Introduction
Cervical cancer (CC) is one of the most common
gynecological malignancies with high rates of morbidity
(0.001–0.05%) and mortality (0.0024–0.0175%), endangering the
health and quality of life of women globally (1). Although the decreased incidence of CC
has primarily been attributed to the widespread use of cytological
screening, invasive CC remains an issue regarding the mortality
rates of patients with CC. Thus, the elucidation of the molecular
pathogenesis of CC is urgently required. The pathogenesis of CC
remains enigmatic and may be connected with numerous factors,
including infection with high-risk human papillomaviruses (HPVs)
(2).
Thymic stromal lymphopoietin (TSLP) is primarily
produced by stromal cells, epithelial cells, fibroblasts,
keratinocytes, basophils and other types of cells, including mast
cells, smooth muscle cells, fibroblasts, dendritic cells,
trophoblasts, and cancer or cancer-associated cells (3–5). TSLP may
trigger helper T-cell 2 cytokines, and is associated with airway
inflammatory disease, immunoglobulin E production, allergic
responses and eosinophilia (3,4,6). The TSLP receptor (TSLPR) is a typical
heterodimeric cytokine receptor, including a TSLP binding subunit
(TSLPRα) and α-subunit of the interleukin-7 receptor (7,8). A
previous study reported that an aberrantly high level of TSLP
present in cancer lesions, potentially mediated by hypoxia, was a
notable regulator of the progression of CC by recruiting and
licensing tumor-associated eosinophils (EOS) to CC lesions
(9). In addition, TSLP produced by CC
cells promotes angiogenesis in an EOS-dependent and -independent
manner (10,11). Watanabe et al (12) reported that high TSLP expression
levels indicate a poor prognosis in patients with gastric cancer.
However, whether and how TSLP regulates the proliferation and
invasion of CC cells remains unknown.
Previously, an increasing number of studies have
focused on the effect of microRNA (miRNA/miR) on CC (13). Zhao et al (14) reported that miR-132 expression was
decreased in CC tissues compared with that in adjacent
non-cancerous tissues. Transforming growth factor (TGF)-β is a
multifunctional cytokine and may induce numerous important
signaling pathways in several types of cancer cells (15,16).
Furthermore, TGF-β may regulate the expression of TSLP in the
intervertebral disc tissue (17) and
regulate the expression of miR-132 in glioma cells (18). However, it remains unknown whether
TSLP regulates the biological behaviors by modulating the
expression of miR-132 in CC.
Therefore, the present study investigated the effect
of TSLP on the expression of miR-132, and the proliferation and
invasion of the CC HeLa and SiHa cell lines in vitro.
Materials and methods
Cell culture
Cervical epidermal carcinoma HeLa and SiHa cells
were obtained from the Institute of Biochemistry and Cell Biology
of the Chinese Academy of Sciences (Shanghai, China), and grown in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 5% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified
atmosphere containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following treatment with a range of concentrations
of recombinant human TSLP (rhTSLP; 1, 10, 100 ng/ml; R&D
Systems, Inc., Minneapolis, MN, USA), the anti-human TSLP
neutralizing antibodies (α-TSLP) or anti-human TSLPR neutralizing
antibodies (α-TSLPR) (5 µg/ml; R&D Systems, Inc.) at 37°C in a
humidified incubator containing 5% CO2 for 48 h, with 1%
PBS used as a control. RT-qPCR was performed in order to determine
the expression levels of miR-132 in the HeLa and SiHa cells. Total
RNA was extracted from these cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. The total miRNA of HeLa and SiHa cells
were harvested using the PureLink™ miRNA Isolation kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. miR-132 expression was detected using the TaqMan MicroRNA
Assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The parameters of the PCR
reaction were as follows: 94°C for 2 min, 1 cycle; 94°C for 20 sec,
60°C for 34 sec for 40 cycles. The relative expression levels of
miR-132 were analyzed using the 2−ΔΔCq relative
quantification method with human U6 small nuclear RNA used as an
internal control (19). The sequences
of primers and TaqMan probes are presented in Table I. All assays were performed in
triplicate.
 | Table I.Sequences of the primers and TaqMan
probes. |
Table I.
Sequences of the primers and TaqMan
probes.
| A, Primer
sequence |
|---|
|
|---|
| Gene | Sequence
(5′-3′) |
|---|
| miR-132 |
|
|
Forward |
TGGATCCCCCCCAGTCCCCGTCCCTCAG |
|
Reverse |
TGAATTCGGATACCTTGGCCGGGAGGAC |
| U6 |
|
|
Forward |
CTCGCTTCGGCAGCACA |
|
Reverse |
AACGCTTCACGAATTTGCGT |
|
| B, TaqMan probe
sequence |
|
| Gene | Sequence
(5′-3′) |
|
| miR-132 |
FAM-TGGATACGACCGACCAT-BHQ1 |
| U6 |
FAM-TGCGCAAGGATGACACGCA-BHQ1 |
Overexpression of miR-132 in HeLa and
SiHa cells
The miR-132 mimic lentivirus (miR-132-mimic) and its
corresponding miRNA lentivirus negative control (miR-NC) were
constructed by Shanghai GenePharma Co., Ltd. (Shanghai, China).
miR-132 overexpression and corresponding control stable cell lines
were then established. The recombinant virus was packaged using the
Lentivector Expression system (GeneChem Co., Ltd.), and HeLa and
SiHa cells were infected. For lentivirus infection,
3×105 HeLa or SiHa cells were cultured in 6-well plates
and incubated for 24 h prior to be infected. Next, miR-132-mimic
lentivirus or miR-NC lentivirus at a multiplicity of infection
(MOI) of 20, was added to the cells. After three days, the
efficiency of miR-132 overexpression was verified using RT-qPCR
analysis. PCR cycling conditions were as follows: 94°C for 2 min, 1
cycle; 94°C for 20 sec, 60°C for 34 sec for 40 cycles. The
subsequent experiments were performed using viruses at the
aforementioned MOIs.
