Introduction
Lung cancer is the leading cause of
cancer-associated mortality globally (1). Although surgical resection is considered
the best chance for curing patients with early-stage disease and
certain patient subsets with locally advanced disease, the
prognosis for these patients is unfavorable, with poor five-year
survival rates and high recurrence rates (2,3).
Hematogenous dissemination of circulating tumor cells (CTCs) is
important in initiating metastasis and disease recurrence (4). In addition to tumor biological features,
intraoperative manipulation may also cause CTC shedding into the
blood and accelerate metastasis subsequent to surgery (5,6).
Over the past decade, the CELLSEARCH system (Veridex
LLC, Raritan, NJ, USA), based on immunomagnetic enrichment of
epithelial cells and identification by fluorescently labeled
antibodies (7), has been widely used
to evaluate CTC numbers, and by extension, to assess treatment
response and predict prognosis in cancer, including non-small cell
lung cancer (NSCLC) (8–10). Using CELLSEARCH, two studies on lung
cancer surgery evaluated CTC counts in pulmonary venous (PV) blood
and peripheral blood during surgery (11,12).
Although these studies reported the presence of CTCs in PV blood
(CTC-PV) and increased CTC-PV following intraoperative
manipulation, whether their conclusions can be used to standardize
and guide intraoperative surgical behavior for lung cancer is
unclear. In addition, whether CTCs detected in PV blood are viable
and tumorigenic is also unknown.
The present study was conducted using CELLSEARCH to
assess the extent to which surgical manipulation causes tumor cell
dissemination, to analyze the associated risk factors and to
explore the biological features of tumor cells shed in the blood.
The present study aimed to provide evidence for confirming
oncological principles in lung cancer surgery and guiding
perioperative treatment.
Materials and methods
Patients
The present study corresponds to a prospective study
conducted at the Second Department of Thoracic Surgery of Peking
University Cancer Hospital (Beijing, China). Between October 2013
and March 2014, a total of 33 consecutive patients were enrolled in
the present study, including 32 patients with lung cancer and 1
with pulmonary hamartoma. In total, 18 malignant tumors were
diagnosed by bronchoscopy or CT-guided needle biopsy prior to
surgery and the corresponding patients underwent lobectomy; the
remaining patients, including the patient with hamartoma, did not
undergo wedge resection for biopsy prior to lobectomy during
surgery since the tumor was either too large or too close to the
hilum. Resection consisted of 5 pneumonectomies (15.2%), 3 sleeve
lobectomies (9.1%), 1 bilobectomy (3.0%) and 24 lobectomies
(72.7%). All cases met complete resection (R0) criteria (13). The 32 patients with lung cancer
consisted of 19 males (59%) and 13 females (41%), with the age
ranging between 32 and 78 years (mean, 59.5±8.7 years). The patient
clinical characteristics are listed in Table I. Prior to surgery, brain magnetic
resonance imaging studies, abdomen and supraclavicular lymph node
ultrasound examination, and bone scans were performed to exclude
distant metastases. Positron emission tomography (PET)-computed
tomography (CT) was used as a diagnostic and staging method.
Patients were excluded if they had concurrent or prior malignancy
treated within the previous 5 years. The Institutional Ethics Board
of Peking University Cancer Hospital approved the study protocol,
and patients who participated in the study provided written
informed consent.
 | Table I.Patient characteristics and
correlation with CTCs/CTM. |
Table I.
Patient characteristics and
correlation with CTCs/CTM.
|
|
| P-value |
|---|
|
|
|
|
|---|
| Characteristics | No. of patients with
lung cancer (n=32) | CTC-peri | CTCs-PV | CTMs-PV |
|---|
| Sex |
|
|
|
|
| Male | 19 (59%) | 0.668 | 0.050 | 0.440 |
|
Female | 13 (41%) |
|
|
|
| Age (range) | 32-78 (years) | 0.219 | 0.664 | 0.829 |
| Histological
type |
|
|
|
|
|
Adenocarcinoma | 16 (50%) | 0.442 | 0.734 | 0.811 |
| Non
adenocarcinoma | 16 (50%) |
|
|
|
| Tumor site |
|
|
|
|
|
Central | 8 (25%) | 0.764 | 0.281 | 0.789 |
|
Peripheral | 24 (75%) |
|
|
|
| Visceral pleural
involvement |
|
|
|
|
| Yes | 6 (19%) | 0.182 | 0.176 | 0.331 |
| No | 26 (81%) |
|
|
|
| Vessel
invasion |
|
|
|
|
|
Yes | 6 (19%) | 0.182 | 0.059 | 0.232 |
| No | 26 (81%) |
|
|
|
| Tumor size range
(cm) | 1.5–9.0 | 0.016 | 0.012 | 0.028 |
| Lymph node
metastasis |
|
|
|
|
|
Yes | 13 (41%) | 0.143 | 0.038 | 1.000 |
| No | 19 (59%) |
|
|
|
| Pathological TNM
staging |
|
|
|
|
| I | 13 (41%) | 0.149 | 0.048 | 0.641 |
| II | 12 (37%) |
|
|
|
|
III | 7 (22%) |
|
|
|
| CT-guided needle
biopsya |
|
|
|
|
|
Yes | 8 (33%) | 0.259 | 0.646 | 0.139 |
| No | 16 (67%) |
|
|
|
| SUVb range | 2.6–20.5 | 0.691 | 0.863 | 0.537 |
Clinical information
The same surgical team performed all surgeries, and
the following clinical information was carefully recorded in
detail: Age, sex, histological type, tumor site, visceral pleural
involvement, vessel invasion, tumor size and primary tumor standard
uptake value (SUV). The pathological stage was determined according
to the 7th edition of the tumor-node-metastasis (TNM)
classification system (3).
Perioperative treatment, which included neoadjuvant chemotherapy,
adjuvant chemotherapy and targeted therapy, was also recorded.
