Introduction
The ameloblastoma is the most common and clinically
relevant odontogenic tumor (1). Our
institution, Osaka Dental University (Osaka, Japan) treated 111
cases of biopsy-proven ameloblastoma from 2005–2014. Ameloblastoma
is typically benign while locally invasive, with a considerable
tendency to recur when not adequately removed (2).
In 1986, Muller and Slootweg (3) observed that clear cell differentiation
may occur as a histological feature of ameloblastoma; the clear
cells contain glycogen-rich cytoplasm. Mari et al (4) stated that the clear cell appearance in
ameloblastoma could be considered as a new histological variant
(4). Waldron et al (5) reported 2 cases of a lesion that they
designated as ‘clear-cell ameloblastoma’ or CCAM, an odontogenic
carcinoma. The 2 cases demonstrated an unusual biphasic pattern,
with areas of typical follicular ameloblastoma together with a
conspicuous clear-cell component. The clinical course indicated
that these lesions should be considered as low-grade odontogenic
carcinomas. Braunshtein et al (6) reviewed the reported features of clear
cell odontogenic carcinoma and CCAM and suggested that these two
types represented a continuum of the same neoplasm. Thus, CCAM is
well-documented in the English-language literature; however, it is
not officially recognized as a neoplasm by the World Health
Organization.
To the best of our knowledge, details of the
features of CCAM identified from cross-sectional imaging
modalities, including computed tomography (CT) and magnetic
resonance (MR) imaging, are unclear at present, due to the rarity
of the disease and the past underdevelopment of these imaging
modalities. Therefore, the present study reports a solid tumor in
the mandible of a 40-year-old male, which exhibited features that
were notably similar to a desmoplastic ameloblastoma on CT and MR
imaging. The unique process of reaching the correct diagnosis is
presented, with a brief review of the literature on this topic.
Case report
A 40-year-old Japanese male was referred to Osaka
Dental University by his dentist in 2015 for the inspection of a
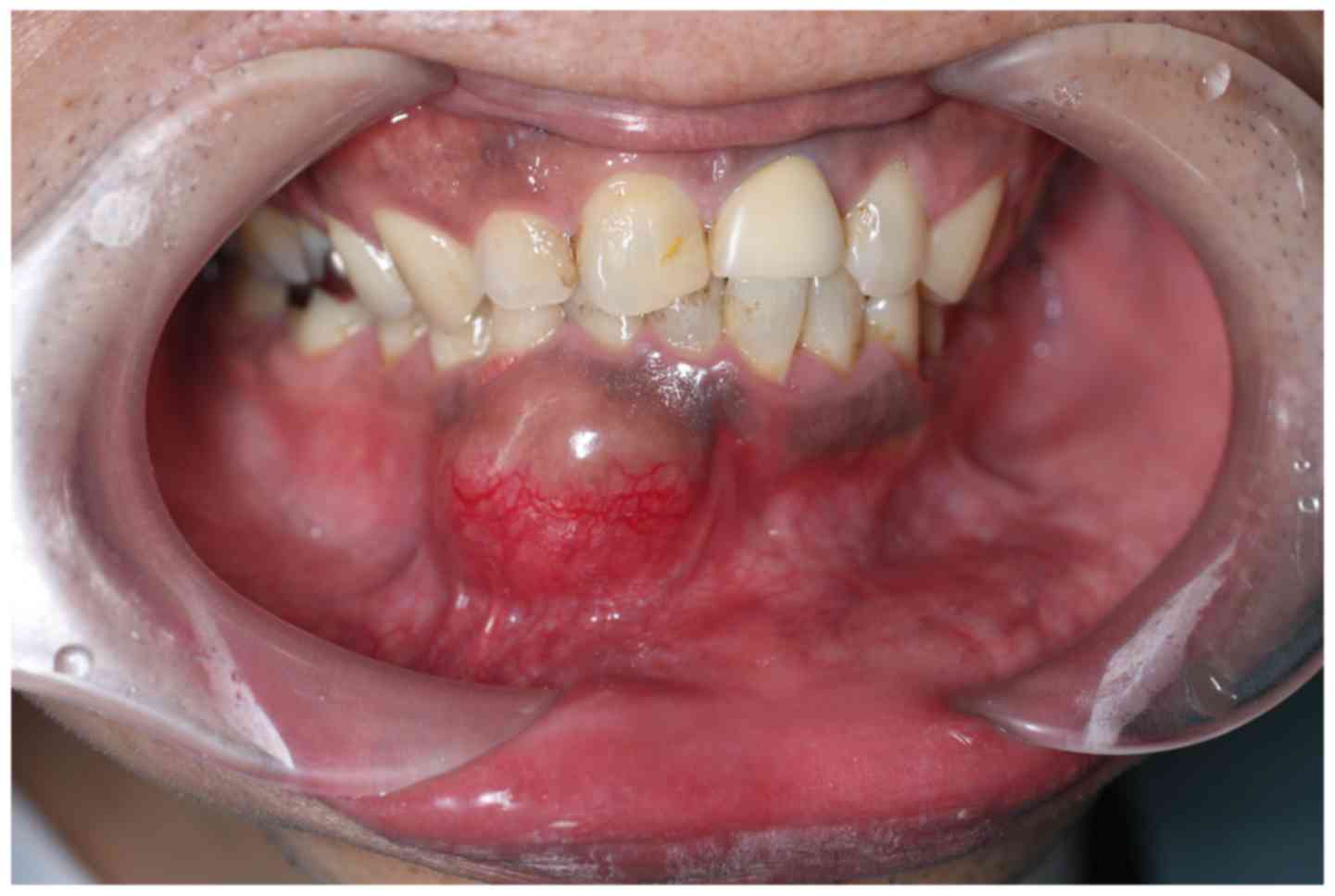
swelling in the area of the right canine of the mandible (Fig. 1). The patient had noticed the swelling
for 4 months. An initial clinical examination presented a
localized, elastic, hard swelling in the right buccal vestibule
around teeth 42–44. The overlying mucosa was normal. An electric
pulp test was performed due to the newly onset tenderness
associated with the tooth, and the pulp was observed to be vital.
