Introduction
Thyroid cancer is a kind of malignant endocrine
system tumor with the highest incidence rate (1), which consists of 4 different
pathological types: papillary thyroid carcinoma (PTC), medullary
carcinoma, follicular carcinoma and undifferentiated carcinoma.
Approximately 60–89% of thyroid cancer is PTC (2) that is highly differentiated (3) and often occurs in children or female
patients aged 20–50 years. The pathogenesis of PTC is very complex,
which is closely related to the heredity, environment and endocrine
levels; moreover, the radioactive substances, Hashimoto thyroiditis
and iodine deficiency can lead to the occurrence and development of
PTC (4). In recent years, the
incidence rate of PTC has risen continuously (5,6), and it
has aroused great concern of scholars world-wide. The combined
application of treatment methods, such as surgery and nuclide, can
significantly improve the survival of PTC patients, and the 10-year
survival rate of 70% patients can be up to 10 years (7). Thyroid stimulating hormone (TSH)
receptor exists in PTC cells, and the PTC cell growth and
differentiation depend on TSH secreted by pituitary gland to a
certain extent. Therefore, the routine application of levothyroxine
(TH) for TSH inhibition therapy after PTC operation can reduce the
postoperative recurrence of patients and obtain a better prognosis
(8). At the same time, the serum TSH
level can be used as a clinical index of predicting PTC in patients
with thyroid nodules (9,10). In this study, the in vitro cell
experiment was performed to observe the effects of TSH and TH
intervention on the proliferation of PTC cells, and the role of TSH
in the pathogenesis of PTC and the therapeutic effect of TH were
investigated from the cytological level, so as to provide an
experimental basis for the clinical prevention and treatment of
PTC.
Materials and methods
Experimental materials and
reagents
Human PTC cells (TPC-1) (BNCC, Beijing, China);
cattle TSH and TH (Sigma, San Francisco, CA, USA); methyl thiazolyl
tetrazolium (MTT) (Sigma); RPMI-1640 medium and fetal calf serum
(Gibco, Grand Island, NY, USA); cell cycle assay kit (Beyotime,
Shanghai, China); TRIzol RNA extraction reagent (Takara, Shiga,
Japan); reverse transcription kit (Toyobo, Osaka, Japan); human
cyclin D1 and β-actin primers (Shanghai Sangon Biomedical
Engineering Co., Ltd., Shanghai, China); SYBR Green PCR Master mix
(Takara); cyclin D1 enzyme-linked immunosorbent assay (ELISA) kit
(R&D Systems, Inc., Minneapolis, MN, USA).
Laboratory equipment
CO2 incubator (Thermo Fisher Scientific,
Inc., Waltham, MA, USA); clean bench (Suzhou Purification Equipment
Co., Ltd., Suzhou, China); inverted fluorescence microscope (Nikon,
Tokyo, Japan); flow cytometer (Becton-Dickinson, Franklin Lakes,
NJ, USA); continuous-wavelength multi-function microplate reader
(Tecan Austria, Grodig, Austria); real-time fluorescence
quantitative polymerase chain reaction (PCR) instrument (Eppendorf,
Hamburg, Germany).
TPC-1 cell culture
TPC-1 cells were cultured in RPMI-1640 medium
containing 100 µg/ml streptomycin, 100 U/ml penicillin and 10%
fetal bovine serum, and the liquid was replaced every other day,
followed by passage after cells grew to 80% confluence. The
3–5-generation cells in the logarithmic growth phase were used for
experiments.
Experimental grouping
1) Single application of 0.1, 1.0 and 10 U/l TSH; 2)
single application of 10−2, 10−4 and
10−6 mol/l TH; according to the appropriate
concentration 1) and 2) screened in the early experiment, cells
were further grouped and used for experiment: 1) Normal group:
RPMI-1640 medium + 10% fetal calf serum; 2) TSH group: 10U/l TSH
intervention; 3) TH group: 10−2 mol/l TH intervention;
4) combined group: (TSH+TH group): 10 U/l TSH+10−2 mol/l
TH intervention. TPC-1 cells were treated in different culture
solution for 48 h. The study was approved by the Ethics Committee
of Shandong Institute Hospital. Signed written informed consents
were obtained from all participants before the study.
