Introduction
Thyroid cancer is the most common subtype of
endocrine malignancy, with 300,000 new cases per year, and ~40,000
mortalities per year, worldwide (1).
The incidence of thyroid cancer has increased in recent decades
(2). It can be categorized into four
major histologic groups, including papillary thyroid cancer (PTC),
follicular thyroid cancer, poorly differentiated carcinoma and
anaplastic thyroid cancer (3). PTC
accounts for 80–90% of all thyroid cancer cases (4).
A number of factors have been demonstrated to be
associated with PTC progression, including genetic factors,
environmental exposure, epigenetic alteration, nodular disease of
the thyroid and radiation exposure (5,6). The
prognosis of PTC patients is associated with their age, tumor size,
lymph node invasion and distant metastasis (7). Currently, the standard therapeutic
treatment for PTC is complete thyroidectomy followed by radioiodine
and levothyroxine therapy (8).
However, ~10% of patients with PTC develop recurrence and
metastasis, which are associated with poor prognosis (9). Thus, it is necessary to characterize the
molecular mechanisms of PTC initiation and development, and to
develop novel, targeted therapies for PTC.
The abnormal expression of microRNAs (miRNAs/miRs)
has been implicated in the pathogenesis of numerous tumor types,
including PTC (10–12). miRNAs are a group of short,
endogenous, non-protein-coding and single-stranded RNA molecules
18–25 nucleotides in length (13).
They function as negative regulators for target mRNA expression
through binding to the 3′-untranslated region (3′UTR) of mRNAs in a
base-pairing manner, resulting in mRNA degradation or translational
repression (14–17). miRNAs have been demonstrated to serve
a crucial function in various biological processes, including cell
growth, cell cycle, development, differentiation, metabolism and
metastasis (18–21). The expression of specific miRNAs may
be upregulated in particular types of cancer and downregulated in
others; this conflicting observation is predominantly a result of
differences in the mechanisms of oncogenesis between tumor types
(22,23). Upregulated miRNAs in cancer act as
oncogenes by negatively regulating tumor suppressor genes, whereas
downregulated miRNAs act as tumor suppressors via the blockade of
oncogenes in tumor progression (24,25).
Therefore, identifying the targets of miRNAs is essential for
understanding the functions of miRNAs in cancer.
In the present study, it was demonstrated that
miR-335 was significantly downregulated in PTC tissue and cell
lines. In addition, it was identified that miR-335 inhibited PTC
cell growth, migration and invasion in vitro. miR-335 may
act as a tumor suppressor in PTC by targeting zinc finger E-box
binding homeobox 2 (ZEB2). Taken together, the present study
revealed a novel perspective on how miR-335 affects PTC.
Materials and methods
Human tissue specimens and ethics
The present study was approved by the research
ethics committee of Henan Provincial People's Hospital (Zhengzhou,
China). Written informed consent was obtained from the patients
with PTC recruited into the study. A total of 59 pairs of human PTC
tissues and adjacent normal tissues (NATs) were obtained from Henan
Provincial People's Hospital between June 2013 to February 2015.
All patients (21 male and 38 female; age range, 32–77 years; mean
age, 53 years) enrolled in the study had not received any
preoperative treatments, including radiotherapy, chemotherapy and
levothyroxine, prior to the thyroidectomy. The tissue samples were
immediately snap-frozen in liquid nitrogen and stored at −80°C
until use.
Cell culture
The human PTC cell lines (TPC-1, HTH83, K1 and
BCPAP) and normal human thyroid cell line (HT-ori3) were purchased
from American Type Culture Collection (ATCC; Manassas, VA, USA).
Although K1 cells are contaminated with GLAG-66 (26), the resulting phenotypic and genotypic
differences between K1 and GLAG-66 cells were considered unlikely
to affect the outcome of the present study. 293T cells, used for
the luciferase reporter assay, were obtained from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). All
cell lines were maintained in RPMI-1640 or Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin (all Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), in a humidified
incubator with 5% CO2 at 37°C.
miRNA/siRNA transfection
Chemically synthesized miRNA mimics and siRNA
[miR-335 mimic, negative control (NC), ZEB2 siRNA and siRNA-ctrl]
were acquired from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
The miR-335 mimics sequence was 5′-UCAAGAGCAAUAACGAAAAAUGU-3′ and
the NC sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. The ZEB2 siRNA
sequence was 5′-GGACACAGGUUCUGAAACAdTd T-3′ and the siRNA-ctrl
sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. miRNA and siRNA
transfection were performed using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cell lines
using the mirVana miRNA Isolation kit (Ambion; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
miR-335 expression, the total RNA was reverse transcribed into cDNA
by using a TaqMan® MicroRNA Reverse Transcription kit
(cat. no., 4366596; Applied Biosystems; Thermo Fisher Scientific,
Inc.). A TaqMan miRNA assay (cat. no., 4324018; Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for the quantification of
miR-335; U6 was used as an internal control for miR-335 expression.
To determine ZEB2 mRNA expression, an M-MLV Reverse Transcription
system (Promega Corporation, Madison, WI, USA) was used for reverse
transcription according to the manufacturer's protocol, followed by
qPCR with SYBR Green Master mix (Takara, Biotechnology Co., Ltd.,
Dalian, China). The thermocycling conditions for qPCR were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The primers were designed as follows: miR-335,
5′-AGCCGTCAAGAGCAATAACGAA-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′
(reverse); U6, 5′-CTCGCTTCGGCAGCACA-3′ (forward) and
5′-AACGCTTCACGAATTTGCGT-3′ (reverse); ZEB2,
5′-AGTCCTCCCCACACGTGAGCC-3′ (forward) and
5′-TGCGGTCTGGATCGTGGCTTC-3′ (reverse); and GAPDH,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ (forward) and
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (reverse). GADPH was used as an
internal control for ZEB2 mRNA expression. Each sample was analyzed
in triplicate. The relative expression of miRNA and mRNA was
analyzed with the 2−∆∆Ct method (27).
