Introduction
Colorectal cancer (CRC) is the third most common
type of diagnosed cancer and a major cause of mortality in western
countries (1). Although resection of
primary CRC can be curative when the disease is localized, distant
metastasis remains the primary cause of treatment failure and
mortality from cancer. The existing standardized pathological
staging systems do not reflect the exact biological behavior of
carcinoma, which may be associated with tumor metastasis and
recurrence. In the past decades, treatment and molecular
characteristics relating to prognosis of CRC have been focused
mainly on malignant cancer cells. However, recent studies revealed
that CRC progression was mediated in many aspects by tumor stroma
(2), and improvements in survival
have been observed in patients with metastatic CRC using the
therapeutic strategy of targeting tumor stroma (3).
Insulin-like growth factor II mRNA-binding protein 3
(IMP3; IGF2BP3) is a member of the insulin-like growth factor II
mRNA-binding proteins. IMP3 is known as an oncofetal protein, which
has been only been detected in human cancer tissues and early human
embryogenesis, but not in normal tissues of adults (4). Two known functions of IMP3 are
regulation of mRNA stability and subcellular localization (5), and further studies have indicated that
IMP3 promotes tumor cell proliferation, adhesion and invasion
(6–8).
Recent studies revealed that IMP3 expression in tumor cells was a
marker of poor prognosis in CRC patients (9–12).
However, limited information is known about whether stromal
expression of IMP3 is of any clinical relevance in CRC.
Tumor stroma is a circuitous ecosystem consisting of
an extracellular matrix (ECM) scaffold that is populated by
cancer-associated fibroblasts (CAF), vascular space-related cells,
and diverse innate and adaptive immune response cells (13). According to the ‘seed and soil’
hypothesis, tumor cells and stromal cells co-evolve (14). Metastasis is determined by a number of
complex interactions between tumor cells (the ‘seed’) and their
stroma (the ‘soil’) (15). Tumor
stoma is pivotal not only to tumor initiation, malignant
progression and metastasis, but also to response to therapy
(16). Massoner et al
(17) demonstrated that stroma was
the main source of IMP3 in the prostate, suggesting that IMP3 acted
as a mediator of stromal-epithelial interactions. Epithelial
mesenchymal transition (EMT) is proposed as a critical mechanism
for the acquisition of malignant phenotypes by epithelial cells
(18). In colorectal cancer, tumor
cells that have undergone EMT are characterized histologically by
the presence of tumor buds, which are defined as single cells or
small clusters of de-differentiated tumor cells at the invasive
front (19). Tumor budding is
predictive of lymph node metastasis, vascular and lymphatic
invasion, distant metastasis, local recurrence and poor
disease-specific survival time (20).
In the present study, expression of IMP3 in CRC was
evaluated by immunohistochemistry, and it was observed that IMP3
expression was not only abundant in the cytoplasm of tumor cells,
but expression was also detected in stroma cells. The pattern of
IMP3 staining in CRC specimens was examined in order to investigate
its role in the progression and survival of patients with CRC.
Materials and methods
Patients and tumor samples
Formalin-fixed, paraffin-embedded samples were
obtained following surgical resection from 130 patients (70 males
and 60 females; mean age, 61.1 years; range, 24–88 years) with
primary colorectal carcinoma who did not undergo any radiotherapy
and chemotherapy prior to surgery in the Department of Pathology at
the Third Affiliated Hospital of Southern Medical University
(Guangzhou, China) between January 2004 and December 2014. During
follow-up, 58 patients had been followed for >60 months or until
mortality at the end of follow-up, 27 patients succumbed to disease
and 103 patients survived. Clinical data was obtained from
follow-up records including age, gender, tumor location, tumor size
and survival time. The experimental protocol was approved by the
Human Ethics Review Committee of the Third Affiliated Hospital of
Southern Medical University. Informed consent was obtained from all
patients.
Histological examination and tumor
stage
The tumor located on the right side [30 cases, cecum
(12 cases), ascending colon (12 cases) and hepatic flexure (6
cases)] and left side [100 cases, splenic flexure (16 cases),
descending colon (23 cases) and sigmoid colon (61 cases)],
respectively. All specimens were routinely fixed in 10% buffered
formalin and embedded in paraffin at 70°C, put on ice for 10 min,
and 4 µm thick sections were cut and stained with hematoxylin and
eosin (H and E) for 2–3 min at room temperature. All H and E slides
of specimens were re-evaluated, and the diagnosis was confirmed by
two experienced pathologists. Histological subtype, presence of
lympho-vascular invasion, lymph node metastasis, tumor border and
tumor budding were assessed. The histological grade was assessed
according to the World Health Organization 2010 criteria (21). The tumor stage was based on
pathological findings according to the American Joint Committee on
Cancer (AJCC) guidelines (22). Tumor
stage was classified as stage I (T1-2 N0 M0, 16 cases), II (T3-4 N0
M0, 47 cases), III (T1-4 N1-2 M0, 38 cases) and IV (T1-4 N0-3 M1,
29 cases).
