 (81.5) | 0.526 |
| ≥5 | 24 | 9 (14.5) | 15 (18.5) |
|
| Recurrence |
|
|
|
|
| No | 102 | 38 (61.3) | 64 (79.0) | 0.020 |
|
Yes | 41 | 24 (38.7) | 17 (21.0) |
|
| Mortality |
|
|
|
|
| No | 121 | 45 (72.6) | 76 (93.8) | <0.001 |
|
Yes | 22 | 17 (27.4) | 5 (6.2) |
|
Chemotherapy regimen
The patients were divided into two groups based on
the different chemotherapy regimens (FOLFOX4 or FOLFOXU). The
FOLFOX4 regimen comprised oxaliplatin (85 mg/m2) as a
2-h infusion on day 1, LV (75–90 mg/m2) administered as
a 2-h infusion on days 1 and 2, followed by a loading dose of 5-FU
(400 mg/m2) intravenous bolus, and then 5-FU (600
mg/m2) administered via ambulatory pump for a period of
22 h on days 1 and 2, all of which were repeated every 2 weeks. A
total of 62 patients received only FOLFOX4 adjuvant treatment
(FOLFOX4, biweekly × 12 cycles for 6 months), and 81 patients
received FOLFOXU treatment (FOLFOX4 biweekly × 12 cycles for 6
months followed by oral UFUR/LV for an additional 6 months). Oral
UFUR and LV were administered for 6 months at a dose of 400 mg/day
for UFUR, and 100 mg/day for LV, respectively. Subsequent to
detailed information on potential benefits or disadvantages, the
patients provided consent to receive FOLFOXU.
If Common Toxicity Criteria (34) grade 3 stomatitis, diarrhea or
dermatitis occurred, the dose of oxaliplatin was reduced by 25%.
The same reduction was made for grades 3 and 4 neutropenia and in
the case of persistent (>14 days) paresthesia, temporary (7–14
days) painful paresthesia or functional impairment. Chemotherapy
was discontinued in the case of unacceptable toxicity, disease
progression or the refusal of the patient for additional
treatment.
Patient follow-up
The clinical records for each patient were
retrospectively reviewed. The characteristics of the patients that
were recorded included age, gender, the type of chemotherapy
administered and any observed recurrence encountered following
chemotherapy. The two regimens were continued until one of the
following occurred: Recurrence of the disease and/or unacceptable
adverse effects, or the patient was lost to follow-up. The median
follow-up period was 31 months (range, 7.5–60 months).
Statistical analysis
All data were analyzed using the Statistical Package
for the Social Sciences version 17.0 software (SPSS, Inc., Chicago,
IL, USA). χ2 testing was used to compare distributions
for categorical variables, and t-tests were utilized to compare any
differences in the continuous variables between FOLFOX4 and
FOLFOXU. Using the calculator for survival probability (the
Kaplan-Meier method), DFS was defined as the time elapsed between
the administration of FOLFOX4 and the date of tumor recurrence, the
date of mortality from any cause, or the date at which the last
follow-up data was obtained. OS was defined as the time elapsed
from the administration of FOLFOX4 until mortality from any cause
or until the loss of follow-up date. The DFS and OS values were
calculated by the Kaplan-Meier method, and the differences were
analyzed by the log-rank test. A logistic regression model was used
to identify risk factors for recurrence and mortality. Cox
proportional hazard regression model results (for DFS and OS) were
applied to estimate the HR of time/probability of
mortality/recurrence. A reference group is a group to which another
group is compared. P<0.05 was considered to indicate a
statistically significant difference.
Results
The characteristics of these 143 patients with stage
III colon cancer are summarized in Table
I. All 143 patients were classified into two groups (FOLFOX4
and FOLFOXU), according to the two different regimens of adjuvant
chemotherapy. The median age ± standard deviation was 64.8±10.9
years in the FOLFOX4 group (range, 33–81 years) and 63.4±13.2 years
in the FOLFOXU group (range, 20–84 years). A total of 24 patients
(38.7%) in the FOLFOX4 group who underwent adjuvant FOLFOX4
chemotherapy and 17 patients (21.0%) in the FOLFOXU group who
underwent sequential FOLFOX4 and oral UFUR/LV chemotherapy had a
recurrence of cancer (P=0.020). The recurrence and survival
statuses of these patients are summarized in Table I. Among the 62 patients in the FOLFOX4
group, survival was observed in 45 cases (72.6%). Of the 81
patients in the FOLFOXU group, 76 patients survived (93.8%;
P<0.001).
