Introduction
Granulocyte colony stimulating factor (G-CSF) is a
cytokine mainly produced by macrophages, fibroblasts and
endothelial cells in an inflammatory milieu. G-CSF stimulates
neutrophil precursors, resulting in an increase in neutrophils, and
recruits neutrophils from the bone marrow to peripheral blood.
G-CSF is an important factor in infection prophylaxis, and
recombinant G-CSF is universally used to treat neutropenia
(1). G-CSF is also produced by
non-hematologic malignancies with high leukocyte counts, consisting
predominantly of neutrophils, in patients without infectious
diseases. Most of these G-CSF producing tumors are present in the
lungs (2), with tumors in the oral
regions being rare. This report describes a patient with a tongue
carcinoma producing G-CSF, as well as showing diffuse uptake of FDG
in the bone marrow.
Case report
In July 2013, a 78-year-old man visited the
Department of Oral Surgery, Hokuto Hospital (Obihiro, Japan) with a
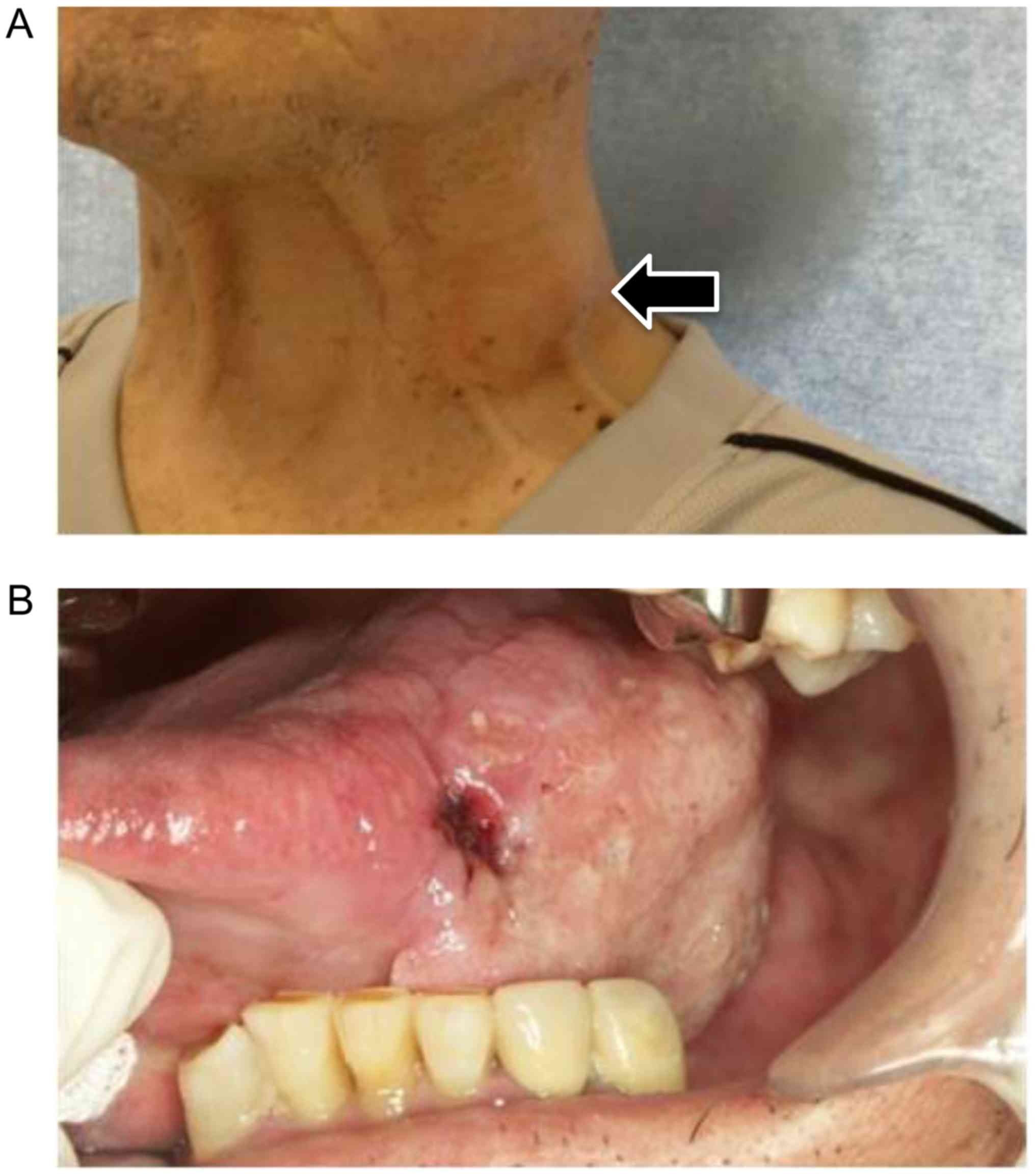
2-week history of painless swelling on the left neck and tongue.