Detection of cell proliferation via
MTT assay
TPC-1 cells were inoculated onto a 96-well plate
with 104 cells in each well. After the normal culture
for 24 h, the cells were synchronously incubated in the serum-free
culture solution for 24 h, and then 200 µl different media were
added for intervention for 48 h according to the above grouping. At
the same time, the blank control group that was only added with
complete medium without cells was set for zero setting. Six control
wells were set for each group. After 48 h, the original culture
solution was removed from each well, and then 100 µl 0.5 mg/ml MTT
solution was added into each well for continuous incubation at 37°C
for 4 h. Then the MTT solution was absorbed, and 100 µl dimethyl
sulfoxide was added into each well, followed by vibration on the
shaking table to dissolve the crystal. The continuous-wavelength
multi-function microplate reader was used to measure the optical
density (OD) value at 492 nm.
Detection of TPC-1 cell proliferation
cycle
TPC-1 cells were inoculated onto a 6-well plate with
105 cells in each well. After normal culture for 24 h,
the control group, TSH group, TH group and TSH+TH group received
drug intervention for 48 h. Then cells were collected, pre-cooled
using 75% alcohol at −20°C, and fixed overnight. Then cells were
washed with phosphate buffered saline (PBS) 3 times, and then RNase
and PI dye were added successively. Finally, the cell cycle in each
group was measured using the flow cytometer, and the proportions of
cells in G1 phase and S phase in each group were observed.
Total RNA extraction and reverse
transcription
TPC-1 cells in control group, TSH group, TH group
and TSH+TH group received the drug intervention for 48 h. Then the
cells were collected and added with appropriate amount of TRIzol
reagent. The total RNA was extracted according to the instructions
of TRIzol kit, and its concentration was determined using the
ultraviolet spectrophotometer. Both RNA concentration and purity
reached the experimental requirements. The cDNA was prepared via
RNA reverse transcription according to the instructions and stored
at −80°C.
Detection of mRNA expression level of
cyclin D1 in each group via real-time PCR
The primer sequences of cyclin D1 and β-actin gene
are shown in Table I. The reaction
system was as follows: 2 µl cDNA, 12.5 µl 2X SYBR Green PCR master
mixes, 0.5 µl forward primer and 0.5 µl reverse primer; finally
ultra-pure water was added until the total volume was 25 µl.
Amplification procedure: 95°C for 30 sec, 95°C for 5 sec and 60°C
for 30 sec, a total of 40 cycles. After the amplification, the
amplification curve and Ct value were read. With β-actin as a
reference, the relative quantitative 2−∆∆Ct method was
used to compare the expression difference of each gene.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Detection gene | Amplification
length | Primer sequence |
|---|
| Cyclin D1 | 146 bp | Forward primer:
5′-CAATGACCCCGCACGATTTC-3′ |
|
|
| Reverse primer:
5′-CATGGAGGGCGGATTGGAA-3′ |
| β-actin | 200 bp | Forward primer:
5′-CGCACCACTGGCATTGTCAT-3′ |
|
|
| Reverse primer:
5′-TTCTCCTTGATGTCACGCAC-3′ |
Detection of cyclin D1 in TPC-1
cells
After the drug intervention for TPC-1 cells
according to the above grouping for 48 h, the supernatant was
collected and centrifuged at 3,627 × g for 10 min at 4°C. The
supernatant was separated for ELISA. Sample loading and treatment
were performed strictly according to the instructions of the kit
and the OD value was measured at 490 nm using the
continuous-wavelength multi-function microplate reader. Besides,
the standard curve was drawn and the content of cyclin D1 in each
sample was calculated.
Statistical analysis
Experimental results are presented as mean ± SD, and
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used for
statistical analysis of data. Independent sample t-test was used
for the comparison between the two groups, one-way analysis of
variance was used for the comparison among groups, and LSD method
was used for the pairwise comparison. P<0.05 is considered to
indicate a statistically significant difference.