As shown in Table II,
in seeking to identify the risk factors of recurrence and mortality
in the 143 patients with stage III colon cancer using a logistic
regression model, a statistically significant association between
lower recurrence rate and mortality rate was observed in the
FOLFOXU group compared with the FOLFOX4 group (recurrence: OR,
0.312; 95% CI, 0.131–0.714; P=0.008; mortality: OR, 0.072; 95% CI,
0.014–0.358; P=0.001). In addition, patients with lower
post-operative serum carcinoembryonic antigen (CEA) levels (<5
ng/ml) were prominently associated with lower recurrence and
mortality rates (recurrence: OR, 0.134; 95% CI, 0.039–0.462,
P=0.001; mortality: OR, 0.053; 95% CI, 0.007–0.392; P=0.004).
 | Table II.Risk factors of recurrence and
mortality in 143 stage III colon cancer patients as determined by
logistic regression model. |
Table II.
Risk factors of recurrence and
mortality in 143 stage III colon cancer patients as determined by
logistic regression model.
|
| Recurrence | Mortality |
|---|
|
|
|
|
|---|
| Factor | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| CT formula |
|
|
|
|
| FOLFOX4
(Ref. group) |
|
|
|
|
|
FOLFOXU | 0.312
(0.131–0.741) | 0.008 | 0.072
(0.014–0.358) | 0.001 |
| Sex |
|
|
|
|
| Male
(Ref. group) |
|
|
|
|
|
Female | 0.725
(0.315–1.668) | 0.450 | 0.932
(0.286–3.034) | 0.907 |
| Age |
|
|
|
|
| <60
years (Ref. group) |
|
|
|
|
| ≥60
years | 1.140
(0.492–2.643) | 0.760 | 1.536
(0.469–5.036) | 0.478 |
| Tumor size |
|
|
|
|
| <5
cm (Ref. group) |
|
|
|
|
| ≥5
cm | 1.239
(0.506–3.033) | 0.639 | 2.732
(0.756–9.870) | 0.125 |
| Histology |
|
|
|
|
| PD (Ref.
group) |
|
|
|
|
|
WD+MD | 0.469
(0.142–1.546) | 0.213 | 0.179
(0.031–1.029) | 0.054 |
| T status |
|
|
|
|
| T3+T4
(Ref. group) |
|
|
|
|
|
T1+T2 | 0.660
(0.174–2.497) | 0.540 | 0.622
(0.073–5.266) | 0.663 |
| N status |
|
|
|
|
| N2
(Ref. group) |
|
|
|
|
| N1 | 1.574
(0.600–4.131) | 0.357 | 0.727
(0.224–2.356) | 0.595 |
| Vascular
invasion |
|
|
|
|
| Yes
(Ref. group) |
|
|
|
|
| No | 0.796
(0.318–1.994) | 0.627 | 0.620
(0.184–2.090) | 0.441 |
| Perineural
invasion |
|
|
|
|
| Yes
(Ref. group) |
|
|
|
|
| No | 0.604
(0.241–1.517) | 0.284 | 0.357
(0.096–1.330) | 0.125 |
| Pre-op CEA,
ng/ml |
|
|
|
|
| ≥5
(Ref. group) |
|
|
|
|
|
<5 | 1.020
(0.380–2.736) | 0.968 | 1.580
(0.351–7.113) | 0.552 |
| Post-op CEA,
ng/ml |
|
|
|
|
| ≥5
(Ref. group) |
|
|
|
|
|
<5 | 0.134
(0.039–0.462) | 0.001 | 0.053
(0.007–0.392) | 0.004 |
Cox proportional hazard regression model results
were applied to identify the prognostic factors for DFS and OS time
(Table III). Through multivariate
analyses, it was observed that the FOLFOXU regimen was an
independent factor of DFS and OS time (DFS: HR, 0.367; 95% CI,
0.190–0.709; P=0.003; OS: HR, 0.155; 95% CI, 0.054–0.450; P=0.001).
Similarly, lower post-operative serum CEA levels (<5 ng/ml) were
another independent factor of DFS and OS time (DFS: HR, 0.279; 95%
CI, 0.115–0.676; P=0.005; OS: HR, 0.173; 95% CI, 0.050–0.594;
P=0.005).
 | Table III.Univariate and multivariate analysis
of prognostic indicators on disease-free survival and overall
survival for 143 patients with stage III colon cancer. |
Table III.