Clinical response to neoadjuvant chemotherapy was evaluated by CT
according to the Response Evaluation Criteria in Solid Tumors
(14). Histopathological response to
neoadjuvant chemotherapy was assessed according to the percentage
of residual tumor, which was estimated using hematoxylin and eosin
(H&E)-stained slides of gross residual tumor sections. The
result for each slide was averaged to obtain a mean value.
Follow-up information was collected every 3 months within 2 years
after surgery.
Blood collection
All patients underwent lobectomy, sleeve lobectomy,
bilobectomy or pneumonectomy directly. Neither segmentectomy nor
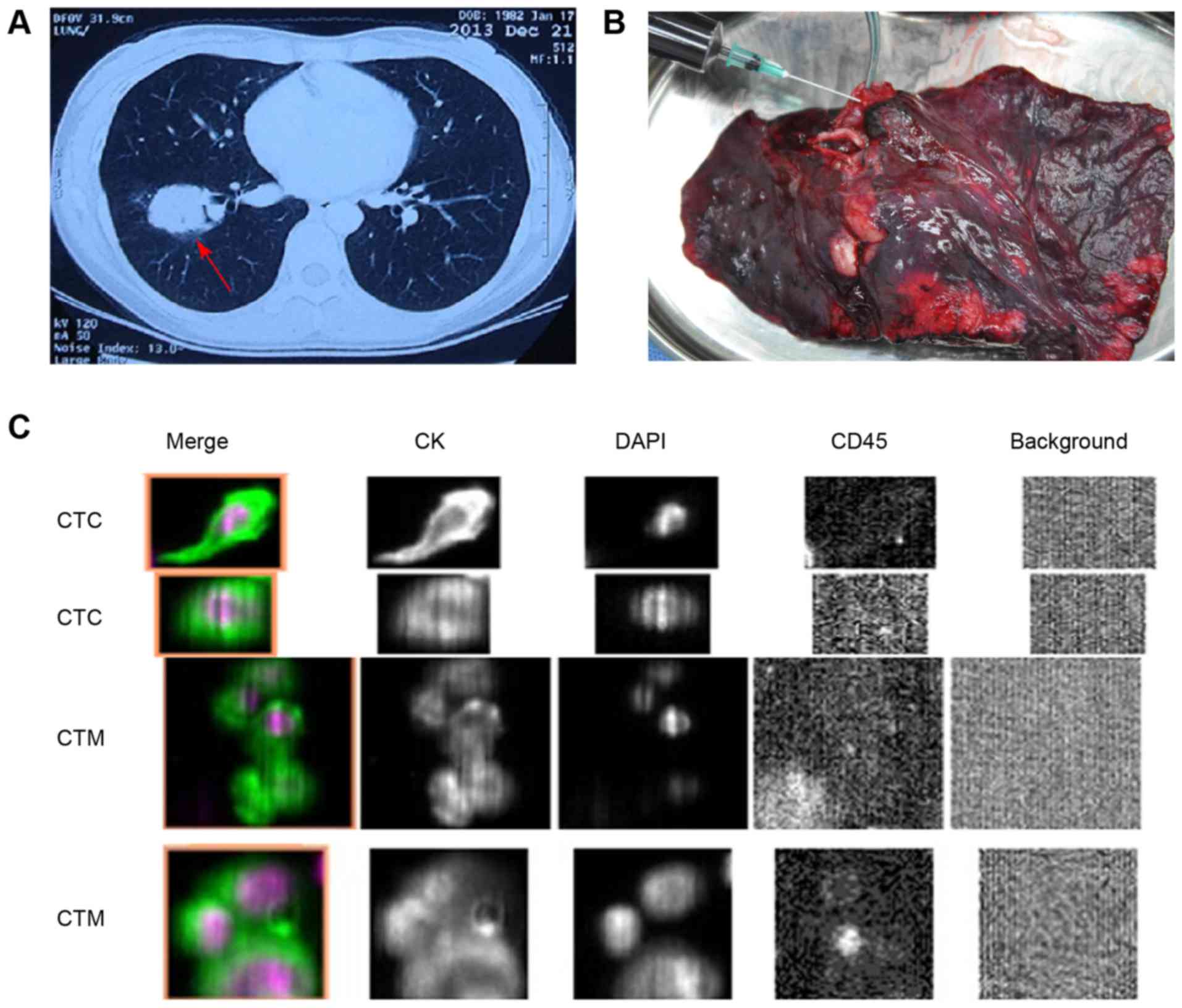
wedge resection was performed during the operation (Fig. 1A). For accurate N staging (15), systematic mediastinal lymph node
dissection, and lobar, segmental and subsegmental node resection
were performed. Following thoracotomy, the PV was first isolated
and stapled using Endo GIA (Covidien Surgical Solutions, Boulder,
CO, USA). When the lung was resected, PV blood samples were
immediately obtained with a needle (syringe) inserted into the vein
(Fig. 1B). Next, the blood sample was
gently injected into a CellSave Preservative Tube (Janssen
Diagnostics, LLC, Raritan, NJ, USA). Peripheral blood samples were
also collected from the radial artery opposite to the surgical side
during the operation, which were referred to as CTC-peri and served
as controls.
Determination of CTCs and circulating
tumor microemboli (CTM)
Blood samples (7.5 ml) were collected in 10-ml
CellSave Preservative Tubes (Janssen Diagnostics, LLC), stored at
room temperature and processed within 96 h of collection. CTCs were
evaluated quantitatively using the USA Food and Drug Administration
(FDA)-approved CELLSEARCH (Janssen Diagnostics, LLC) as described
previously (7) and without knowledge
of the patients' clinical characteristics. CTCs were defined as
epithelial cell adhesion molecule-isolated cells with round to oval
morphology and DAPI-stained nuclei expressing cytokeratins (CKs) 8,
18 and 19 but not the white blood cell-surface marker cluster of
differentiation 45. To avoid identifying mitotic CTC as
microemboli, CTM were identified as CTC clusters containing ≥3
distinct nuclei (16). Fig. 1C depicts the representative images of
CTCs and CTM from two patients.