Tumor budding was defined as dedifferentiated single
cells or clusters of <5 cells at the invasive tumor front. The
extent of tumor budding was assessed on caudal type homeobox 2
(CDX2)-stained whole tissue sections as described previously by
Karamitopoulou et al (23).
The CDX2-stained whole tissue sections were first examined at low
magnification (×4) and the area with the highest density of
peritumoral budding was selected for counting. The average number
of buds was counted in ten high power fields (magnification, ×40).
Specimens were divided into two groups according to the average
number of budding: Low grade (≤10 buds) and high grade (>10
buds).
Immunohistochemical analysis
Immunohistochemical staining was performed on 4 µm
thick sections. In brief, the sections were deparaffinized and
rehydrated. Heat-induced antigen retrieval was performed at 95°C
for 15 min in citrate buffer (pH 6.0). Endogenous peroxidase
activity was quenched with 3% hydrogen peroxide solution. The
sections were immunostained using a monoclonal mouse anti-human
antibody against IMP3 (cat. no. M362629-2; clone 69.1; dilution,
1:100; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) and
CDX2 (cat. no. GT201907; clone EPR2764Y; dilution, 1:100; Gene Tech
Company, Ltd., Shanghai, China). The GTVision™ Detection system
(Gene Tech Company, Ltd.) and 3,3′-diamino-benzidine (Gene Tech
Company, Ltd.) were used to detect antibody-conjugated peroxidase
activity. The sections were then counterstained with hematoxylin
for 2–3 min at room temperature, dehydrated and mounted.
Evaluation of immunohistochemical
staining
Immunostaining was assessed by two experienced
pathologists, who were blinded to the clinical data of the
patients. Stromal cells positive for cytoplasmic IMP3 staining were
considered IMP3-positive. IMP3 expression in tumor cells was
considered positive if IMP3 staining in tumor cells was >10%.
Nuclear staining for CDX2 was considered positive in resection
specimens (i.e. a majority of cancer cells exhibited widespread
nuclear expression of CDX2; those exhibiting faint or no nuclear
expression were considered CDX2-negative).
Statistical analysis
Statistical analyses were performed using SPSS
(version 13.0; SPSS, Inc., Chicago, IL, USA). The association
between IMP3 expression in tumor stroma and tumor cells was
analyzed using Pearson χ2 test. The association between
IMP3 expression (in tumor stroma and tumor cells) and
clinicopathological parameters were evaluated by Pearson
χ2 and Fisher's exact tests. The Kaplan-Meier method and
the log-rank test were used to analyze the associations between
IMP3 expression (in tumor stroma and tumor cells) and the overall
survival time of patients. A Cox regression model was used for
univariate and multivariate analyses to determine the independent
significance of relevant clinical covariates. Two tailed P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient results
A total of 130 patients with CRC were available for
review between 2004 and 2014 (mean age, 61.1 years; range, 24–88
years). The end date of the follow-up study for conducting the
analysis was 1 January 2015 (mean duration of follow-up, 52.5
months; range, 1–132 months). A total of 58 patients were followed
for >60 months or until mortality. At the end of follow-up, 27
patients succumbed to disease and 103 patients survived.
Clinicopathological characteristics of the patients and tumor
characteristics are summarized in Table
I.
 | Table I.Association between stromal and
tumoral expression of IMP3 and clinicopathological characteristics
in 130 patients with colorectal cancer. |
Table I.
Association between stromal and
tumoral expression of IMP3 and clinicopathological characteristics
in 130 patients with colorectal cancer.