Univariate and multivariate analysis
of prognostic indicators on disease-free survival and overall
survival for 143 patients with stage III colon cancer.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Chemotherapy
formula |
|
|
|
|
|
|
|
|
| FOLFOX4
(Ref. group) |
|
|
|
|
|
|
|
|
|
FOLFOXU | 0.520
(0.278–0.975) | 0.042 | 0.367
(0.190–0.709) | 0.003 | 0.240
(0.087–0.665) | 0.006 | 0.155
(0.054–0.450) | 0.001 |
| Sex |
|
|
|
|
|
|
|
|
| Male
(Ref. group) |
|
|
|
|
|
|
|
|
|
Female | 0.884
(0.476–1.639) | 0.695 | 0.690
(0.348–1.365) | 0.286 | 1.264
(0.525–3.042) | 0.602 | 0.798
(0.311–2.048) | 0.639 |
| Age |
|
|
|
|
|
|
|
|
| <60
years (Ref. group) |
|
|
|
|
|
|
|
|
| ≥60
years | 1.100
(0.599–2.018) | 0.759 | 0.977
(0.503–1.898) | 0.945 | 1.344
(0.588–3.070) | 0.484 | 1.033
(0.439–2.434) | 0.940 |
| Tumor size |
|
|
|
|
|
|
|
|
| <5
cm (Ref. group) |
|
|
|
|
|
|
|
|
| ≥5
cm | 0.799
(0.434–1.469) | 0.470 | 1.070
(0.548–2.088) | 0.843 | 0.829
(0.364–1.887) | 0.656 | 1.689
(0.654–4.361) | 0.279 |
| Histology |
|
|
|
|
|
|
|
|
| PD
(Ref. group) |
|
|
|
|
|
|
|
|
|
WD+MD | 0.583
(0.285–1.190) | 0.138 | 0.688
(0.276–1.710) | 0.420 | 0.380
(0.162–0.888) | 0.026 | 0.502
(0.112–2.243) | 0.367 |
| T status |
|
|
|
|
|
|
|
|
| T3+T4
(Ref. group) |
|
|
|
|
|
|
|
|
|
T1+T2 | 0.515
(0.165–1.609) | 0.254 | 0.846
(0.230–3.117) | 0.802 | 0.341
(0.046–2.529) | 0.293 | 0.549
(0.052–5.757) | 0.617 |
| N status |
|
|
|
|
|
|
|
|
| N2
(Ref. group) |
|
|
|
|
|
|
|
|
| N1 | 1.123
(0.569–2.217) | 0.739 | 1.419
(0.701–2.872) | 0.330 | 0.603
(0.255–1.429) | 0.251 | 0.698
(0.280–1.739) | 0.440 |
| Vascular
invasion |