Bromodeoxyuridine (BrdU) cell
proliferation and Matrigel invasion assays
The miR-NC and miR-132-mimic HeLa and SiHa cells
were treated with rhTSLP (10 ng/ml) in a humidified incubator
containing 5% CO2 at 37°C for 48 or 72 h. In addition,
the vehicle (1% PBS) for the negative control was added. Next, the
proliferation ability of the HeLa and SiHa cells was detected using
a BrdU cell proliferation assay kit (Merck KGaA, Darmstadt,
Germany) according to the manufacturer's protocol. The experiments
were performed in triplicate, and repeated 4 times.
In addition, the invasion ability of HeLa and SiHa
cells was analyzed using a Matrigel invasion assay. Briefly, the
cells inserts (8-µm pore size, 6.5-mm diameter; Corning
Incorporated, Corning, NY, USA) coated with 25 µl Matrigel were
placed in a 24-well plate. The miR-NC and miR-132-mimics HeLa and
SiHa cells, at 2×104 cells per well, were plated in the
upper chamber with 100 µl RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.). RhTSLP (10 ng/ml) or the vehicle was added. The
lower chamber was filled with 800 µl RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) including 5% FBS. The cells were
then incubated at 37°C for 48 h. The inserts were removed, washed
in phosphate-buffered saline, and the non-invading cells together
with the Matrigel were removed from the upper surface of the filter
by wiping with a cotton bud. The inserts were then fixed in
methanol for 10 min at room temperature and stained with
hematoxylin for 30 min at room temperature. The result was observed
under an Olympus BX51+P70 microscope (Olympus Corporation, Tokyo,
Japan). The cells that migrated to the lower surfaces were counted
in 5 predetermined fields using light microscopy at a magnification
of ×200. Each experiment was performed in triplicate, and repeated
3 times.
Flow cytometry (FCM)
The expression of marker of proliferation Ki-67 and
proliferating cell nuclear antigen (PCNA) in HeLa and SiHa cells
was analyzed using FCM. Specifically, these cells were fixed with
4% paraformaldehyde and permeabilized with 70% ethanol. After
centrifugation at 150 × g for 10 min at room temperature, the
precipitate was resuspended in 1 ml of 0.9% physiological saline
and centrifuged at 150 × g for 10 min at room temperature. The
precipitate was then resuspended in 150 µl 0.9% physiological
saline and blocked with human AB serum (Sigma-Aldrich; Merck KGaA)
at 4°C for 30 min. Samples were then stained with
allophycocyanin-conjugated anti-human Ki-67 antibody (1:30, cat.
no. 350514; BioLegend, Inc., San Diego, CA, USA) and
phycoerythrin-conjugated anti-human PCNA antibody (1:30, cat. no.
307908; BioLegend, Inc.) for 30 min at room temperature.
Thereafter, the cells were washed twice, and resuspended in PBS for
FCM analysis. In parallel, APC-conjugated Mouse IgG1, κ Isotype
Control antibody (1:30, cat. no. 400119; BioLegend, Inc.) and
PE-conjugated Mouse IgG2a, κ Isotype Control antibody (1:30, cat.
no. 400211; BioLegend, Inc.) were used as controls. Samples were
analyzed in a FACS Calibur flow cytometer (Becton Dickinson, New
York, NY, USA) using Becton Dickinson CellQuest software (version
7.1; Becton Dickinson). Statistical analysis was conducted by using
isotype matched controls as references.
Enzyme-linked immunosorbent assay
(ELISA)
miR-NC and miR-132-mimic HeLa and SiHa cells were
cultured in a humidified incubator containing 5% CO2 at
37°C for 72 h. Next, the cell culture supernatant was harvested,
centrifuged at 300 × g at 4°C for 10 min to remove cellular debris,
and stored at −80°C until assayed using an ELISA. The secretion
level of matrix metalloproteinase (MMP)2 and MMP9 using the
supernatant was detected using human MMP2 and MMP9 ELISA kits
(Shanghai ExCell Biology, Inc., Shanghai, China) according to the
manufacturer's protocols. The experiment was repeated three
times.
Statistics
All data are presented as the mean ± the standard
error of the mean. The data were analyzed using GraphPad Prism
version 5 (GraphPad Software, Inc., La Jolla, CA, USA) using an
unpaired Student's t-test, or one-way analysis of variance followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TSLP downregulates the expression of
miR-132 in HeLa and SiHa cells
In order to evaluate the effect of TSLP on the
expression of miR-132 in CC cells, HeLa and SiHa cells were treated
with with rhTSLP, α-TSLP or α-TSLPR. rhTSLP was revealed to
downregulate the expression of miR-132 in HeLa and SiHa cells, with
a signficant difference observed at concentrations of 10 and 100
ng/ml (P<0.05 or P<0.01; Fig. 1A
and B). By contrast, blocking TSLP signaling using α-TSLP or
α-TSLPR significantly upregulated the expression of miR-132 in the
HeLa and SiHa cells (P<0.05 or P<0.01; Fig. 1A and B). These results indicate that
exogenous and endogenous TSLP decrease the expression of miR-132 in
CC cells, and may further regulate the biological behaviors of CC
cells.