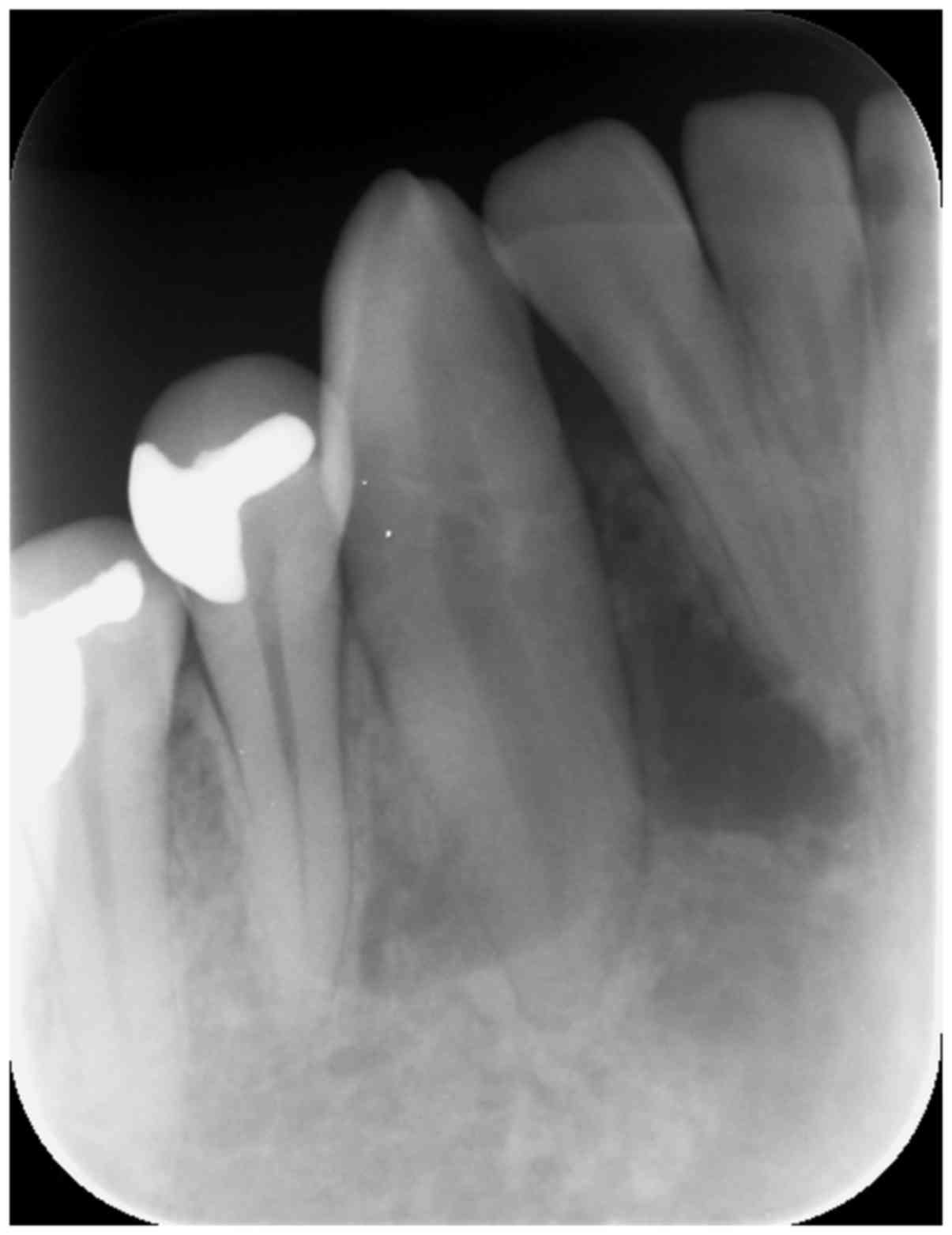
An initial intraoral x-ray demonstrated a radiolucent lesion with
delicate septa and margins near the apexes of teeth 42, 43 and 44,
mildly dislocating 42 and 43 (Fig.
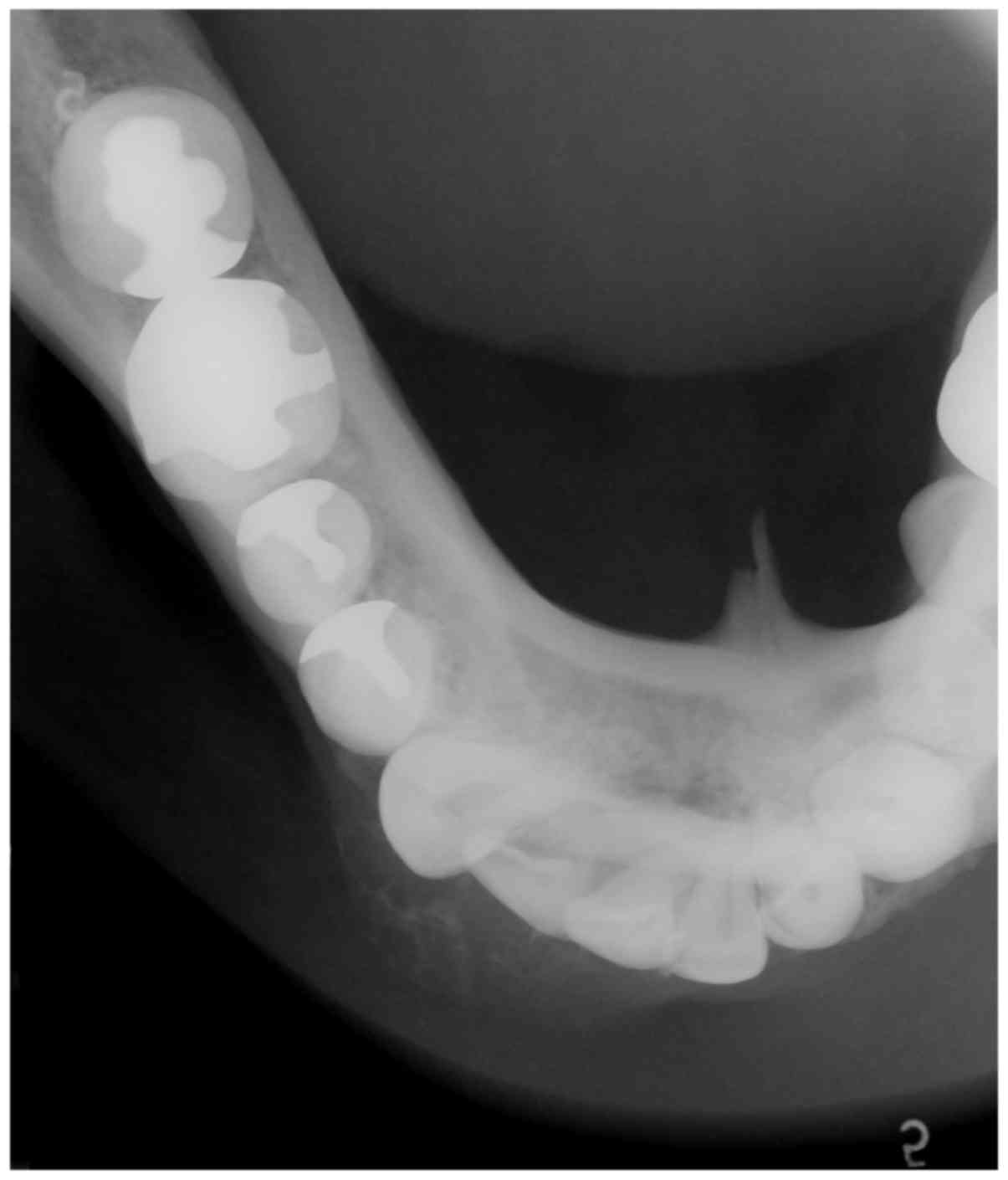
2). An occlusal x-ray revealed a radiolucent-radiopaque lesion
with a buccal bony expansion in the right lateral incisor and
canine area of the mandible, illustrating an indistinct soap
bubble-like appearance (Fig. 3). A