The patient had no systemic complications and no significant family
history. Some cervical lymph nodes on both sides were palpable
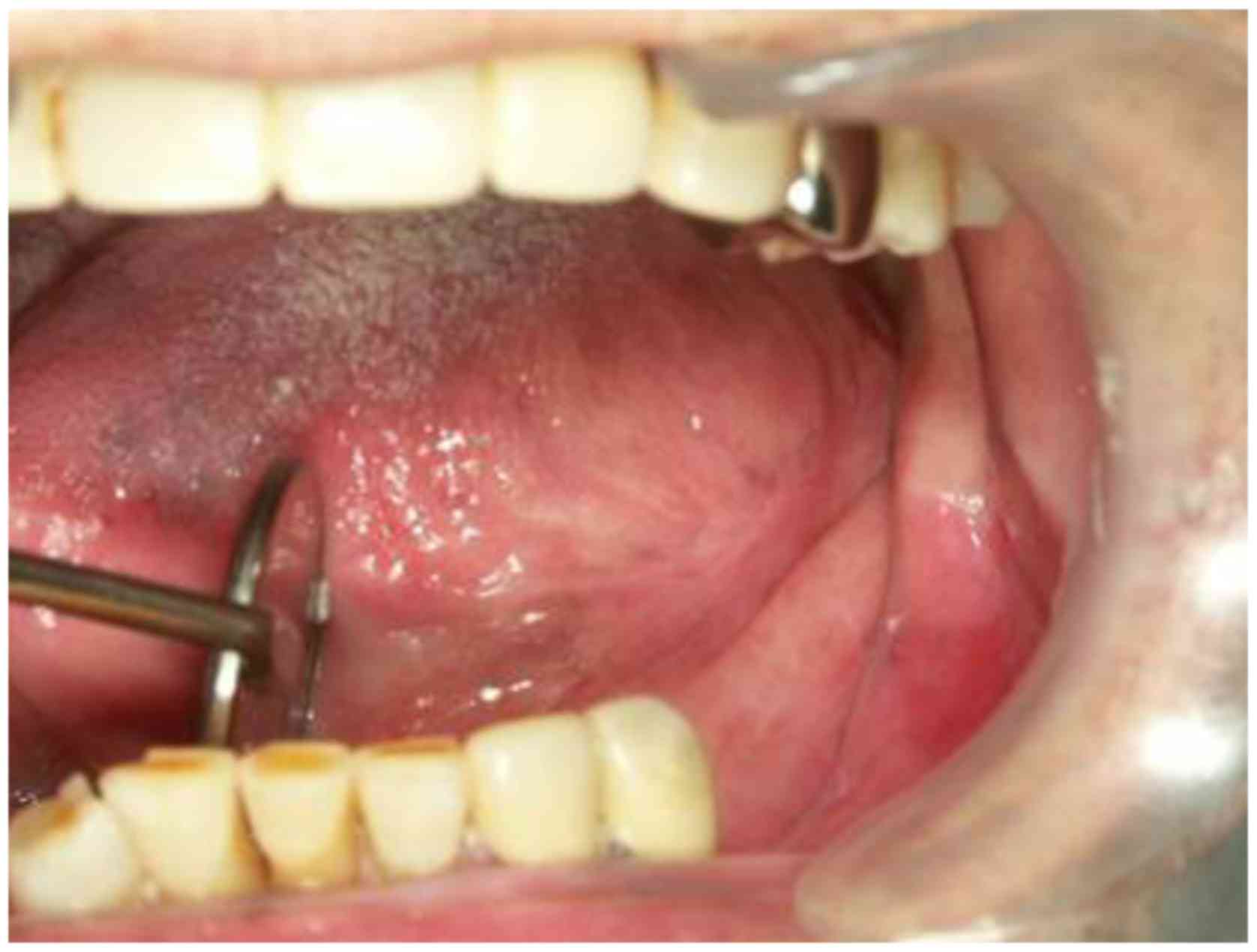
(Fig. 1A). Intra-oral examination
showed a tumor with induration about 40 mm in diameter on the left
side of the tongue (Fig. 1B).
