Introduction
Stomach adenocarcinoma or gastric cancer (GC) is the
fourth most common cancer and the second highest cause of
cancer-related mortality worldwide (1). Despite improvements in surgical and
adjuvant treatment approaches, the prognosis of patients with GC
continues to be dismal, with a 5-year overall survival lower than
25% (2). The success of personalized
therapy depends on the identification and inhibition of the
oncogene(s) on which the tumor depends. Thus, it is of great
clinical significance to identify genes that determine the severity
of GC and assess their predictive value in the prognosis of GC
(3,4).
Previous studies have shown that most GC patients die due to
metastasis and treatment failure. Therefore, there is an urgent
need to improve the understanding of the mechanisms that lead to GC
so that new treatment strategies may be developed to target the
associated pathways. Accumulating reports have shown that the
extracellular signal-regulated kinase (ERK) signaling pathway is
associated with increased motility, invasion and metastasis of
cancer cells. Moreover, the ERK signaling pathway is frequently
found to be amplified in GC (5–8). Since ERK
signaling is also controlled by spatiotemporal regulatory
mechanisms (9,10), it is of great interest to determine if
there is a key gene that contributes to the deregulation of ERK in
GC. Hence, elucidating the mechanism of activation of the ERK
signaling pathway will further provide a novel approach to treat GC
and thereby improve the survival of GC patients.
Interestingly, several studies have reported that
cluster of differentiation (CD)133 contributes to the activation of
the ERK signaling pathway (11). The
transmembrane protein CD133 is of particular interest and a
controversial subject. However, the physiological function of CD133
remains unclear. CD133 is the most commonly expressed cancer stem
cell (CSC) marker in various cancer types such as colon, lung,
brain, pancreas, and GC (12–15). It has been shown that
CD133+ cells exhibit a higher degree of activation of
the ERK signaling pathway than CD133− cells (8). However, the biological function of CD133
in the activation of the ERK signaling pathway in GC cells is still
unknown.
CD133 has been reported to be a useful marker for
predicting recurrence and chemotherapy efficacy in not only breast
cancer but also GC (16,17). Accumulating evidence strongly suggests
the functional association of CD133+ CSCs with the ERK
signaling pathway. CD133+ tumor cells derived from
hepatoma (18), colon cancer
(19), melanoma (20), malignant peripheral nerve sheath tumor
(21) and neuroblastoma (22) samples consistently displayed increased
phospho-ERK (p-ERK) levels compared with matched CD133−
tumor cells. In addition, the overexpression of CD133 has been
shown to promote the phosphorylation of Erk in U87MG human
glioblastoma cells. These results strongly imply that CD133
facilitates the activation of the ERK signaling pathway in many
tumor cells. However, the role of CD133 in GC cells has not yet
been studied.
The transforming growth factor (TGF)-β family plays
a pivotal role in regulating a variety of cellular processes such
as differentiation, proliferation and apoptosis. TGF-β1 has also
been shown to mediate the activation of a certain downstream
targets of the PI3K signaling pathway, such as Jnk and Erk
(23–26). Moreover, the TGF-β1 signaling pathway
has been shown to regulate the function of CD133+ CSCs
in human brain tumors (27).
Vangipuram demonstrated that TGF-β1 stimulation can enhance CD133
expression in a time- and dose-dependent manner in Huh7 HCC cells
(28).
In the present study, we investigated whether CD133
can mediate the TGF-β1-induced activation of the ERK/P70S6K
signaling pathway in GC cells. This study may provide insights into
the molecular mechanism(s) responsible for the activation of the
TGF-β1-mediated ERK/P70S6K signaling pathway and enable the
development of effective anticancer therapies.
Materials and methods
Chemicals
TGF-β1 was purchased from PeproTech, Inc. (Rocky
Hill, NJ, USA), and U0126, a small molecular inhibitor of the ERK
pathway, was purchased from Sigma-Aldrich, Inc. (St. Louis, MO,
USA).
Cell lines and cultures
The human GC cell lines MKN45 and SGC7901 were
provided by the Shanghai Institute of Cell Biology, CAS (Shanghai,
China). The cells were cultured in Roswell Park Memorial Institute
(RPMI) 1640 culture medium (HyClone, Logan, UT, USA) supplemented
with 100 g/ml streptomycin, 100 U/ml penicillin, and 10% fetal
bovine serum (HyClone) at 37°C in a humidified environment
containing 5% carbon dioxide.
Immunomagnetic cell sorting
The cells were subcultured every 2 to 3 days. The
third to fifth subcultures were harvested, and the CD133
immunomagnetic cell sorting kit (Miltenyi Biotec, Bergisch
Gladbach, Germany) was used to isolate the CD133+ GC
cells. The CD133+ cells were maintained in serum-free
RPMI 1640 medium at 37°C in a humidified environment containing 5%
carbon dioxide (29,30).