|
|
| IMP3 expression in
tumor stroma | IMP3 expression in
tumor cells |
|---|
|
|
|
|
|
|---|
| Characteristic | Total | Positive (%) | Negative (%) | P-value | Positive (%) | Negative (%) | P-value |
|---|
| Colorectal
cancer | 130 | 24 (18.5) | 106 (81.5) |
| 94 (72.3) | 36 (27.7) |
|
| Age, years |
|
|
| 0.244 |
|
| 0.445 |
| ≤45 | 23 | 2 (8.7) | 21 (91.3) |
| 15 (65.2) | 27 (34.8) |
|
|
>45 | 107 | 22 (20.6) | 85 (79.4) |
| 79 (73.8) | 28 (26.2) |
|
| Gender |
|
|
| 0.181 |
|
| 0.432 |
|
Female | 60 | 8 (13.3) | 52 (86.7) |
| 41 (68.3) | 19 (31.7) |
|
| Male | 70 | 16 (22.9) | 54 (77.1) |
| 53 (75.7) | 17 (24.3) |
|
| Tumor location |
|
|
| 0.104 |
|
| 0.247 |
| Left
sided | 100 | 21 (19.6) | 85 (85.0) |
| 75 (75.0) | 25 (25.0) |
|
| Right
sided | 30 | 3 (13.0) | 21 (87.0) |
| 19 (63.3) | 11 (36.7) |
|
| Tumor size,
diameter (cm) |
|
|
| 0.497 |
|
| 0.171 |
| ≤4 | 59 | 15 (15.0) | 85 (85.0) |
| 39 (66.1) | 20 (33.9) |
|
|
>4 | 71 | 9 (30.0) | 21 (70.1) |
| 55 (77.5) | 16 (22.5) |
|
| Histological
grade |
|
|
| 0.566 |
|
| 0.122 |
| Well
and moderately differentiated | 107 | 9 (15.3) | 50 (84.7) |
| 74 (69.2) | 33 (30.8) |
|
| Poorly
differentiated | 23 | 15 (21.1) | 56 (78.9) |
| 20 (87.0) | 3 (13.0) |
|
| Histological
subtype |
|
|
| 0.130 |
|
| 0.759 |
|
Non-mucinous | 115 | 24 (20.9) | 91 (79.1) |
| 82 (71.3) | 33 (28.7) |
|
|
Mucinous | 15 | 0 (0) | 15 (100) |
| 12 (80.0) | 3 (20.0) |
|
| T
classification |
|
|
| 0.121 |
|
| 0.027 |
|
T1-T2 | 20 | 1 (50) | 19 (95.0) |
| 10 (53.4) | 10 (46.6) |
|
|
T3-T4 | 110 | 23 (20.9) | 87 (79.1) |
| 84 (76.4) | 26 (23.6) |
|
| TNM stage |
|
|
| 0.003 |
|
| 0.011 |
|
I–II | 63 | 5 (7.9) | 58 (92.1) |
| 39 (61.9) | 24 (38.1) |
|
|
III–IV | 67 | 19 (28.4) | 48 (71.6) |
| 55 (82.1) | 12 (17.9) |
|
| Lymph node
metastasis |
|
|
| 0.006 |
|
| 0.048 |
|
Absent | 66 | 6 (9.1) | 60 (90.9) |
| 43 (65.2) | 23 (34.8) |
|
|
Present | 64 | 18 (28.1) | 46 (71.9) |
| 52 (81.2) | 12 (18.8) |
|
| Tumor border |
|
|
| 0.013 |
|
| 0.055 |
|
Infiltrating | 90 | 22 (24.4) | 68 (75.6) |
| 70 (77.8) | 20 (22.2) |
|
|
Pushing | 40 | 2 (5.0) | 38 (95.0) |
| 24 (60.0) | 16 (40.0) |
|
| Tumor budding |
|
|
| 0.371 |
|
| 0.005 |
| Low
(≤10 buds) | 71 | 11 (15.5) | 60 (84.5) |
| 44 (62.0) | 27 (38) |
|
| High
(>10 buds) | 59 | 13 (22.0) | 46 (78.0) |
| 50 (84.7) | 9 (15.3) |
|
| Lympho-vascular
invasion |
|
|
| 0.003 |
|
| 0.116 |
|
Absent | 57 | 4 (7.0) | 53 (93.0) |
| 37 (64.9) | 20 (35.1) |
|
|
Present | 73 | 20 (27.4) | 53 (72.6) |
| 57 (78.1) | 16 (21.9) |
|
Immunohistochemical staining in CRC
tissue samples
Immunostaining of IMP3 was performed in CRC tissue
samples (Fig. 1). IMP3 expression was
not only detected in the tumor cells region, but also in the
cytoplasm of tumor stroma cells (Fig.
1B). Notably, it was observed that stromal expression of IMP3
was primarily expressed in the spindle cells of the tumor stroma
(Fig. 1C). Of the 24 specimens
positive for IMP3 expression in the stroma, positive expression in
the tumor cells was also detected in 18 cases. However, no
statistically significant association was identified between IMP3
expression in the tumor cells and tumor stroma (0.807; Table II). Positive stromal expression of
IMP3 (stroma cells positive for cytoplasmic IMP3 staining) was
detected in 24/130 patients (18.5%), and negative expression was
observed in 106/130 patients (81.5%). By contrast, positive tumoral
expression of IMP3 (IMP3 staining in >10% of tumor cells) was
detected in 94/130 cases of CRC (72.3%), and negative expression
was detected in 36/130 patients (27.7%) (Table I). High tumor budding (Fig. 1E) was observed in 59/130 (45.4%)
patients, whereas low tumor budding (Fig.
1H) was detected in 71/130 (54.6%) cases (Table I). High tumor budding with positive
IMP3 expression was also observed in tumor front of CRC (Fig. 1F).