Cytological examination of a swollen left lymph node showed
atypical squamous cells, and pathological examination of a biopsy
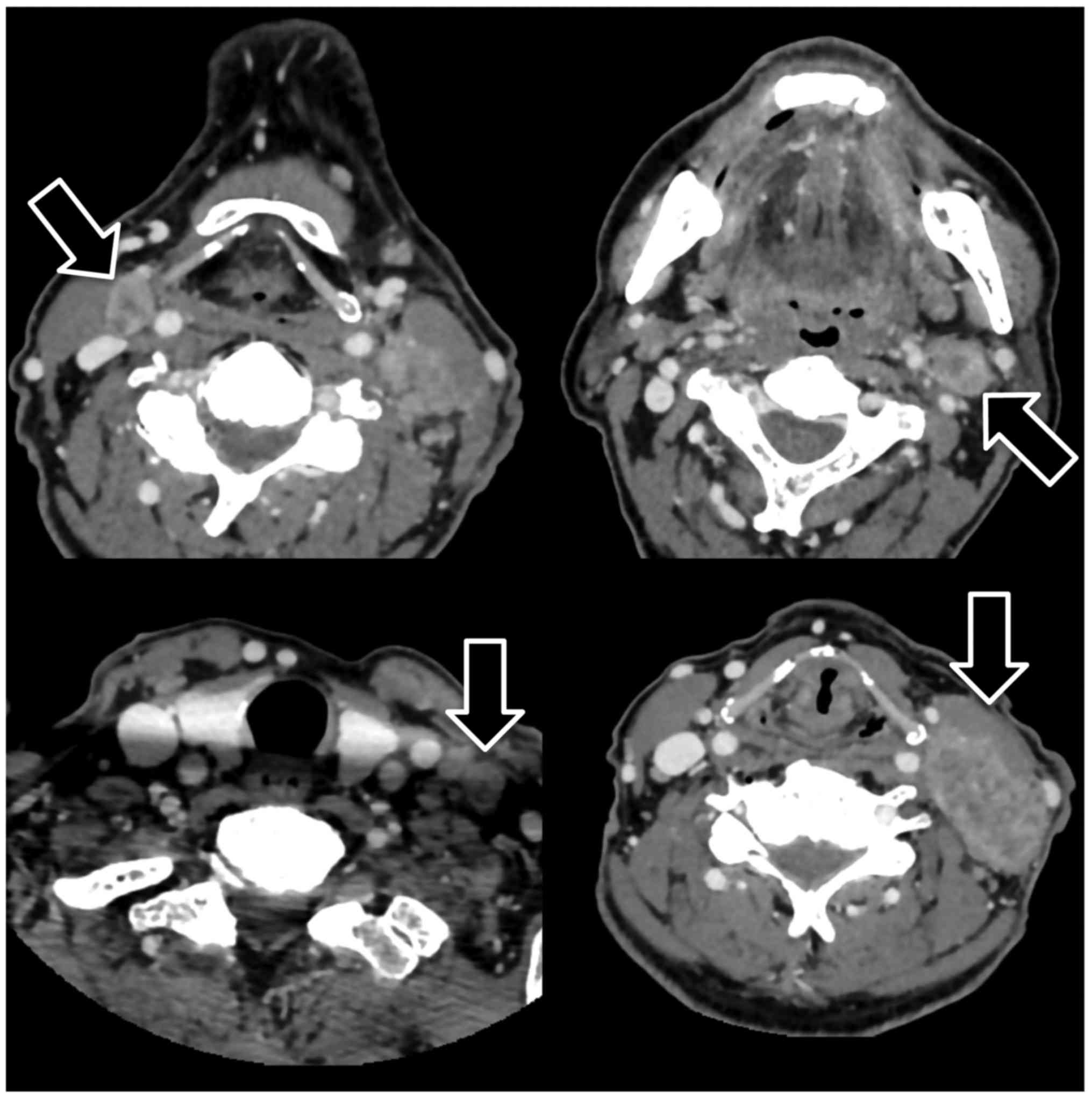
of the tongue tumor revealed a squamous cell carcinoma. A computed
tomography (CT) scan with contrast demonstrated a large lateral
oral tongue tumor of diameter 42 mm, without extension to the
extrinsic muscles of the tongue; and some metastatic cervical lymph
nodes that were enlarged, nonhomogeneously enhanced, and partially
necrotic. Metastatic disease of left middle jugular lymph node was
>30 mm in maximum diameter (Fig.