|
|
|
|
|
|
|
|
| Yes
(Ref. group) |
|
|
|
|
|
|
|
|
| No | 0.662
(0.356–1.231) | 0.192 | 0.859
(0.373–1.976) | 0.721 | 0.443
(0.189–1.036) | 0.060 | 0.680
(0.210–2.199) | 0.520 |
| Perineural
invasion |
|
|
|
|
|
|
|
|
| Yes
(Ref. group) |
|
|
|
|
|
|
|
|
| No | 0.947
(0.495–1.813) | 0.869 | 0.760
(0.392–1.472) | 0.416 | 0.715
(0.300–1.704) | 0.449 | 0.598
(0.227–1.575) | 0.298 |
| Pre-op CEA,
ng/ml |
|
|
|
|
|
|
|
|
| ≥5
(Ref. group) |
|
|
|
|
|
|
|
|
|
<5 | 0.463
(0.250–0.858) | 0.014 | 0.754
(0.328–1.735) | 0.507 | 0.425
(0.184–0.986) | 0.046 | 1.012
(0.289–3.545) | 0.985 |
| Post-op CEA,
ng/ml |
|
|
|
|
|
|
|
|
| ≥5
(Ref. group) |
|
|
|
|
|
|
|
|
|
<5 | 0.294
(0.158–0.547) | <0.001 | 0.279
(0.115–0.676) | 0.005 | 0.251
(0.110–0.572) | 0.001 | 0.173
(0.050–0.594) | 0.005 |
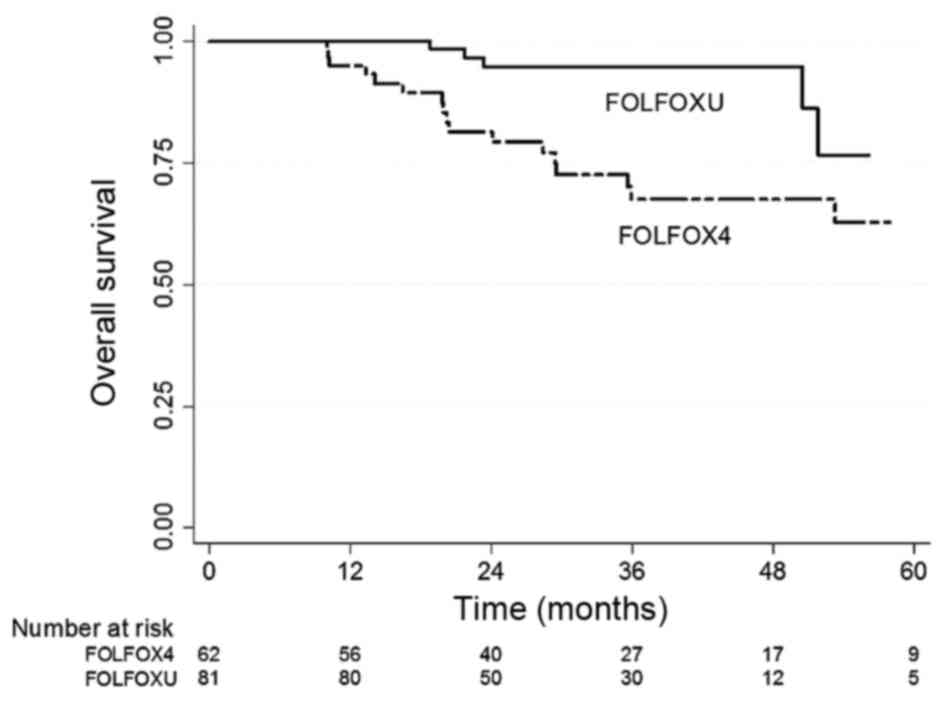
Fig. 1 shows the OS
Kaplan-Meier curves of these two different regimen groups. A
statistically significant difference was observed between the OS
values of the two groups (P=0.001). The 5-years OS rate of the
FOLFOXU group was 76.9%, which was superior to that of the FOLFOX4
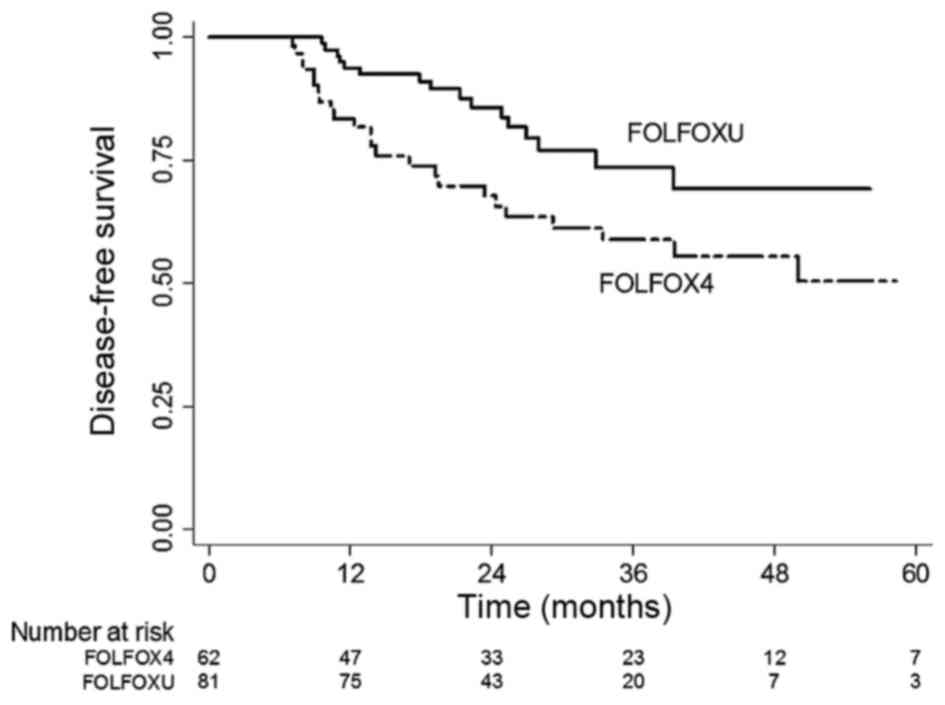
group (63.8%). Fig. 2 shows the DFS
Kaplan-Meier curves of the two regimen groups. Similarly, a
statistically significant difference was observed between the DFS
values of the two groups (P=0.003). The 3-year DFS rate of the
FOLFOXU group was 74.3%, which was significantly better compared
with that of the FOLFOX4 group (59.9%). In terms of efficacy, these
results showed that FOLFOX4 and subsequent oral UFUR chemotherapy
was better than the FOLFOX4 regimen alone.