CTC isolation and tumorigenic
assay
To explore the tumorigenic features of CTC-PV, blood
samples were collected from several patients if they had residual
PV blood after CELLSEARCH analysis. A total of three of
4–6-week-old female non-obese diabetic/severe combined
immunodeficient (Nod-SCID) mice weighing ~20 g were purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China) and maintained in specific pathogen-free
conditions (19–22°C, 12 h light and sterile food and water).
Hematopoietic cells were depleted according to the RosetteSep™
Human Circulating Epithelial Tumor Cell Enrichment Cocktail
procedure (cat. no. 15167; Stemcell Technologies, Inc., Vancouver,
BC, Canada). Next, cells were mixed with Matrigel and directly
injected subcutaneously into the flanks of these immunodeficient
mice. The Peking University Cancer Hospital and Institute Animal
Care and Use Committee approved the animal protocols.
Xenograft tumor analysis
Xenograft tumors were collected, fixed in formalin
and paraffin-embedded, and 4-µm thick sections were obtained
immediately prior to H&E staining. To identify the histological
types and to analyze the expression of drug resistance-associated
proteins in the xenograft model, the Department of Pathology of
Peking University Cancer Hospital performed an immunohistochemistry
(IHC) staining series and compared the primary tumor of the
patients with the xenograft tumor. Antibodies against the following
proteins were used: CK7 (cat. no. IR61961; dilution, 1:50), CK20
(cat. no. IR77761; dilution, 1:50), excision repair
cross-complementing 1 (cat. no. IR09161; dilution, 1:300),
thymidylate synthase (cat. no. M361401; dilution, 1:300),
topoisomerase II (cat. no. M718601; dilution, 1:100), epidermal
growth factor receptor (cat. no. M729829; dilution, 1:200; all from
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA),
ribonucleotide reductase M1 (cat. no. ZA-0373; dilution, 1:100),
thyroid nuclear factor-1 (cat no. ZM-0250; dilution, 1:50),
β-tubulin (cat. no. ZA-0581; dilution, 1:100), multidrug
resistance-associated protein (cat. no. ZM-0345; dilution, 1:50)
and P170 (cat. no. ZM-0189; dilution, 1:100; all from OriGene
Technologies, Inc., Beijing, China). Immunohistochemical staining
was performed on the 4-µm formalin-fixed (at room temperature for
24 h) paraffin-embedded tissue slides. Briefly, following
deparaffinization and rehydration, using xylene and descending
alcohol series, antigen retrieval was performed between 95 and
100°C citrate buffer (10 µmol/l, pH 6.0) for 15 min. Subsequently,
3% H2O2 in methanol was used to block
endogenous peroxidase activity for 20 min. Following blocking with
goat serum (cat. no. ZLI-9022; OriGene Technologies, Inc.) in room
temperature for 30 min, the primary antibodies were added to the
slides overnight at 4°C. Subsequently, tissue sections were
incubated with a secondary goat anti-mouse horseradish peroxidase
(HRP)-conjugated antibody (cat. no. SAB3701044; dilution, 1:1,000;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in room temperature
for 30 min. HRP activity was visualized using the Liquid DAB Plus
Substrate kit (cat. no. ZLI-9018; OriGene Technologies, Inc.)
according to manufacturer's protocol. Automated hematoxylin was
used to counterstain the nucleus for 3 min at room temperature.
Sections were examined under light microscope (magnification,
×20).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
for Windows (SPSS, Inc., Chicago, IL, USA). The association between
CTC/CTM counts and clinical variables was analyzed with the
Mann-Whitney (two groups) or Kruskal-Wallis (≥3 groups) test for
discrete variables, and with Spearman's correlation analysis for
continuous variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Detection of CTC-PV/CTM-PV and
CTC-peri/CTM-peri counts
The majority the 32 patients with lung cancer had
CTCs in their PV blood (n=29, 90.6%; range, 1–8,000; median, 18;
mean, 617). CTM-PV were also detected in 12 patients (37.5%; range,
1–100; median, 4; mean, 17). Only 8 patients had CTCs in their
peripheral blood (25%; range=1–14), whereas no CTM-peri were
detected. Neither CTCs nor CTM were detected in the PV or
peripheral blood of the patient with pulmonary hamartoma.
