Introduction
Through screening for naturally occurring antitumor
medicines, an increasing number of natural products have been
identified for use in clinical tumor therapy. A number of these
natural products were originally used in traditional Chinese
medicine and other forms of folk medicine (1).
Angiogenesis is associated with tumor recurrence and
metastasis. In the 1970s, Folkman advanced the theory of inhibiting
the formation of new blood vessels as a strategy against cancer
(2). Since then, anti-angiogenesis
has been a key subject in anticancer research. At present, the FDA
has approved a range of drugs with anti-angiogenic activity,
including sorafenib, sunitinib, pazopanib and everolimus (3–5).
Anti-angiogenic natural extracts are also being considered in
anticancer drug screening.
Livistona chinensis, the Chinese fan palm or
fountain palm, which belongs to the monocotyledonous
Palmaceae family, has been used for centuries as a medicinal
herb in eastern Asia. It is native to southern Japan, Taiwan, the
Ryukyu Islands and the Guangdong region of southern China (6). Extracts from the seeds of Livistona
chinensis have been used to treat a range of types of cancer in
traditional Chinese medicine including HCC and colon cancer
(7,8).
Previous studies screening for naturally occurring angiogenesis
inhibitors have identified that the extracts from the shells of
Livistona chinensis seeds were candidates for use in
anti-angiogenic and antitumor therapy (8,9). Extracts
of the Livistona chinensis seed have been demonstrated to
suppress cancer cell growth (10).
However, the precise mechanisms for its antitumor activity have yet
to be characterized. In the present study, the anti-angiogenic
effect of the extract from Livistona chinensis seeds will be
considered.
Materials and methods
Reagents
RPMI-1640, fetal bovine serum (FBS), penicillin,
streptomycin, and SuperScript II reverse transcriptase were
obtained from Promega Corporation (Madison, WI, USA); trypsin-EDTA
and TRIzol® reagent were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). An angiogenesis
assay kit was purchased from EMD Millipore (Billerica, MA, USA).
Human vascular endothelial growth factor (VEGF)-A and VEGF receptor
2 (VEGFR)-2 Quantikine ELISA kits were obtained from R&D
Systems (Minneapolis, MN, USA). All other chemicals were obtained
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Preparation of ethanol extract from
Livistona chinensis seeds
A refluxing process was used to extract the
Livistona chinensis seeds (500 g) with 85% ethanol (5,000
ml) and the extract was filtered. The extract was concentrated to a
relative density of 1.05. The extraction liquid was condensed by a
rotary spray dryer (Model B-290; Buchi, Flawil, Switzerland) to
produce solid powder. The solid powder of EELC was initially
diluted to 300 mg/ml in saline; further dilutions were performed
with dimethyl sulfoxide (DMSO). DMSO was also used as the control
treatment.
In vivo angiogenesis assay with a
chorioallantoic membrane (CAM) model
The anti-angiogenic activity of EELC was detected
in vivo with a CAM assay. Briefly, 5-day old chicken embryos
in eggs were purchased from Dabeinong Biotech Co., Ltd. (Fuzhou,
China) and incubated for 2 days at 37°C with a relative humidity of
80%. Subsequent to the incubation, holes were cut in the shells to
expose the CAMs and the embryos were placed in plastic culture
dishes (Merck KGaA) according to an established shell-less culture
technique: A chick embryo was removed from an eggshell and cultured
in an automatic biochemical incubator (XiHeng Biological Co., Ltd.,
Shanghai, China) at a temperature of 38°C and constant air humidity
of 70% after which time angiogenesis could be quantified via image
analysis This is an important technique for the generation of
transgenic chickens that produce useful substances in their eggs,
and for various embryonic manipulations. A 5-mm diameter Whatman
filter paper circle sterilized by high pressure was loaded with 10
µl EELC (10 mg/ml). The filter paper was then placed on the CAMs.
Images were captured (using a phase-contrast microscope at a
magnification of ×50) of the treated areas and the extent of
angiogenesis was evaluated at 24 h. The total number of vessels
that had sprouted from the primary vessel of the CAM was counted in
the area within 2.5 mm from the edge of the filter paper, and the
total length of neoangiogenesis was evaluated with Saisam software
(version 2.0; Microvision Instruments, Lisses, France). Ten
replicates of the experiment were performed.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from the cell bank of the Chinese Academy of Science
(Shanghai, China). The cells were cultured in RPMI-1640
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C.
Evaluation of cell viability by MTT
assay
An MTT colorimetric assay was used to detect cell
viability. HUVECs (1×104 cells/well) were seeded into
96-well plates and treated with different concentrations of EELC
(0, 0.125, 0.25 and 0.5 mg/nl) for 24 h in the previously described
cell culture conditions. MTT solution (20 µl, 5 mg/ml) was added to
each well of the plate. The MTT solution was discarded following 4
h of incubation in the previously described cell culture
conditions, and 100 µl DMSO was added to each well. An ELISA plate
reader (EXL800; BioTek Instruments, Inc., Winooski, VT, USA) was
used to measure the relative absorbance of the samples at 570 nm.