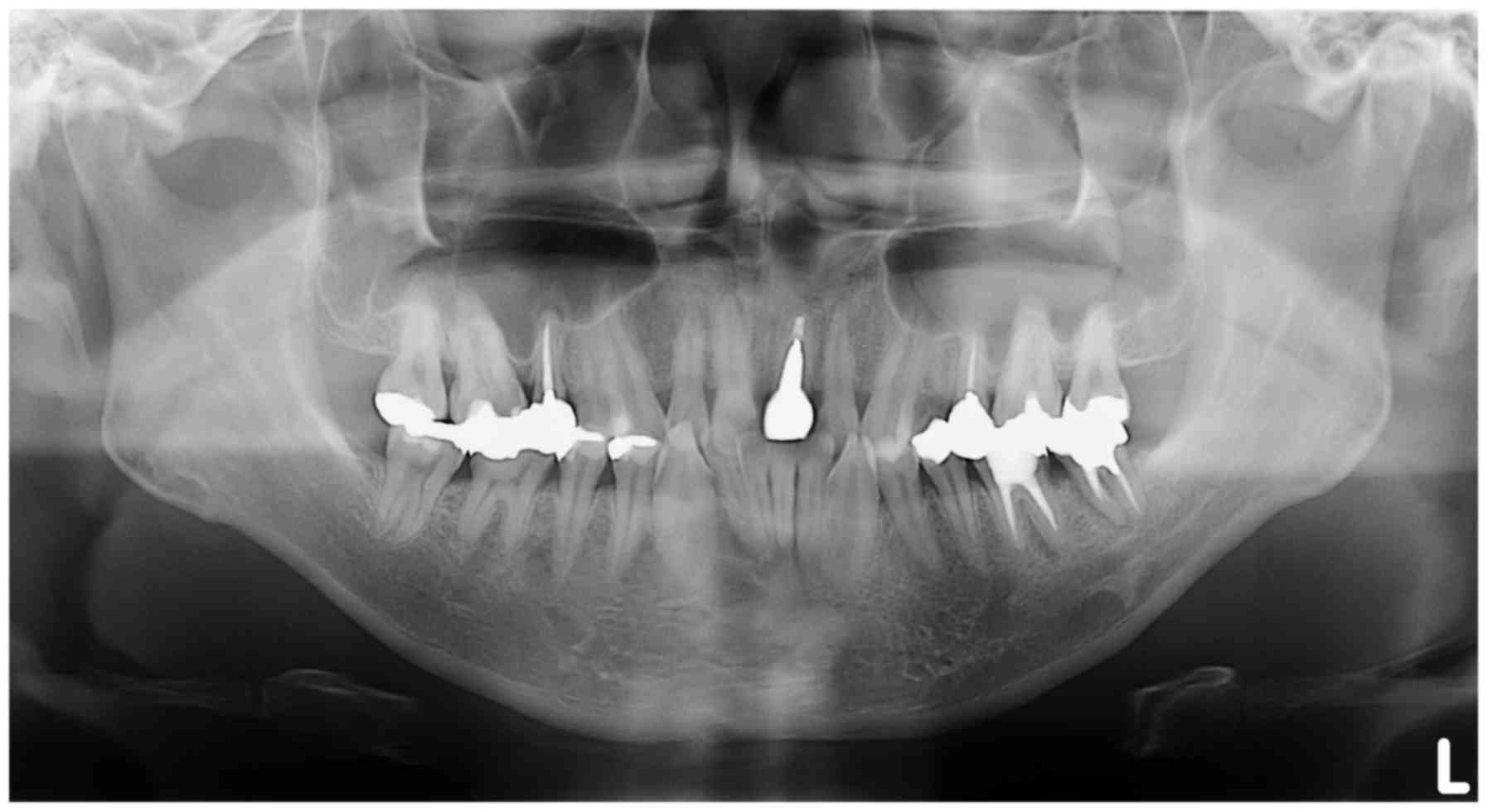
panoramic x-ray image detected nothing relevant to the primary
complaint of the patient and was affected by ghost images from the
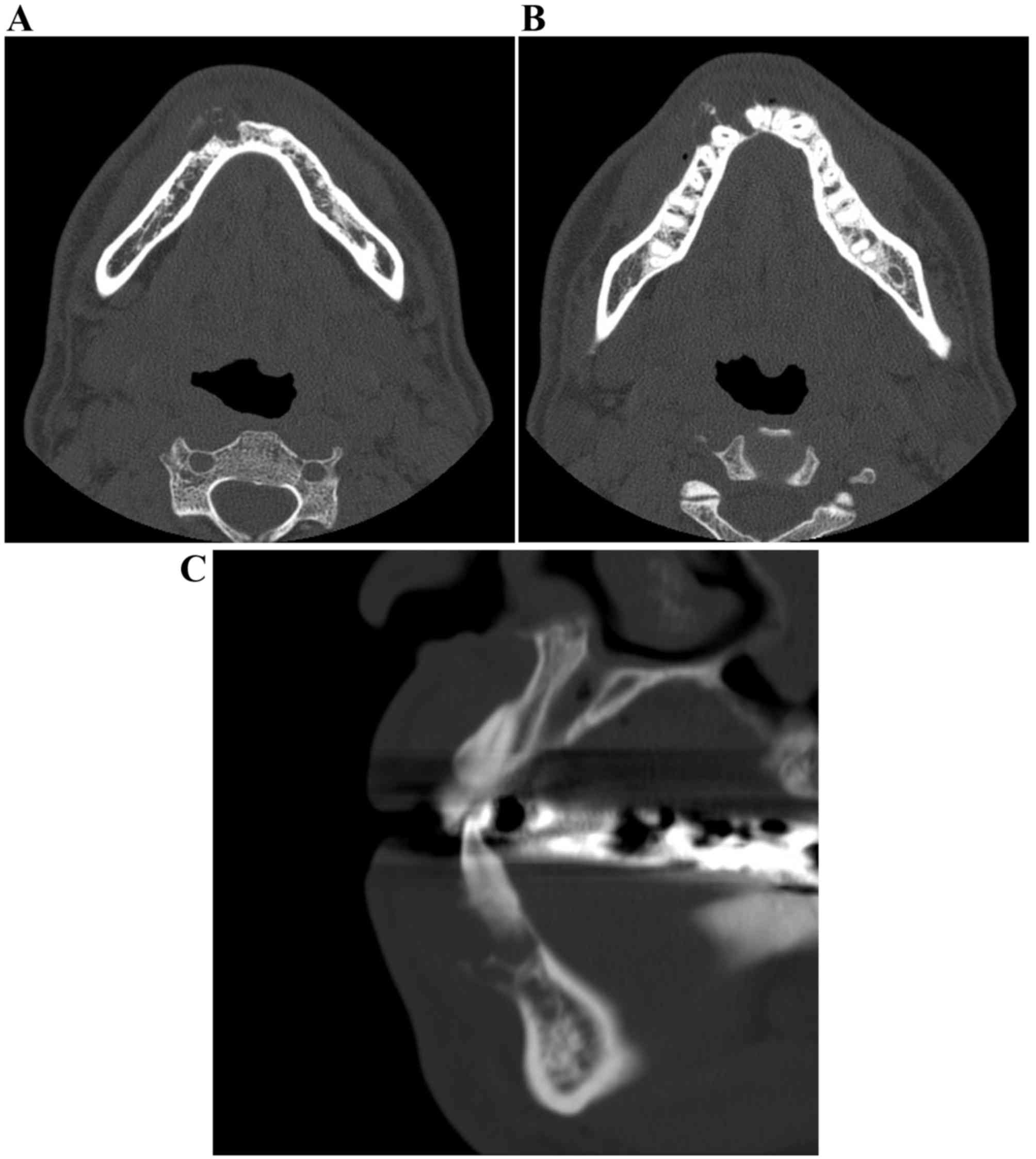
cervical vertebrae (Fig. 4). CT
images were obtained using a CT scanner (BrightSpeed Elite; GE
Healthcare, Chicago, IL, USA) at 120 kV. The electrical current was
automatically optimized for the object thickness (maximum, 120 mA).