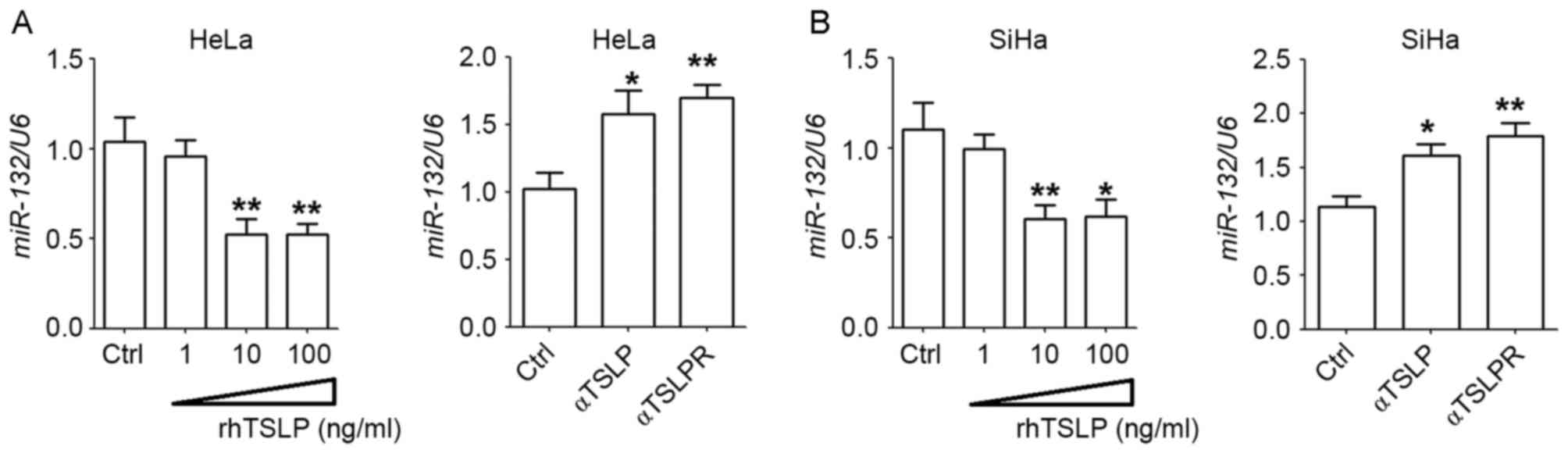 | Figure 1.TSLP downregulates the expression
levels of miR-132 of HeLa and SiHa cells. Following treatment with
differing concentrations of rhTSLP (1, 10 and 100 ng/ml), α-TSLP or
α-TSLPR (5 µg/ml), or no treatment for 48 h, the mRNA expression
levels of miR-132 in (A) HeLa and (B) SiHa cells were analyzed
using reverse transcription-quantitative polymerase chain reaction
analysis. *P<0.05 or **P<0.01 vs. the control. TSLP, thymic
stromal lymphopoietin; rhTSLP, recombinant human TSLP; α-TSLP,
anti-human TSLP neutralizing antibody; α-TSLPR, anti-human TSLP
receptor neutralizing antibody; miR, microRNA; Ctrl, control. |
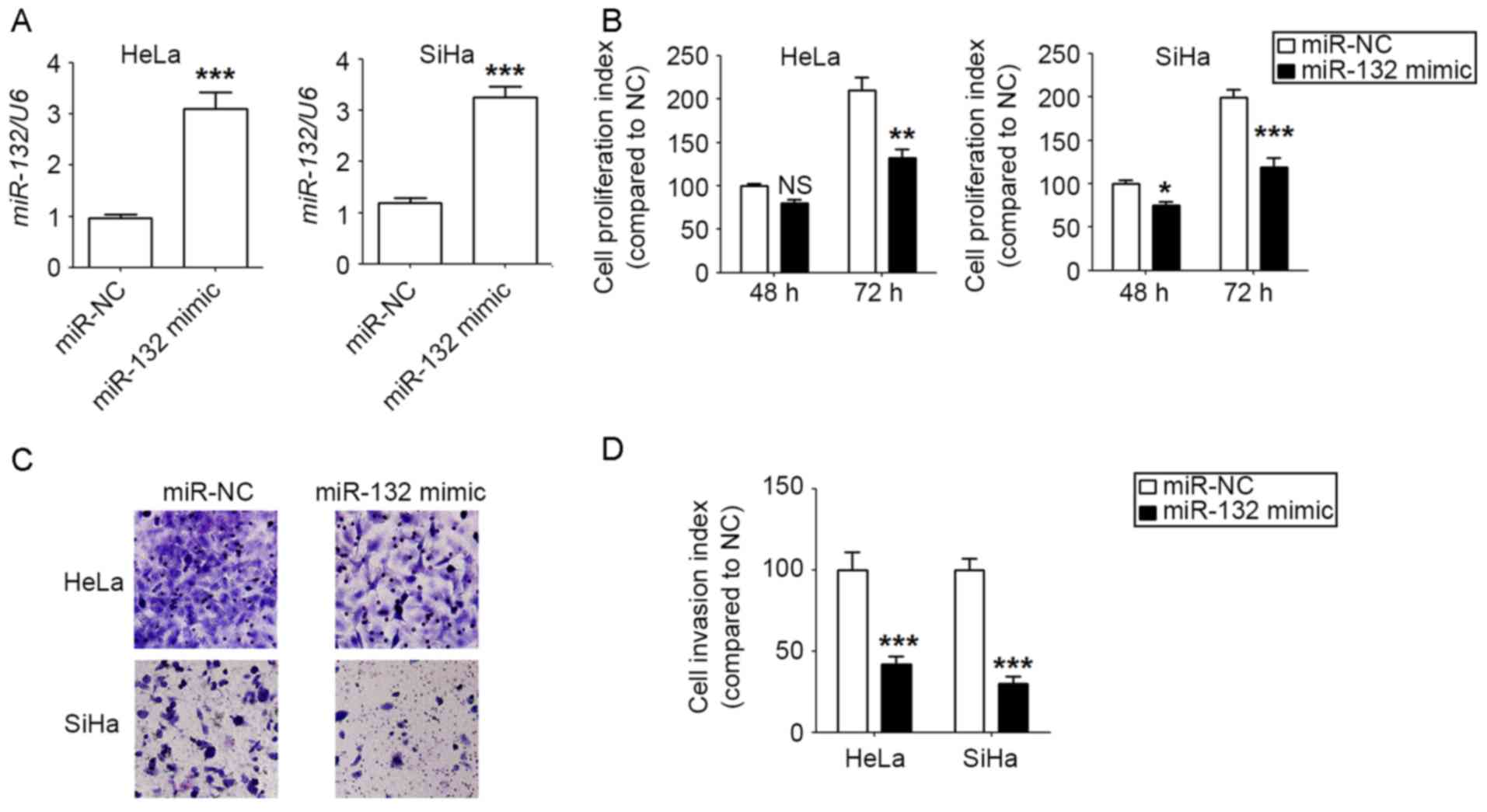
miR-132 inhibits the proliferation and
invasion of HeLa and SiHa cells
To investigate the potential function of miR-132 in
CC cells, miR-132 was first overexpressed in HeLa and SiHa cells by
transfection, and miR-132 overexpression was obtained in
miR-132-mimic stable HeLa and SiHa cell lines, compared with the
negative control (P<0.001; Fig.
2A). Next, it was revealed that there were lower levels of