Experiments were repeated in triplicate.
Quantification of cell migration by a
wound-healing assay
HUVECs were grown to confluence in 6-well plates,
washed with serum-free medium and wounded with a 200 µl pipette
tip. The wounded monolayers were then incubated, as previously
described, for 24 h. The cells were observed with phase-contrast
microscopy (Olympus Corporation, Tokyo, Japan) and images were
captured with ×100 magnification. The experiment was performed in
triplicate.
Capillary-like tube formation
assay
Tube formation by HUVECs was detected in
vitro with the previously described Angiogenesis Assay kit,
used according to the manufacturer's protocol. HUVECs were treated
with different concentrations of EELC (0, 0.125, 0.25 and 0.5
mg/nl) in starvation RPMI-1640 medium for 24 h in the previously
described incubation conditions. The cells were harvested with
trypsin, resuspended with fresh assay medium from the angiogenesis
assay kit and seeded (5×103/well) into 24-well plates
coated with ECMatrix gel (EMD Millipore). They were then cultured
at 37°C for 3 h.
Cellular morphology and the development of tube
formation were evaluated with phase-contrast microscopy at ×100
magnification. The length of capillary tube formation was measured
in three randomly chosen fields from each well with Image-Pro Plus
software (version 5.0; Media Cybernetics, Inc., Rockville, MD,
USA). The treated groups were compared with the untreated groups.
The experiment was performed in triplicate.
RNA extraction and reverse
transcription-semi-quantitative polymerase chain reaction (PCR)
analysis
HUVECs (2×105) were seeded onto 6-well
plates and treated with different concentrations of EELC (0, 0.125,
0.25 and 0.5 mg/nl) for 24 h in the previously described cell
culture conditions. Total RNA was then extracted from the HUVECs
with TRIzol reagent, used according to the manufacturer's protocol.
A total of 1 µg RNA was reverse transcribed according to the
manufacturer's protocol with the previously described SuperScript
II reverse transcriptase. The produced cDNA was used for the
evaluation of VEGF-A and VEGFR-2 mRNA by semi-quantitative PCR with
Taq DNA polymerase (Fermentas; Thermo Fisher Scientific, Inc.). The
primer sequences were as follows: VEGF-A forward,
5′-GCCTTGCCTTGCTGCTCTA-3′ and reverse, 5′-GATGTCCACCAGGGTCTCG-3′;
VEGFR2 forward, 5′-ACGCCGATTATGTGAGA-3′ and reverse,
5′-AGGCAGGAGTTGAGTATGT-3′; GAPDH forward,
5′-GTCATCCATGACAACTTTGG-3′ and reverse, 5′-GAGCTTGACAAAGTGGTCGT-3′.
The thermocycler conditions were as follows: 1 cycle of
denaturation at 95°C for 5 min, then denaturation at 94°C for 30
sec, annealing at 54°C for 30 sec, elongation at 72°C for 45 sec,
for 35 cycles, and 1 cycle of extension at 72°C for 10 min. For
quantification, samples were electrophoresed in 1.5% agarose gel
containing GoldView™ in Tris-acetate/EDTA buffer (Sigma-Aldrich;
Merck KGaA), and PCR product images were analyzed using the
ChemiDoc™ system and Quantity One 4.62 software (Bio-Rad
Laboratories, Hercules, CA, USA). All cDNA samples were synthesized
in parallel, and PCR reactions were run in triplicate. mRNA levels
of each gene were normalized to GAPDH mRNA levels using the
2−ΔΔCq methods (11).
ELISA analysis
HUVECs cells (2×105) were seeded into
6-well plates and treated with different concentrations of EELC (0,
0.125, 0.25 and 0.5 mg/nl) for 24 h. Cells were lysed using
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA)
on ice for 10–15 min. Then, the supernatant from each well was
collected by centrifugation at 1,000 × g for 25 min at 4°C. The
instructions from the Quantikine ELISA kit were followed and the
VEGF-A and VEGFR-2 levels were measured with the ELISA plate
reader. Experiments were repeated in triplicate.