Cell viability assay
Cell viability was evaluated with an MTT assay.
Cells were seeded into 96-well plates at a density of 3,000 cells
per well. Subsequent to incubation overnight, cells were
transfected with miRNA or siRNA. At a range of time points
subsequent to transfection (24, 48, 72 and 96 h), MTT assays were
performed. In brief, 20 µl 5 mg/ml MTT solution (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was added to each well. The plates
were incubated at 37°C for an additional 4 h. Subsequently, the
medium was removed and 100 µl dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA) was added to each well to dissolve the purple crystal.
The absorbance at 490 nm was detected using an ELISA plate reader.
Each sample was analyzed in triplicate.
Transwell migration and invasion
assay
The ability of cells to migrate and invade was
evaluated with transwell chambers (Corning Incorporated, Corning,
NY, USA) with an 8-µm pore polycarbonate membrane insert. miRNA or
siRNA-transfected cells were treated with trypsin/EDTA solution,
washed once with serum-free culture medium and re-suspended in
serum-free culture medium. 5×104 cells in 200 µl
serum-free medium were seeded into the upper chamber, and 500 µl
medium containing 20% FBS was added into the lower chamber. A cell
invasion assay was performed with the same procedure, with the
exception that the transwell chamber membranes were pre-coated with
Matrigel (BD Biosciences, San Jose, CA, USA). Subsequent to
incubation at 37°C for 48 h, cells that migrated or invaded to the
bottom surface of the transwell chambers were fixed with 100%
methanol at room temperature for 10 min, stained with 0.5% crystal
violet at room temperature for 10 min and washed with PBS three
times. Cells on the top surface of the transwell chamber were
removed with a cotton swab. The migration and invasion abilities
were quantified by counting the number of migrated and invaded
cells in five fields per transwell chamber, using an inverted
microscope (×200 magnification; Olympus Corporation, Tokyo, Japan).
Each experiment was repeated three times.
Western blotting
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing protease inhibitors. Total
protein concentration was detected with a Bicinchoninic Acid
Protein assay kit (Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Equivalent amounts of protein (20 µg per
lane) were separated via 10% SDS-PAGE and transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA) using a semidry transfer system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Subsequent to blocking with 5% skimmed milk in
Tris buffered saline with Tween-20 (TBST) at room temperature for 1
h, the membranes were probed with the primary antibodies, including
monoclonal mouse anti-human ZEB2 (dilution, 1:500; cat. no.,
sc-271984) and β-actin (dilution, 1:500; cat. no., sc-47778; both
Santa Cruz Biotechnology, Dallas, TX, USA) antibodies, overnight at
4°C. Subsequent to washing with TBST three times, the membranes
were incubated with a goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (dilution, 1:5,000; cat.
no., sc-2005; Santa Cruz Biotechnology) at room temperature for 2
h, followed by development with ECL Plus reagent (Pierce; Thermo
Fisher Scientific, Inc.). β-actin was used as an internal control.
This assay was repeated three times.
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/) was used to predict
targets of miR-335. Luciferase reporter plasmids, pGL3-ZEB2-3′UTR
wild type (Wt) and pGL3-ZEB2-3′UTR mutant (Mut), were synthesised
by Shanghai GenePharma Co., Ltd (Shanghai, China). For the
luciferase reporter assay, 293T cells were seeded into 24-well
plates at a density of 1.5×105 cells per well. Following
incubation overnight, cells were co-transfected with
pGL3-ZEB2-3′UTR Wt or pGL3-ZEB2-3′UTR, and miR-335 or NC, using
Lipofectamine 2000. At 48 h post-transfection, firefly and
Renilla luciferase activity were measured using a
Dual-Luciferase Reporter assay system (Promega Corporation). The
Renilla luciferase activity was measured as an internal
control for each well. The assay was performed in triplicate.
Statistical analysis
Data were expressed as the mean ± standard
deviation, and compared with a Student's t-test or one-way analysis
of variance using SPSS 17 software (SPSS Inc., Chicago, IL, USA).