proliferation in the miR-132-overexpressed HeLa and SiHa cells
compared with the control cells, with a greater signficant
difference observed at 72 h (P<0.05, P<0.01 or P<0.001;
Fig. 2B). Furthermore, miR-132
overexpression resulted in a lower invasiveness of HeLa and SiHa
cells compared with that of the control cells (P<0.001; Fig. 2C and D). This data suggest that
miR-132 may suppress the proliferation and invasion of CC cells
in vitro.
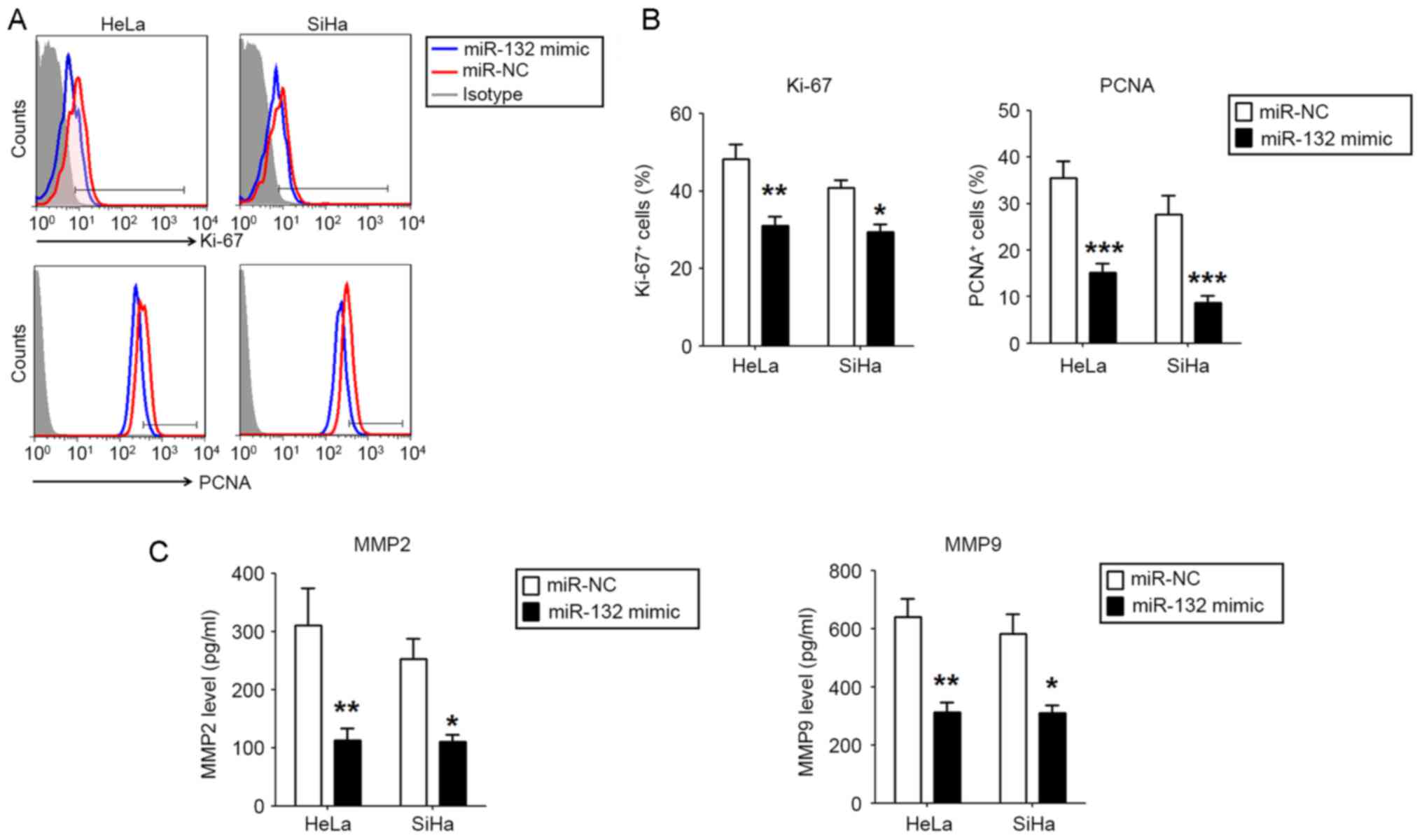
miR-132 downregulates the expression
of Ki-67, PCNA, MMP2 and MMP9 in HeLa and SiHa cells
To explore the potential mechanism of miR-132 in
regulating the proliferation and invasion of CC cells, the
expression of proliferation-associated molecules Ki-67 and PCNA,
and invasion-associated molecules MMP2 and MMP9 were analyzed
between miR-132-overexpressed HeLa and SiHa cells and control
cells. As presented, the overexpression of miR-132 resulted in the
significantly lower expression of Ki-67 and PCNA (P<0.05,
P<0.01 or P<0.001; Fig. 3A and
B), and MMP2 and MMP9 (P<0.05 or P<0.01; Fig. 3C) in the HeLa and SiHa cells.