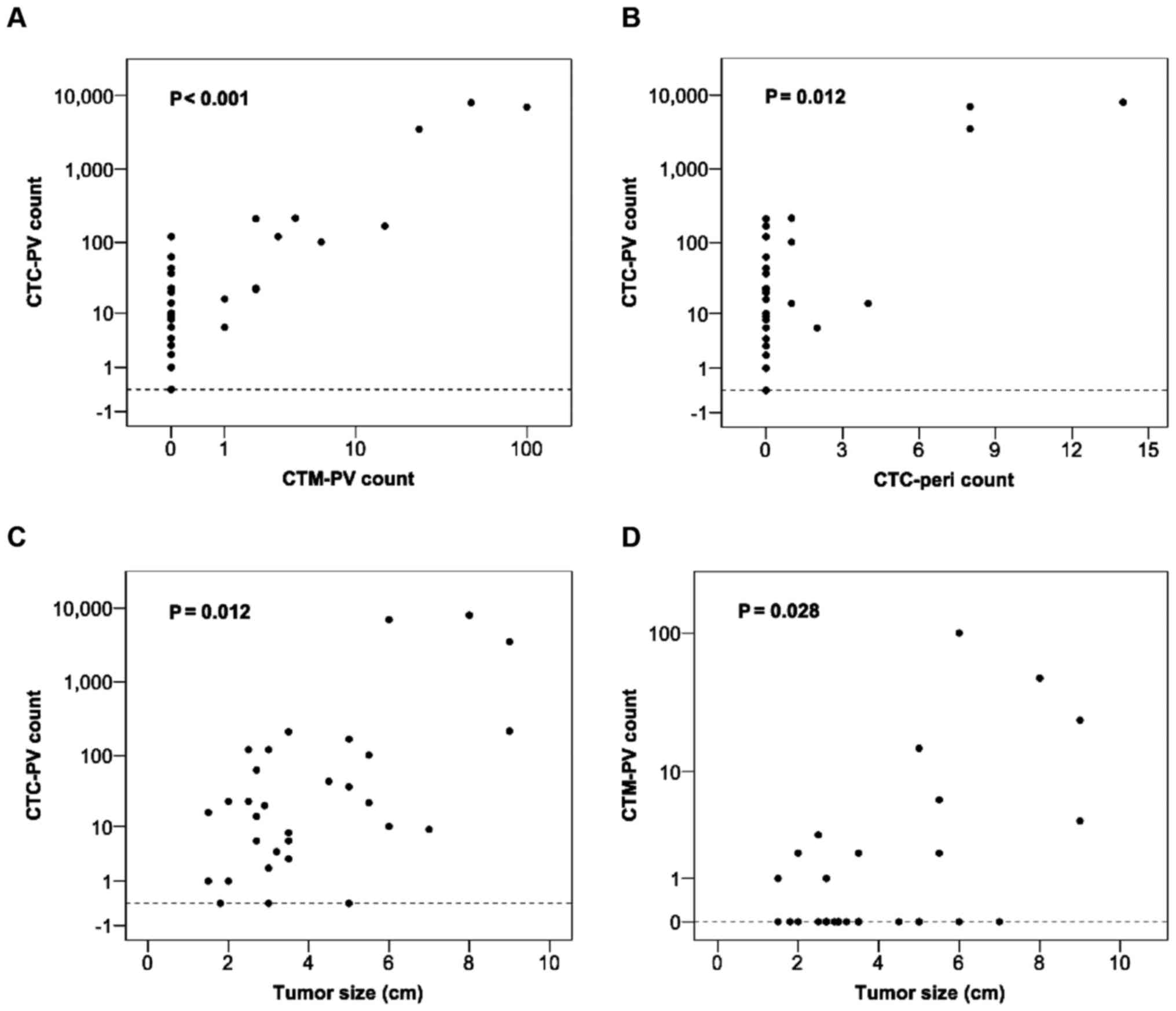
Correlation analysis revealed a significant association between the
CTC-PV counts and CTM-PV (P<0.001) and CTC-pericounts (P=0.012)
(Fig. 2A and B, respectively).
Correlation between CTC-PV/CTM-PV
counts and clinical variables
Table I lists the
association between CTC-PV/CTM-PV counts and the corresponding
clinical parameters. Statistical analysis indicated that tumor
size, lymph node metastasis and pathological staging were
significantly correlated with increased CTC-PV counts. Furthermore,
tumor size was also positively associated with increased CTM counts
(Fig. 2C and D). Although patients
with vessel invasion tended to have higher CTC-PV counts, this
association was not significant (P=0.059). In addition, pre-surgery
CT-guided needle biopsy and PET-CT evaluation of primary tumor SUV
had no impact on CTC count (Table I).
All 3 patients with >1,000 CTC-PV had large tumors (>6.0 cm).
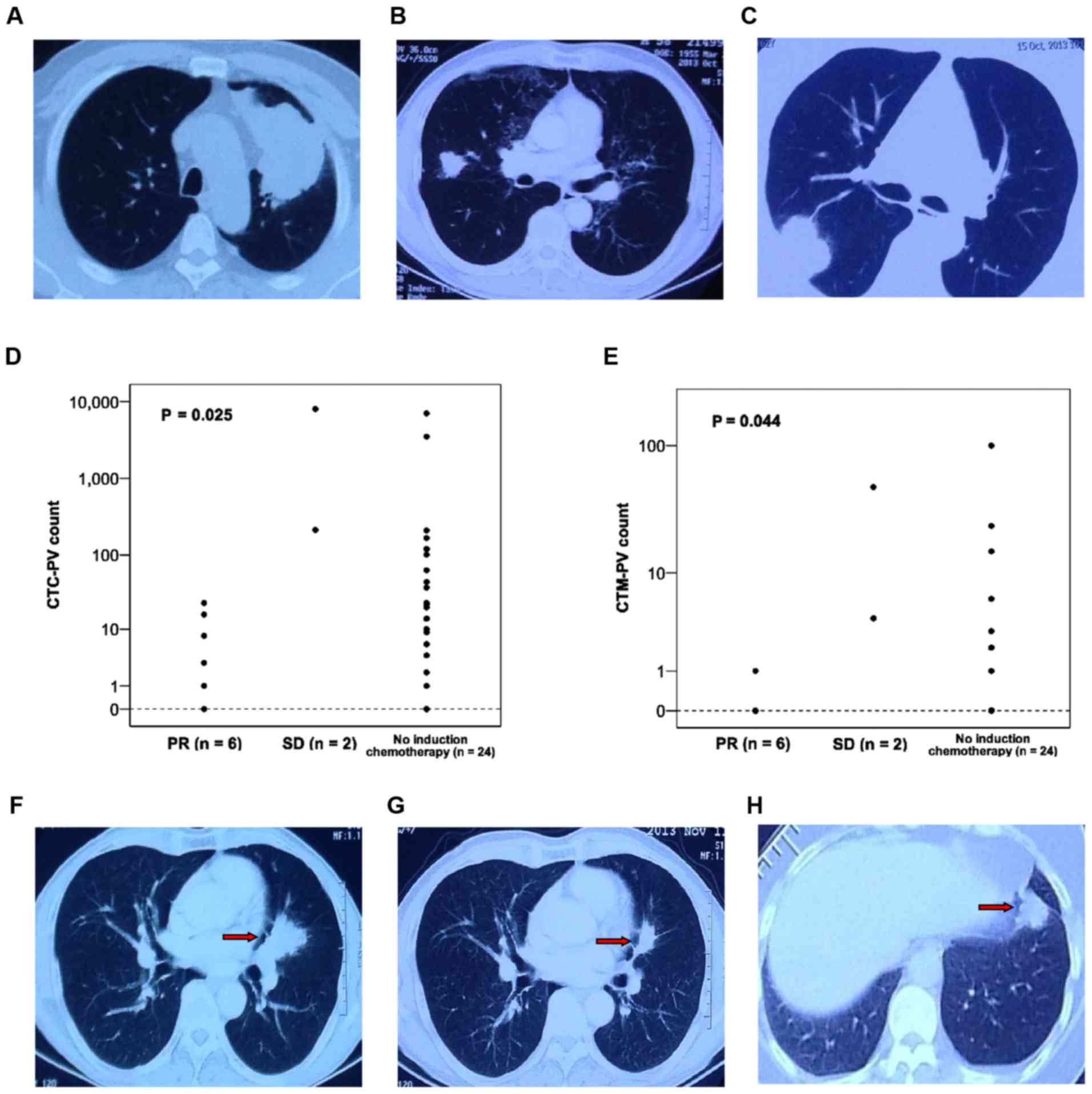
Fig. 3A demonstrates the CT images of
a patient with a large primary tumor. However, patients with small
tumors also had considerable CTC counts in their PV blood
regardless of their pathological stage (P=0.495; Table II). Table
II lists the CTC-PV/CTM-PV counts and clinical characteristics
of 12 patients with tumors <3.0 cm. The median and mean CTC-PV
counts of these 12 patients were 15 and 25, respectively. Fig. 3B and C demonstrate the CT images of
patients with varying tumor sizes.