Results
Effect of TSH on the proliferation of
TPC-1 cells
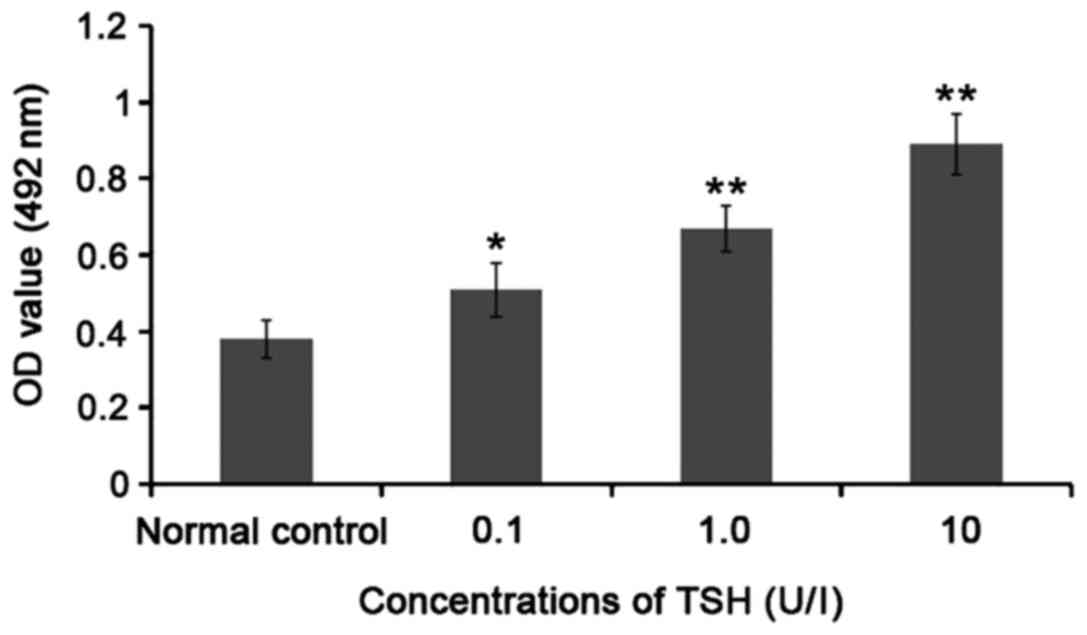
Compared with that in normal control group, the OD
value was significantly increased (P<0.05 or P<0.01) after
0.1, 1.0 and 10 U/l TSH acted on TPC-1 cells for 48 h; with the
increase in TSH concentration, the OD value was significantly
increased, suggesting that TSH can significantly promote the TPC-1
cell proliferation, and its proliferation reached the peak at 10
U/l. Thus, 10 U/l TSH was selected for the subsequent experiments
(Fig. 1).
Effect of TH on the proliferation of
TPC-1 cells
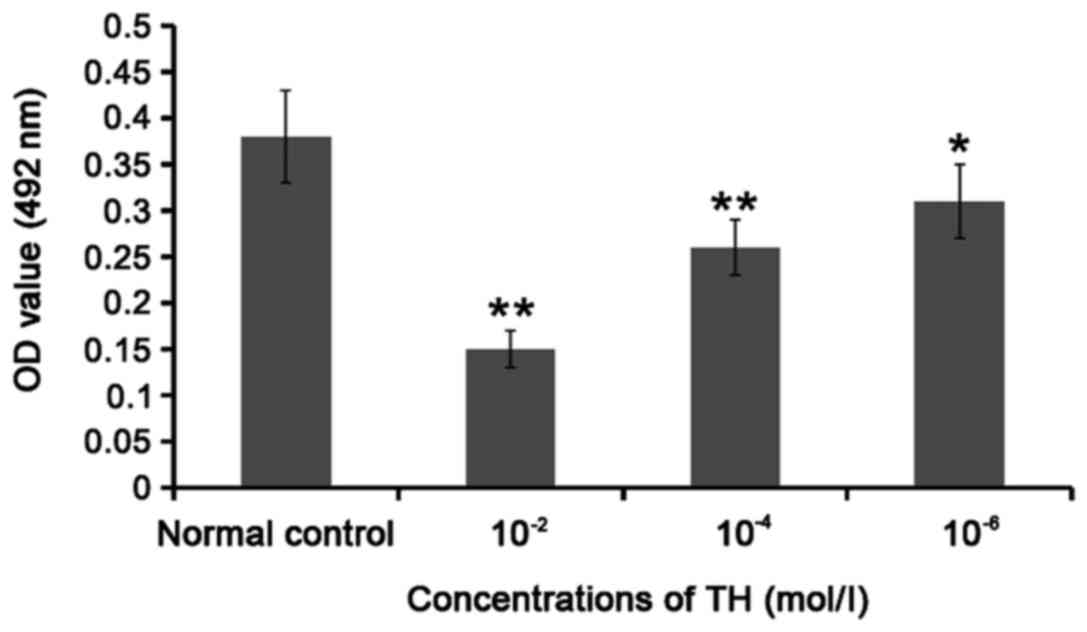
Compared with that in normal control group, the OD
value was significantly decreased (P<0.05 or P<0.01) after
10−2, 10−4 and 10−6 mol/l TH acted