Together, these data suggest that miR-132 may inhibit the
proliferation and invasion of CC cells, potentially via the
downregulation of Ki-67, PCNA, MMP2 and MMP9 in vitro.
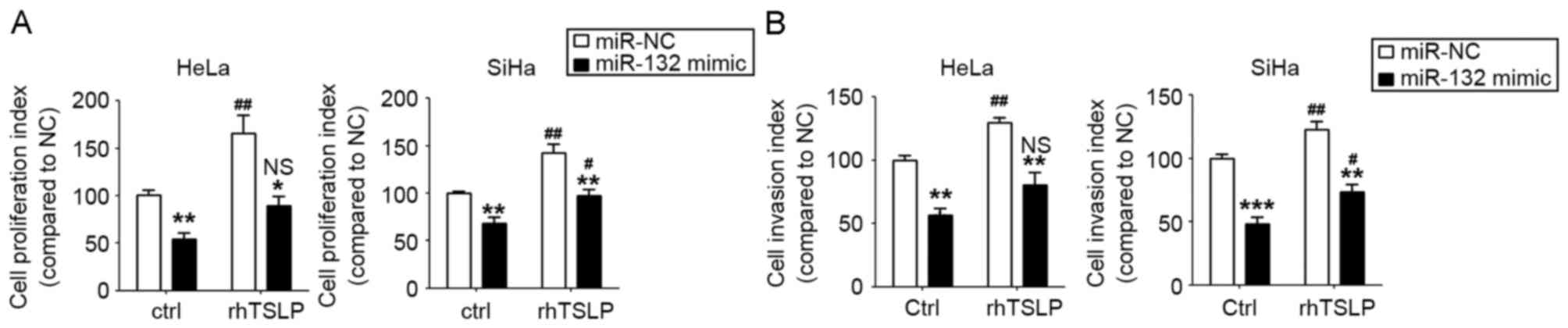
TSLP stimulates the proliferation and
invasion of HeLa and SiHa cells by the downregulation of
miR-132
To further explore the function and mechanism of
TSLP in CC cells, the miR-132-overexpressed HeLa and SiHa cells and
the control cells were incubated with rhTSLP or were left
untreated. As presented, rhTSLP markedly stimulated the
proliferation and invasion of HeLa and SiHa cells (P<0.05 or
P<0.01; Fig. 4A and B). However,
the overexpression of miR-132 could partly abrogate the effects of
rhTSLP on the proliferation and invasion ability of the HeLa and
SiHa cells (Fig. 4A and B). The
results of the present study suggest that TSLP promotes the
proliferation and invasion of CC cells, and that these effects are
partly dependent on the downregulation of miR-132.
Discussion
As a class of small, highly-conserved non-coding
RNAs, miRNAs are known to regulate gene expression at the
post-transcriptional level by complementarity with the biding sites
in the 3′-untranslated region of the target mRNA (20). It has been reported that miRNAs serve
functions in numerous physiological and pathological processes,
including cell development, differentiation, proliferation,
apoptosis and metastasis. An accumulating body of evidence has
indicated that miRNAs serve vital functions in the progression of
CC and that they directly contribute to the growth and metastasis
of CC cells via the targeting of a large number of critical
protein-coding genes. miR-132 is located on human chromosome
17p13.3, which is associated with various types of human cancer
including osteosarcoma, gastric cancer (21), colorectal cancer (22), prostate cancer (23), breast cancer (24,25),
hepatocellular carcinoma (26),
pancreatic cancer (27,28) and glioma (29). Zhao et al (14) reported that the expression levels of
miR-132 in CC tissues were lower compared with those in adjacent
non-cancerous tissues, and it was revealed that miR-132
downregulated SMAD family member (SMAD)2 expression in order to
suppress the G1/S phase transition of the cell cycle and the
epithelial to mesenchymal transition (EMT) in CC cells. However,
the mechanism resulting in the low expression of miR-132 in CC
remains largely unknown.
Previous research has established that TSLP is
aberrantly highly expressed in CC cells, indirectly promoting their
growth by recruiting and regulating tumor-associated EOS, and
stimulating angiogenesis in CC lesions (9–11).