Statistical analysis
A Student's t-test was used for comparisons between
two groups; an analysis of variance followed by Tukey's post-hoc
test was used for more than two groups. The data were analyzed
using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
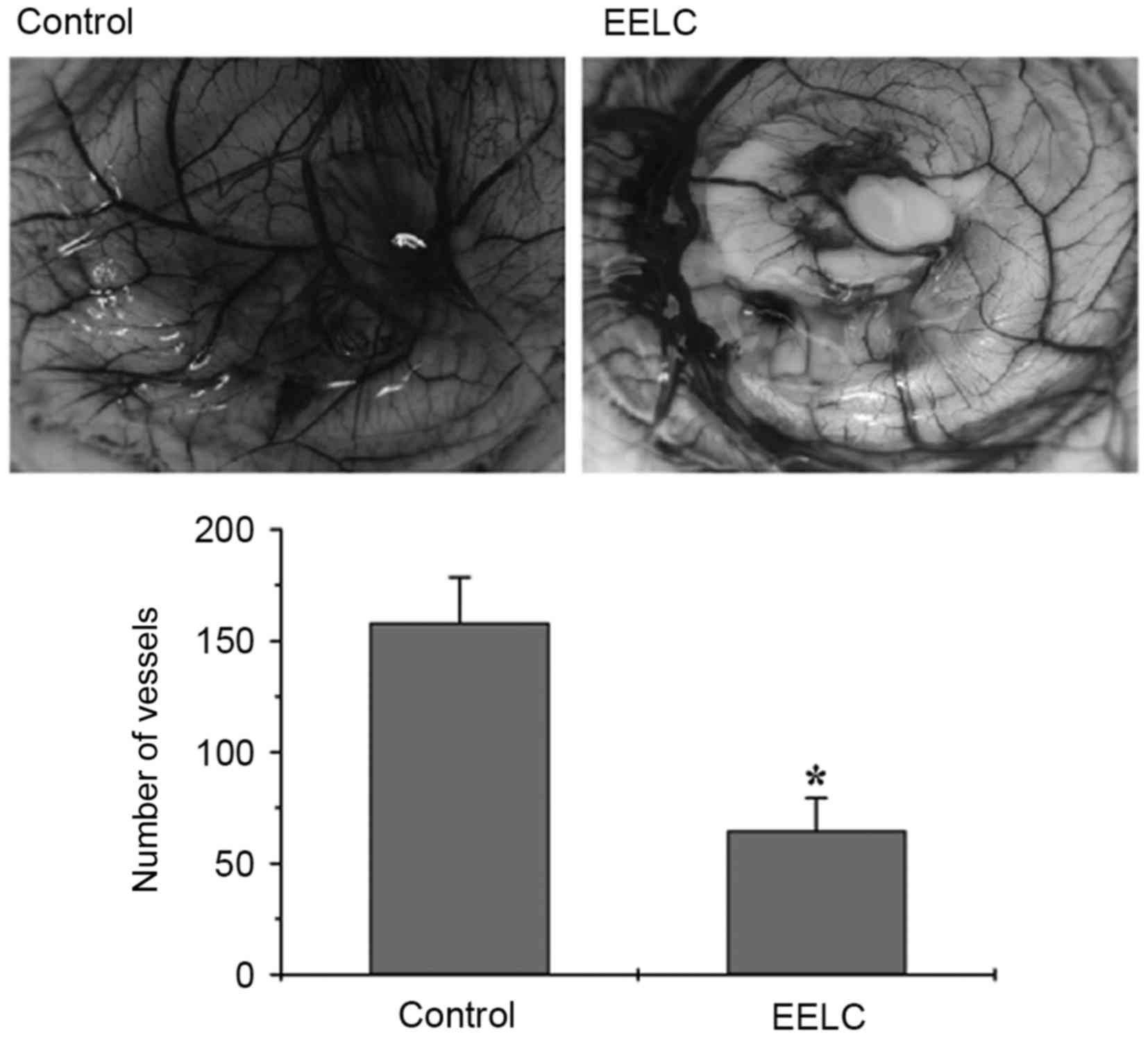
EELC inhibits the angiogenesis of CAMs
in vivo
A CAM assay was used to determine the in vivo
anti-angiogenic activity of EELC. Following 24 h of EELC treatment,
the total number of blood vessels sprouting from the primary
vessels in the CAMs was significantly reduced compared with the
untreated control (Fig. 1;
P<0.05). This result indicated that angiogenesis is suppressed
by EELC in vivo.