on TPC-1 cells for 48 h; with the increase in TH concentration, the
OD value was significantly decreased, suggesting that TH can
significantly inhibit the TPC-1 cell proliferation, and its
proliferation reached the bottom at 10−2 mol/l. Thus,
10−2 mol/l TH was selected for the subsequent
experiments (Fig. 2).
Effect of TH combined with TSH on the
proliferation of TPC-1 cells
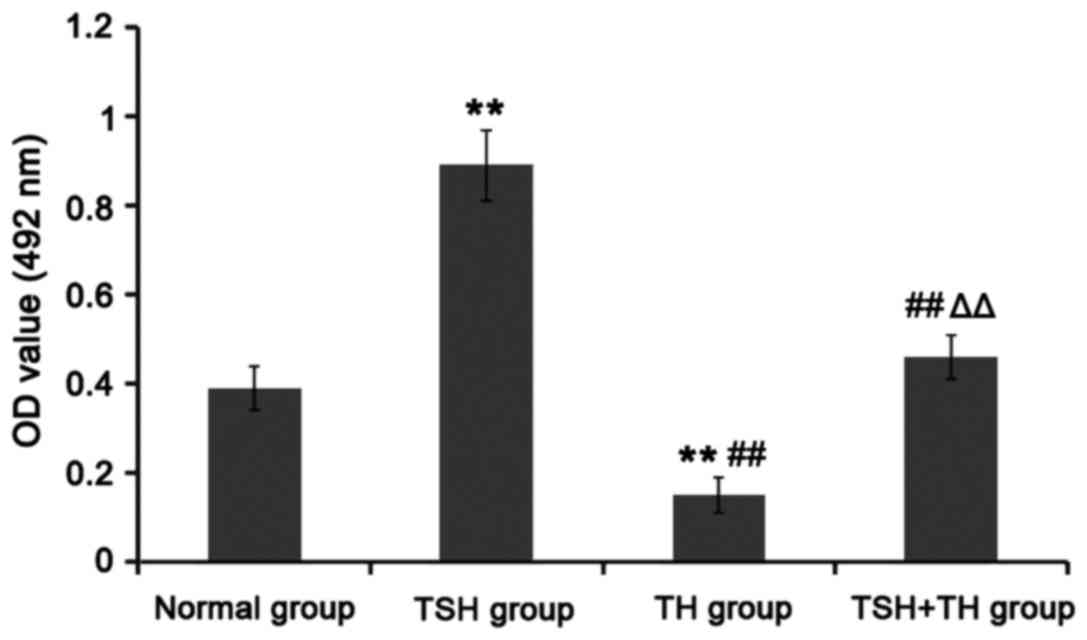
Compared with that in normal group, the OD value was
significantly increased in TSH group (P<0.01), but significantly
decreased in TH group (P<0.01). Compared with that in TSH group,
the OD values in TH group and TSH+TH group were significantly
decreased (P<0.01). The OD value in TSH+TH group was
significantly increased compared with that in TH group (P<0.01).