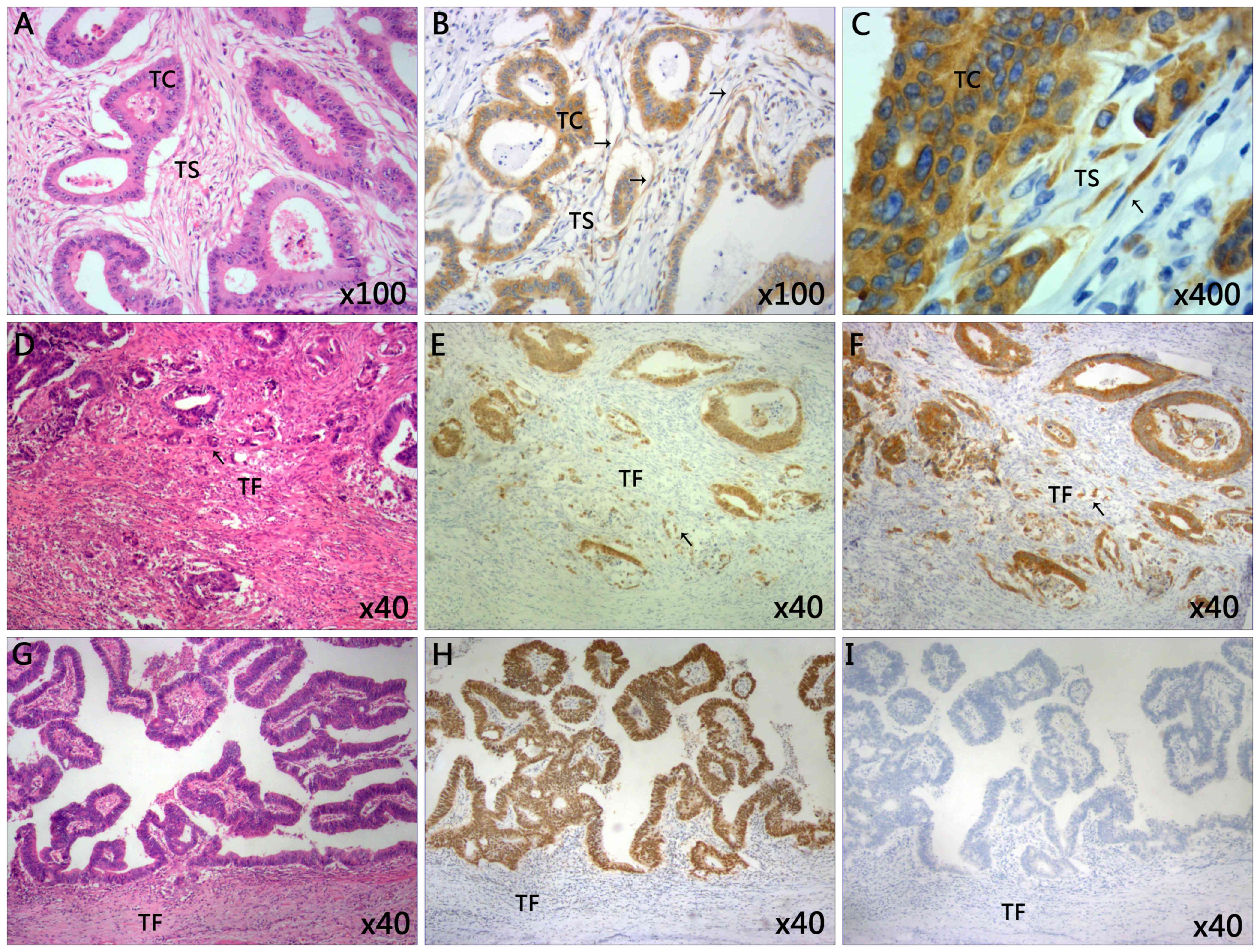 | Figure 1.Representative images of
immunohistochemical staining of tissues from patients with CRC. (A)
The classic microscopic features of colorectal cancer with
hematoxylin and eosin staining. The chromatin in the nucleus was
stained violet blue and the cytoplasm and extracellular matrix were
stained red. Magnification, ×100. (B) High expression of IMP3 in
tumor cells and tumor stroma cells. Magnification, ×100. (C) The
IMP3-positive cells in tumor stroma closely resemble spindle cells.
Magnification, ×400. (D) High grade of tumor budding stained with
hematoxylin and eosin in the TF. Magnification, ×40. (E) The
present study observed the tumor buds using CDX2 staining. CDX2
stained the nucleus brown. The mean number of buds was counted
using ten high power fields. Specimens were divided into two groups
according to the average number of buds: Low grade (≤10 buds) and
high grade (>10 buds). Here the image revealed the high grade
tumor budding with CDX2 staining in the TF. Magnification, ×40. (F)
High expression of tumor budding stained with IMP3 in tumor front
of CRC. Magnification, ×40. (G) Low grade tumor budding with
hematoxylin and eosin staining in the TF. Magnification, ×40. (H)
Low grade of tumor budding stained with CDX2 in tumor front of CRC.
Magnification, ×40. (I) Negative expression of IMP3 in tumor tissue
and tumor front. Magnification, ×40. Slides A, D and G were stained
with hematoxylin and eosin. CDX2, caudal type homeobox 2; CRC,
colorectal cancer; IMP3, insulin-like growth factor II mRNA-binding
protein 3; TC, tumor cells; TS, tumor stroma; TF, tumor front. |
 | Table II.Concordance of IMP3 expression
between tumor cells and tumor stroma. |
Table II.