In addition, CT imaging was performed with the following
parameters: Slice thickness, 0.65 mm; pitch and tube voltage,
0.625:1; and field of view, 16.8 cm2. CT imaging
revealed an expanding, mixed radiolucent-radiopaque appearance with
poorly defined borders, including irregularly thinned cortical
plates (Fig. 5A-C). The mass was a
multiloculated, honeycomb-like lytic lesion containing a high
number of septa. The CT value of the radiolucency inside the lesion
was 30 Hounsfield units (HU), indicating fluid, and those of the
septa were ~120 HU, suggesting that there was an extent of
calcification. Teeth 42 and 43 were slightly displaced, without
root resorption. Neither a destructive condition nor inflammatory
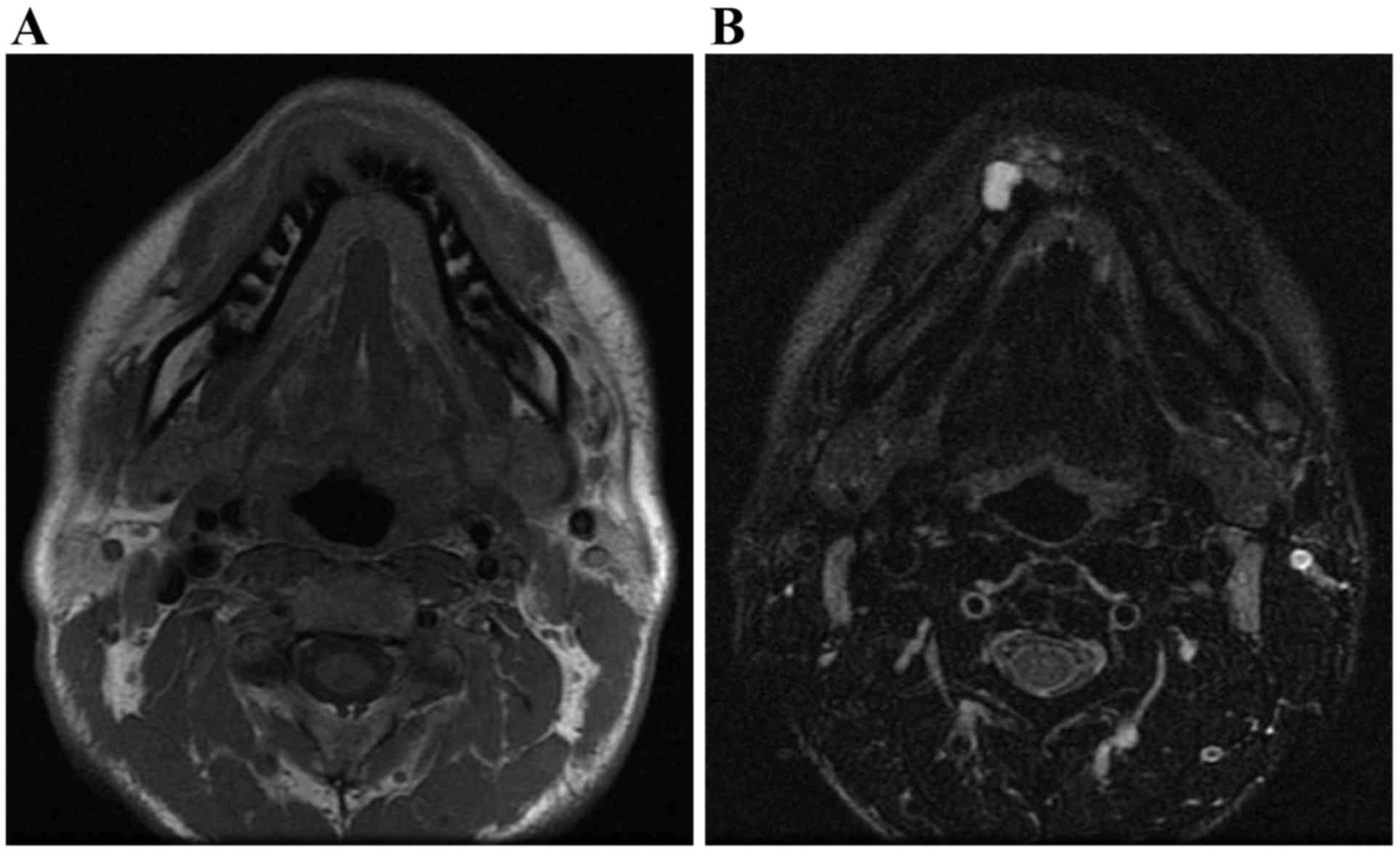
osteosclerosis were observed. MR examination was performed using a
1.5-T superconducting magnet (SIGNA™; GE Healthcare) with an
8-channel head and neck coil. T1-weighted spin-echo sequences
[repetition time (TR)/echo time
(TE)=760–800/8-10], T2-weighted fast spin-echo sequences
(TR/TE =5,000–5,250/100-200) and T2-weighted
fast spin-echo sequences with the fat-suppression technique of the
chemical shift selective method
(TR/TE=2,500–3,000/80-120) were obtained with
a 20.0×20.0 cm field of view and slice thickness of 4 mm, with 1 mm
spacing. Axial T1-weighted images (T1WI), axial T2-weighted images
(T2WI), axial T2-weighted fat-suppressed images (T2WI-fat), coronal
T1WI, and coronal T2WI were obtained. MR imaging revealed
nonhomogeneous mixed signal intensities in the equivalent region.