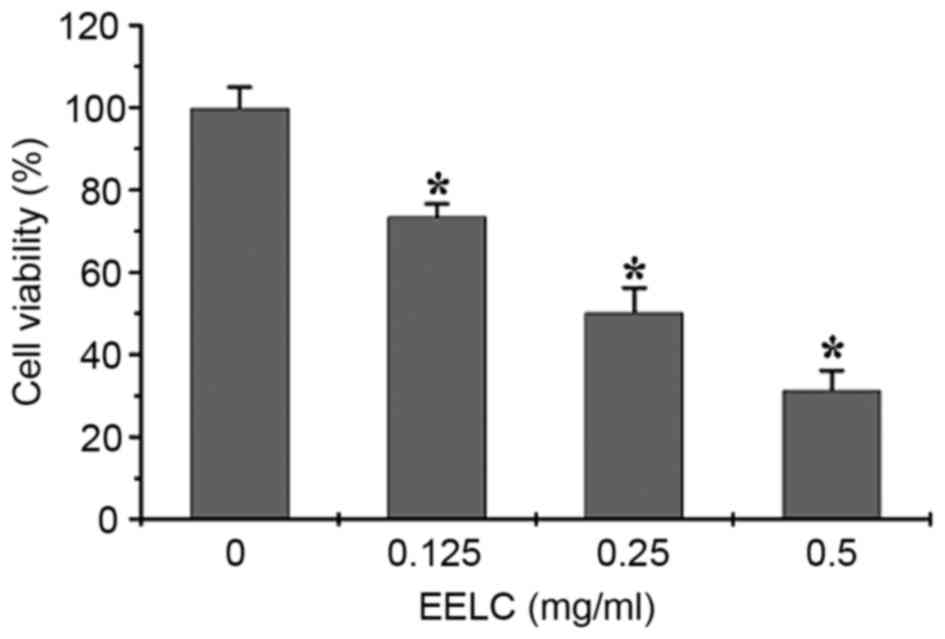
EELC inhibits the proliferation of
HUVECs
The effect of EELC on the growth of HUVECs was
evaluated. HUVECs were incubated with 0, 0.125, 0.25 or 0.5 mg/nl
EELC for 24 h. As presented in Fig.
2, the growth of HUVECs treated with EELC was significantly
inhibited compared with untreated control cells, in a
dose-dependent manner (P<0.05).
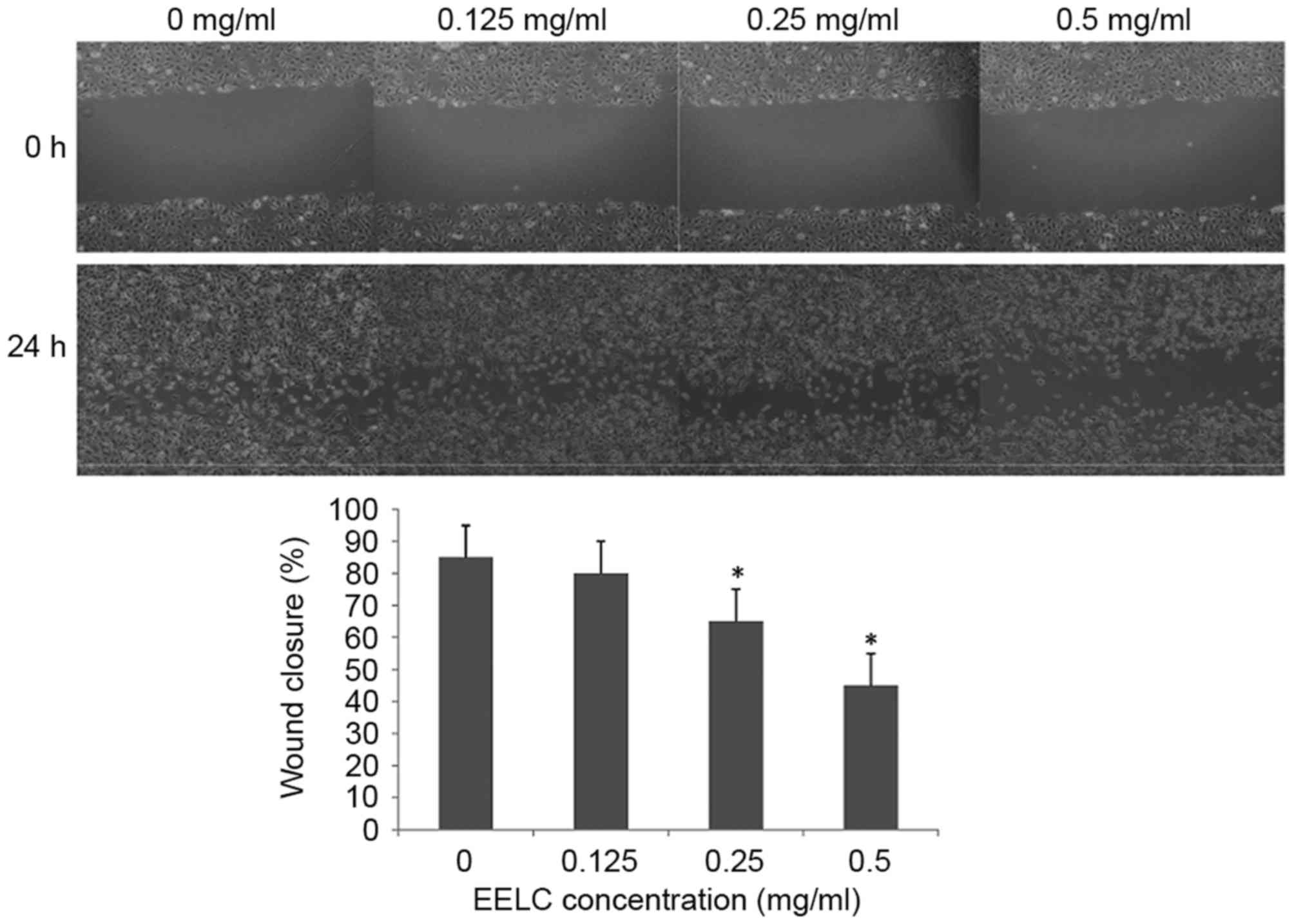
EELC inhibits the migration of
HUVECs
As it had been demonstrated that EELC reduced cell
viability, the effect of EELC on the migration abilities of HUVECs
were assessed at 0, 0.125, 0.25 or 0.5 mg/nl EELC with a wound
healing assay. As presented in Fig.