Transient transfection of CD133 small
interfering RNA (siRNA). CD133-specific siRNA oligonucleotides were
purchased based on the CD133 gene sequence (Shanghai GenePharma
Co., Ltd., Shanghai, China). The sequences of the double-stranded
siRNA oligonucleotides were 5′-GUCCUUCCUAUAGAACAAUTT-3′ (sense) and
5′-AUUGUUCUAUAGGAAGGACTT-3′ (antisense). The negative control siRNA
sequences were 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and
5′-ACGUGACACGUUCGGAGAATT-3′ (antisense). Human GC SGC7901 cells
were transfected with siRNA (100 nM) using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
protocol.
Stable transfection of CD133
A plasmid extraction kit (Qiagen, Düsseldorf,
Germany) was used to extract the CD133 complementary
deoxyribonucleic acid (cDNA)-encoding plasmid according to the
manufacturer's protocol. The human GC SGC7901 cells, which have
been confirmed to have low CD133 expression, were stably
transfected using Lipofectamine® LTX Reagent
(Invitrogen, Tokyo, Japan) in accordance with the manufacturer's
instructions.
Western blotting and antibodies
Quantified protein lysates were resolved via sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, transferred
onto polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA), and incubated with primary antibodies (CD133/1 mouse mAb
1:100, Miltenyi Biotec; and phospho-P70S6 kinase (p-P70S6K),
P70S6K, p-ERK and ERK, rabbit mAb 1:1,000, Cell Signaling
Technology, Inc., Boston, MA, USA), followed by incubation with the