In T1WI it demonstrated an intermediate signal intensity (Fig. 6A), whereas in T2WI-fat it exhibited
variable intermediate and high signal intensities (Fig. 6B). These findings suggested a mixed
distribution of solid and cystic components. The interpretation of
the imaging was that the patient exhibited a desmoplastic
ameloblastoma.
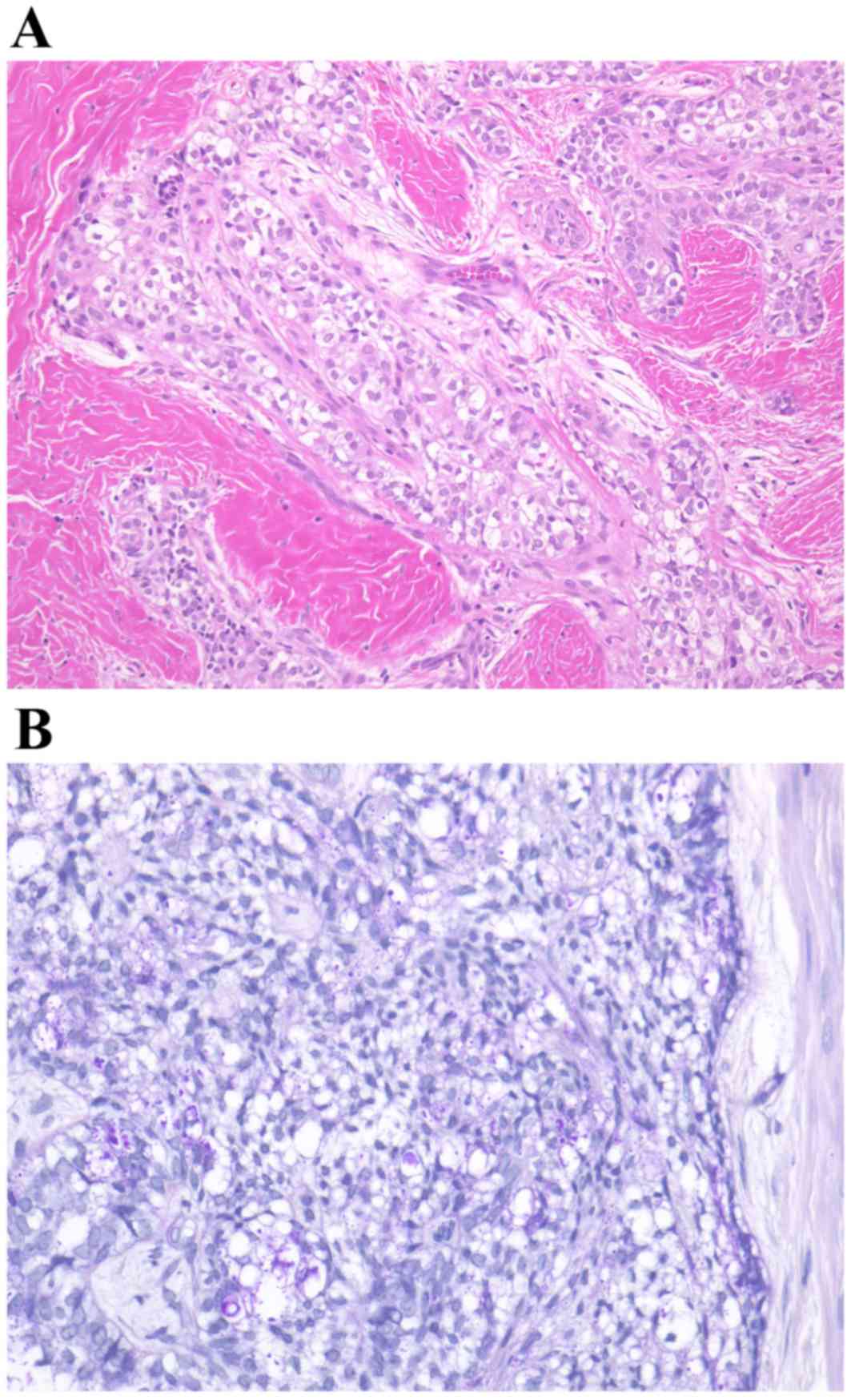
A biopsy was performed with local anesthesia,
resulting in the diagnosis of clear cell odontogenic carcinoma
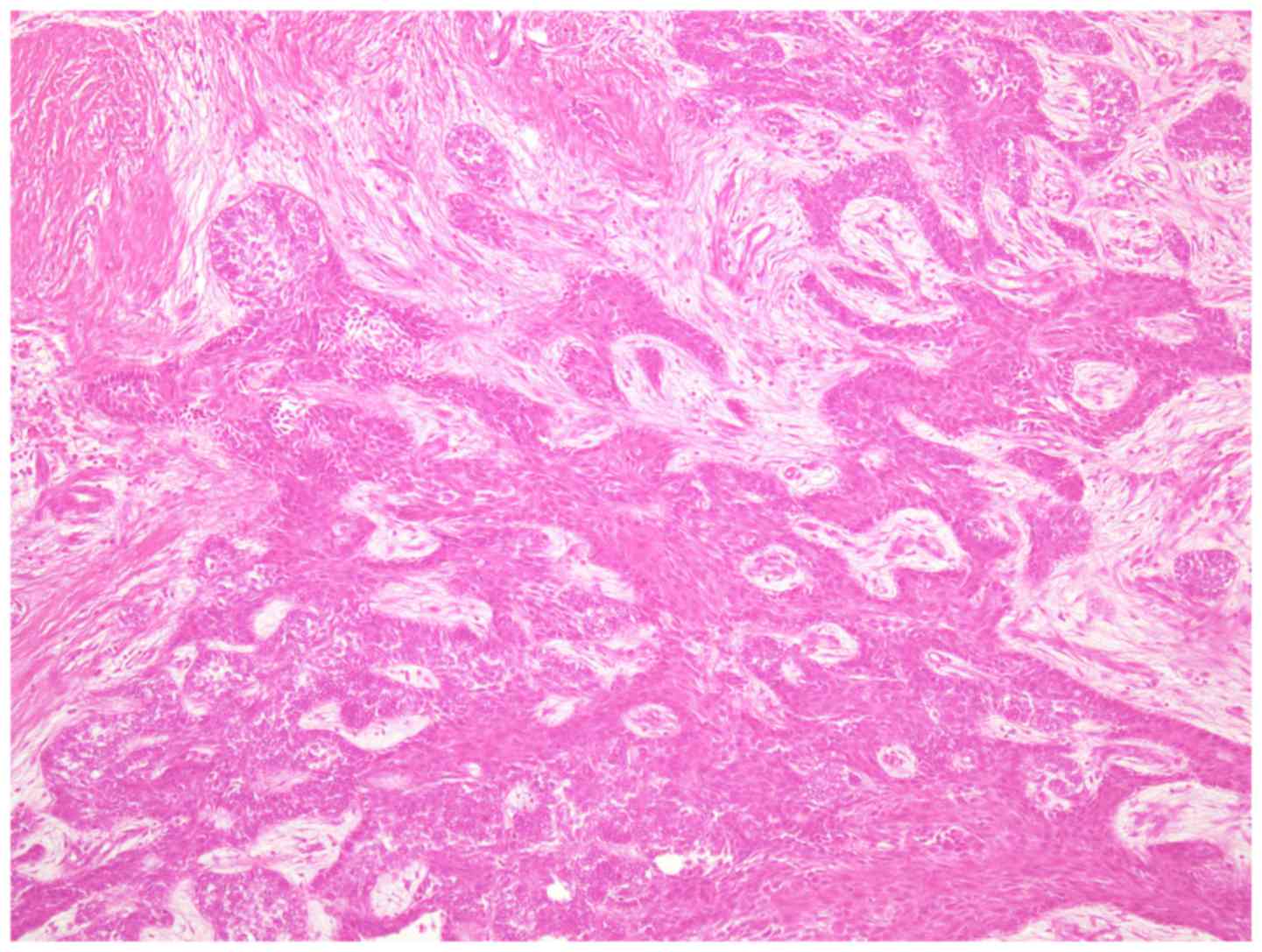
accompanied by ameloblastoma. Numerous clear cells were observed in
tumor islands without an inconspicuous stellate reticulum (Fig. 7). The clear cells were periodic
acid-Schiff positive and resistant to diastase digestion; however,
the clear cells did not uptake alcian blue staining.
Immunohistochemical examination was performed as follows:
Formalin-fixed paraffin-embedded tissues were cut at 2 µm
thickness. Ki-67, p53, and p63 proteins were retrieved by
autoclaving at 121°C for 15 min in retrieval buffer (0.1 M citrate
buffer; Mitsubishi Yatron, Tokyo, Japan). The S-100-protein was
retrieved without autoclaving.
The sections were incubated with the following
diluted primary antibodies: Rabbit anti-human S-100-protein
polyclonal antibody (1:1; cat. no. 422091; Nichirei, Tokyo, Japan),
mouse anti-human Ki-67 monoclonal antibody (1:100; cat. no.