Student-Newman-Keuls test was used as a post hoc test following
ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-335 is downregulated in PTC
tissues and cell lines
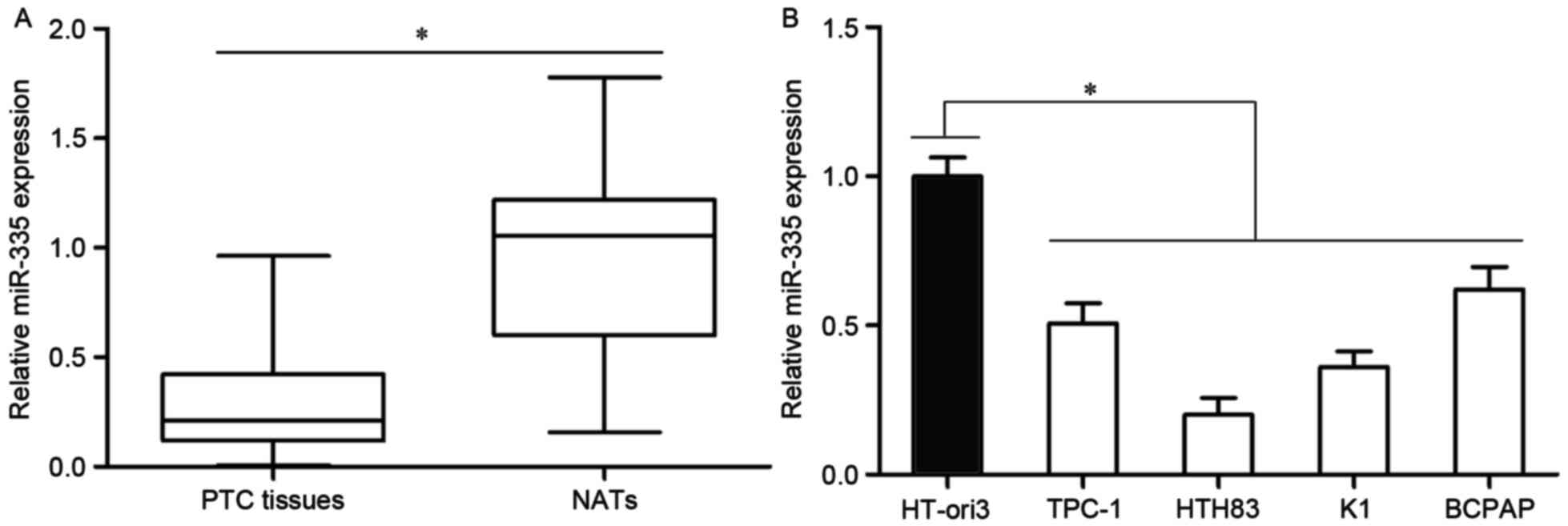
In order to confirm the association of miR-335 with
PTC, miR-335 expression levels were investigated in PTC tissue and
matched NATs. RT-qPCR analysis revealed that miR-335 was
significantly downregulated in PTC tissue compared with the matched
NATs (Fig. 1A; P<0.05).
In addition, the expression levels of miR-335 in
four PTC cell lines and a normal human thyroid cell line, HT-ori3,
were also quantified. The results indicated that the miR-335
expression levels were reduced in the four PTC cell lines relative
to the expression in HT-ori3 (Fig.
1B; P<0.05). These results suggested that miR-335 may serve
a role in the development of PTC.
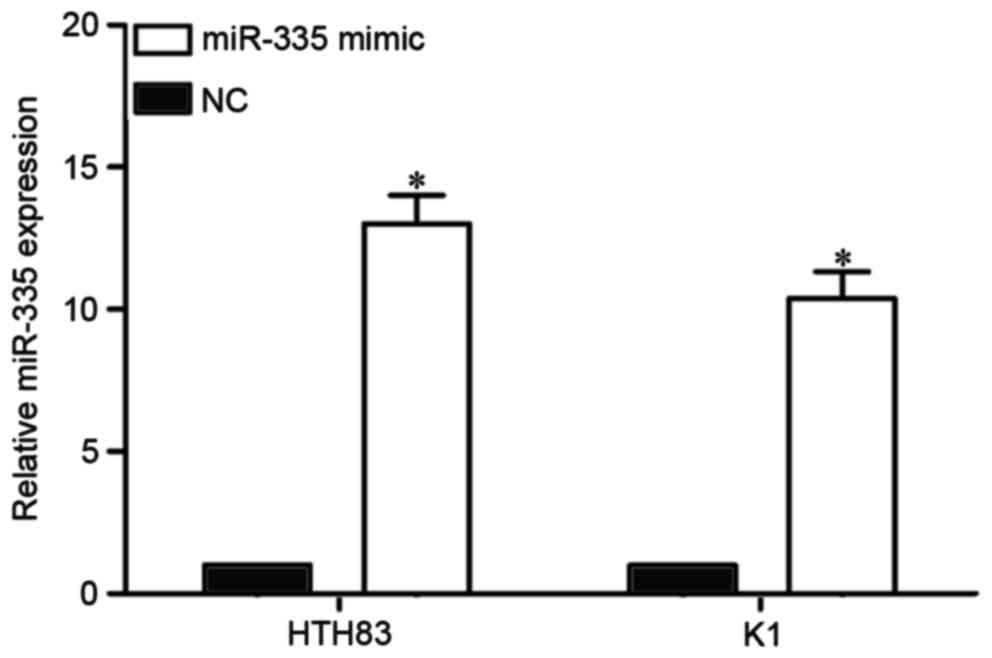
miR-335 is upregulated in HTH83 and K1
cells following transfection with an miR-335 mimic
miR-335 expression was the lowest in HTH83 and K1
cells. Therefore, HTT83 and K1 cells were selected for functional
studies. To explore the function of miR-335 in PTC, an miR-335
mimic or NC was transfected into HTT83 and K1 cells. The
transfection efficiency was assessed with RT-qPCR. The result
indicated that miR-335 was significantly upregulated in HTH83 and
K1 cells following transfection with an miR-335 mimic compared with
transfection with the NC (Fig. 2;
P<0.05).