There was no significant difference between TSH+TH group and normal
group (P>0.05). It can be seen that TH can significantly inhibit
the proliferation of TPC-1 cells (Fig.
3).
Effects of TH, TSH and combined
application on the TPC-1 cell cycle
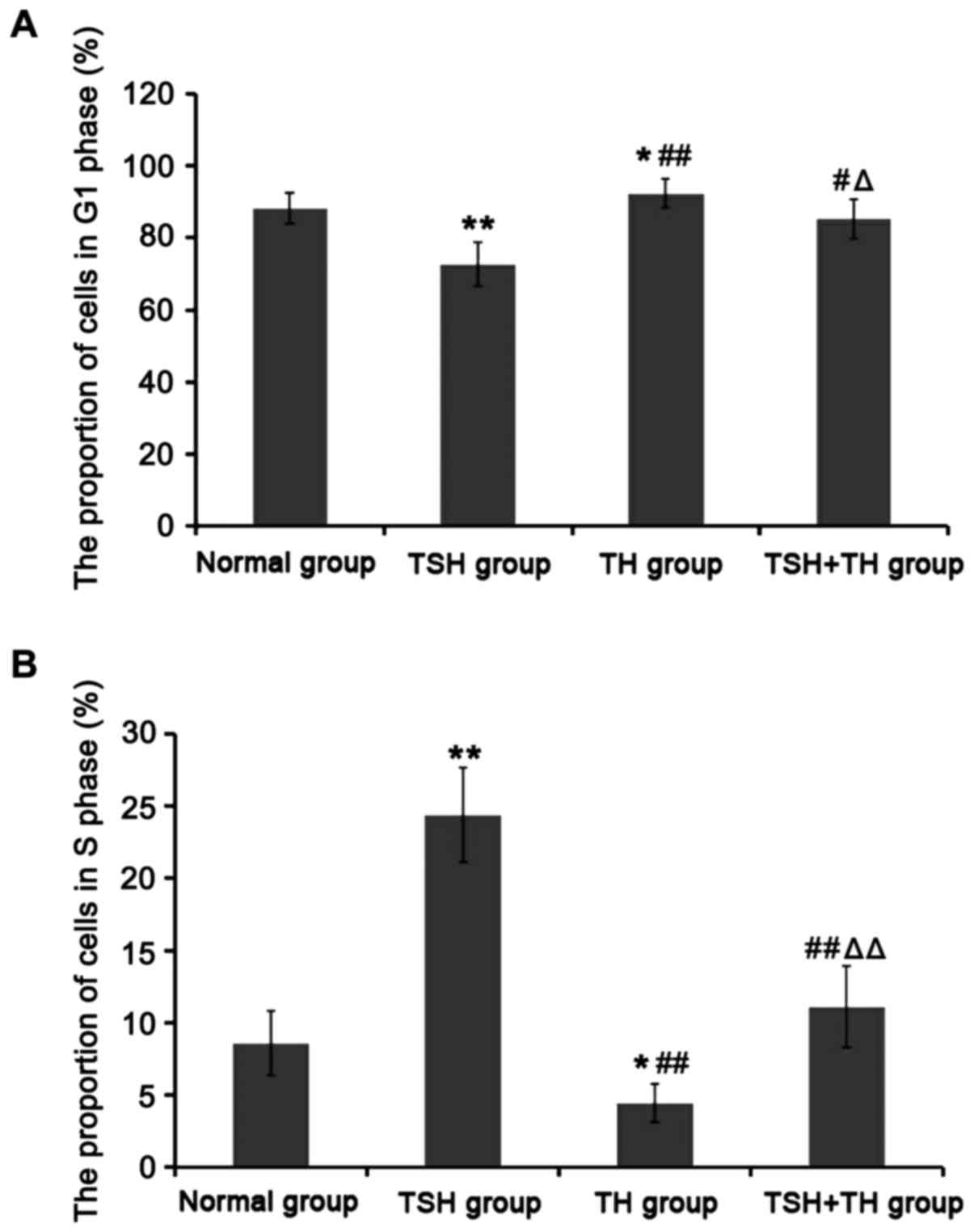
Compared with those in normal group, the proportion
of cells in G1 phase in TSH group was significantly decreased, but
that in S phase was significantly increased (P<0.01); the
proportion of cells in G1 phase in TH group was significantly
increased, but that in S phase was significantly decreased
(P<0.05). Compared with those in TSH group, the proportions of
cells in G1 phase in TH group and TSH group were significantly
increased, but those in S phase were significantly decreased
(P<0.05 or P<0.01). Compared with those in TH group, the
proportion of cells in G1 phase in TSH+TH group was significantly
decreased, but that in S phase was significantly increased
(P<0.05 or P<0.01). There was no significant difference
between TSH+TH group and normal group (P>0.05) (Fig. 4).