Concordance of IMP3 expression
between tumor cells and tumor stroma.
|
| Tumoral IMP3
expression, n |
|
|
|---|
|
|
|
|
|
|---|
| Stromal IMP3
expression | Positive | Negative | Total, n | P-value |
|---|
| Positive | 18 | 6 | 24 | 0.807 |
| Negative | 76 | 30 | 106 |
|
| Total | 94 | 36 | 130 |
|
Association between stromal expression
of IMP3 and clinicopathological characteristics
Stromal expression of IMP3 was associated with TNM
stage (stage III–IV, P=0.003), lymph node metastasis (P=0.006),
lympho-vascular invasion (P=0.003) and tumor border (infiltrating
vs. pushing, P=0.013). No statistically significant associations
were observed between IMP3 expression in tumor stroma and age
(P=0.244), gender (P=0.181), tumor location (P=0.104), tumor size
(P=0.497), histological grade (P=0.566), histological subtype
(P=0.130), T-classification (T1-2 vs. T3-4, P=0.121) and tumor
budding (P=0.371) (Table I).
Associations between tumoral
expression of IMP3 and clinicopathological characteristics
Tumoral expression of IMP3 was significantly
associated with T-classification (T1-2 vs. T3-4, P=0.027; Table I), TNM stage (stage I–II vs. III–IV,
P=0.011), lymph node metastasis (absent vs. present, P=0.048) and
tumor budding (low vs. high, P=0.005). Additionally, IMP3
expression was significantly associated with CDX2 expression in
tumor cells and tumor budding (all P=0.005), which means tumor
budding with CDX2 staining was also positive for IMP3, (Fig. 1E, F, H and I). Furthermore, there was
no significant association between IMP3 expression and age
(P=0.445), gender (P=0.432), tumor location (P=0.247), tumor size
(P=0.171), histological grade (P=0.122), histological subtype
(P=0.759), lympho-vascular invasion (P=0.116) and tumor border
(P=0.055) (Table I).
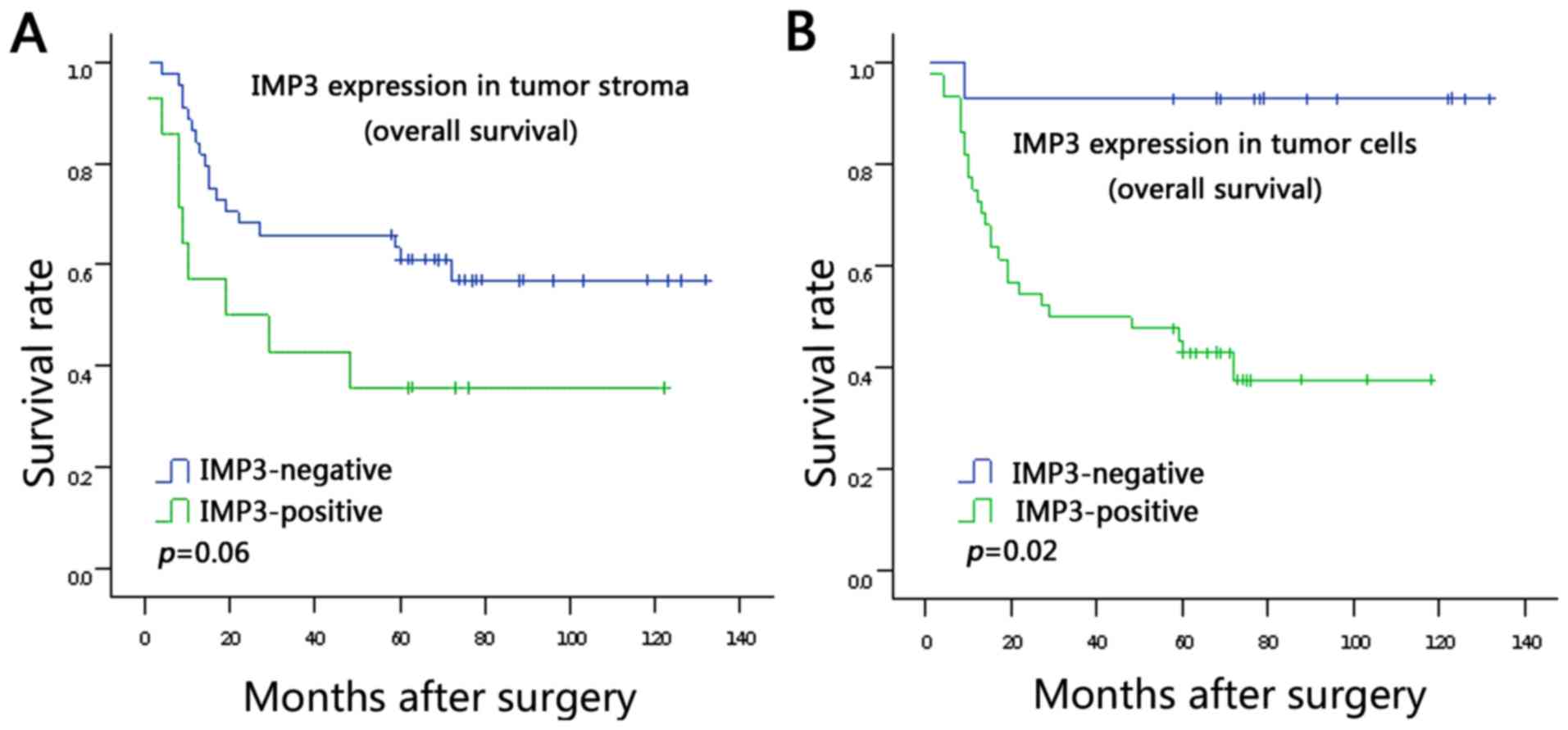
Survival analysis
The associations between IMP3 expression in the
tumor stroma and tumor cells and survival rate were analyzed using
Kaplan-Meier analysis (Fig. 2A and
B). It was demonstrated that patients with positive IMP3
expression in tumor cells had a poorer survival rate compared with
patients with negative IMP3 expression in tumor cells (log rank
P=0.02; Fig. 2B). The data did not
show statistically significant association between stromal
expression of IMP3 and survival rate, but a trend is evident from
the survival curve: That patients with stromal expression of IMP3
tend to have poor survival rates (log rank P=0.06; Fig. 2A). Moreover, in a univariate analysis
based on the Cox regression model, tumoral expression of IMP3
(P=0.015), TNM stage (P<0.001), lymph node metastasis (P=0.002)
and lympho-vascular invasion (P=0.006) were observed to be
associated with poor prognosis (Table
III). However, multivariate survival analysis using the Cox
regression model indicated that tumoral expression of IMP3
(P=0.029) and TNM stage (P=0.045) were independent prognostic
factors (Table III).
 | Table III.Univariate and multivariate analyses
of the associations between prognostic variables in 130 patents
with colorectal carcinoma. |
Table III.