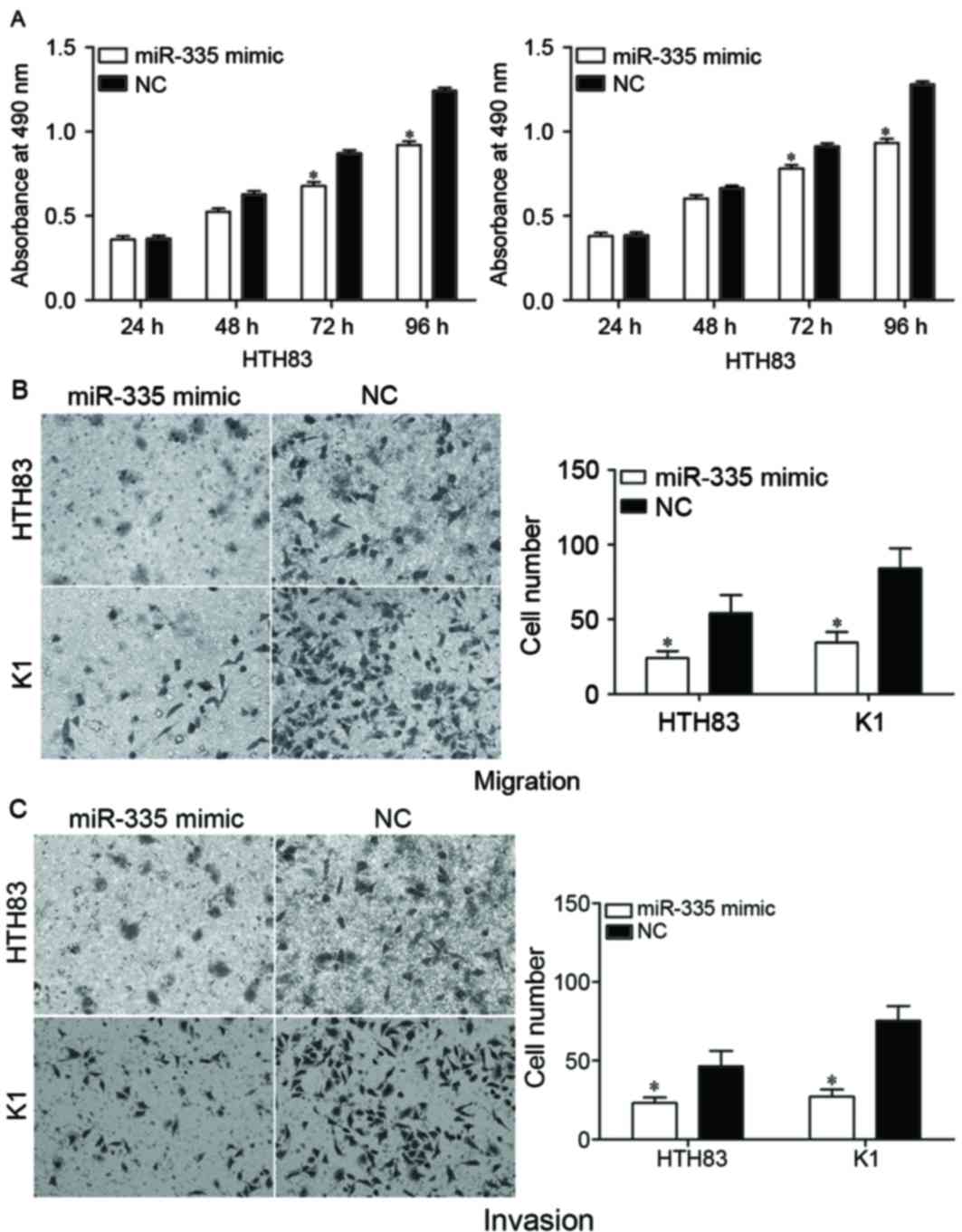
Overexpression of miR-335 suppresses
the proliferation, migration and invasion of PTC cells
An MTT assay was performed to evaluate the effect of
miR-335 on PTC cell proliferation. The induced overexpression of
miR-335 in HTH83 and K1 cells resulted in a reduced proliferation
rate compared with the NC groups (Fig.
3A; P<0.05).
The effect of miR-335 on cell migration and invasion
was assessed by using transwell migration and invasion assays. The
results indicated that the upregulation of miR-335 decreased the
migration (Fig. 3B; P<0.05) and
invasion (Fig. 3C; P<0.05)
abilities of HTH83 and K1 cells. Collectively, the data suggested
that miR-335 was a regulator of proliferation, migration and
invasion in PTC cells.