Effects of TH, TSH and combined
application on the mRNA and protein expression levels of cyclin D1
in TPC-1 cells
Compared with those in normal group, the mRNA and
protein expression levels of cyclin D1 in TSH group were
significantly increased (P<0.01), and those in TH group were
significantly decreased (P<0.05). Compared with those in TSH
group, the mRNA and protein expression levels of cyclin D1 in TH
group and TSH+TH group were significantly decreased (P<0.05 or
P<0.01). Compared with those in TH group, the mRNA and protein
expression levels of cyclin D1 in TSH+TH group were significantly
increased (P<0.05). There was no significant difference between
TSH+TH group and normal group (P>0.05) (Table II).
 | Table II.Effects of TH, TSH and combined
application on the mRNA and protein expression levels of cyclin
D1. |
Table II.
Effects of TH, TSH and combined
application on the mRNA and protein expression levels of cyclin
D1.
| Group | mRNA | Protein (ng/l) |
|---|
| Normal group | 1.02±0.11 | 1.89±0.25 |
| TSH group |
2.18±0.34b |
3.07±0.38b |
| TH group |
0.63±0.20a,d |
1.42±0.27a,d |
| TSH+TH group |
1.32±0.32c,e |
2.34±0.36c,e |
Discussion
The largest endocrine gland in human body is the
thyroid gland, and hypothalamus-anterior pituitary system regulates
the growth, development and functions of thyroid gland (11). TSH is secreted by the anterior
pituitary, its function is to promote the synthesis of thyroid
hormone. Human TSH is a kind of glycoprotein in the body, mainly
composed of 211 amino acids, 15% of which in the whole molecule is
the sugar. Moreover, the entire TSH molecule consists of two
peptide chains: α and β chains. Moreover, the secretion of TSH has
significant rhythm: TSH gradually increases at 2 h after sleep and
its level in serum reach the peak at 2–4 o'clock in the morning
(12,13). TSH receptor belongs to the
G-protein-coupled receptor, mainly expressed on the thyroid
follicular epithelial cell membrane. The encoding gene of the TSH
receptor is located on chromosome 14 and approximately 60 kb in
length, which consists of 10 exons (14). TSH binds to the extracellular amino
terminal of its receptor to regulate the thyroid function by
enhancing the iodine pump activity and tyrosine iodination,
promoting the thyroglobulin synthesis and enhancing the peroxidase
activity (15).
In recent years, a large number of basic and
clinical studies world-wide have found that when the thyroid
hormone is lacking in the body, it can secrete a large amount of
TSH into the blood, leading to high TSH and increasing the risk of
thyroid tumor (16). Under the
long-term stimulation of TSH, thyroid tissues will suffer from
diffuse enlargement, and the thyroid nodules are gradually formed
with the course of disease, ultimately developing into thyroid
cancer without timely treatment (17). In patients with thyroid nodules, the
probability of suffering from thyroid cancer increases with the
increase in serum TSH level (9). In
animal experiments, it is found that the appropriate supplement of
thyroxine can reduce the incidence of thyroid cancer (18).