Univariate and multivariate analyses
of the associations between prognostic variables in 130 patents
with colorectal carcinoma.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumoral expression
of IMP3 |
|
|
|
|
| 0.029 |
|
Negative vs. positive | 11.891 | 1.606–88.074 | 0.015 | 9.356 | 1.250–70.048 |
|
| TNM stage |
|
|
|
|
| 0.045 |
| I- II
vs. III -IV | 7.195 | 2.471–20.949 | <0.001 | 6.178 | 1.037–36.800 |
|
| Lymph node
metastasis |
|
|
|
|
| 0.567 |
| No vs.
yes | 4.068 | 1.708–9.689 | 0.002 | 0.511 | 0.051–5.0913 |
|
| Lympho-vascular
invasion |
|
|
|
|
| 0.402 |
| No vs.
yes | 3.973 | 1.499–10.530 | 0.006 | 0.402 | 0.358–12.913 |
|
Discussion
Distant metastasis and recurrence are still the main
causes of mortality in patients with CRC (1). Investigation of metastatic mechanisms
and identification of molecular targets remains a key issue faced
in CRC research. Tumor stroma has a crucial role in tumor
progression, which provides an interface between malignant cells
and host tissue (24). The balance of
host-tumor interdependency is able to modulate the phenotype of a
tumor to influence the outcome of the disease (25). IMP3 expression has been verified to be
present predominantly in the tumor cells of multiple malignancies
including CRC, and its presence has been associated with poor
survival following surgical resection (9–12).
However, little is known about the role of stromal IMP3 in CRC
progression.
To the best our knowledge, the present study
elucidated for the first time that IMP3 expressed in tumor stroma
cells in CRC was associated with TNM stage, lymph node metastasis,
lympho-vascular invasion and tumor border. These findings indicated
that stromal expression of IMP3 may be used as a potential marker
for lymph node metastasis and TNM stage. The stromal expression of
IMP3 may be useful in identifying CRC patients with a poor
prognosis though the present data did not indicate a statistical
significant association between stromal expression of IMP3 and a
poor survival (P=0.06), which may be due to a small sample size
(follow-up >60 month, 58 cases) used in the study. Therefore,
large scale studies are warranted. IMP3 expression has been
detected in the stroma, particularly reactivated stroma which was
the main source of IMP3 in the prostate, suggesting that this
peptide acts as a mediator of stromal-epithelial interaction
(17). Further study showed that IMP3
was essential for transforming growth factor β1-mediated
differentiation. The dysregulation of the stromal IGF axis, in
particular elevated IMP3 expression, has a crucial role in
fibroblast-to-myofibroblast differentiation in the diseased
prostatic stroma (26). Since the
abundance of myofibroblasts in cancer-associated stroma may be an
useful indicator of disease recurrence in CRC patients (27), the findings in the present study lead
to the hypothesis that IMP3 may contribute to the recruitment of
CAFs to promote tumor invasion and metastasis. The molecular
mechanism of stromal IMP3 in the CRC progression would be the focus
of further study.