 | Table II.CTC-PV/CTM-PV counts of 12 patients
with lung cancer and small tumors (<3.0 cm). |
Table II.
CTC-PV/CTM-PV counts of 12 patients
with lung cancer and small tumors (<3.0 cm).
| Patient no. | Tumor size
(cm) | LN | Stage | CTC-PVa | CTM-PVb |
|---|
| 1 | 1.8 | N0 | IA | 0 | 0 |
| 2 | 1.5 | N0 | IA | 1 | 0 |
| 3 | 2.0 | N0 | IA | 1 | 0 |
| 4 | 2.7 | N0 | IA | 6 | 1 |
| 5c | 2.0 | N0 | IA | 23 | 2 |
| 6d | 2.5 | N0 | IA | 120 | 3 |
| 7 | 1.5 | N0 | IB | 16 | 1 |
| 8 | 2.5 | N0 | IB | 23 | 0 |
| 9 | 2.7 | N1 | IIA | 14 | 0 |
| 10 | 2.7 | N1 | IIA | 14 | 0 |
| 11 | 2.7 | N1 | IIA | 63 | 0 |
| 12 | 2.9 | N2 | IIIA | 20 | 0 |
Perioperative treatment, CTC/CTM
counts and survival result
Of the 32 patients, 8 received platinum-based
chemotherapy prior to surgery, and partial response (PR) was noted
in 6 patients. Table III lists the
CTC/CTM counts in 8 patients who received neoadjuvant chemotherapy.
Statistical analysis revealed that the CTC-PV and CTM-PV counts in
the PR cases were significantly lower than those in patients with
stable disease (SD) or patients who did not receive induction
therapy. (P=0.025 and P=0.044, respectively; Fig. 3D and E). Fig. 3F-H demonstrate the CT images of
patients who had or had not undergone induction chemotherapy. Upon
surgery, 11 patients received four cycles of platinum-based
adjuvant chemotherapy based on their pathological stage, while
another 5 patients diagnosed with stage II–IIIA adenocarcinoma with
EGFR mutation were enrolled in an ongoing phase-II study and
received adjuvant targeted therapy (icotinib). The median follow-up
time was 12 months; 4 patients recurred postoperatively, 3 of whom
succumbed to the disease. All 4 patients had received perioperative
therapy, and their CTC-PV and CTM-PV counts are listed in Table IV. Compared with the patients who did
not experience a recurrence, no significant correlation was
observed between CTC-PV/CTM-PV counts and disease recurrence
(P=0.266 and P=0.394, respectively; Table IV).
 | Table III.CTC-PV/CTM-PV counts of 8 patients
with lung cancer who received induction chemotherapy. |
Table III.
CTC-PV/CTM-PV counts of 8 patients
with lung cancer who received induction chemotherapy.
| Patient no. | Histology | cTNM | Chemotherapy
regimen | No. of cycles | Response | ypTNM | Residual tumor
(%) | CTC-PV | CTM-PV |
|---|
| 1 | Squamous | cT2bN0M0 | GP | 1 | PR | ypT1bN0M0 | 20 | 0 | 0 |
| 2 | Adenocarcinoma | cT2aN0M0 | TP | 3 | PR | ypT1aN0M0 | 95 | 1 | 0 |
| 3 | Squamous | cT3N1M0 | GP | 2 | PR | ypT3N0M0 | 95 | 3 | 0 |
| 4a | Squamous | cT2aN2M0 | GP | 2 | PR | ypT2aN2M0 | 95 | 8 | 0 |
| 5 | Squamous | cT3N0M0 | GP | 2 | PR | ypT2aN0M0 | 95 | 16 | 1 |
| 6 | Squamous | cT3N2M0 | GP | 2 | PR | ypT2aN0M0 | 80 | 23 | 0 |
| 7 | Large cell | cT3N1M0 | GP | 2 | SD | ypT3N0M0 | 30 | 214 | 4 |
| 8 | Squamous | cT3N1M0 | GP | 2 | SD | ypT3N1M0 | 80 | 8,000 | 48 |
 | Table IV.CTC-PV/CTM-PV counts of 4 patients
with lung cancer andpostoperative recurrence. |
Table IV.