appropriate HRP-conjugated secondary antibodies (1:2,000; Jackson,
Mukilteo, WA, USA) at room temperature. Immunoreactive proteins
were detected using an enhanced chemiluminescence detection kit
(Amersham Biosciences, Inc., Piscataway, NJ, USA).
Statistical analyses
Student's t-test and ANOVA were used to compare the
results, when appropriate. All statistical analyses were performed
using the software SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). Values
of P<0.05 were considered statistically significant.
Results
TGF-β1 upregulates the level of CD133
and activates the ERK/P70S6K signaling pathway
Recent studies have shown that CD133 expression is
regulated by microenvironmental changes within the CSC niche
(31,32). We hypothesized that CD133 expression
is regulated by known growth factors, such as TGF-β1, which are
highly expressed in GC. To confirm our hypothesis, SGC7901 and
MKN45 cells were treated with 5 ng/ml TGF-β1 and analyzed via
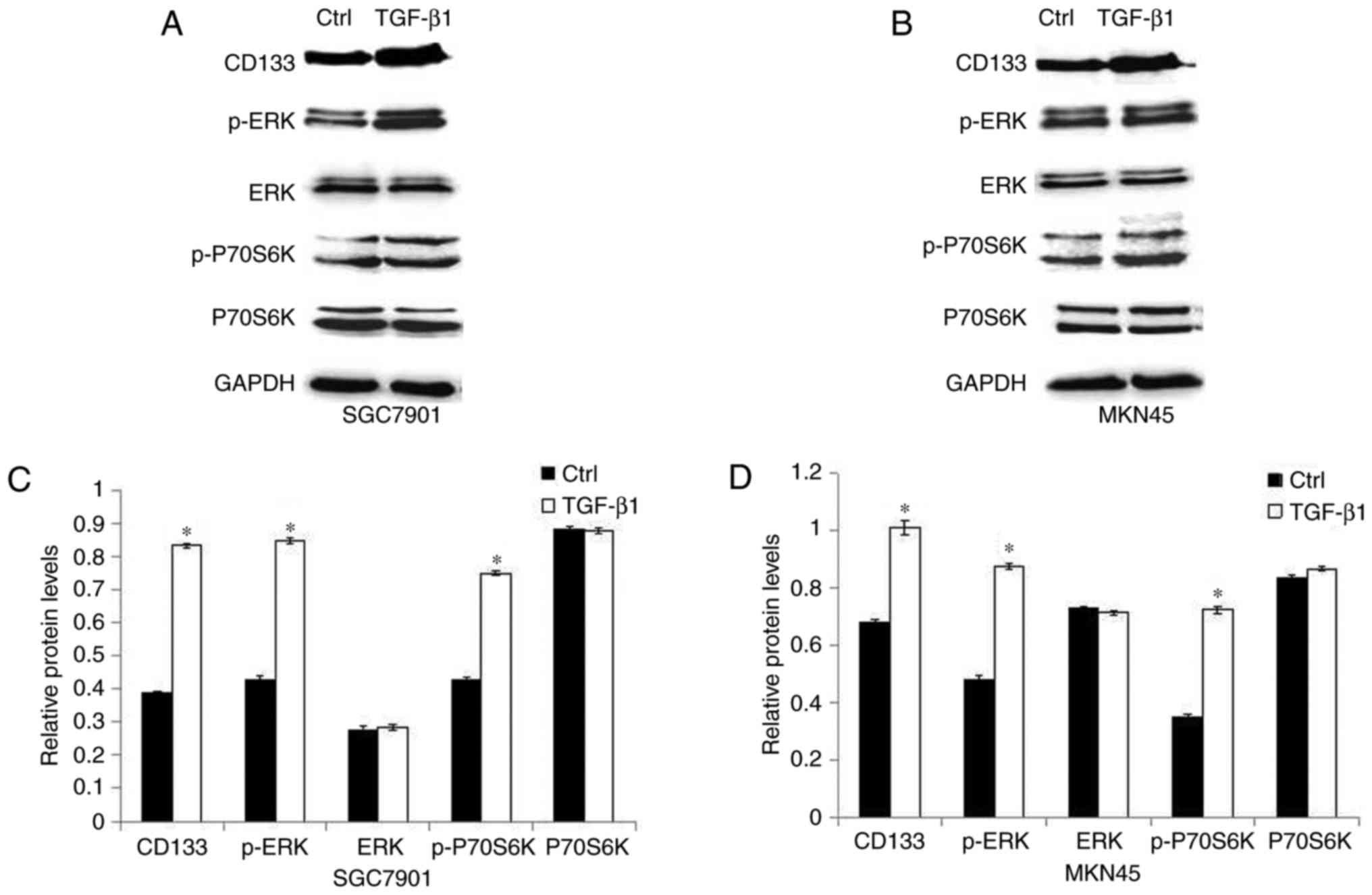
immunoblotting. Fig. 1A-D shows that
the expression of CD133 protein was enhanced by TGF-β1 treatment.
In addition, the expression level of p-ERK and p-P70S6K was induced
in GC cells treated with TGF-β1, while the expression of ERK and
P70S6K was not affected.
CD133+ GC cells display a
higher degree of activation of ERK/P70S6K signaling
To test whether TGF-β1 activates the ERK/P70S6K
pathway by regulating CD133 expression, we examined ERK, p-ERK,
P70S6K and p-P70S6K expression levels in CD133+ and