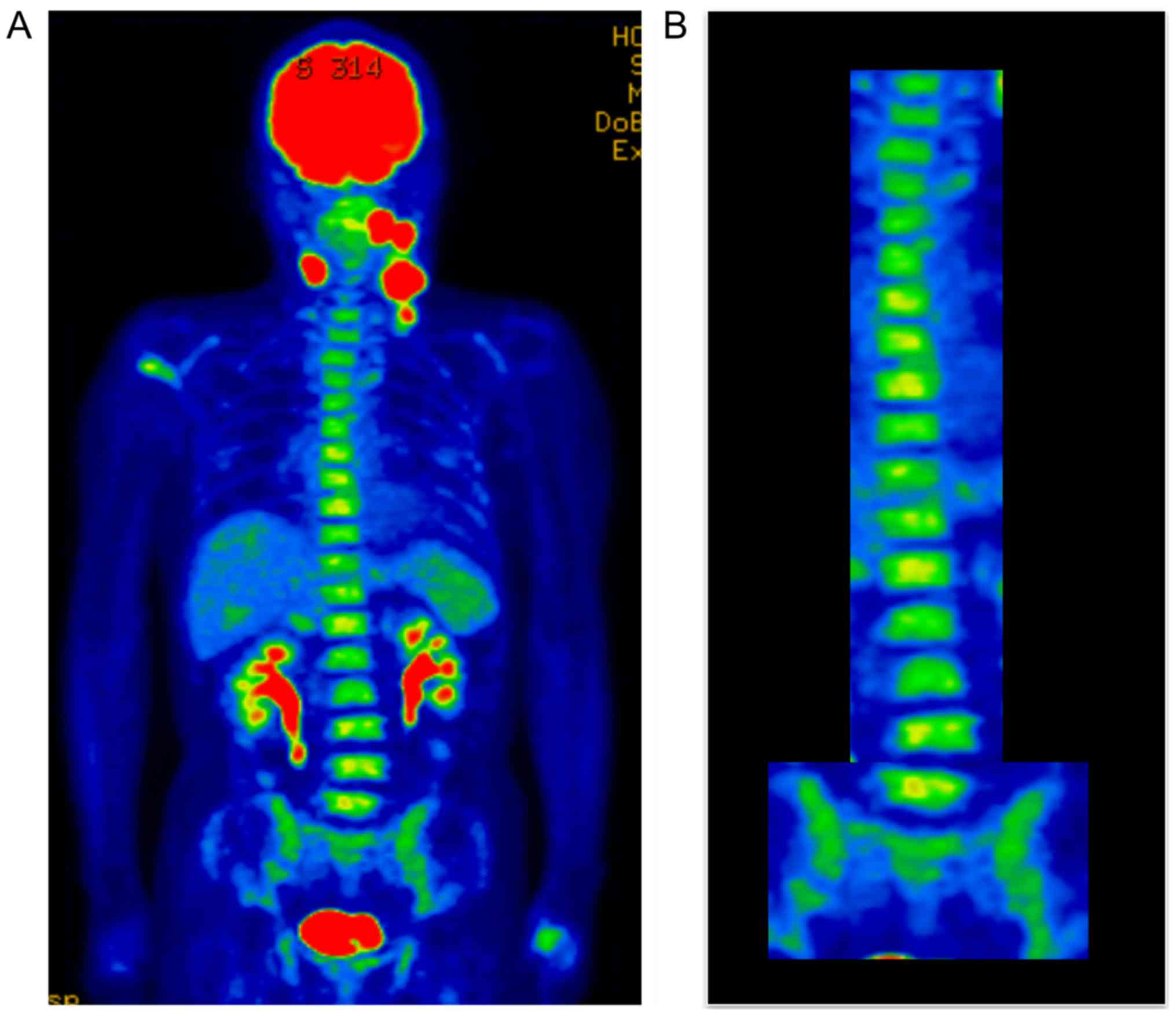
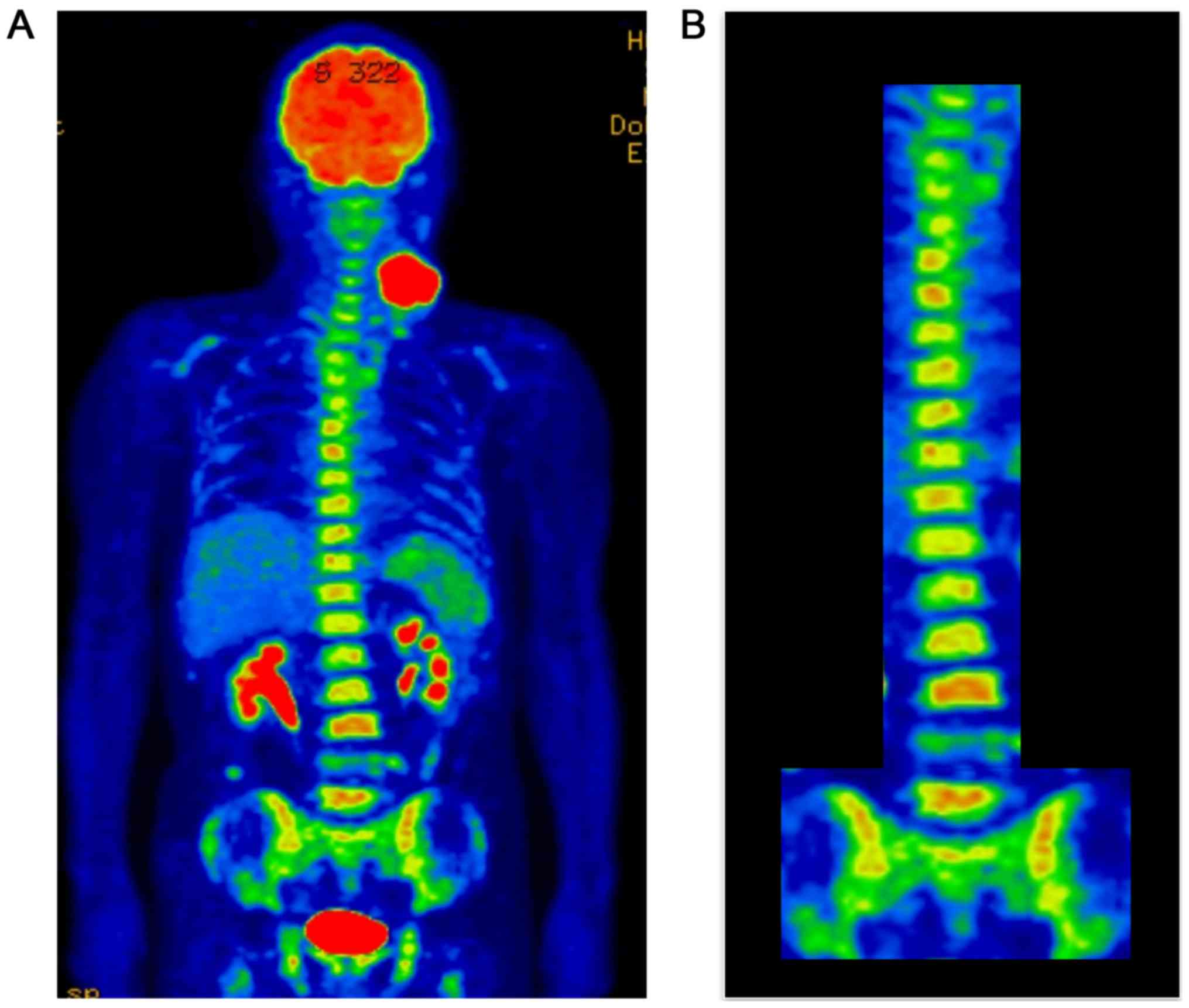
2). 18F-fluorodeoxyglucose-positron emission
tomography (FDG-PET)/CT showed abnormally high uptake by the tongue
tumor (maximum standardized uptake value [SUVmax] 22.19) and by the
four large metastatic nodes, with the large left middle jugular
node having an SUVmax of 14.43 (Fig.