3, the migration of HUVECs was significantly inhibited
following treatment with EELC at doses above 0.25 mg/nl
(P<0.05).
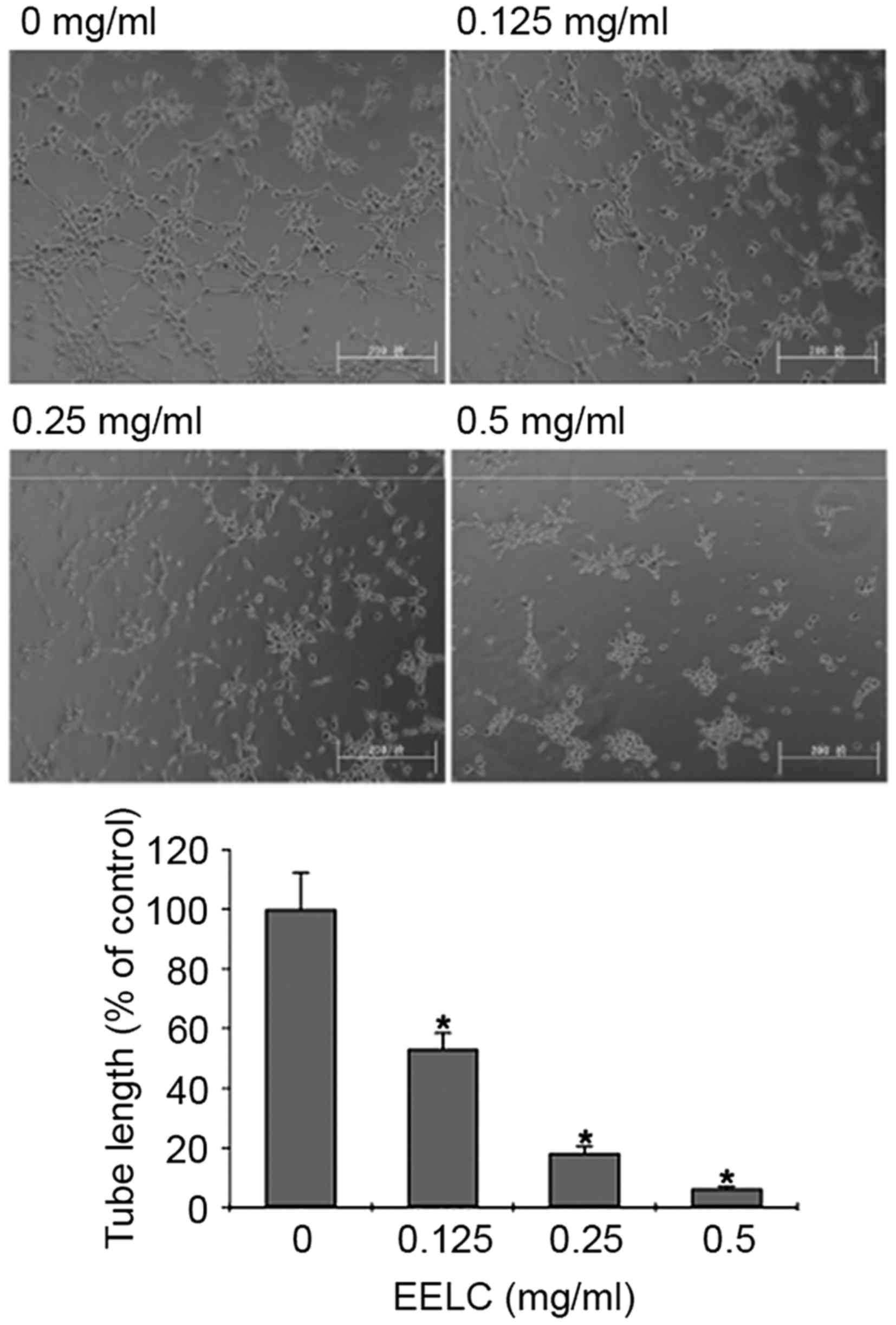
EELC inhibits the tube formation of
HUVECs
To determine the effect of EELC on endothelial
capillary tube formation, HUVECs were seeded on a solid ECMatrix
gel containing mouse basement membrane proteins, which allowed
endothelial cells to rapidly align to form hollow tube-like
structures. HUVECs treated with EELC exhibited a dose-dependent
decreased capacity for capillary tube formation compared with
untreated HUVECs (Fig. 4;
P<0.05).