CD133− GC cells. As shown in Fig. 2A, B, D and E, there was no significant
difference in the expression of total ERK and P70S6K between
CD133+ and CD133− cells, but the expression
of p-ERK and p-P70S6K was significantly higher in the
CD133+ cells compared to that in the CD133−
cells. To clarify whether CD133 is upstream of the ERK/P70S6K
signaling pathway, SGC7901 GC cells were treated with the specific
inhibitor of the ERK pathway U0126. Western blotting showed that
U0126 treatment clearly decreased p-ERK and p-P70S6K expression,
while the expression of CD133 was unchanged (Fig. 2C and F). Taken together, our results
indicate that CD133 is likely upstream of the ERK/P70S6K signaling
pathway. Given that TGF-β1 both activates the ERK/P70S6K pathway
and upregulates CD133 expression, CD133 might be a mediator of the
TGF-β1-induced activation of the ERK/P70S6K signaling pathway.
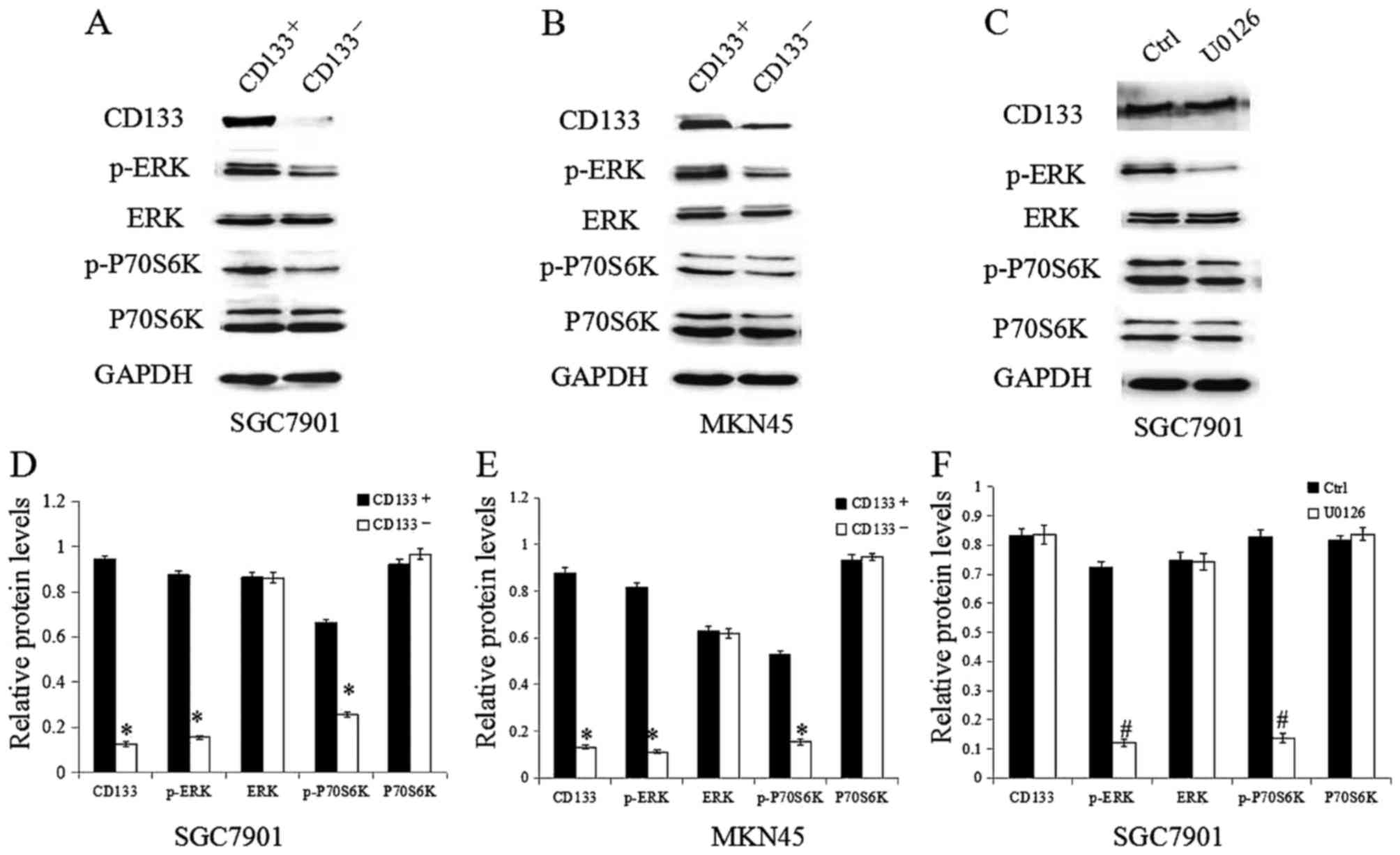 | Figure 2.CD133+ GC cells display a
greater extent of activation of ERK/P70S6K signaling.
CD133+ and CD133− GC cells were isolated. (A
and B) Western blotting was used to confirm the expression levels
of CD133, p-ERK, ERK, p-P70S6K and P70S6K. (C) The effect of U0126
treatment on the expression of CD133, p-ERK, ERK, p-P70S6K and
P70S6K. (D and E) Quantification of the target protein bands
relative to GAPDH levels is shown in the panels. (F) Quantification
of the target protein bands relative to GAPDH levels is shown in
the panels *P<0.05 vs. CD133+; #P<0.05
vs. Ctrl. CD, cluster of differentiation; GC, gastric cancer; ERK,