Consistent with previous findings (9–12), the
present study demonstrated that positive IMP3 expression in tumor
cells predicted a poor survival in CRCs (P=0.02). Moreover, to the
best of our knowledge, the present study was the first to associate
IMP3 expression in tumor cells with tumor budding (P=0.005). Tumor
budding is not a static, histological feature; it represents a
snapshot of a dynamic process undertaken by an aggressive tumor
with the potential to disseminate and metastasize (28). Tumor budding was recommended as an
additional prognostic factor according to the AJCC (29). Furthermore, it was detected in the
present study that tumor budding in the tumor front was also
positive for IMP3 staining. Taken together, the strong association
indicated that IMP3 may have a vital role in EMT to promote cancer
invasiveness.
Furthermore, it was identified that the prognostic
value of IMP3 expression in tumor cells was better than that of
lymph node metastasis. Therefore, IMP3 may be a novel prognostic
marker in CRC. The modern evolution of CRC treatment has tended
toward multidisciplinary management combining multiple types of
treatment, including chemotherapy, surgery and radiotherapy
(30). Advances in preoperative
chemotherapy and radiation have reduced disease recurrence and
increased survival in high-risk diseases (31). However, preoperative chemotherapy and
radiotherapy can lead to partial or complete pathological
regression (10–27%) of CRC (32). The
present data suggested that IMP3 expression in in biopsy specimens
form patients with CRC may be used as a parameter to select
patients that are most likely to benefit from preoperative
chemotherapy and radiotherapy.
Finally, the present results suggested that CRCs may
benefit from a targeted anti-IMP3 therapy on the basis of the
finding that IMP3 was expressed mainly in tumor cells (72.3%).
Since benefit was seen in survival in metastatic CRC with the
therapeutic strategy of targeting tumor stroma (2), the finding from the present study that
stromal expression of IMP3 is associated with advanced tumor TNM
stage and metastasis may suggest that stromal therapy may be a
viable approach for CRC. We hypothesize that targeting a tumor as
an organ (i.e. the tumor and stroma together) would be more
effective than targeting the tumor cells alone.
Here, the findings suggested that IMP3 expression
was upregulated in the tumor cells and stromal compartments of
patients with CRC compared with that in the normal colonic mucosal
epithelium and the colonic adenoma. A significant association was
identified between stromal IMP3 expression and lymph node
metastasis and advanced tumor TNM stage. Moreover, IMP3
overexpression in tumor cells can be used as an independent factor
for predicting poor prognosis of patients with CRC. However,
although the results presented here may be useful as a biological
marker, the findings may not specifically reflect the biological
nature of cancer is only a preliminary phenomenological study and
does not investigate the specific molecular mechanism (33). Further research of the underlying
interactive mechanism of IMP3 in primary tumor and stroma should
allow an improved understanding of more specific interactions
between tumor cells and microenvironment. A larger scale
prospective study will be necessary to verify the prognostic
significance of IMP3.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isella C, Terrasi A, Bellomo SE, Petti C,
Galatola G, Muratore A, Mellano A, Senetta R, Cassenti A, Sonetto
C, et al: Stromal contribution to the colorectal cancer
transcriptome. Nat Genet. 47:312–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giordano G, Febbraro A, Venditti M,
Campidoglio S, Olivieri N, Raieta K, Parcessepe P, Imbriani GC,
Remo A and Pancione M: Targeting angiogenesis and tumor
microenvironment in metastatic colorectal cancer: Role of
aflibercept. Gastroenterol Res Pract. 2014:5261782014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Müeller-Pillasch F, Lacher U, Wallrapp C,
Micha A, Zimmerhackl F, Hameister H, Varga G, Friess H, Büchler M,
Beger HG, et al: Cloning of a gene highly overexpressed in cancer
coding for a novel KH-domain containing protein. Oncogene.