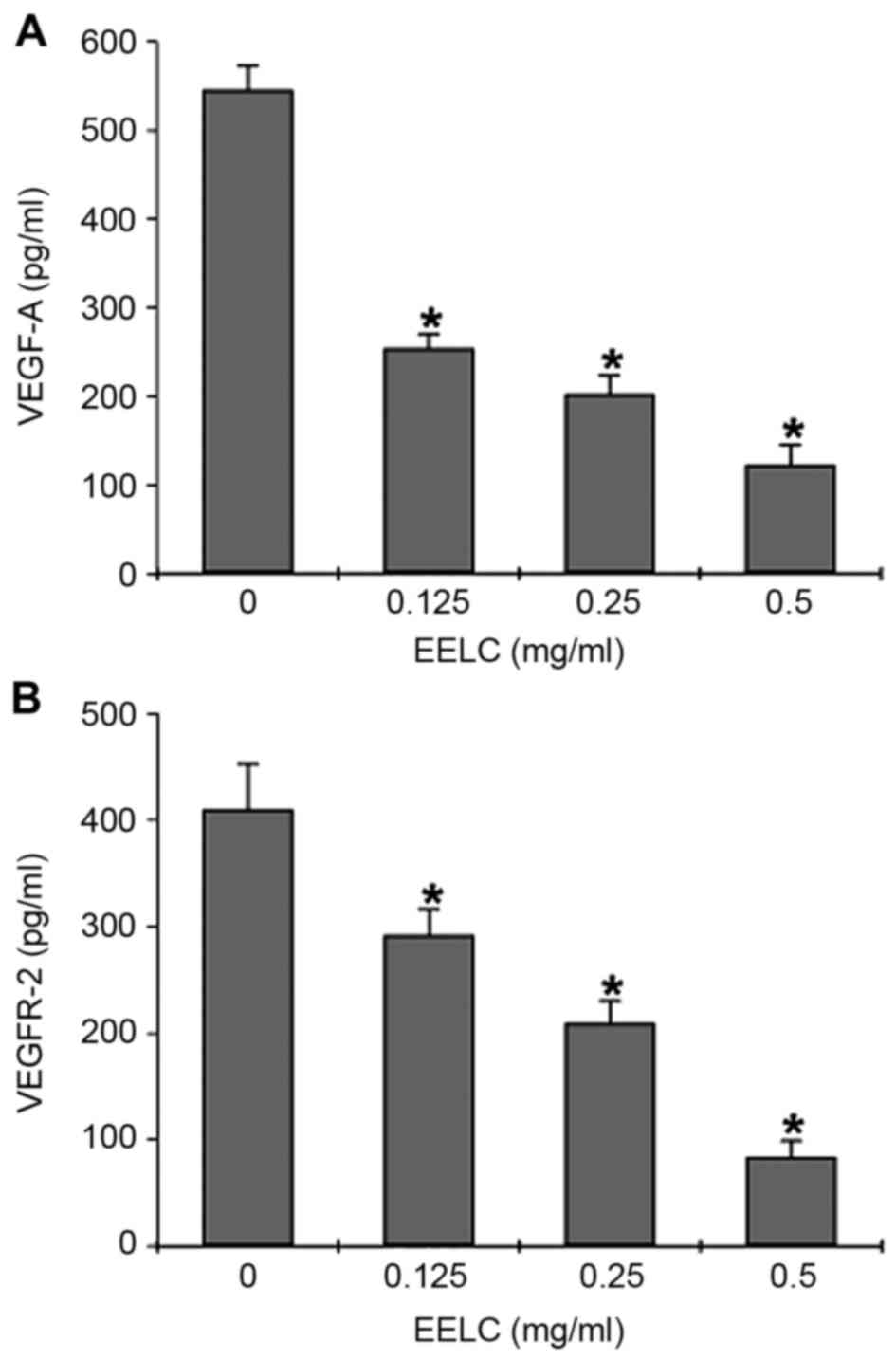
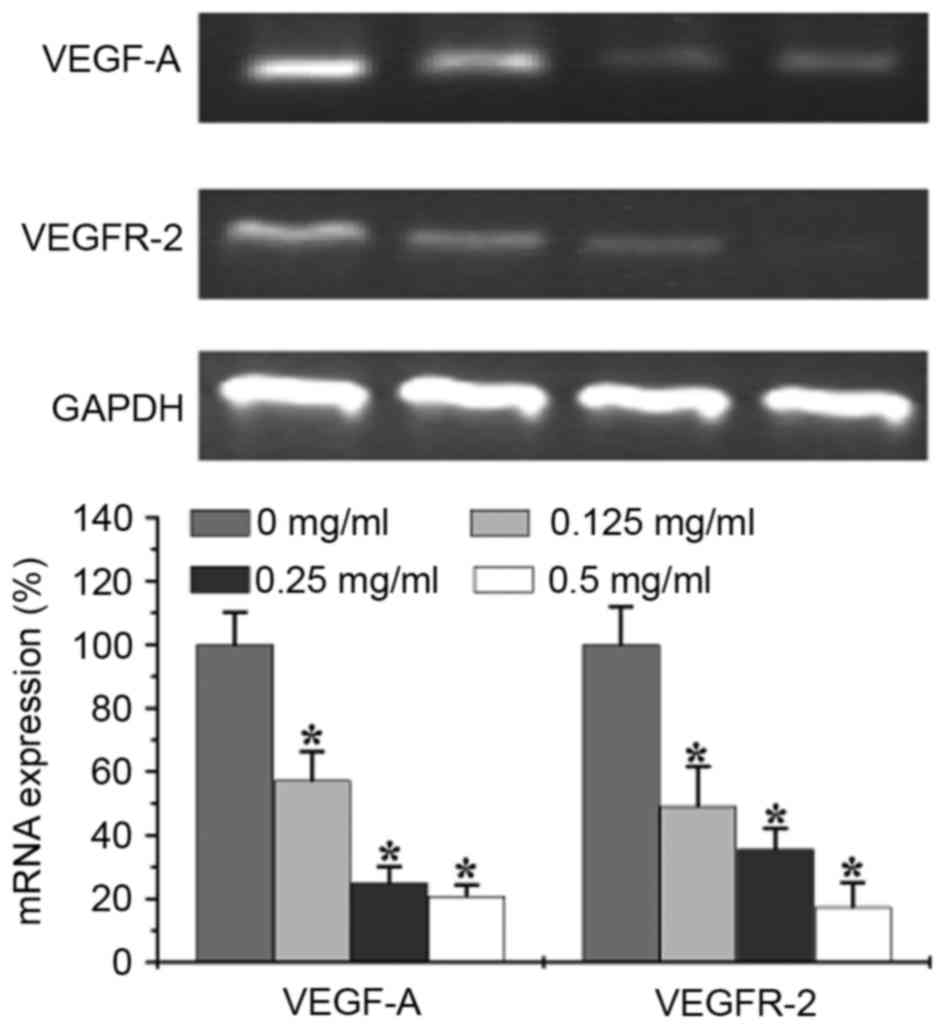
Effects of EELC on VEGF-A and VEGFR-2
mRNA and protein expression
The effects of EELC on VEGF-A secretion and mRNA,
and VEGFR-2 protein and mRNA expression in HUVECs were determined
by ELISA and semi-quantitative PCR. The ELISA revealed that VEGF-A
secretion was dose-dependently reduced in HUVECs following
treatment with EELC (Fig. 5A,
P<0.05). EELC treatment also suppressed VEGFR-2 protein
expression in HUVECs (Fig. 5B,
P<0.05). The mRNA levels of VEGFR-2 and VEGF-A were evaluated by
semi-quantitative PCR. The results revealed that the relative mRNA
levels of VEGFR-2 and VEGF-A were reduced following treatment with
EELC (Fig. 6, P<0.05).
Discussion
Anti-angiogenesis research began >35 years ago
with the study of the late Folkman (12–14).
Though tumors may stimulate the growth of new vessels with
inflammation, mutation/overexpression or mechanical stress, the
principal stimulus for angiogenesis is oxygen deprivation (15). Once angiogenesis has been activated,
tumors express pro-angiogenic factors and tumor vascularization
increases. Pro-angiogenic paracrine factors, including angiogenin,
VEGF, fibroblast growth factor, and transforming growth factor-β,
are released by tumor cells to stimulate the growth of new vessels
as a response to the stimulus (16).