Additionally, hypoxia may contribute to the increase in the TSLP
expression level in CC cells. In the present study, it was revealed
that exogenous and endogenous TSLP decreased the level of miR-132
expression in HeLa and SiHa cells, and further stimulated the
proliferation and invasion of CC cells in vitro. In
addition, the inhibitory effects of miR-132 on the proliferation
and invasion of CC cells were associated with the regulation of
Ki-67, PCNA, MMP2 and MMP9.
TGF-β may upregulate the miR-132 expression level in
dose- and time-dependent manners, and may further enhance the
activation of TGF-β signaling by inhibiting SMAD7 expression in
glioma cells (18). TGF-β
additionally serves important regulatory functions in CC, as for
example, the oncoproteins of HPVs may stimulate TGF-β1 expression
in CC cells, which in turn suppresses the host immune surveillance
towards CC, and triggers the EMT process, migration and metastasis
of CC cells (30–33). Endogenous TGF-β activity has been
reported to limit the expression in of TSLP in intervertebral disc
tissue in a steady state by suppressing nuclear factor-κB
activation (17). Therefore, TGF-β
upregulates the expression of miR-132, potentially by suppressing
TSLP production, and the association between TGF-β, TSLP and
miR-132 in CC cells requires further research.
The high level of TSLP production is associated with
local hypoxia (9). Notably, hypoxia
also results in the upregulation of miR-132 (34,35). Thus,
hypoxia may regulate CC cells in a dual-directional manner by
upregulating TSLP and miR-132. The precise association and
mechanism of hypoxia with TSLP and miR-132 in CC should be further
studied for clarification.
Antigen Ki-67 is a nuclear protein that is
associated with, and may be necessary for, cellular proliferation.
Ki-67 and PCNA are considered to be prognostic markers in CC
(36,37). A number of studies have demonstrated
that miR-132 inhibits the growth of tumor cells by suppressing the
expression of Ki-67 (26,38). Cancer cell migration from the tissue
of origin to either the surrounding or distant organs is vital for
the progression of tumors. An association has been found between
MMP2 and MMP9 and the processes of tumor cell invasion and
metastasis in multiple types of human cancer, including uterine
neoplasms (39). Jasińska et
al (40) reported that miR-132
regulated the structural plasticity of dendritic spines through
directly repressing the expression of MMP9. In the present study,
miR-132 significantly downregulated the expression of
proliferation-associated proteins Ki-67 and PCNA, and
invasion-associated enzymes MMP2 and MMP9 in CC cells, and further
suppressed the proliferation and invasion of CC cells in
vitro.
Based on the results of the present study and other
studies, as presented in Fig. 5, it
may be concluded that the high level of TSLP may be attributed to
hypoxia and/or TGF-β. This high level increases EOS infiltration
and tumor angiogenesis, and downregulates the expression level of
miR-132 in CC cells. miR-132 may decrease the expression of Ki-67,
PCNA, MMP2 and MMP9, and limit the proliferation and invasion of CC
cells. Therefore, these numerous effects of TSLP contribute to the
development of CC. The results of the present study further
contribute to the present understanding on the biological function
and manner of TSLP/miR-132 signaling in CC progression.
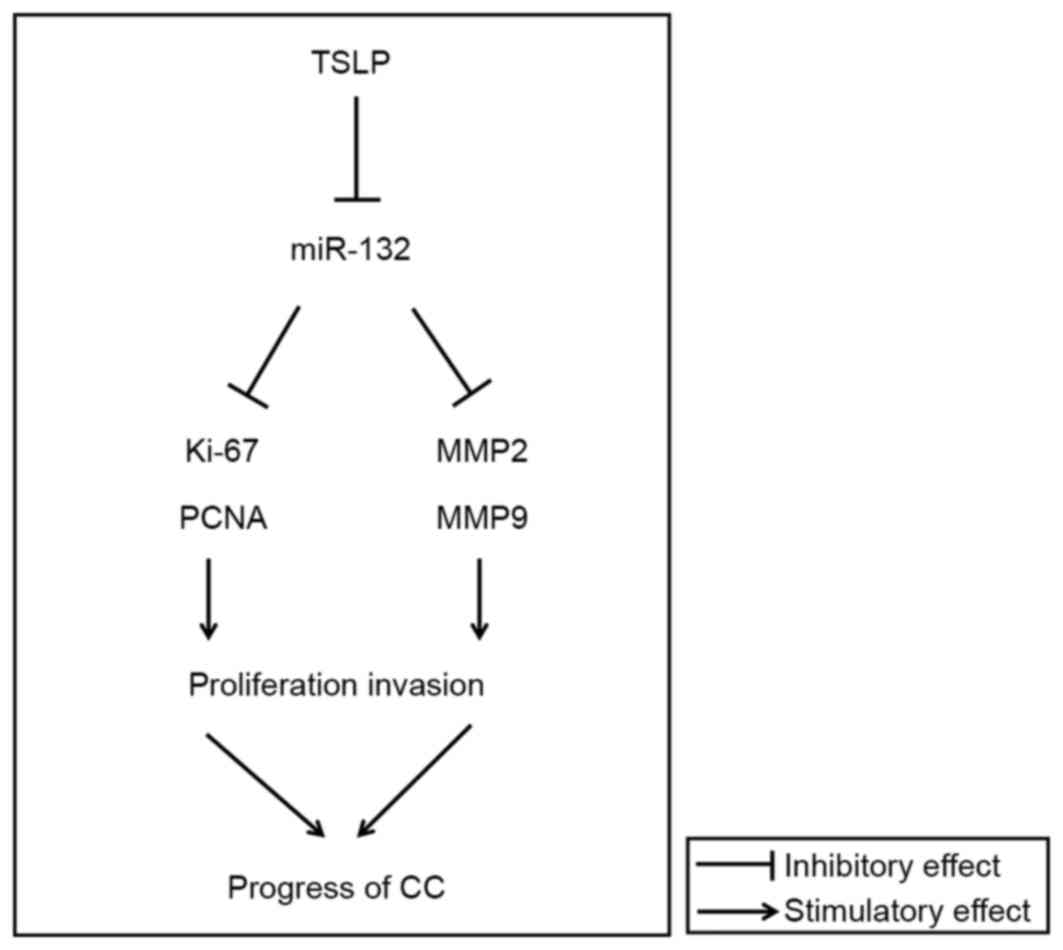 | Figure 5.Function of TSLP/miR-132 signaling in
CC cells. CC cells secrete high levels of TSLP. TSLP then
downregulates the level of miR-132 in CC cells. As a result,
miR-132 suppresses the expression of proliferation-associated
molecules Ki-67 and PCNA, and invasion-associated molecules MMP2