extracellular signal-regulated kinase; p-ERK, phospho-ERK;
p-P70S6K, phospho-P70S6 kinase. |
CD133 activation enhances ERK/P70S6K
activity
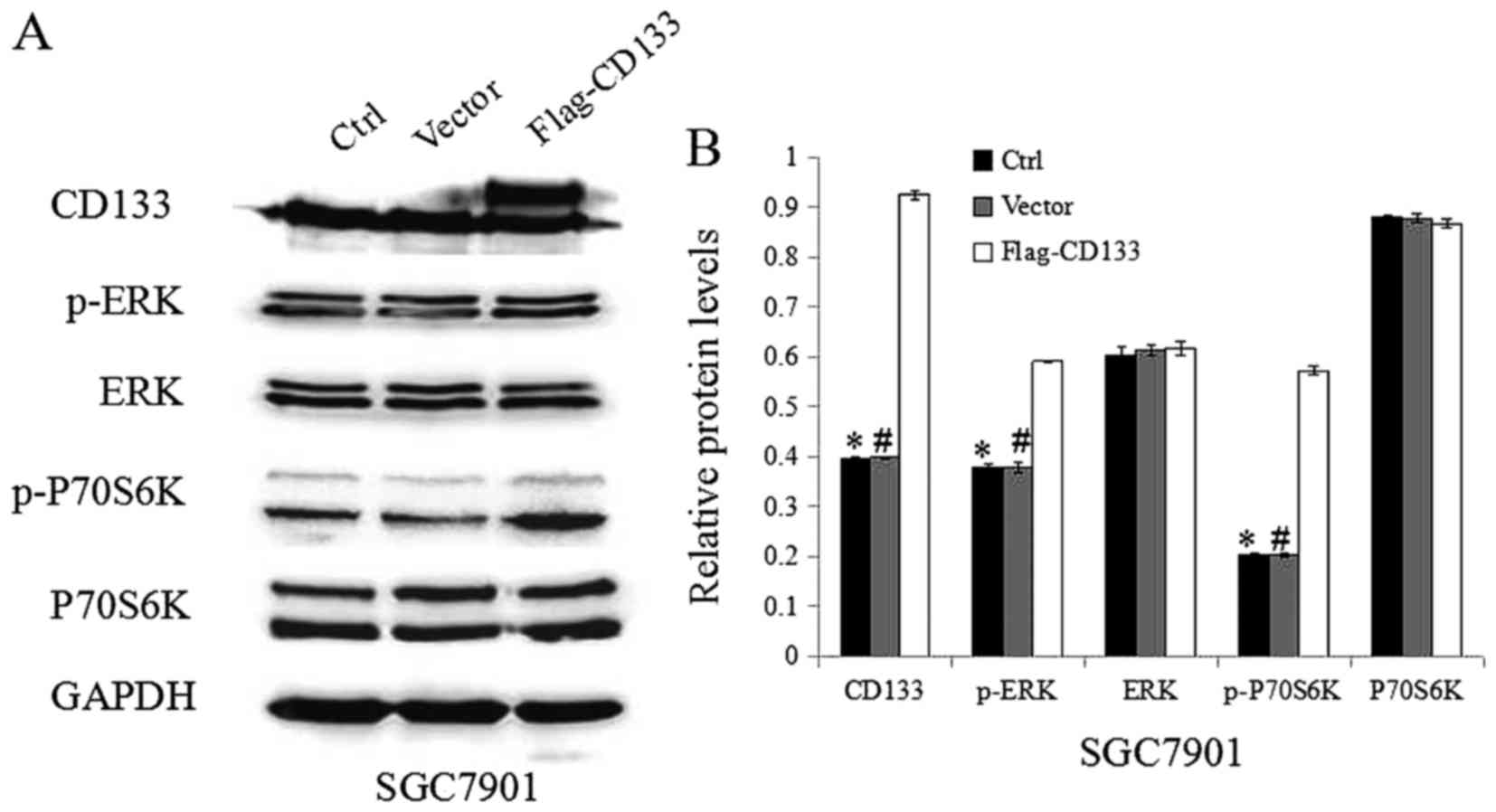
To confirm the role of CD133 in the TGF-β1-induced
activation of ERK/P70S6K signaling, the expression of CD133 was
increased by transfecting the CD133-expression construct into
SGC7901 cancer cells. Western blotting demonstrated that CD133
expression was clearly upregulated in the CD133-overexpressing
cells compared with that in the vector-transfected cells (Fig. 3A and B). Meanwhile, the expression of
p-ERK and p-P70S6K was upregulated in the CD133-overexpressing
cells compared to that in the empty vector-transfected cells, while
the expression of ERK and P70S6K was not affected.
Inhibition of CD133 in SGC7901 GC
cells inhibits the activation of ERK/P70S6K signaling
To further confirm the significance of CD133, siRNAs
targeting CD133 were used. As indicated in Fig. 4A and B, the expression of CD133 in GC
cells was successfully inhibited by siRNAs targeting CD133. Western
blotting showed that the downregulation of CD133 contributed to a
reduction in the level of p-ERK and p-P70S6K in cells transfected
with siRNAs targeting CD133 compared to that in cells transfected
with control siRNAs. In contrast, the expression of ERK and P70S6K
was not significantly altered after CD133 inhibition. Taken
together, CD133 likely plays an important role in the
TGF-β1-induced activation of the ERK/P70S6K signaling pathway.