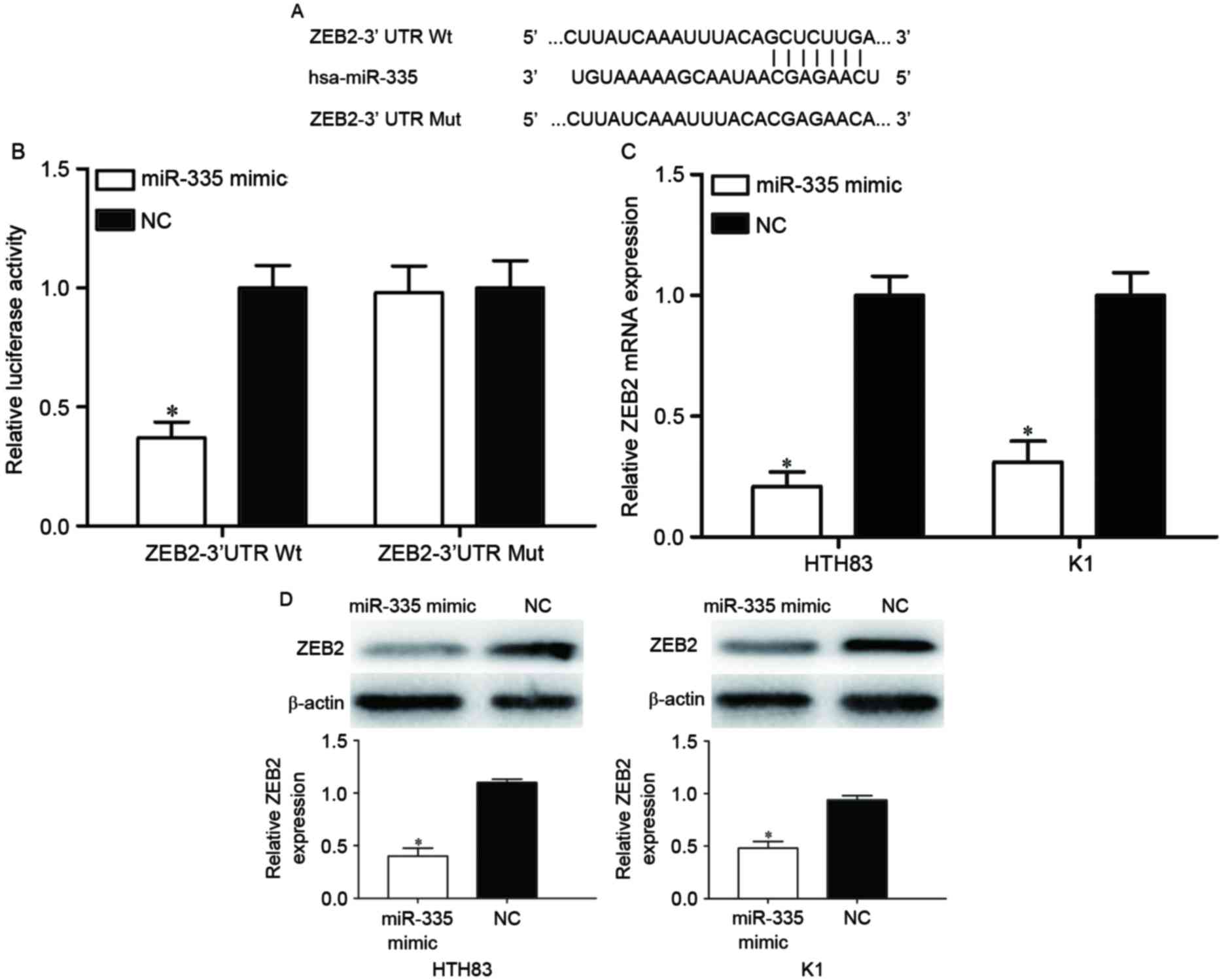
ZEB2 is a direct target of
miR-335
TargetScan was used to predict the direct target
genes of miR-335. As illustrated in Fig.
4A, the ZEB2 mRNA 3′UTR contained a predicted binding site for
miR-335. To explore whether miR-335 directly targeted the 3′UTR of
ZEB2, a dual-luciferase reporter assay was performed. The
luciferase activity was significantly reduced in 293T cells
co-transfected with ZEB2-3′UTR Wt and the miR-335 mimic compared
with the cells co-transfected with ZEB2-3′UTR Mut and the miR-335
mimic (Fig. 4B; P<0.05).
To determine whether there was an effect of miR-335
expression on ZEB2 expression, HTH83 and K1 cells transfected with
miR-335 mimics were evaluated with RT-qPCR and western blot.
RT-qPCR analysis indicated that the overexpression of miR-335
reduced ZEB2 mRNA levels in HTH83 and K1 cells (Fig. 4C; P<0.05). The western blot
analysis revealed that the expression of ZEB2 protein was
downregulated in miR-335 mimic-transfected HTH83 and K1 cells
(Fig. 4D; P<0.05). Taken together,
the results demonstrated that ZEB2 is a direct target of miR-335 in
PTC.
miR-335 inhibits the proliferation,
migration and invasion of PTC cells via the regulation of ZEB2
ZEB2 was demonstrated to be a direct target of
miR-335 in PTC. Therefore, we hypothesized that miR-335 may have
decreased the proliferation, migration and invasion of HTH83 and K1
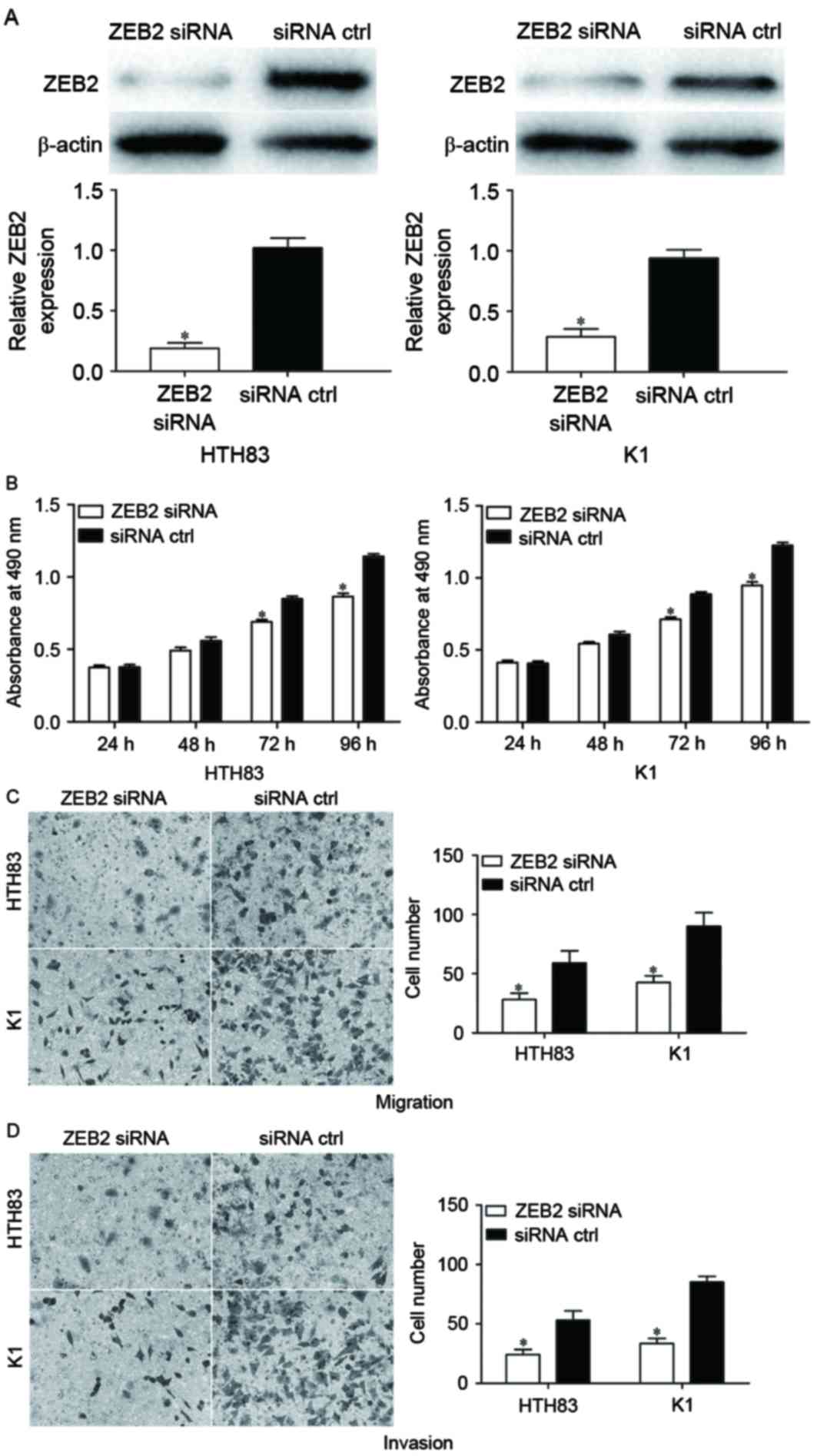
cells by the downregulation of ZEB2. To confirm this, ZEB2 siRNA
was used to knockdown ZEB2 expression. Following transfection,
western blot analysis demonstrated that ZEB2 was significantly
downregulated in HTH83 and K1 cells (Fig.
5A; P<0.05). The effect of ZEB2 siRNA on the proliferation,
migration and invasion of PTC cells was also measured. The results
indicated that ZEB2 siRNA significantly suppressed cellular
proliferation (Fig. 5B; P<0.05),
migration (Fig. 5C; P<0.05) and
invasion (Fig. 5D; P<0.05)
compared with negative control siRNA groups. These data suggested
that the overexpression of miR-335 inhibited the proliferation,
migration and invasion of PTC cells at least partially by the
knockdown of ZEB2 expression.
Discussion
It has previously been demonstrated that alterations
in the expression of miRNAs occur in a large variety of tumor types
in humans (28). There may be a
connection between the functions of miRNAs and carcinogenesis,