The rates of various toxicities in the two groups
were similar (all P>0.05; Table
IV). In terms of grade 3 or 4 events, 57.4% (39/68) of events
among the FOLFOXU group patients vs. 53.1% (26/49) of events among
the FOLFOX4 groups patients were non-hematological toxicities. The
most frequently observed severe non-hematological toxicities were
fatigue and diarrhea. In each group, 9.8% of the patients
experienced grade 3 or 4 fatigue during therapy. In the FOLFOXU
group, 7.4% of the patients had grade 3 diarrhea, compared with
4.8% in the FOLFOX4 group (P=0.913). A total of 12 of 62 (19.3%)
patients in the FOLFOX4 group and 14 of 81 (17.3%) patients in the
FOLFOXU group had elevated liver function. In total, 11 FOLFOX4
patients (17.7%) and 15 FOLFOXU patients (18.5%) experienced
peripheral sensory neuropathy (grade 3 in 1 FOLFOX4 patient and in
2 FOLFOXU patients) (P=0.931). The grade 3/4 hematological
toxicities, which included neutropenia in 17/10 (21%/12.3%)
patients in the FOLFOXU group and 13/8 (21%/12.9%) patients in the
FOLFOX4 group, were comparable between the two groups
(P=0.940).
 | Table IV.Common toxicities of the FOLFOX4
group and FOLFOXU group regimens in 143 patients with stage III
colon cancer. |
Table IV.
Common toxicities of the FOLFOX4
group and FOLFOXU group regimens in 143 patients with stage III
colon cancer.
|
| FOLFOX4, n (%)
(n=62) | FOLFOXU, n (%)
(n=81) |
|
|---|
|
|
|
|
|
|---|
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P-value |
|---|
| Total events | 156 | 92 | 39 | 10 | 212 | 125 | 56 | 12 | 0.985 |
| Nausea | 24 (38.7) | 8 (12.9) | 2 (3.2) | 0 (0) | 33 (40.7) | 11 (13.6) | 3 (3.7) | 0 (0) | 0.996 |
| Vomiting | 11 (17.7) | 8 (12.9) | 2 (3.2) | 0 (0) | 16 (19.8) | 11 (13.6) | 3 (3.7) | 0 (0) | 0.994 |
| Anorexia | 11 (17.7) | 6 (9.7) | 1 (1.6) | 0 (0) | 15 (18.5) | 8 (9.9) | 2 (2.5) | 0 (0) | 0.952 |
| Stomatitis | 11 (17.7) | 7 (11.3) | 1 (1.6) | 0 (0) | 15 (18.5) | 10 (12.3) | 2 (2.5) | 0 (0) | 0.956 |
| Diarrhea | 18 (29) | 9 (14.5) | 3 (4.8) | 0 (0) | 26 (32.1) | 14 (17.3) | 6 (7.4) | 0 (0) | 0.913 |
| Abdominal pain | 7 (11.3) | 6 (9.7) | 3 (4.8) | 0 (0) | 10 (12.3) | 9 (11.1) | 4 (4.9) | 0 (0) | 0.992 |
| Constipation | 11 (17.7) | 4 (6.5) | 1 (1.6) | 0 (0) | 14 (17.3) | 5 (6.2) | 1 (1.2) | 0 (0) | 0.987 |
| Hand-foot
syndrome | 3 (4.8) | 1 (1.6) | 1 (1.6) | 0 (0) | 3 (3.7) | 1 (1.2) | 1 (1.2) | 0 (0) | 1.000 |
| Pyrexia | 10 (16.1) | 5 (8.2) | 1 (1.6) | 0 (0) | 14 (17.3) | 6 (7.4) | 1 (1.2) | 0 (0) | 0.959 |
| Paresthesia | 14 (22.6) | 4 (6.5) | 2 (3.2) | 0 (0) | 18 (22.2) | 5 (6.2) | 3 (3.7) | 0 (0) | 0.986 |
| Asthenia | 4 (6.5) | 4 (6.5) | 2 (3.2) | 0 (0) | 6 (7.4) | 5 (6.2) | 3 (3.7) | 0 (0) | 0.977 |
| Peripheral sensory
neuropathy | 8 (12.9) | 2 (3.2) | 1 (1.6) | 0 (0) | 10 (12.3) | 3 (3.7) | 2 (2.5) | 0 (0) | 0.931 |
| Fatigue | 11 (17.7) | 10 (16.1) | 5 (8.2) | 1 (1.6) | 15 (18.5) | 14 (17.3) | 7 (8.6) | 1 (1.2) | 0.997 |
| Elevated liver
function | 9 (14.5) | 3 (4.8) | 0 (0) | 0 (0) | 12 (14.8) | 2 (2.5) | 0 (0) | 0 (0) | 0.635 |
| Neutropenia | 2 (3.2) | 8 (12.9) | 13 (21.0) | 8 (12.9) | 3 (3.7) | 11 (13.6) | 17 (21.0) | 10 (12.3) | 0.982 |
|
Thrombocytopenia | 2 (3.2) | 7 (11.3) | 1 (1.6) | 1 (1.6) | 2 (2.5) | 10 (12.3) | 1 (1.2) | 1 (1.2) | 0.918 |
Discussion
The recent introduction of drugs, including
oxaliplatin, oral capecitabine and UFUR, has increased the
treatment options available for these patients (22,26,28,31).