00095324; M1B-1, Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA), mouse anti-human p53 monoclonal antibody (1:100; cat. no.
20023361; DO-7, Dako; Agilent Technologies, Inc.), and mouse
anti-human p63 monoclonal antibody (1:25; cat. no. 147817; 7JUL,
Novocastra; Leica Biosystems (Newcastle) Ltd., Newcastle, UK) for
30 min at room temperature. For the secondary antibody incubation,
S-100-protein was incubated with peroxidase conjugated anti-rabbit
antibody (1:1; cat. no. 10097631; Dako; Agilent Technologies,
Inc.); Ki-67, p53, and p63 were incubated with a peroxidase
conjugated anti-mouse antibody (1:1; cat. no. 10037259; Dako;
Agilent Technologies, Inc.). The signals were then visualized using
3,3′-diaminobenzidine tetrahydrochloride and staining was
considered to be positive if it was yellowish-brown. As negative
control, normal mouse IgG and rabbit IgG were used instead of
primary antibodies. This analysis revealed that the clear cells
were negative for S-100-protein and Ki-67. p53-positive cells were
infrequent, and p63 was expressed in the nuclei of the tumor
cells.
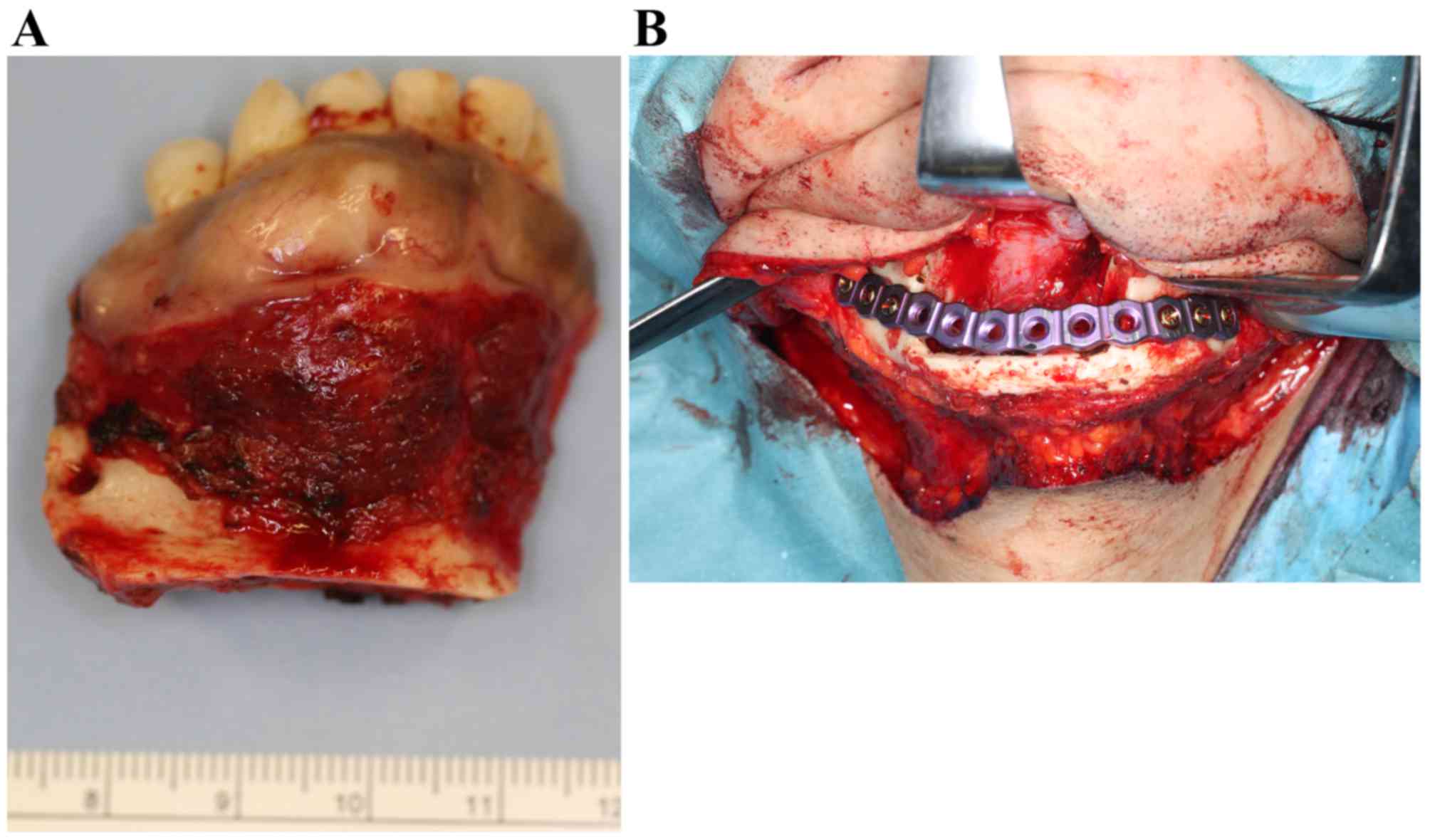
With a tentative diagnosis of clear cell odontogenic
carcinoma, the tumor was excised with a continuity of the lower
border of the mandible (Fig. 8A). The
remaining mandible was immediately reinforced with a mandibular
reconstruction titanium plate (Fig.