and MMP9, and further inhibits the proliferation and invasion of CC
cells. Therefore, TSLP/miR-132 signaling contributes to the
progression of CC. TSLP, thymic stromal lymphopoietin; miR,
microRNA; CC, cervial cancer; Ki-67, marker of proliferation Ki-67;
PCNA, proliferating cell nuclear antigen; MMP, matrix
metalloproteinase. |
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81302260 and
31600735) and the Shanghai Natural Science Foundation (grant no.
17ZR1403200), and the Program for Zhuoxue of Fudan University.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
zur Hausen H: Human papillomaviruses in
the pathogenesis of anogenital cancer. Virology. 184:9–13. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YJ, Soumelis V, Watanabe N, Ito T,
Wang YH, Malefyt Rde W, Omori M, Zhou B and Ziegler SF: TSLP: An
epithelial cell cytokine that regulates T cell differentiation by
conditioning dendritic cell maturation. Annual Annu Rev Immunol.
25:193–219. 2007. View Article : Google Scholar
|
|
4
|
Sokol CL, Barton GM, Farr AG and Medzhitov
R: A mechanism for the initiation of allergen-induced T helper type
2 responses. Nat Immunol. 9:310–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rochman Y and Leonard WJ: Thymic stromal
lymphopoietin: A new cytokine in asthma. Curr Opin Pharmacol.
8:249–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YJ: Thymic stromal lymphopoietin and
OX40 ligand pathway in the initiation of dendritic cell-mediated
allergic inflammation. J Allergy Clin Immunol. 120:238–246. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park LS, Martin U, Garka K, Gliniak B, Di
Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr
AG, et al: Cloning of the murine thymic stromal lymphopoietin
(TSLP) receptor: Formation of a functional heteromeric complex
requires interleukin 7 receptor. J Exp Med. 192:659–670. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reche PA, Soumelis V, Gorman DM, Clifford
T, Liu Mr, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, et
al: Human thymic stromal lymphopoietin preferentially stimulates
myeloid cells. J Immunol. 167:336–343. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie F, Liu LB, Shang WQ, Chang KK, Meng
YH, Mei J, Yu JJ, Li DJ and Li MQ: The infiltration and functional
regulation of eosinophils induced by TSLP promote the proliferation
of cervical cancer cell. Cancer Lett. 364:106–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie F, Meng YH, Liu LB, Chang KK, Li H, Li
MQ and Li DJ: Cervical carcinoma cells stimulate the angiogenesis
through TSLP promoting growth and activation of vascular
endothelial cells. Am J Reprod Immunol. 70:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang B, Wei CY, Chang KK, Yu JJ, Zhou WJ,
Yang HL, Shao J, Yu JJ, Li MQ and Xie F: TSLP promotes angiogenesis
of human umbilical vein endothelial cell by strengthening crosstalk
between cervical cancer cells and eosinophils. Oncol Lett. DOI:
10.3892/ol.2017.7121.
|
|
12
|
Watanabe J, Saito H, Miyatani K, Ikeguchi
M and Umekita Y: TSLP expression and high serum TSLP level indicate
a poor prognosis in gastric cancer patients. Yonago Acta Med.
58:137–143. 2015.PubMed/NCBI
|
|
13
|
Deftereos G, Corrie SR, Feng Q, Morihara
J, Stern J, Hawes SE and Kiviat NB: Expression of mir-21 and
mir-143 in cervical specimens ranging from histologically normal
through to invasive cervical cancer. PLoS One. 6:e284232011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao JL, Zhang L, Guo X, Wang JH, Zhou W,
Liu M, Li X and Tang H: miR-212/132 downregulates SMAD2 expression
to suppress the G1/S phase transition of the cell cycle and the
epithelial to mesenchymal transition in cervical cancer cells.