CTC-PV/CTM-PV counts of 4 patients
with lung cancer andpostoperative recurrence.
| Patient no. | Histology | Tumor size
(cm) | Stage | CTC-PV | CTM-PV | DFS (months) | OS (months) |
|---|
| 1 | Squamous | 7.0 | pT2bN0M0 | 9 | 0 | 7 | 10 |
| 2 | Squamous | 2.5 | ypT2aN0M0 | 23 | 0 | 3 | 4 |
| 3 | Adenocarcinoma | 5.5 | pT2bN2M0 | 101 | 6 | 2 | 6 |
| 4a | Adenocarcinoma | 9.0 | pT3N0M0 | 3,500 | 24 | 13 | Alive |
Xenograft assay and tumor
analysis
PV blood (15 ml) was collected from 3 patients in
the overall cohort to perform a xenograft assay. Table V lists the patient characteristics and
CTC/CTM counts. The PV blood of one of the patients, who had 167
CTCs and 15 CTM per 7.5 ml PV blood as detected by CELLSEARCH,
formed a subcutaneous xenograft tumor in the injected mouse.
Fig. 4A depicts CT images of a
patient and a mouse xenograft tumor. H&E staining, and CK7,
CK20 and TTF-1 IHC results revealed concordant typical
adenocarcinoma between the primary tumor and the matched xenograft.
In addition, there was similar positive expression of drug
resistance-associated proteins in the xenograft tumor compared with
that in the patient. Fig. 4B and C
demonstrate a series of H&E and IHC results of a patient
primary tumor and the xenograft tumor.
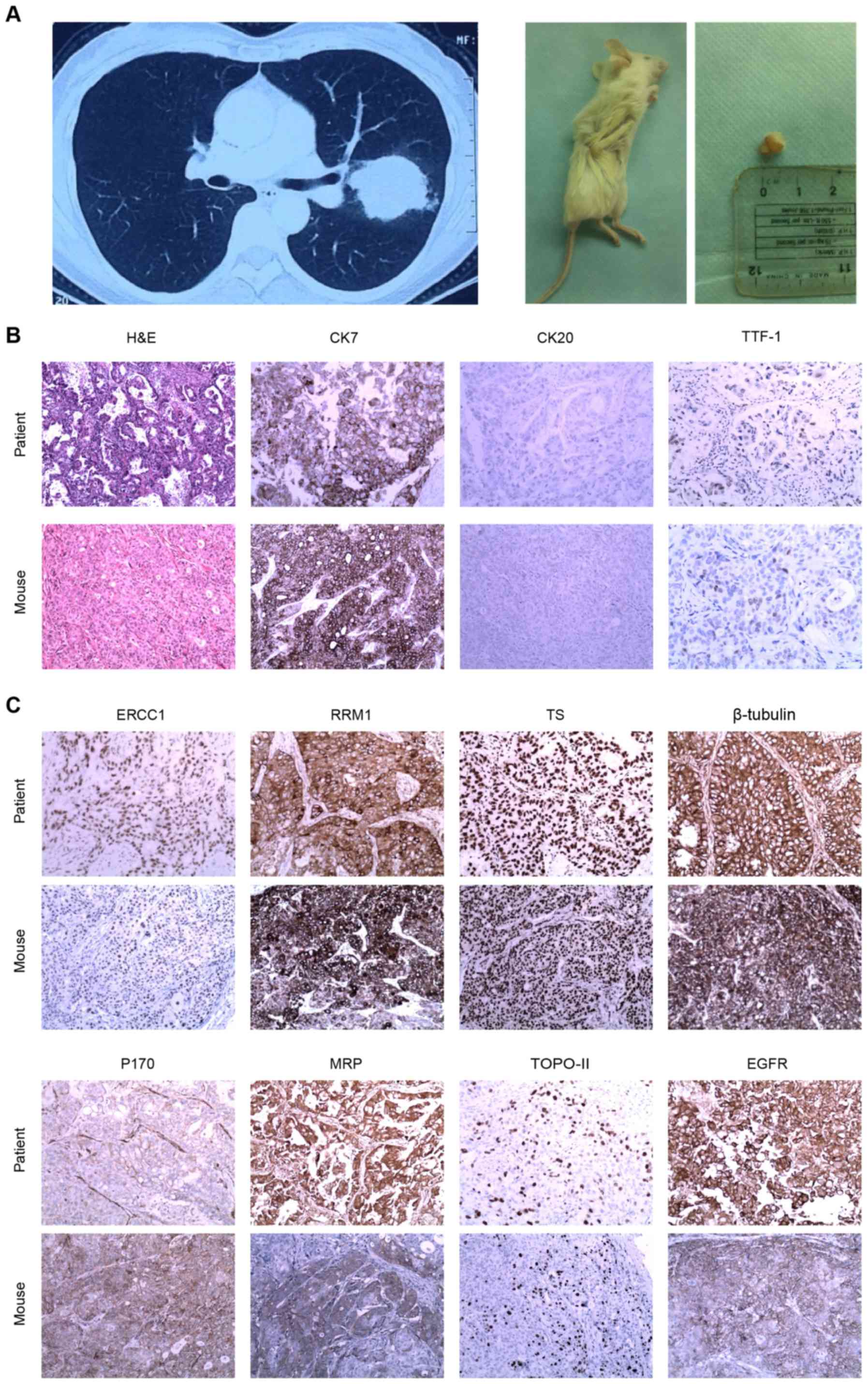 | Figure 4.Xenograft assay and IHC staining. (A)
Patient with a tumor in the left upper lobe of the lung (left
panel) and mouse xenograft tumor formed following injection with
circulating tumor cells from the pulmonary vein of the patient
(right panel). (B) H&E and CK7-positive, CK20-negative and
TTF-1-positive IHC staining of the primary tumor from the patient
and the mouse xenograft tumor. (C) IHC staining of a series of drug
resistance-associated proteins in the primary and xenograft tumors
(magnification, ×20). H&E, hematoxylin and eosin; CK,
cytokeratin; TTF-1, thyroid nuclear factor-1; ERCC1, excision
repair cross-complementing 1; RRM1, ribonucleotide reductase M1;
TS, thymidylate synthase; MRP, multi-drug resistance-associated
protein; TOPO-II, topoisomerase II; EGFR, epidermal growth factor
receptor; IHC, immunohistochemistry. |
 | Table V.CTC-PV/CTM-PV counts of 3 cases of
lung cancer whose PV blood was used for xenograft assay. |
Table V.