3A). Diffuse FDG uptake was also observed in the bone marrow of
the spine and pelvis (Fig. 3B). These
findings suggested that hematopoietic capacity was enhanced.
Hematological examination showed leukocytosis (WBC count 21,680
µl−1), dominated by neutrophils (86.4%), and a high
serum concentration of C-reactive protein (CRP), 4.47 mg/dl
(Table I). Because we suspected that
these findings were due to G-CSF produced by the tumor, additional
analyses were performed. Although immunohistochemical staining of a
paraffin-embedded tongue biopsy specimen with monoclonal anti-G-CSF
antibody yielded negative results, the patient's serum G-CSF level
was increased, to 117.0 pg/ml (normal, <39.0 pg/ml). He had no
symptoms of infectious disease, including fever, and no blast cells
in his peripheral blood.
 | Table I.Hematological examination at first
visit. |
Table I.
Hematological examination at first
visit.
| Variables | Values |
|---|
| WBC | 21,680/µl |
| Neut | 86.4% |
| Eos | 1.0% |
| Bas | 0.2% |
| Mon | 4.2% |
| Lym | 8.2% |
| RBC |
297×104/µl |
| Hb | 9.0 g/dl |
| Hct | 27.4% |
| Plt | 44.8×103/µl |
| TP | 6.7 g/dl |
| Alb | 2.7 g/dl |
| AST | 12
U/l |
| ALT | 12
U/l |
| LDH | 109 U/l |
| γ-GTP | 33
U/l |
| BUN | 8.9 mg/dl |
| Cr | 0.67 mg/dl |