which is supported by investigations of the expression and
functions of miRNAs in cancer tissue specimens (29). In the present study, it was identified
that that miR-335 was significantly downregulated in PTC tissues
and cell lines compared with matched NATs and normal thyroid cells,
respectively. Functional studies revealed that miR-335 may have
functioned as a tumor suppressor in PTC cells, as its expression
was associated with the inhibition of cell growth, migration and
invasion. In addition, ZEB2 was identified as a direct target gene
of miR-335 in PTC. These results suggest that miRNA-335 may be a
candidate in the therapy of patients with PTC.
miR-335, located at 7q32.2, has also been
demonstrated as a tumor suppressor in other types of cancer. For
example, the overexpression of miR-335 was observed to suppress
breast cancer cell proliferation, cell cycle progress, colony
formation and cell invasion through the negative regulation of
Paired box 6 (30). Xu et al
(31) reported that the expression
level of miR-335 was relatively low in gastric cancer tissues, and
that a low miR-335 expression level was correlated with lymph node
metastasis, an advanced pT and pN stage, and the invasion of
lymphatic vessels. The upregulation of miR-335 may have inhibited
gastric cancer cell invasion and metastasis in vitro and
in vivo by directly targeting SP1, as well as acting
indirectly through the Bcl-W-induced phosphoinositide
3-kinase-Akt-SP1 pathway (31). The
reduced expression of miR-335 was also identified in human prostate
cancer tissues and cell lines; miR-335 expression level was
associated with a high Gleason Score, advanced clinical stage and
metastasis in patients with prostate cancer (32). Enforced miR-335 expression decreased
the growth and metastatic characteristics of prostate cancer cells
in vitro (32). Sun et
al (33) identified that miR-335
was downregulated in more invasive colorectal cancer tissues and
cell lines. Kaplan-Meier survival analysis suggested that
colorectal cancer tumors with a low miR-335 expression level were
associated with a reduced overall survival time. Furthermore, the
upregulation of miR-335 suppressed cancer cell migration and
invasion in vitro and metastasis to the lung and liver in
vivo via the inhibition of ZEB2 (33). These findings indicated that miR-335
may serve as a potential therapeutic target in the treatment of
these cancers.