CTC-PV/CTM-PV counts of 3 cases of
lung cancer whose PV blood was used for xenograft assay.
| Patient no. | Histology | Tumor size
(cm) | Stage | CTC-PV | CTM-PV | Successful
xenograft tumor |
|---|
| 1 | Adenocarcinoma | 5.5 | pT2bN2M0 | 101 | 6 | No |
| 2 | Adenocarcinoma | 5.0 | pT2aN1M0 | 167 | 15 | Yes |
| 3 | Squamous | 3.5 | ypT3N0M0 |
3 | 0 | No |
Discussion
The present study detected numerous CTCs and CTM in
7.5 ml PV blood compared with those in peripheral blood. Although
previous studies have demonstrated that intraoperative manipulation
increases hematogenous dissemination in patients with NSCLC
(5,6,17), whether
the PV should be ligated first remains controversial. Hashimoto
et al reported that the increased CTC-PV count prior and
subsequent to surgical manipulation for lobectomy was not
significantly associated with the sequence of vessel interruption
(12). Refaely et al also
demonstrated that the sequence of vessel interruption was not a
risk factor for recurrence (18).
Therefore, ligating the pulmonary artery first remains an option
during lobectomy based on the preference of the surgeon (11) or the minimally invasive surgery
technique used, particularly for upper lobectomy (19). Different from a previous study
(11), the PV was stapled prior to
other surgical manipulation in the present study. Following
lobectomy, a large number of CTCs and CTM were retained and
detected in PV blood. Okumura et al and Hashimoto et
al reported no significant correlation between CTC-PV count and
patient characteristics (11,12). However, the present study observed an
apparent increase in CTC-PV in patients with large primary tumors
and lymph node metastasis, which indicated that, for these
patients, surgical behavior such as retracting the lobe, exposing
the hilum or manipulating the tumor may present a high risk for
disseminating tumor cells into the blood during surgery. The
present study also analyzed the CTC-PV count in 12 patients with
small tumors (<3.0 cm), and noticed that the majority of
patients had CTCs in their PV blood (n=11, 91.7%). Furthermore, 8
of these 12 patients were diagnosed as pathological stage I (stage
IA, 6; stage IB, 2), but 50% of them were CTM-positive (n=4,
50.0%). Although the immune system would clear the majority of
tumor cells shed into the blood, and only a small portion of CTCs
can develop metastases (20), several
studies have demonstrated that CTCs detected in PV blood predict
poor clinical outcome (21,22). Therefore, even though the impact of
intraoperative manipulation on survival remains unclear, lobectomy
may be recommended for lung cancer of any tumor size and stage
according to oncological principles, in addition to ligating the
PV, if possible, prior to any other treatment.
Besides surgical resection, the correlation between
CTC-PV counts and the outcome of perioperative treatment,
particularly induction chemotherapy, was also analyzed in the
present study. Despite the fact that only 8 patients received
platinum-based chemotherapy prior to surgery, a promising tendency
for the CTC count to markedly decrease was noted in patients who
achieved response through induction therapy compared with that in
other patients. The pathological result revealed that the majority
of patients had >80% of residual tumor, but the patient whose
tumor size was 3.0 cm with only 20% of residual tumor had no CTCs
or CTM in his PV blood. Previous studies have reported that the
percentage of viable tumor cells is a significant predictor of
overall survival and disease-free survival in patients with
neoadjuvant-treated NSCLC, but not in patients who undergo surgery
alone (23,24). It can be hypothesized that the
neoadjuvant chemotherapy-mediated inhibition of tumor cell
metastatic characteristics and changes in the percentage of tumor
cells in primary disease reduces CTC shedding into the blood during
surgery. In addition, it has been reported that tumor cells within
CTM have survival advantage and relative resistance to cytotoxic
drugs (16). Consistently, the
present study revealed that, among 6 PR cases, 5 were free from
CTM-PV. The CTM-PV count in PR cases was significantly less than
that in SD cases or patients who did not receive induction therapy.
This finding implies that CTCs/CTM in PV blood may indirectly
determine the response to induction therapy, and suggests to a
certain degree, that neoadjuvant treatment contributes to the
reduction of intraoperative hematogenous dissemination in patients
with locally advanced disease or heavy tumor burden. As the
follow-up period was short, the correlation between CTC-PV count
and disease recurrence or the postoperative result of adjuvant
therapy was not determined. It should be mentioned that CTC/CTM
counts are not always consistent with clinical characteristics,
which indicates that tumor cell shedding into the blood is not only
influenced by TNM stage, but is also associated with tumor
biological behavior. Therefore, in addition to TNM staging, future
studies should determine whether CTC and CTM counts could guide
adjuvant chemotherapy following surgery.
Although the CELLSEARCH system has been approved in
the USA by the FDA for monitoring hematogenous metastasis in
patients with cancer, no direct evidence indicates that the
captured CTCs are true tumor cells and that they are able to form
distant metastases (11). In the
present study, the results of a xenograft assay demonstrated that
the CTCs and CTM detected in the PV blood of patients were
tumorigenic. Similar human CTC-derived xenograft models have been
reported for breast cancer, hepatocellular carcinoma and SCLC
(25–27). In addition, CTC culture has been
established successfully from patients with early-stage NSCLC
(28). An additional assay was
performed in the present study to compare differences in protein
expression between the primary tumor and the xenograft model in
order to explore whether the mouse xenograft tumor derived from
CTC-PV differed from that of the patient in terms of drug
resistance. However, there were various limitations, as the
xenograft assay was performed using cells from only 3 patients, and
only 1mouse developed a xenograft tumor. To confirm these results,
future studies should enroll additional patients for xenograft
assay and cell culture to explore CTC-PV tumorigenicity and to
analyze its tumor biological features.