One study found that the 3-year DFS rate was significantly improved
in patients who had undergone resection with curative intent for
stage II or III colon cancer and received bolus plus
continuous-infusion 5-FU plus LV (LV5FU2), with the addition of
oxaliplatin (FOLFOX4) (26). In
general, adjuvant chemotherapy should be routinely offered to
medically fit patients with stage III colon cancer.
In the present retrospective study, patients were
enrolled for FOLFOXU subsequent to receiving detailed information
regarding the advantages and disadvantages. The present study has
shown that the recurrence rates and mortality rates for the FOLFOX4
and FOLFOXU groups were significantly different. Among the patients
who received FOLFOX4 chemotherapy without subsequent oral UFUR/LV
chemotherapy, 24 patients (38.7%) had tumor recurrence and 45
patients (72.6%) were alive; whereas in the FOLFOXU group, 64
patients (79%) experienced no recurrence and 76 out of 81 (93.8%)
patients were alive, both of which represent considerably improved
clinical outcomes. Furthermore, the sequential FOLFOX4 and oral
UFUR/LV adjuvant chemotherapy resulted in superior DFS and OS rates
when compared with FOLFOX4 adjuvant chemotherapy alone, and the
present DFS and OS of FOLFOX4 groups were compatible with the
results of a previous study from Western countries (26). Despite the finding that patients
treated with FOLFOXU chemotherapy experienced somewhat increased
gastrointestinal toxicity, including diarrhea, stomatitis and
vomiting, the differences in such toxicities were not statistically
significant and they were well tolerated in the two groups. In
addition, the beneficial prognostic role of sequential FOLFOX4 and
oral UFUR/LV adjuvant chemotherapy with the 1-year maintenance
therapy remains a crucial issue in clinical practice, since ~50% of
recurrence or metastasis occurred within 1 year of radical
resection.
The mean OS time of the patients in the FOLFOXU
group with a high postoperative CEA level was 64.28 months, which
was superior compared with patients in the FOLFOX4 group with a
high postoperative CEA level (26.76 months). A statistically
significant difference was observed between the OS values of the
two groups (P=0.002). The average DFS time of the patients in the
FOLFOXU group with high postoperative CEA level was 41.54 months,
which was superior compared with patients in the FOLFOX4 group with
high postoperative CEA level (19.47 months). A borderline
significant difference was observed between the DFS values of the
two groups (P=0.056). Our future prospective study will investigate
the benefit of FOLFOXU regimen in the patients with stage III colon
cancer with high postoperative CEA level.
The major limitation of the present study was that
the comparison of the FOLFOX4 adjuvant chemotherapy alone vs. the
sequential FOLFOX4 and oral UFUR/LV adjuvant chemotherapy was not
based on a prospective randomized design, but was accomplished via
a retrospective review. Another limitation was that only the 5-year
OS rates were available, due to the limited follow-up period. The
validation of these findings with larger sample sizes with a longer
follow-up period from multicenter sources would be crucial in our
future prospective study. A future prospective, randomized study
may investigate the potential benefit of FOLFOXU regimen in
patients with stage III colon cancer to verify the current
retrospective study.