These factors activate endothelial cell proliferation, migration
and invasion, resulting in new vessel formation from neighboring
blood vessels.
Livistona chinensis is used as a folk
medicine in China to treat various types of tumor (6). The crude aqueous extract of Livistona
chinensis seeds can inhibit the growth of HUVEC, breast cancer
and colon adenocarcinoma (Ht-29) cells (9). Our previous study demonstrated that the
ethanol extract of EELC inhibited tumor growth in an HCC mouse
model in vivo, and induced the apoptosis of HepG2 cells
in vitro. The apoptosis following EELC treatment was
associated with the loss of mitochondrial membrane potential, the
activation of caspase-9 and caspase-3, and an increase in the
Bax/Bcl-2 ratio (10). In the present
study, EELC inhibited angiogenesis in vivo and in
vitro, and inhibited the growth and migration of HUVECs. EELC
treatment also reduced the VEGF-A secretion and VEGFR-2 expression
of HUVECs. Our previous study identified that EELC may affect
angiogenesis via the inhibition of VEGFR-2 through the Notch
signaling pathway (17). Other
previous studies have indicated that the activated Notch pathway
reduced the level of VEGFR-2 by affecting transcriptional
regulation in mouse endothelial cells (18,19). EELC
may therefore inhibit the VEGFR-2 through notch signaling pathway.