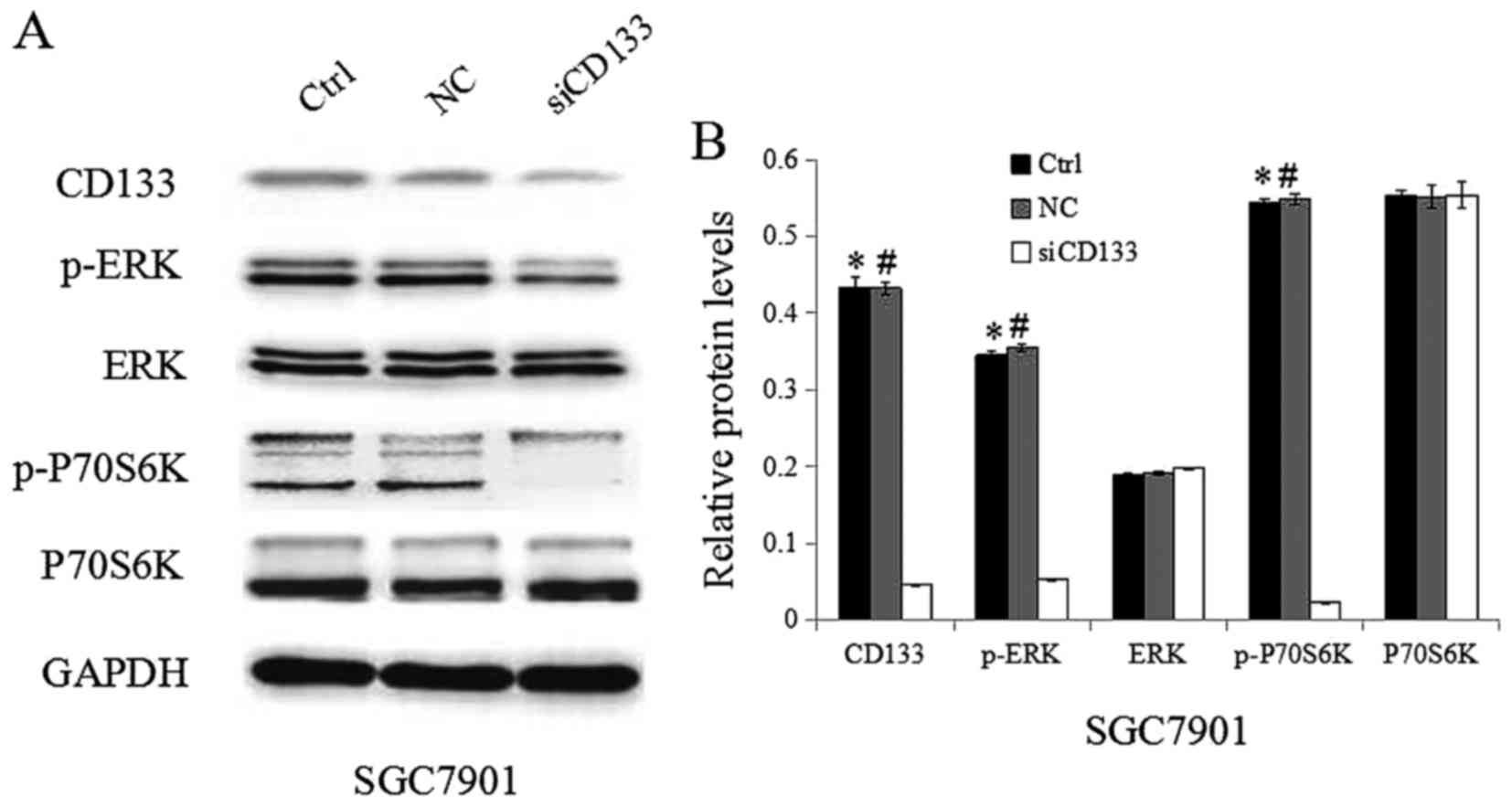 | Figure 4.Inhibition of CD133 in SGC7901 GC
cells inhibits the activation of ERK/P70S6K signaling. (A) Protein
expression was analyzed via western blotting using antibodies
against CD133, p-ERK, ERK, p70S6K, p-p70S6K, and glyceraldehyde
3-phosphate dehydrogenase. (B) Quantification of the target protein
bands relative to GAPDH levels is shown in the panels, *P<0.05
vs. Ctrl; #P<0.05 vs. NC. CD, cluster of
differentiation; GC, gastric cancer; ERK, extracellular
signal-regulated kinase; p-ERK, phospho-ERK; p-P70S6K,
phospho-P70S6 kinase. |
Discussion
CD133 is a transmembrane protein that is well
adapted to participate in ERK signaling regulated by TGF-β1.
Better understanding of the relevance and function
of CSCs may provide novel insights into the underlying mechanislms
and possible targets for GC therapies. Increasing evidence has
demonstrated that in addition to being a biomarker in tumors, CD133
regulates the growth and development of tumor cells. Recently,
CD133 has been reported to be involved in the activation of the ERK
signaling pathway in various cancer cells (18–22).
Moreover, increasing evidence has indicated that the enhanced
motility, invasion and metastasis of cancer cells are associated
with the ERK signaling pathway. Although the activation of the ERK
signaling pathway has been reported to be associated with GC, we
are the first to report that CD133 involved in the activation of
the ERK signaling pathway induced by TGF-β1 in GC. Our current
study demonstrates the correlation between CD133 and the
TGF-β1-mediated activation of the ERK/P70S6K signaling pathway in
GC cells.
In the current study, CD133 protein expression was
induced by TGF-β1 treatment. In addition, the expression level of
p-ERK and p-P70S6K was upregulated in GC cells treated with TGF-β1,
while the expression of ERK and P70S6K was not changed. The above
results showed that TGF-β1 might activate CD133 as well as the
ERK/P70S6K signaling pathway. However, the correlation among
TGF-β1, CD133 and ERK/P70S6K signaling pathway remained
unclear.