Studies have also indicated that miR-335 is
upregulated in meningioma (34) and
glioma (35) tumors. The ectopic
expression of miR-335 promoted cell growth and prevented cell cycle
arrest in the G0/G1 phase through directly targeting the Rb1
signaling pathway in meningioma (34). Jiang et al (35) identified that a relatively high
miR-335 expression level was associated with advanced tumor
progression in glioma. Furthermore, miR-335 expression was verified
for the first time as an independent prognostic marker for
predicting the clinical outcome of patients with glioma (35). These results appear to be conflicting,
as miR-335 was demonstrated as an oncogene in certain types of
cancer and a tumor suppressor in others. This contradiction may be
explained by the ‘imperfect complementarity’ of the interactions
between miRNAs and target mRNAs (36).
The identification of cancer-specific miRNAs and
their target genes may provide therapeutic targets for PTC. To
investigate the molecular mechanism of miR-335 in PTC,
bioinformatics analysis was performed to predict potential target
genes. ZEB2 was predicted as a target gene for miR-335. To
determine whether miR-335 directly targets ZEB2, a dual-luciferase
reporter assay was performed; it was revealed that miR-335
significantly decreased the luciferase activity of cells
transfected with ZEB2-3′UTR Wt compared with cells transfected with
ZEB2-3′UTR Mut. The upregulation of miR-335 suppressed the mRNA and
protein expression levels of ZEB2 in PTC cells. The effect of ZEB2
siRNA in PTC cells was similar to the effect of miR-335, which
suggested that ZEB2 may be a functional target of miR-335 in
PTC.
ZEB2, a member of the zinc finger family, functions
as a transcriptional repressor of E-cadherin (37). ZEB2 has been identified as upregulated
in various types of human cancer, including breast, gastric, head
and neck, hepatocellular, ovarian and non-small cell lung
carcinoma, and glioma (38–44). ZEB2 was previously identified as
upregulated in PTC cell lines compared with normal thyroid cancer
cells (45). ZEB2 was also previously
identified as contributing to thyroid cancer migration and invasion
(3). In the present study, it was
demonstrated that the downregulation of ZEB2 significantly
inhibited the growth, migration and invasion of PTC cells. These
results indicate that ZEB2 may require scrutiny as a potential
target for the inhibition of PTC growth and metastasis.
In conclusion, miR-335 was downregulated in PTC
tissue samples and cell lines compared with matched NATs and normal
thyroid cells, respectively. The overexpression of miR-335
suppressed the proliferation, migration and invasion of PTC cells.
Furthermore, ZEB2 may be a functional target of miR-335 in PTC. All
the results obtained in the present study suggest that miR-335 may
serve as a tumor suppressor gene in the tumorigenesis and
progression of PTC. The upregulation of miR-335 may be a novel
potential therapeutic strategy in the treatment of patients with
PTC.
References
|
1
|
Sondermann A, Andreghetto FM, Moulatlet
AC, da Silva Victor E, de Castro MG, Nunes FD, Brandão LG and
Severino P: MiR-9 and miR-21 as prognostic biomarkers for
recurrence in papillary thyroid cancer. Clin Exp Metastasis.
32:521–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing M, Haugen BR and Schlumberger M:
Progress in molecular-based management of differentiated thyroid
cancer. Lancet. 381:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan H, Liang W, Xie Z, Li H, Liu J, Liu
L, Xiu L and Li Y: Down-regulation of miR-144 promotes thyroid
cancer cell invasion by targeting ZEB1 and ZEB2. Endocrine.
48:566–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geraldo MV, Fuziwara CS, Friguglieti CU,
Costa RB, Kulcsar MA, Yamashita AS and Kimura ET: MicroRNAs
miR-146-5p and let-7f as prognostic tools for aggressive papillary
thyroid carcinoma: A case report. Arq Bras Endocrinol Metabol.
56:552–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng H, Wang M, Jiang L, Chu H, Hu J,
Ning J, Li B, Wang D and Xu J: BRAF-activated long noncoding RNA
modulates papillary thyroid carcinoma cell proliferation through
regulating thyroid stimulating hormone receptor. Cancer Res Treat.
48:698–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider AB and Sarne DH: Long-term risks
for thyroid cancer and other neoplasms after exposure to radiation.
Nat Clin Pract Endocrinol Metab. 1:82–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang
X and Fan Y: MiR-146b-5p promotes metastasis and induces
epithelial-mesenchymal transition in thyroid cancer by targeting
ZNRF3. Cell Physiol Biochem. 35:71–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chou CK, Yang KD, Chou FF, Huang CC, Lan
YW, Lee YF, Kang HY and Liu RT: Prognostic implications of miR-146b
expression and its functional role in papillary thyroid carcinoma.
J Clin Endocrinol Metab. 98:E196–E205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lang BH, Wong KP, Wan KY and Lo CY:
Significance of metastatic lymph node ratio on stimulated
thyroglobulin levels in papillary thyroid carcinoma after
prophylactic unilateral central neck dissection. Ann Surg Oncol.
19:1257–1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi J, Rice SJ, Salzberg AC, Runkle EA,
Liao J, Zander DS and Mu D: MiR-365 regulates lung cancer and
developmental gene thyroid transcription factor 1. Cell Cycle.
11:177–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang HG, Luo X, Wu S and Jian B: MiR-99a
inhibits cell proliferation and tumorigenesis through targeting
mTOR in human anaplastic thyroid cancer. Asian Pac J Cancer Prev.