In conclusion, patients with stage III colon cancer
may be more prone to benefit from sequential FOLFOX4 and oral
UFUR/LV adjuvant chemotherapy than from the adjuvant FOLFOX4
chemotherapy alone; however, a prospective, randomized clinical
trial is required to confirm the findings of the current
retrospective study.
Acknowledgements
The present study was supported by grants from the
Excellence for Cancer Research Center Grant through funding by the
Ministry of Science and Technology (grant no.
MOST105-2325-B-037-001), the Ministry of Health and Welfare (grant
no. MOHW106-TDU-B-212-144007; Health and Welfare Surcharge of
Tobacco Products, Taiwan, R.O.C.), the Kaohsiung Medical University
Hospital (grant nos. KMUH104-4M46, KMUH105-5R26, KMUHS10601,
KMUHS10608 and KMUHA10664). In addition, the present study was
supported by Kaohsiung Medical University ‘Aim for the Top 500
University Grant’ (grant nos. KMU-TP105C01, KMU-TP105C02 and
KMU-TP105C11) and ‘Aim for the top University Grant’ [grant nos.
KMU-TP105A14 and SH000113 (Give2Asia)] and the Grant of
Biosignature in Colorectal Cancers, Academia Sinica, Taiwan, R.O.C.
(grant no. T106-002).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer incidence and mortality
worldwide. IARC CancerBase no. 11, Lyon. 2013.
|
|
2
|
Pestana C, Reitemeier RJ, Moertel CG, Judd
ES and Dockerty MB: The natural history of carcinoma of the colon
and rectum. Am J Surg. 108:826–829. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falterman KW, Hill CB, Markey JC, Fox JW
and Cohn I Jr: Cancer of the colon, rectum, and anus: A review of
2313 cases. Cancer. 34 Suppl:S951–S959. 1974. View Article : Google Scholar
|
|
4
|
Olson RM, Perencevich NP, Malcolm AW,
Chaffey JT and Wilson RE: Patterns of recurrence following curative
resection of adenocarcinoma of the colon and rectum. Cancer.
45:2969–2974. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA,
Tormey DC, Glick JH, et al: Levamisole and fluorouracil for
adjuvant therapy of resected colon carcinoma. N Engl J Med.
322:352–358. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ha GS, Kim YW, Choi EH and Kim IY: Factors
associated with the lack of adjuvant chemotherapy following
curative surgery for stage II and III colon cancer: A Korean
National Cohort Study. Anticancer Res. 37:915–922. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bos AC, van Erning FN, van Gestel YR,
Creemers GJ, Punt CJ, van Oijen MG and Lemmens VE: Timing of
adjuvant chemotherapy and its relation to survival among patients
with stage III colon cancer. Eur J Cancer. 51:2553–2561. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
No authors listed: Efficacy of adjuvant
fluorouracil and folinic acid in colon cancer. International
Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT)
investigators. Lancet. 345:939–944. 1995.PubMed/NCBI
|
|
9
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey
DC, Glick JH, et al: Fluorouracil plus levamisole as effective
adjuvant therapy after resection of stage III colon carcinoma: A
final report. Ann Intern Med. 122:321–326. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Connell MJ, Laurie JA, Kahn M,
Fitzgibbons RJ Jr, Erlichman C, Shepherd L, Moertel CG, Kocha WI,
Pazdur R, Wieand HS, et al: Prospectively randomized trial of
postoperative adjuvant chemotherapy in patients with high-risk
colon cancer. J Clin Oncol. 16:295–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolmark N, Rockette H, Fisher B, Wickerham
DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ,
et al: The benefit of leucovorin-modulated fluorouracil as
postoperative adjuvant therapy for primary colon cancer: Results
from National Surgical Adjuvant Breast and Bowel Project protocol
C-03. J Clin Oncol. 11:1879–1887. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
NIH consensus conference. Adjuvant therapy
for patients with colon and rectal cancer. JAMA. 264:1444–1450.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peters GJ, Van der Wilt CL, van Groeningen
CJ, Smid K, Meijer S and Pinedo HM: Thymidylate synthase inhibition
after administration of fluorouracil with or without leucovorin in
colon cancer patients: Implications for treatment with
fluorouracil. J Clin Oncol. 12:2035–2042. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelsen DP, Saltz L, Cohen AM, Yao TJ,
Enker W, Tong W, Tao Y and Bertino JR: A phase I trial of immediate
postoperative intraperitoneal floxuridine and leucovorin plus
systemic 5-fluorouracil and levamisole after resection of high risk
colon cancer. Cancer. 74:2224–2233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Department of Health, the Executive Yuan,
. Cancer registry annual report. Taiwan, Republic of China:
2012
|
|
16
|
International Union Against Cancer, . TNM
classification of malignant tumors. Sobin LH and Wittekind C: 6th.