To test whether TGF-β1 activates the ERK/P70S6K
pathway by regulating CD133 expression, we examined the expression
of ERK, p-ERK, P70S6K and p-P70S6K in CD133+ and
CD133− GC cells. As demonstrated, there was no obvious
difference in total ERK and P70S6K levels between CD133+
and CD133− cells, but p-ERK and p-P70S6K levels were
significantly higher in the CD133+ cells compared to
those in the CD133− cells. To clarify whether CD133 is
upstream of the ERK/P70S6K signaling pathway, SGC7901 GC cells were
treated with the specific inhibitor of the ERK signaling pathway
U0126. Western blotting showed that U0126 treatment clearly
decreased the expression of p-ERK and p-P70S6K, while the
expression of CD133 was unchanged. Taken together, our results
indicate that CD133 is likely upstream of the ERK/P70S6K signaling
pathway. Given that TGF-β1 activates the ERK/P70S6K pathway and
upregulates CD133 expression, CD133 is the mediator of the
TGF-β1-induced activation of the ERK/P70S6K signaling pathway.
To confirm the role of CD133 in the TGF-β1-induced
activation of ERK/P70S6K signaling, gene modulation was used. In
our current study, the function of CD133 on the activation of the
ERK/P70S6K signaling pathway was confirmed by upregulating and
downregulating CD133 in SGC7901 cells. It was found that silencing
CD133 in cells via CD133-siRNAs resulted in a reduction in the
level of p-ERK and p-P70S6K compared to that in control
siRNA-transfected cells. In addition, the activation of CD133
increased the expression of p-ERK and p-P70S6K in cells transfected
with siRNAs targeting CD133 compared to that in cells transfected
with control siRNA, while the expression of ERK and P70S6K was not
affected. Taken together, these observations clearly suggest that
CD133 plays an important role in the TGF-β1-induced activation of
the ERK/P70S6K signaling pathway.
In conclusion, the results of the present study
suggest that concurrent blocking of CD133 and the ERK/P70S6K
pathway might be an effective approach for improving the prognosis
of GC patients. In addition, our results provide important avenues
for future research in GC. However, studies investigating the
association between CD133 and the TGF-β1-induced activation of the
ERK/P70S6K signaling pathway in GC cells using human GC specimens
and animal models are necessary to validate the usefulness of this
approach. What's more, a limitation of the present study is that we
did not assess whether TGF-beta1-induced CD133 and subsequently
activation of PI3K enhances cell growth. In the future, we will
conduct a functional study aimed to demonstrate the correlation
between the PI3K pathway and cell growth. Furthermore, we did not
prove whether the activation of the PI3K pathway by CD133 is
mediated by the phosphorylation of the regulatory subunits p85
and/or p110. Obviously, the absence of data on PI3K/PTEN and
PI3K/AKT signaling was also a limitation of this study. And our
future study will solve the above problems.
Acknowledgements
The present study was supported by funds from the
Hospital Foundation of Xuzhou Central Hospital, which is affiliated
with the Medical College of Southeast University (grant no.
XZS201673).
References
|
1
|
Coupland VH, Lagergren J, Lüchtenborg M,
Jack RH, Allum W, Holmberg L, Hanna GB, Pearce N and Møller H:
Hospital volume, proportion resected and mortality from oesophageal
and gastric cancer: A population-based study in England, 2004–2008.
Gut. 62:961–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camargo MC, Kim WH, Chiaravalli AM, Kim
KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R,
Meneses-Gonzalez F, et al: Improved survival of gastric cancer with
tumour Epstein-Barr virus positivity: An international pooled
analysis. Gut. 63:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shibata W, Ariyama H, Westphalen CB,
Worthley DL, Muthupalani S, Asfaha S, Dubeykovskaya Z, Quante M,
Fox JG and Wang TC: Stromal cell-derived factor-1 overexpression
induces gastric dysplasia through expansion of stromal
myofibroblasts and epithelial progenitors. Gut. 62:192–200. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Wu X, Zhang J, Chen Y, Xu J, Xia
X, He S, Qiang F, Li A, Shu Y, et al: CHIP functions as a novel
suppressor of tumour angiogenesis with prognostic significance in
human gastric cancer. Gut. 62:496–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paterson AL, Shannon NB, Lao-Sirieix P,
Ong CA, Peters CJ, O'Donovan M and Fitzgerald RC: A systematic
approach to therapeutic target selection in oesophago-gastric
cancer. Gut. 62:1415–1424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang M, Qiu Z, Zhang S, Fan X, Cai X, Xu
B, Li X, Zhou J, Zhang X, Chu Y, et al: Elevated O-GlcNAcylation
promotes gastric cancer cells proliferation by modulating cell
cycle related proteins and ERK 1/2 signaling. Oncotarget.