PTC belongs to the thyroid cancer with a higher
degree of differentiation. Compared with that in normal thyroid
follicle cells, the TSH receptor transcription in PTC cells is
reduced, but some functions are still retained. When TSH binds to
its receptor, it can regulate the expression of thyroid peroxidase
antibody, sodium/iodide symporter and other thyroid-specific genes
through the adenosine cyclophosphate and other signaling pathways,
while it can also regulate the thyroid cell proliferation and
differentiation, ultimately accelerating the deterioration of PTC
(19). Experimental studies have
confirmed that the appropriate amount of TSH can promote the growth
of thyroglobulin and thyroid cell proliferation (1), but the excessive increase in TSH can
induce the PTC progression (20).
After the PTC operation, the routine supplement of thyroxine can
effectively inhibit the serum TSH level in the body and
significantly reduce the recurrence and mortality rates of patients
(21). It can be seen that the serum
TSH level is closely related to the occurrence and progression of
PTC, so reducing the serum TSH level can lower the incidence rate
of PTC. In this study, the cell experiment proved that TSH can
significantly promote the proliferation of PTC cells cultured in
vitro, and the appropriate supplement of TH can inhibit its
proliferation.
Cyclin D1 is a kind of important regulatory protein
in G1 phase, and its encoded D1 protein plays an important role in
regulating the transition from G1 phase to S phase. After D1
protein binds to cyclin-dependent kinase 4P6 (CDK4P6), the
transcription and expression of a series of cell cycle-related
genes are induced, thus promoting the proliferation and
differentiation of the cell cycle from G1 phase to S phase, which
accelerates the whole cell cycle (22). Cyclin D1 is overexpressed in many
malignant tumors of human, such as breast cancer and thyroid cancer
(22,23). In this study, it was also found that
TSH can promote the mRNA and protein expressions of cyclin D1 in
PTC cells, thus promoting the transition of cell cycle from G1
phase to S phase, which can be inhibited by the supplement of
TH.
In conclusion, it was confirmed in this study
through in vitro cell experiments that TSH can promote the
proliferation of PTC cells, and the appropriate supplement of TH
can inhibit its proliferation, providing an experimental basis for
clinical medication. The occurrence and progression of PTC is a
complex pathological process involving a variety of cell signaling
pathways, and its relevant pathways need to be further studied.
References
|
1
|
van der Zwan JM, Mallone S, van Dijk B,
Bielska-Lasota M, Otter R, Foschi R, Baudin E and Links TP:
RARECARE WG: Carcinoma of endocrine organs: Results of the RARECARE
project. Eur J Cancer. 48:1923–1931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu TR, Su X, Qiu WS, Chen WC, Men QQ, Zou
L, Li ZQ, Fu XY and Yang AK: Thyroid-stimulating hormone receptor
affects metastasis and prognosis in papillary thyroid carcinoma.
Eur Rev Med Pharmacol Sci. 20:3582–3591. 2016.PubMed/NCBI
|
|
3
|
Ibitoye R and Wilkins A: Thyroid papillary
carcinoma after alemtuzumab therapy for MS. J Neurol.
261:1828–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Min XS, Huang P, Liu X, Dong C, Jiang XL,
Yuan ZT, Mao LF and Chang S: Bioinformatics analyses of significant
prognostic risk markers for thyroid papillary carcinoma. Tumour
Biol. 36:7457–7463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceresini G, Corcione L, Michiara M, Sgargi
P, Teresi G, Gilli A, Usberti E, Silini E and Ceda GP: Thyroid
cancer incidence by histological type and related variants in a
mildly iodine-deficient area of Northern Italy, 1998 to 2009.
Cancer. 118:5473–5480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Xu Z, Li Z, An C, Liu J, Zhu Y,
Ni S, Tang P, Sayan A and Ilankovan V: Minimally-invasive
endoscopically-assisted neck dissection for lateral cervical
metastases of thyroid papillary carcinoma. Br J Oral Maxillofac
Surg. 52:793–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee T, Cha YJ, Ahn S, Han J and Shim YM: A
rare case of tumor-to-tumor metastasis of thyroid papillary
carcinoma within a pulmonary adenocarcinoma. J Pathol Transl Med.