8B). The postoperative course was uneventful; there has been no
evidence of recurrence or metastatic disease for 2 years and 3
months subsequent to surgery.
Following the marginal resection of the mandible,
histological examination of the tumor identified features
inconsistent with those of the biopsy, including features of a
typical plexiform-type ameloblastoma (Fig. 9). No clear cells were evident in the
intraoperative biopsy or in a thorough examination of the entire
specimen. Taking all the observations into account, the final
diagnosis of CCAM was made. The patient provided informed consent
for the publication of the present study, which was approved by the
Ethics Review board of Osaka Dental University.
Discussion
The course of this patient indicated two notable
aspects of ameloblastoma. Firstly, detailed CT and MRI findings of
CCAM in the mandible of a patient were reported; to the best of our
knowledge, these imaging characteristics were not previously
described. Secondly, not until the histological examination of the
surgical specimen could a definite diagnosis of CCAM be
produced.
The imaging features closely resembled those of
desmoplastic ameloblastoma. On intraoral and occlusal images, the
lesion was radiolucent and multilocular, with a honeycombed or
bubble-like appearance. The tumor expanded onto the cortex of the
mandible, with subsequent tumor extension into the adjacent soft
tissues that could be observed in the axial T1WI MR images.
However, based purely on MR image findings, the osseous nature of
the tissue would likely have remained undetected. In CT images,
bony expansion with mildly scalloped marginal sclerosis,
nonhomogeneous septum and displacement of the teeth were observed;
these findings would typically be indicative of an ameloblastoma
desmoplastic variant.
To the best of our knowledge, in previous
literature, just 9 cases of CCAM have been reported, for which the
imaging characteristics of intraoral or panoramic X-rays have been
described as an ill-defined area of bone destruction and an
extensive radiolucent lesion displacing the roots of the second
premolar and the second molar, involving the floor of the left
maxillary sinus as well as producing buccal expansion into the
vestibule (5), an ill-defined,
multilocular radiolucency (3), an
irregular bone destruction with truncation of the roots of related
teeth (7), a poorly defined
radiolucency with a regional resorption of the teeth (8) and a multilocular radiolucency (9). Only the study by Mari et al
(4) described the CT findings from
CCAM, and the images were limited to a recurrent tumor. In the
first recurrence, a CT scan revealed a well-demarcated tumor that
filled the maxillary sinus, affecting the nasal septum and floor of
the right orbit. In the second recurrence, a CT scan showed a
massive recurrence affecting the orbital cavity and the anterior
cranial fossa (4). The present case
exhibited a number of the previously described distinguishing
characteristics, as it was ill-defined with multilocular
radiolucency and the displacement of the associated teeth. In
particular, the intraoral image from the present case (Fig. 2) and Muller and Slootweg (3) bear a resemblance, in presenting a
displacement of the teeth without resorption. Additionally, the CT
images in this case confirmed that the tumor exhibited a locally
invasive nature, consistent with the description by Mari et
al (4).
It remains a matter of speculation why the histology
results of the incisional biopsy and the surgical specimen were
entirely different. One possibility is that sections prepared from
the surgical specimen failed to contain any clear cells. The clear
cells may have been lost between the incisional biopsy and the
histologic examination of the surgical specimen; numerous clear
cells were observed in sheets of epithelium in the former, whereas
only solid hyperplasia of ameloblastic nests could be observed in
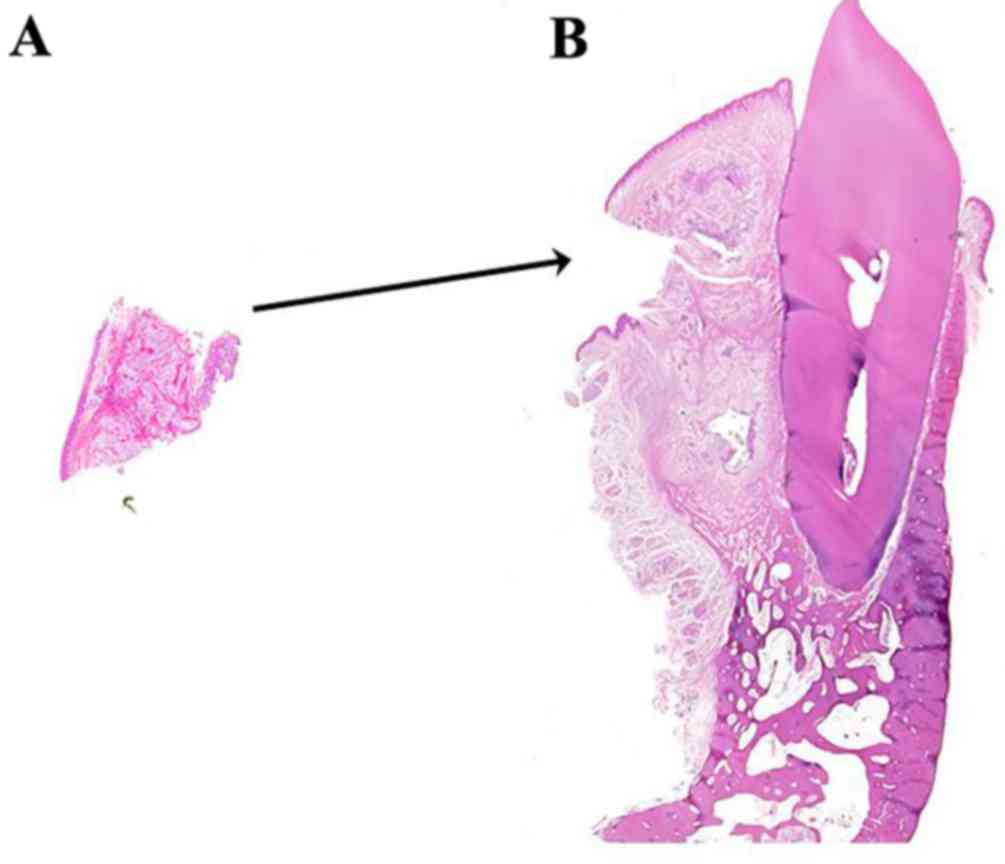
the latter. A further possibility is that the biopsy-section
(Fig. 10A) contained the entire
clear cell component of the tumor. As a consequence, the remaining
section prepared from the surgical specimen (Fig. 10B) did not include any further clear
cells. If this was the case, an additional biopsy obtained from a
different site should have been performed to offset the error. In
either case, sampling error, the pitfall inherently existing in
histopathological diagnosis, was likely a contributing factor.