IUBMB Life. 67:380–394. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazono K, Suzuki H and Imamura T:
Regulation of TGF-beta signaling and its roles in progression of
tumors. Cancer Sci. 94:230–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massagué J: TGFβ in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Ohba T, Ando T, Fujita K, Koyama K,
Nakamura Y, Katoh R, Haro H and Nakao A: Endogenous TGF-β activity
limits TSLP expression in the intervertebral disc tissue by
suppressing NF-κB activation. J Orthop Res. 31:1144–1149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang ZH, Zhang QS, Duan YL, Zhang JL, Li
GF and Zheng DL: TGF-β induced miR-132 enhances the activation of
TGF-β signaling through inhibiting SMAD7 expression in glioma
cells. Biochem Biophys Res Commun. 463:187–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Yu H, Cai H and Wang Y: The
expression and clinical significance of miR-132 in gastric cancer
patients. Diagn Pathol. 9:572014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng YB, Luo HP, Shi Q, Hao ZN, Ding Y,
Wang QS, Li SB, Xiao GC and Tong SL: miR-132 inhibits colorectal
cancer invasion and metastasis via directly targeting ZEB2. World J
Gastroenterol. 20:6515–6522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Formosa A, Lena AM, Markert EK, Cortelli
S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P,
Finazzi-Agrò E, et al: DNA methylation silences miR-132 in prostate
cancer. Oncogene. 32:127–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang ZG, Chen WX, Wu YH, Liang HF and
Zhang BX: MiR-132 prohibits proliferation, invasion, migration, and
metastasis in breast cancer by targeting HN1. Biochem Biophys Res
Commun. 454:109–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Meng H, Zhou F, Zhai L, Zhang L, Gu
F, Fan Y, Lang R, Fu L, Gu L and Qi L: MicroRNA-132 is frequently
down-regulated in ductal carcinoma in situ (DCIS) of breast and
acts as a tumor suppressor by inhibiting cell proliferation. Pathol
Res Pract. 209:179–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Tang W, Li R, He R, Gan T, Luo Y,
Chen G and Rong M: Downregulation of microRNA-132 indicates
progression in hepatocellular carcinoma. Exp Ther Med.
12:2095–2101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang S, Hao J, Xie F, Hu X, Liu C, Tong
J, Zhou J, Wu J and Shao C: Downregulation of miR-132 by promoter
methylation contributes to pancreatic cancer development.
Carcinogenesis. 32:1183–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo G, Long J, Cui X, Xiao Z, Liu Z, Shi
S, Liu L, Liu C, Xu J, Li M and Yu X: Highly lymphatic metastatic
pancreatic cancer cells possess stem cell-like properties. Int J
Oncol. 42:979–984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Q, Liao F, Wu H, Cai T, Yang L, Wang
ZF and Zou R: Upregulation of miR-132 expression in glioma and its
clinical significance. Tumor Biol. 35:12299–12304. 2014. View Article : Google Scholar
|
|
30
|
Zhu H, Luo H, Shen Z, Hu X, Sun L and Zhu
X: Transforming growth factor-β1 in carcinogenesis, progression,
and therapy in cervical cancer. Tumor Biol. 37:7075–7083. 2016.
View Article : Google Scholar
|
|
31
|
Sun SH, Liu D, Deng YT, Zhang XX, Wan DY,
Xi BX, Huang W, Chen Q, Li MC, Wang MW, et al: SIX1 coordinates
with TGFβ signals to induce epithelial-mesenchymal transition in
cervical cancer. Oncol Lett. 12:1271–1278. 2016.PubMed/NCBI
|
|
32
|
Torres-Poveda K, Bahena-Román M,
Madrid-González C, Burguete-García AI, Bermúdez-Morales VH,
Peralta-Zaragoza O and Madrid-Marina V: Role of IL-10 and TGF-β1 in
local immunosuppression in HPV-associated cervical neoplasia. World
J Clin Oncol. 5:753–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin H, Huang CC, Ou YC, Huang EY,
Changchien CC, Tseng CW, Fu HC, Wu CH, Li CJ and Ma YY: High
immunohistochemical expression of TGF-β1 predicts a poor prognosis
in cervical cancer patients who harbor enriched endoglin
microvessel density. Int J Gynecol Pathol. 31:482–489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao C, Shi X, Zhang Z, Zhou S, Qian T,
Wang Y, Ding F, Gu X and Yu B: Hypoxia-Induced upregulation of
miR-132 promotes schwann cell migration after sciatic nerve injury
by targeting PRKAG3. Mol Neurobiol. 53:5129–5139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong S, Lee J, Seo HH, Lee CY, Yoo KJ, Kim
SM, Lee S, Hwang KC and Choi E: Na(+)-Ca(2+) exchanger targeting
miR-132 prevents apoptosis of cardiomyocytes under hypoxic
condition by suppressing Ca(2+) overload. Biochem Biophys Res
Commun. 460:931–937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piri R, Ghaffari A, Azami-Aghdash S,
Ali-Akbar YP, Saleh P and Naghavi-Behzad M: Ki-67/MIB-1 as a
prognostic marker in cervical cancer-a systematic review with
meta-analysis. Asian Pac J Cancer Prev. 16:6997–7002. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Astudillo H, Lopez T, Castillo S, Gariglio
P and Benitez L: p53, Bcl-2, PCNA expression, and apoptotic rates
during cervical tumorigenesis. Ann N Y Acad Sci. 1010:771–774.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo J, Meng C, Tang Y, Zhang S, Wan M, Bi
Y and Zhou X: miR-132/212 cluster inhibits the growth of lung
cancer xenografts in nude mice. Int J Clin Exp Med. 7:4115–4122.
2014.PubMed/NCBI
|
|
39
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (Review). Int J Oncol.
34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jasińska M, Miłek J, Cymerman IA, Łęski S,
Kaczmarek L and Dziembowska M: miR-132 regulates dendritic spine
structure by direct targeting of matrix metalloproteinase 9 mRNA.
Mol Neurobiol. 53:4701–4712. 2016. View Article : Google Scholar : PubMed/NCBI
|