49:78–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haymart MR, Repplinger DJ, Leverson GE,
Elson DF, Sippel RS, Jaume JC and Chen H: Higher serum thyroid
stimulating hormone level in thyroid nodule patients is associated
with greater risks of differentiated thyroid cancer and advanced
tumor stage. J Clin Endocrinol Metab. 93:809–814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiore E, Rago T, Provenzale MA, Scutari M,
Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, et
al: Lower levels of TSH are associated with a lower risk of
papillary thyroid cancer in patients with thyroid nodular disease:
Thyroid autonomy may play a protective role. Endocr Relat Cancer.
16:1251–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Medenica S, Nedeljkovic O, Radojevic N,
Stojkovic M, Trbojevic B and Pajovic B: Thyroid dysfunction and
thyroid autoimmunity in euthyroid women in achieving fertility. Eur
Rev Med Pharmacol Sci. 19:977–987. 2015.PubMed/NCBI
|
|
12
|
McNabb FM: Thyroid hormones, their
activation, degradation and effects on metabolism. J Nutr. 125
Suppl:S1773–S1776. 1995.
|
|
13
|
Glinoer D: The regulation of thyroid
function in pregnancy: Pathways of endocrine adaptation from
physiology to pathology. Endocr Rev. 18:404–433. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomer Y, Barbesino G, Keddache M,
Greenberg DA and Davies TF: Mapping of a major susceptibility locus
for Graves' disease (GD-1) to chromosome 14q31. J Clin Endocrinol
Metab. 82:1645–1648. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerschpacher M, Göbl C, Anderwald C, Gessl
A and Krebs M: Thyrotropin serum concentrations in patients with
papillary thyroid microcancers. Thyroid. 20:389–392. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pacini F, Schlumberger M, Dralle H, Elisei
R, Smit JW and Wiersinga W: European Thyroid Cancer Taskforce:
European consensus for the management of patients with
differentiated thyroid carcinoma of the follicular epithelium. Eur
J Endocrinol. 154:787–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pelizzo MR, Boschin Merante I, Toniato A,
Pagetta C, Casal Ide E, Mian C and Rubello D: Diagnosis, treatment,
prognostic factors and long-term outcome in papillary thyroid
carcinoma. Minerva Endocrinol. 33:359–379. 2008.PubMed/NCBI
|
|
18
|
Uruno T, Miyauchi A, Shimizu K, Tomoda C,
Takamura Y, Ito Y, Miya A, Kobayashi K, Matsuzuka F, Amino N, et
al: Favorable surgical results in 433 elderly patients with
papillary thyroid cancer. World J Surg. 29:1497–1503. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brabant G: Thyrotropin suppressive therapy
in thyroid carcinoma: What are the targets? J Clin Endocrinol
Metab. 93:1167–1169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sfakianakis GN, Skillman TG and George JM:
Thyroxine withdrawal in thyroid cancer. Ohio State Med J. 71:79–82.
1975.PubMed/NCBI
|
|
21
|
Kim SS, Lee BJ, Lee JC, Song SH, Kim BH,
Son SM, Kim IJ, Kim YK and Kang YH: Preoperative serum thyroid
stimulating hormone levels in well-differentiated thyroid carcinoma
is a predictive factor for lateral lymph node metastasis as well as
extrathyroidal extension in Korean patients: A single-center
experience. Endocrine. 39:259–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roy PG, Pratt N, Purdie CA, Baker L,
Ashfield A, Quinlan P and Thompson AM: High CCND1 amplification
identifies a group of poor prognosis women with estrogen receptor
positive breast cancer. Int J Cancer. 127:355–360. 2010.PubMed/NCBI
|
|
23
|
Nakashima M, Meirmanov S, Naruke Y, Kondo
H, Saenko V, Rogounovitch T, Shimizu-Yoshida Y, Takamura N, Namba
H, Ito M, et al: Cyclin D1 overexpression in thyroid tumours from a
radio-contaminated area and its correlation with Pin1 and aberrant
β-catenin expression. J Pathol. 202:446–455. 2004. View Article : Google Scholar : PubMed/NCBI
|