Based on the present case, performing a re-biopsy in a case with
the histological finding of clear cells in addition to the CT/MRI
findings of desmoplastic ameloblastoma may be advisable. Fig. 10A and B demonstrate a
thought-provoking puzzle in the differences between the biopsy and
surgical specimens. Neither the pathologist from the present study
nor previous literature had ever reported this unusual
situation.
As a result, the presence of clear cell elements
presents a diagnostic challenge, and differentiating CCAM from
similar entities may be enigmatic. However, the presence of clear
cells in an ameloblastoma should be expected due to their origin
from the dental lamina, which has been reported to contain clear
cells (10). The proportion of clear
cells in the lesion has varied in previous reports, from tumors
that are composed almost entirely of clear cells (9,11), to
tumors containing a significant portion of other cellular elements
(3,10). When the other component is
ameloblastic, the lesion has been described as CCAM (5,7). In the
present case, the neoplasm demonstrated a consistent biphasic
histological pattern, with some areas resembling a plexiform-type
ameloblastoma, and other areas resembling a clear cell
component.
On immunohistochemical examination, the clear cells
from the present case were negative for S-100-protein and Ki-67
expression. Antigens have been reported as negative (including
vimentin, desmin, enolase, smooth muscle actin, calponin, S-100
protein, human melanoma black-45, α (1)-chymotrypsin, CD10, CD31, CD45, glial
fibrillary acidic protein and chromogranin) or mildly positive in
expression (keratin 13 and epithelial membrane antigen) for clear
cell odontogenic carcinoma cells (11). A small number of previous reports, as
reviewed by Loyola et al (11), have evaluated Ki-67 expression in
clear cell carcinoma; low proliferative activity (<8%) was
exhibited by the tumors. However, it has been established that
S-100 and Ki-67 expression may be negative in clear cell carcinoma
and CCAM. Loyola et al (11)
stated that ‘a challenging scenario appears in the differential
diagnosis of clear cell odontogenic carcinoma from ameloblastoma
with clear cells’. The present case may also have good cause to
misdiagnose CCAM as clear cell carcinoma.
In differential diagnosis, the clear cell
odontogenic tumor described by Hansen et al should be
considered (12); this tumor
exhibited fibroblastic cellular stroma between the epithelial nests
of clear cells. Ameloblastic differentiation, stellate reticulum
and any resemblance to dental lamina were lacking (13). Other types of neoplasm, including the
clear cell variant of calcifying epithelial odontogenic tumor,
odontogenic fibroma, clear cell variant of mucoepidermoid
carcinoma, clear cell squamous carcinoma and metastasis from renal
carcinoma or hypernephroma should also be considered in the
differential diagnosis (12,13). It is always appropriate to rule out
metastasizing disease when clear cell tumors of the jaw are
encountered.
In conclusion, the present case study has
illustrated the CT and MRI findings for CCAM in the mandible of a
patient, including a characteristic example of the process of
reaching a final diagnosis. This case should serve as a valuable
warning that a definitive diagnosis for CCAM should be based on a
combination of clinical and histopathological features.
References
|
1
|
Regezi JA, Kerr DA and Courtney RM:
Odontogenic tumors: Analysis of 706 cases. J Oral Surg. 36:771–778.
1978.PubMed/NCBI
|
|
2
|
Luna MA and Wenig BM: Polymorphous
low-grade adenocarcinoma. In: World Health Organization
Classification of TumorsPathology and Genetics Head and Neck
Tumours. Barnes L, Eveson J, Reichart P and Sidransky D: IARC
Press; Lyon: pp. 223–224. 2005
|
|
3
|
Müller H and Slootweg P: Clear cell
differentiation in an ameloblastoma. J Maxillofac Surg. 14:158–160.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marí A, Escutia E, Carrera M and Pericot
J: Clear cell ameloblastoma or odontogenic carcinoma. A case
report. J Craniomaxillofac Surg. 23:387–390. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waldron CA, Small IA and Silverman H:
Clear cell ameloblastoma-an odontogenic carcinoma. J Oral
Maxillofac Surg. 43:707–717. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braunshtein E, Vered M, Taicher S and
Buchner A: Clear cell odontogenic carcinoma and clear cell
ameloblastoma: A single clinicopathologic entity? A new case and
comparative analysis of the literature. J Oral Maxillofac Surg.
61:1004–1010. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Odukoya O and Arole O: Clear-cell
ameloblastoma of the mandible (a case report). Int J Oral
Maxillofac Surg. 21:358–359. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Aguiar MC, Gomez RS, Silva EC and de
Araújo VC: Clear-cell ameloblastoma (clear-cell odontogenic
carcinoma): Report of a case. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 81:79–83. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shamim T, Varghese Ipe V, Shameena PM and
Sudha S: Clear cell ameloblastoma in a young child-an enigma in
diagnosis. Indian J Pathol Microbiol. 50:362–364. 2007.PubMed/NCBI
|
|
10
|
Wysocki GP, Brannon RB, Gardner DG and
Sapp P: Histogenesis of the lateral periodontal cyst and the
gingival cyst of the adult. Oral Surg Oral Med Oral Pathol.
50:327–334. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loyola AM, Cardoso SV, de Faria PR,
Servato JP, de Paulo Barbosa LF, Eisenberg AL, Dias FL, Gomes CC
and Gomez RS: Clear cell odontogenic carcinoma: Report of 7 new
cases and systematic review of the current knowledge. Oral Surg
Oral Med Oral Pathol Oral Radiol. 120:483–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansen LS, Eversole LR, Green TL and
Powell NB: Clear cell odontogenic tumor-a new histologic variant
with aggressive potential. Head Neck Surg. 8:115–123. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eversole LR: On the differential diagnosis
of clear cell tumours of the head and neck. Eur J Cancer B Oral
Oncol. 29B:173–179. 1993. View Article : Google Scholar : PubMed/NCBI
|