It has been demonstrated that the proliferation, migration and
invasion of endothelial cells are highly dependent on VEGF and
VEGFRs, including in mice and zebrafish (20,21). EELC
may limit the angiogenic behavior of endothelial cells by
suppressing blood vessel formation via the regulation of the VEGF
signal pathway (22,23). In our future study, we will aim to
further characterize the effects of EELC on the Notch signaling
pathway in endothelial cells.
A modern clinical strategy against cancer is the
combination of anti-angiogenesis drugs with other treatments,
including chemotherapy, radiation or other targeted drugs, to
obtain improved therapeutic effects compared with using treatments
individually. For example, monotherapy with bevacizumab failed to
prolong the survival time of patients with cancer, whereas
bevacizumab combined with cytotoxic chemotherapy may increase the
survival time (24,25). The effect of EELC combined with 5-FU
on HCC cells will also be investigated in future study.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81302954 and 81202790) and
the National Natural Science Foundation of FuJian (grant no.
2015J01336, 2017J01542).
Glossary
Abbreviations
Abbreviations:
|
EELC
|
ethanol extract of Livistona chinensis
seed
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
CAM
|
chorioallantoic membrane
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
References
|
1
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis diffusa
Willd suppresses proliferation of human HepG2 cells and potentiates
the anticancer efficacy of low-dose 5-fluorouracil by inhibitingthe
CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shih T and Lindley C: Bevacizumab: An
angiogenesis inhibitor for the treatment of solid malignancies.
Clin Ther. 28:1779–1802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gotink KJ and Verheul HM: Anti-angiogenic
tyrosine kinase inhibitors: What is their mechanism of action?
Angiogenesis. 13:1–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cook KM and Figg WD: Angiogenesis
inhibitors: Current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Li A, Dong XP and Xu XY: Screening
of anti-tumor parts from the seeds of Livistona chinensis and its
anti-angiogenesis effect. Zhong Yao Cai. 31:718–722. 2008.(In
Chinese). PubMed/NCBI
|
|
7
|
Cheueng S and Tai J: In vitro studies of
the dry fruit of Chinese fan palm Livistona chinensis. Oncol Rep.
5:1331–1336. 2005.
|
|
8
|
Sartippour MR, Liu C, Shao ZM, Go VL,
Heber D and Nguyen M: Livistona extract inhibits angiogenesis and
cancer growth. Oncol Rep. 6:1355–1357. 2001.
|
|
9
|
Huang WC, Hsu RM, Chi LM, Leu YL, Chang YS
and Yu JS: Selective downregulation of EGF receptor and downstream
MAPK pathway in human cancer cell lines by active components
partially purified from the seeds of Livistona chinensis R. Brown.
Cancer Lett. 248:137–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L,
Cai Q, Chen D, Wang L, Hong Z and Peng J: Livistona chinensis seed
suppresses hepatocellular carcinoma growth through promotion of
mitochondrial-dependent apoptosis. Oncol Rep. 29:1859–1866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
13
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shweiki D, Itin A, Soffer D and Keshet E:
Vascular endothelial growth factor induced by hypoxia may mediate
hypoxia-initiated angiogenesis. Nature. 359:843–845. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shalaby F, Rossant J, Yamaguchi TP,
Gertsenstein M, Wu XF, Britman ML and Schuh AC: Failure of
blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature. 376:62–66. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L,
Zeng J, Hong Z and Peng J: Livistona chinensis seeds inhibit
hepatocellular carcinoma angiogenesis in vivo via suppression of
the Notch pathway. Oncol Rep. 31:1723–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siekmann AF and Lawson ND: Notch
signalling limits angiogenic cell behaviour in developing zebrafish
arteries. Nature. 445:781–784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suchting S, Freitas C, le Noble F,
Benedito R, Bréant C, Duarte A and Eichmann A: The Notch ligand
Delta-like 4 negatively regulates endothelial tip cell formation
and vessel branching. Proc Natl Acad Sci USA. 104:pp. 3225–3230.
2007, View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
Therapeutic implications. Semin Oncol. 29 6 Suppl 16:S10–S14. 2002.
View Article : Google Scholar
|
|
21
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zachary I and Gliki G: Signaling
transduction mechanisms mediating biological actions of the
vascular endothelial growth factor family. Cardiovasc Res.
49:568–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams CK, Li JL, Murga M, Harris AL and
Tosato G: Up-regulation of the Notch ligand Delta-like 4 inhibits
VEGF-induced endothelial cell function. Blood. 107:931–939. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Ye Y, Zhang X, Liu H and Song J: A
single-arm clinical study of continuous usage of bevacizumab
assecond-line chemotherapy for Chinese patients with metastatic
colorectal cancer. Med Oncol. 32:1632015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|