To conclude, numerous CTC-PV/CTM-PV are tumorigenic
and correlate positively with tumor size and stage. Despite the
limited case number and short follow-up time, the present study
provides evidence for adhering to oncological principles in lung
cancer surgery, and reports a potential biomarker for guiding
perioperative treatment. The impact of surgical behavior on
survival will be evaluated in further studies.
Acknowledgements
The present study was supported by grants from
Peking University (PKU) 985 Special Funding for Collaborative
Research with PKU Hospitals, the National High Technology Research
and Development Program of China (863 Program; grant no.
2012AA02A502) and Beijing Municipal Administration of Hospital
Clinical Medicine Development of Special Funding (grant no.
ZYLX201509).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA-Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee, ; Participating Institutions, :
The IASLC lung cancer staging project: Proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM Classification of malignant tumours. J Thorac Oncol.
2:706–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pantel K, Brakenhoff RH and Brandt B:
Detection, clinical relevance and specific biological properties of
disseminating tumour cells. Nat Rev Cancer. 8:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamashita JI, Kurusu Y, Fujino N, Saisyoji
T and Ogawa M: Detection of circulating tumor cells in patients
with non-small cell lung cancer undergoing lobectomy by
video-assisted thoracic surgery: A potential hazard for
intraoperative hematogenous tumor cell dissemination. J Thorac
Cardiovasc Surg. 119:899–905. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong Q, Huang J, Zhou Y, Li L, Bao G, Feng
J and Sha H: Hematogenous dissemination of lung cancer cells during
surgery: Quantitative detection by flow cytometry and prognostic
significance. Lung Cancer. 37:293–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka F, Yoneda K, Kondo N, Hashimoto M,
Takuwa T, Matsumoto S, Okumura Y, Rahman S, Tsubota N, Tsujimura T,
et al: Circulating tumor cell as a diagnostic marker in primary
lung cancer. Clin Cancer Res. 15:6980–6986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of circulating tumor
cells in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okumura Y, Tanaka F, Yoneda K, Hashimoto
M, Takuwa T, Kondo N and Hasegawa S: Circulating tumor cells in
pulmonary venous blood of primary lung cancer patients. Ann Thorac
Surg. 87:1669–1675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto M, Tanaka F, Yoneda K, Takuwa T,
Matsumoto S, Okumura Y, Kondo N, Tsubota N, Tsujimura T, Tabata C,
et al: Significant increase in circulating tumour cells in
pulmonary venous blood during surgical manipulation in patients
with primary lung cancer. Interact Cardiovasc Thorac Surg.
18:775–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rami-Porta R, Wittekind C and Goldstraw P;
International Association for the Study of Lung Cancer (IASLC)
Staging Committee, : Complete resection in lung cancer surgery:
Proposed definition. Lung Cancer. 49:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mountain CF and Dresler CM: Regional lymph
node classification for lung cancer staging. Chest. 111:1718–1723.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurusu Y, Yamashita J, Hayashi N, Mita S,
Fujino N and Ogawa M: The sequence of vessel ligation affects tumor
release into the circulation. J Thorac Cardiovasc Surg.
116:107–113. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Refaely Y, Sadetzki S, Chetrit A, Simansky
DA, Paley M, Modan B and Yellin A: The sequence of vessel
interruption during lobectomy for non-small cell lung cancer: Is it
indeed important? J Thorac Cardiovasc Surg. 125:1313–1320. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fieira Costa E, Delgado Roel M, Paradela
de la Morena M, Gonzalez-Rivas D, Fernandez-Prado R and de la Torre
M: Technique of uniportal VATS major pulmonary resections. J Thorac
Dis. 6 Suppl 6:S660–S664. 2014.PubMed/NCBI
|
|
20
|
Glaves D: Correlarion between circulating
cancer cells and incidence of metastases. Br J Cancer. 48:665–673.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sienel W, Seen-Hibler R, Mutschler W,
Pantel K and Passlick B: Tumour cells in the tumour draining vein
of patients with non-small cell lung cancer: Detection rate and
clinical significance. Eur J Cardiothorac Surg. 23:451–456. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Funaki S, Sawabata N, Nakagiri T, Shintani
Y, Inoue M, Kadota Y, Minami M and Okumura M: Novel approach for
detection of isolated tumor cells in pulmonary vein using negative
selection method: Morphological classification and clinical
implications. Eur J Cardiothorac Surg. 40:322–327. 2011.PubMed/NCBI
|
|
23
|
Pataer A, Kalhor N, Correa AM, Raso MG,
Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al:
Histopathologic response criteria predict survival of patients with
resected lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 7:825–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
William WN Jr, Pataer A, Kalhor N, Correa
AM, Rice DC, Wistuba II, Heymach J, Lee JJ, Kim ES, Munden R, et
al: Computed tomography RECIST assessment of histopathologic
response and prediction of survival in patients with resectable
non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 8:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baccelli I, Schneeweiss A, Riethdorf S,
Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T,
Wallwiener M, et al: Identification of a population of blood
circulating tumor cells from breast cancer patients that initiates
metastasis in a xenograft assay. Nat Biotechnol. 31:539–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hodgkinson CL, Morrow CJ, Li Y, Metcalf
RL, Rothwell DG, Trapani F, Polanski R, Burt DJ, Simpson KL, Morris
K, et al: Tumorigenicity and genetic profiling of circulating tumor
cells in small-cell lung cancer. Nat Med. 20:897–903. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Shiratsuchi H, Lin J, Chen G,
Reddy RM, Azizi E, Fouladdel S, Chang AC, Lin L, Jiang H, et al:
Expansion of CTCs from early stage lung cancer patients using a
microfluidic co-culture model. Oncotarget. 5:12383–12397. 2014.
View Article : Google Scholar : PubMed/NCBI
|