16:4937–4944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boufraqech M, Zhang L, Jain M, Patel D,
Ellis R, Xiong Y, He M, Nilubol N, Merino MJ and Kebebew E: miR-145
suppresses thyroid cancer growth and metastasis and targets AKT3.
Endocr Relat Cancer. 21:517–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bentwich I, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nikiforova MN, Gandhi M, Kelly L and
Nikiforov YE: MicroRNA dysregulation in human thyroid cells
following exposure to ionizing radiation. Thyroid. 21:261–266.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: MicroRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmittgen TD: Regulation of microRNA
processing in development, differentiation and cancer. J Cell Mol
Med. 12:1811–1819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rottiers V, Najafi-Shoushtari SH, Kristo
F, Gurumurthy S, Zhong L, Li Y, Cohen DE, Gerszten RE, Bardeesy N,
Mostoslavsky R and Näär AM: MicroRNAs in metabolism and metabolic
diseases. Cold Spring Harb Symp Quant Biol. 76:225–233. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bueno MJ and Malumbres M: MicroRNAs and
the cell cycle. Biochim Biophys Acta. 1812:592–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:pp. 2257–2261.
2006, View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang T, Thakur A, Chen T, Yang L, Lei G,
Liang Y, Zhang S, Ren H and Chen M: MicroRNA-15a induces cell
apoptosis and inhibits metastasis by targeting BCL2L2 in non-small
cell lung cancer. Tumour Biol. 36:4357–4365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ribeiro FR, Meireles AM, Rocha AS and
Teixeira MR: Conventional and molecular cytogenetics of human
non-medullary thyroid carcinoma: Characterization of eight cell
line models and review of the literature on clinical samples. BMC
Cancer. 8:3712008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dassow H and Aigner A: MicroRNAs (miRNAs)
in colorectal cancer: From aberrant expression towards therapy.
Curr Pharm Des. 19:1242–1252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng Y, Zou Q, Liu T, Cai X, Huang Y and
Pan J: microRNA-335 inhibits proliferation, cell-cycle progression,
colony formation and invasion via targeting PAX6 in breast cancer
cells. Mol Med Rep. 11:379–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiong SW, Lin TX, Xu KW, Dong W, Ling XH,
Jiang FN, Chen G, Zhong WD and Huang J: MicroRNA-335 acts as a
candidate tumor suppressor in prostate cancer. Pathol Oncol Res.
19:529–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Z, Zhang Z, Liu Z, Qiu B, Liu K and
Dong G: MicroRNA-335 inhibits invasion and metastasis of colorectal
cancer by targeting ZEB2. Med Oncol. 31:9822014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi L, Jiang D, Sun G, Wan Y, Zhang S,
Zeng Y, Pan T and Wang Z: miR-335 promotes cell proliferation by
directly targeting Rb1 in meningiomas. J Neurooncol. 110:155–162.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang J, Sun X, Wang W, Jin X, Bo X, Li Z,
Bian A, Jiu J, Wang X, Liu D, et al: Tumor microRNA-335 expression
is associated with poor prognosis in human glioma. Med Oncol.
29:3472–3477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y,
Yu W, Wu X, Ye J, Yang S, et al: Identification of miR-7 as an
oncogene in renal cell carcinoma. J Mol Histol. 44:669–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bindels S, Mestdagt M, Vandewalle C,
Jacobs N, Volders L, Noël A, van Roy F, Berx G, Foidart JM and
Gilles C: Regulation of vimentin by SIP1 in human epithelial breast
tumor cells. Oncogene. 25:4975–4985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kurashige J, Kamohara H, Watanabe M,
Hiyoshi Y, Iwatsuki M, Tanaka Y, Kinoshita K, Saito S, Baba Y and
Baba H: MicroRNA-200b regulates cell proliferation, invasion and
migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg
Oncol. 19 Suppl 3:S656–S664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chu PY, Hu FW, Yu CC, Tsai LL, Yu CH, Wu
BC, Chen YW, Huang PI and Lo WL: Epithelial-mesenchymal transition
transcription factor ZEB1/ZEB2 co-expression predicts poor
prognosis and maintains tumor-initiating properties in head and
neck cancer. Oral Oncol. 49:34–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qi S, Song Y, Peng Y, Wang H, Long H, Yu
X, Li Z, Fang L, Wu A, Luo W, et al: ZEB2 mediates multiple
pathways regulating cell proliferation, migration, invasion and
apoptosis in glioma. PLoS One. 7:e388422012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cai MY, Luo RZ, Chen JW, Pei XQ, Lu JB,
Hou JH and Yun JP: Overexpression of ZEB2 in peritumoral liver
tissue correlates with favorable survival after curative resection
of hepatocellular carcinoma. PLoS One. 7:e328382012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Q, Guo R, Lin M, Zhou B and Wang Y:
MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration
and invasion by targeting E-cadherin repressor ZEB2. Gynecol Oncol.
122:149–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gemmill RM, Roche J, Potiron VA, Nasarre
P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA and
Drabkin HA: ZEB1-responsive genes in non-small cell lung cancer.
Cancer Lett. 300:66–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Montemayor-Garcia C, Hardin H, Guo Z,
Larrain C, Buehler D, Asioli S, Chen H and Lloyd RV: The role of
epithelial mesenchymal transition markers in thyroid carcinoma
progression. Endocr Pathol. 24:206–212. 2013. View Article : Google Scholar : PubMed/NCBI
|