14:2729–2733. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao B, Hu Y, Herrick D and Brewer G: The
RNA-binding protein IMP-3 is a translational activator of
insulin-like growth factor II leader-3 mRNA during prolifearation
of human K562 leukemia cells. J Biol Chem. 280:18517–18524. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bell JL, Wächter K, Mühleck B, Pazaitis N,
Köhn M, Lederer M and Hüttelmaier S: Insulin-like growth factor 2
mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of
cancer progression? Cell Mol Life Sci. 70:2657–2675. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gong Y, Woda BA and Jiang Z: Oncofetal
protein IMP3, a new cancer biomarker. Adv Anat Pathol. 21:191–200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mueller F, Bommer M, Lacher U, Ruhland C,
Stagge V, Adler G, Gress TM and Seufferlein T: KOC is a novel
molecular indicator of malignancy. Br J Cancer. 88:699–701. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan RH, Wang CC, Chou CC, Chang KJ, Lee
PH and Jeng YM: Diffuse expression of RNA-binding protein IMP3
predicts high-stage lymph node metastasis and poor prognosis in
colorectal adenocarcinoma. Ann Surg Oncol. 16:1711–1719. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Yan D, Tang H, Zhou C, Fan J, Li S,
Wang X, Xia J, Huang F, Qiu G and Peng Z: IMP3 is a novel
prognostic marker that correlates with colon cancer progression and
pathogenesis. Ann Surg Oncol. 12:3499–3506. 2009. View Article : Google Scholar
|
|
11
|
Lochhead P, Imamura Y, Morikawa T, Kuchiba
A, Yamauchi M, Liao X, Qian ZR, Nishihara R, Wu K, Meyerhardt JA,
et al: Insulin-like growth factor 2 messenger RNA binding protein 3
(IGF2BP3) is a marker of unfavourable prognosis in colorectal
cancer. Eur J Cancer. 48:3405–3413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin L, Zhang J, Wang Y, Ju W, Ma Y, Li L
and Chen L: Insulin-like growth factor-II mRNA-binding protein 3
predicts a poor prognosis for colorectal adenocarcinoma. Oncol
Lett. 6:740–744. 2013.PubMed/NCBI
|
|
13
|
Herrera M, Islam AB, Herrera A, Martín P,
García V, Silva J, Garcia JM, Salas C, Casal I, de Herreros AG, et
al: Functional heterogeneity of cancer-associated fibroblasts from
human colon tumors shows specific prognostic gene expression
signature. Clin Cancer Res. 19:5914–5926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhowmick NA and Moses HL: Tumor-stroma
interactions. Curr Opin Genet Dev. 15:97–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J and Liu J: Tumor stroma as targets
for cancer therapy. Pharmacol Ther. 137:200–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massoner P, Haag P, Seifarth C, Jurgeit A,
Rogatsch H, Doppler W, Bartsch G and Klocker H: Insulin-like growth
factor binding protein-3 (IGFBP-3) in the prostate and in prostate
cancer: Local production, distribution and secretion pattern
indicate a role in stromal-epithelial interaction. Prostate.
68:1165–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prall F: Tumour budding in colorectal
carcinoma. Histopathology. 50:151–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hase K, Shatney CH, Mochizuki H, Johnson
DL, Tamakuma S, Vierra M and Trollope M: Long-term results of
curative resection of ‘minimally invasive’ colorectal cancer. Dis
Colon Rectum. 38:19–26. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive system,
Fourth Edition. WHO Classif Tumours Digestive System. 3:2010
|
|
22
|
O'Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
Cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karamitopoulou E, Zlobec I, Kölzer V,
Kondi-Pafiti A, Patsouris ES, Gennatas K and Lugli A: Proposal for
a 10-high-power-fields scoring method for the assessment of tumor
budding in colorectal cancer. Mod Pathol. 26:295–301. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bissell MJ and Radisky D: Putting tumours
in context. Nat Rev Cancer. 1:46–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calorin L and Bianchini F: Environmental
control of invasiveness and metastatic dissenmination of tumor
cells: The role of tumor cell-host cell interations. Cell Commun
Signal. 8:242010.PubMed/NCBI
|
|
26
|
Sampson N, Zenzmaier C, Heitz M, Hermann
M, Plas E, Schäfer G, Klocker H and Berger P: Stromal insulin-like
growth factor binding protein 3 (IGFBP3) is elevated in the
diseased human prostate and promotes ex vivo
fibroblast-to-myofibroblast differentiation. Endocrinology.
154:2586–2599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsujino T, Seshimo I, Yamamoto H, Ngan CY,
Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N and Monden M:
Stromal myofibroblasts predict disease recurrence for colorectal
cancer. Clin Cancer Res. 13:2082–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zlobec I and Lugli A: Epithelial
mesenchymal transition and tumor budding in aggressive colorectal
cancer: Tumor budding as oncotarget. Oncotarget. 1:651–661. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giger OT, Comtesse SC, Lugli A, Zlobec I
and Kurrer MO: Intra-tumoral budding in preoperative biopsy
specimens predicts lymph node and distant metastasis in patients
with colorectal cancer. Mod Pathol. 25:1048–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van de Velde CJ, Aristei C, Boelens PG,
Beets-Tan RG, Blomqvist L, Borras JM, van den Broek CB, Brown G,
Coebergh JW, Cutsem EV, et al: EURECCA colorectal:
Multidisciplinary mission statement on better care for patients
with colon and rectal cancer in Europe. Eur J Cancer. 49:2784–2790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed S, Johnson K, Ahmed O and Iqbal N:
Advances in the management of colorectal cancer: From biology to
treatment. Int J Colorectal Dis. 29:1031–1042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R,
Haustermans K, et al: Long-term outcome in patients with a
pathological complete response after chemoradiation for rectal
cancer: A pooled analysis of individual patient data. Lancet Oncol.
11:835–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishio K, Inoue A, Qiao S, Kondo H and
Mimura A: Senescence and cytoskeleton: Overproduction of vimentin
induces senescent-like morphology in human fibroblast. Histochem
Cell Biol. 116:321–327. 2001. View Article : Google Scholar : PubMed/NCBI
|