7:61390–61402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Q, Wang X, Yu Z, Wu X, Chen X, Li J,
Zhu Z, Liu B and Su L: Transducin (β)-like 1 X-linked receptor 1
promotes gastric cancer progression via the ERK1/2 pathway.
Oncogene. 36:1873–1886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Hua R, Wang X, Huang M, Gan L, Wu
Z, Zhang J, Wang H, Cheng Y, Li J and Guo W: Identification of
stem-like cells and clinical significance of candidate stem cell
markers in gastric cancer. Oncotarget. 7:9815–9831. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kholodenko BN, Hancock JF and Kolch W:
Signalling ballet in space and time. Nat Rev Mol Cell Biol.
11:414–426. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong X, Qian J, Chen LS, Wang YC, Wang JL,
Chen H, Weng YR, Zhao SL, Hong J, Chen YX, et al: Synbindin in
extracellular signal-regulated protein kinase spatial regulation
and gastric cancer aggressiveness. J Natl Cancer Inst.
105:1738–1749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong L, Qi N, Ge RM, Cao CL, Lan F and
Shen L: Overexpression of CD133 promotes the phosphorylation of Erk
in U87MG human glioblastoma cells. Neurosci Lett. 484:210–214.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
15
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aomatsu N, Yashiro M, Kashiwagi S,
Takashima T, Ishikawa T, Ohsawa M, Wakasa K and Hirakawa K: CD133
is a useful surrogate marker for predicting chemosensitivity to
neoadjuvant chemotherapy in breast cancer. PLoS One. 7:e458652012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Yu J, Wang S, Lu R, Wu J and Jiang
B: Overexpression of CD133 enhances chemoresistance to
5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric
cancer cells. Oncol Rep. 32:2437–2444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding W, Mouzaki M, You H, Laird JC, Mato
J, Lu SC and Rountree CB: CD133+ liver cancer stem cells
from methionine adenosyl transferase1 A-deficient mice demonstrate
resistance to transforming growth factor (TGF)-beta-induced
apoptosis. Hepatology. 49:1277–1286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YK, Zhu YL, Qiu FM, Zhang T, Chen ZG,
Zheng S and Huang J: Activation of Akt and MAPK pathways enhances
the tumorigenicity of CD133+ primary colon cancer cells.
Carcinogenesis. 31:1376–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Khattouti A, Selimovic D, Haïkel Y,
Megahed M, Gomez CR and Hassan M: Identification and analysis of
CD133(+) melanoma stem-like cells conferring resistance to taxol:
An insight into the mechanisms of their resistance and response.
Cancer Lett. 343:123–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borrego-Diaz E, Terai K, Lialyte K, Wise
AL, Esfandyari T, Behbod F, Mautner VF, Spyra M, Taylor S, Parada
LF, et al: Overactivation of Ras signaling pathway in
CD133+ MPNST cells. J Neurooncol. 108:423–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vangipuram SD, Wang ZJ and Lyman WD:
Resistance of stem-like cells from neuroblastoma cell lines to
commonly used chemotherapeutic agents. Pediatr Blood Cancer.
54:361–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mucsi I, Skorecki KL and Goldberg HJ:
Extracellular signal-regulated kinase and the small GTP-binding
protein, Rac, contribute to the effects of transforming growth
factor-beta1 on gene expression. J Biol Chem. 271:16567–16572.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atfi A, Djelloul S, Chastre E, Davis R and
Gespach C: Evidence for a role of Rho-like GTPases and
stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK)
in transforming growth factor beta-mediated signaling. J Biol Chem.
272:1429–1432. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhowmick NA, Ghiassi M, Bakin A, Aakre M,
Lundquist CA, Engel ME, Arteaga CL and Moses HL: Transforming
growth factor-beta1 mediates epithelial to mesenchymal
transdifferentiation through a RhoA-dependent mechanism. Mol Biol
Cell. 12:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
You H, Ding W and Rountree CB: Epigenetic
regulation of cancer stem cell marker CD133 by transforming growth
factor-beta. Hepatology. 51:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu RQ, Wu JG, Zhou GC, Jiang HG, Yu JW and
Jiang BJ: Sorting of CD133(+) subset cells in human gastric cancer
and the identification of their tumor initiating cell-like
properties. Zhonghua Wei Chang Wai Ke Za Zhi. 15:174–179. 2012.(In
Chinese). PubMed/NCBI
|
|
30
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Platet N, Liu SY, Atifi ME, Oliver L,
Vallette FM, Berger F and Wion D: Influence of oxygen tension on
CD133 phenotype in human glioma cell cultures. Cancer Lett.
258:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCord AM, Jamal M, Shankavarum UT, Lang
FF, Camphausen K and Tofilon PJ: Physiologic oxygen concentration
enhances the stem-like properties of CD133+ human
glioblastoma cells in vitro. Mol Cancer Res. 7:489–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|