Wiley-Liss Inc; New York: 2002
|
|
17
|
Fleshman JW, Nelson H, Peters WR, Kim HC,
Larach S, Boorse RR, Ambroze W, Leggett P, Bleday R, Stryker S, et
al: Early results of laparoscopic surgery for colorectal cancer.
Retrospective analysis of 372 patients treated by Clinical Outcomes
of Surgical Therapy (COST) study group. Dis Colon Rectum. 39 10
Suppl:S53–S58. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Franklin ME Jr, Rosenthal D, Abrego-Medina
D, Dorman JP, Glass JL, Norem R and Diaz A: Prospective comparison
of open vs. laparoscopic colon surgery for carcinoma. Five-year
results. Dis Colon Rectum. 39 10 Suppl:S35–S46. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bokey EL, Moore JW, Chapuis PH and Newland
RC: Morbidity and mortality following laparoscopic-assisted right
hemicolectomy for cancer. Dis Colon Rectum. 39 10 Suppl:S24–S28.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwenk W, Bohm B and Müller JM:
Postoperative pain and fatigue after laparoscopic or conventional
colorectal resections. A prospective randomized trial. Surg Endosc.
12:1131–1136. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haller DG, Catalano PJ, Macdonald JS,
O'Rourke MA, Frontiera MS, Jackson DV and Mayer RJ: Phase III study
of fluorouracil, leucovorin, and levamisole in high-risk stage II
and III colon cancer: Final report of Intergroup 0089. J Clin
Oncol. 23:8671–8678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panettiere FJ, Goodman PJ, Costanzi JJ,
Cruz AB Jr, Vaitkevicius VK, McCracken JD, Brownlee RW, Laufman L,
Stephens RL, Bonnet J, et al: Adjuvant therapy in large bowel
adenocarcinoma: Long-term results of a Southwest Oncology Group
Study. J Clin Oncol. 6:947–954. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andre T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurihara M, Uchida J, Fujioka A, Kato T,
Ohshimo H, Abe M, Takeda S and Fukushima M: Effect of combination
therapy with UFT plus cisplatin (UFTP) on the survival of mice in
the experimental model for wide-spread metastasis in the peritoneal
cavity of gastrointestinal cancer using colon 26 PMF-15 cells.
Anticancer Res. 17:2217–2220. 1997.PubMed/NCBI
|
|
28
|
Smith RE, Lembersky BC, Wieand HS,
Colangelo L and Mamounas EP: UFT/leucovorin vs 5-FU/leucovorin in
colon cancer. Oncology (Williston Park). 14 10 Suppl 9:S24–S27.
2000.
|
|
29
|
Pazdur R, Lassere Y, Rhodes V, Ajani JA,
Sugarman SM, Patt YZ, Jones DV Jr, Markowitz AB, Abbruzzese JL,
Bready B, et al: Phase II trial of uracil and tegafur plus oral
leucovorin: An effective oral regimen in the treatment of
metastatic colorectal carcinoma. J Clin Oncol. 12:2296–2300. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saltz LB, Leichman CG, Young CW, Muggia
FM, Conti JA, Spiess T, Jeffers S and Leichman LP: A fixed-ratio
combination of uracil and Ftorafur (UFT) with low dose leucovorin.
An active oral regimen for advanced colorectal cancer. Cancer.
75:782–785. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meropol NJ, Rustum YM, Petrelli NJ,
Rodriguez-Bigas M, Frank C, Ho DH, Kurowski M and Creaven PJ: A
phase I and pharmacokinetic study of oral uracil, ftorafur, and
leucovorin in patients with advanced cancer. Cancer Chemother
Pharmacol. 37:581–586. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mukai M, Moriya H, Himeno S, Oida Y,
mukohyama S, Nishi T, Nakasaki H, Satoh S and Makuuchi H: Efficacy
of oral UFT plus leucovorin therapy for colon cancer with ovarian
and multiple liver metastases: Report of two cases. Oncol Rep.
8:1079–1083. 2001.PubMed/NCBI
|
|
33
|
West HJ and Jin JO: JAMA oncology patient
page. Performance status in patients with cancer. JAMA Oncol.
1:9982015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
U.S. Department of Health and Human
Services, National Institutes of Health, National Cancer Institute.
Common terminology criteria for adverse events (CTCAE), version
4.0. 2009.
|