| Na | 136 mEq/l |
| K | 4.0 mEq/l |
| Cl | 102 mEq/l |
| Ca | 7.8 mEq/l |
| CRP | 4.5 mg/dl |
| SCC | 1.3 ng/ml |
| G-CSF | 117.0 pg/ml |
Because the initial diagnosis of the tumor was
cT3N3M0, we thought that it was resectable. However, the patient
refused surgical treatment, radiation therapy and intravenous
chemotherapy. Therefore, he was treated with oral chemotherapy,
consisting of 3-week cycles of 100 mg/day S1 for 2 weeks followed
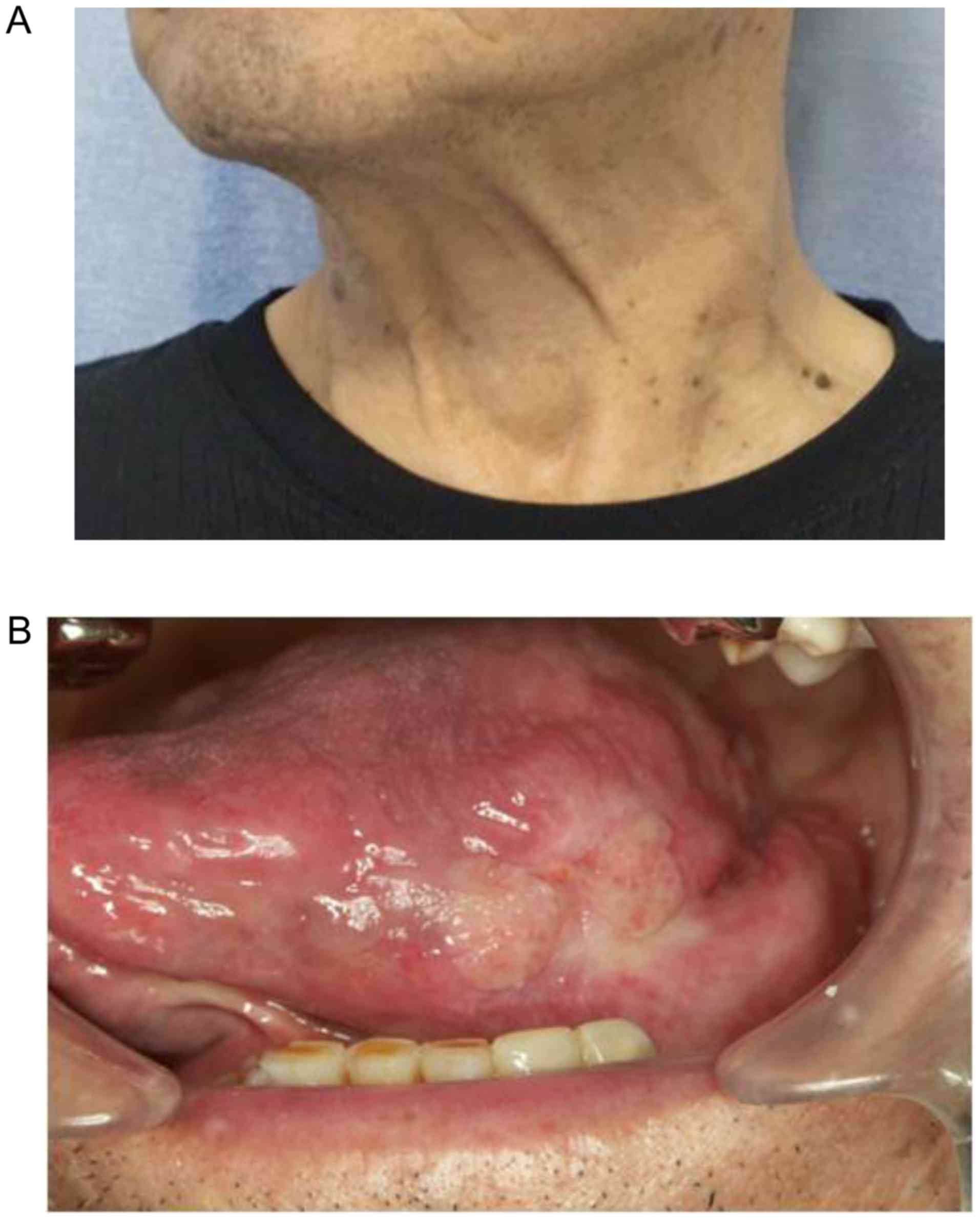
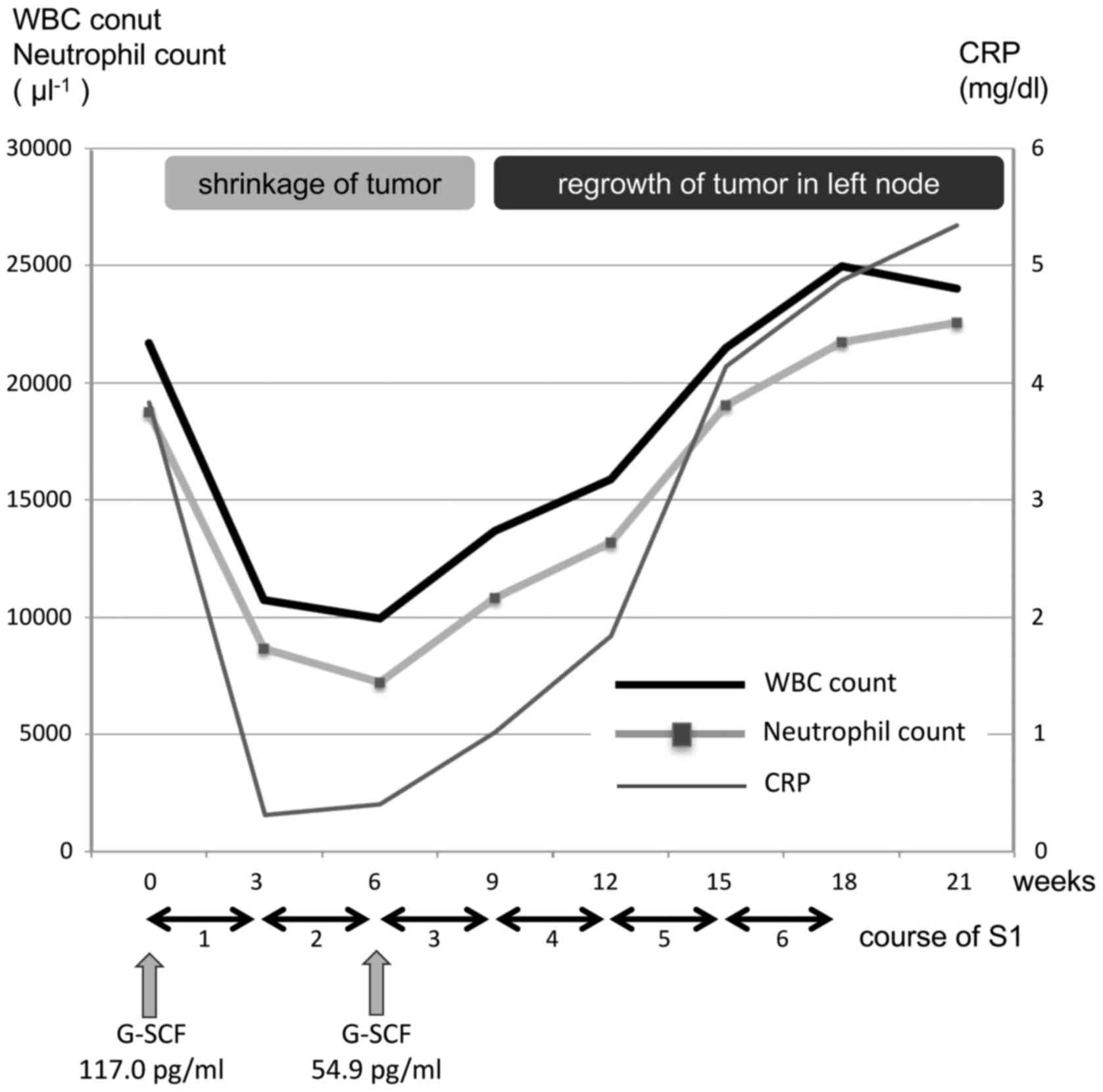
by a 1-week rest. Following the second treatment cycle, we observed
marked shrinkage of the patient's primary tumor and metastatic
cervical lymph nodes (Fig. 4), along
with reductions in his WBC count (9,930 µl−1),
neutrophil count (72.7%) and serum CRP (0.47 mg/dl) and G-CSF (54.9
pg/ml) concentrations (Fig. 5). After
the fourth cycle of chemotherapy, the tongue tumor further
decreased in size (Fig. 6), but
regrowth of the tumor in the left middle jugular node was observed.
In addition, his WBC count (13,670 µl−1), neutrophil
count (79.2%) and CRP concentration (1.19 mg/dl) had again
increased (Fig. 5). At the end of the
sixth cycle of chemotherapy, the tumor in the left node had
increased further and become painful, and his WBC count (24,980
µl−1), neutrophil count (87.2%) and CRP concentration
(3.85 mg/dl) had increased (Fig. 5).
Although FDG uptake was observed in the left metastatic node
(SUVmax 17.52) and the bone marrow, FDG did not accumulate in the
primary tongue tumor and in other node lesions (Fig. 7). Because of neck pain, the patient
consented to chemoradiotherapy as curative treatment with the
possibility of pain relief. He was therefore started on
intensity-modulated radiation therapy with concurrent low-dose
daily CDDP (4 mg/m2), but radiation was discontinued at
10 Gy due to patient refusal. Thereafter, he received supportive
care and died of the disease one year after initial
examination.
Discussion
Leukocytosis in association with non-hematologic
malignancies in the absence of infectious disease is a
paraneoplastic syndrome (1). These
tumors are thought to produce G-CSF, resulting in leukocytosis,
consisting predominantly of neutrophils. G-CSF-producing tumors are
characterized by i) increased WBC counts, predominantly
neutrophils, in the absence of infectious and hematologic diseases;
ii) increased serum G-CSF level; iii) normalization of WBC count
and serum G-CSF level after remission or removal of the tumor; and
iv) presence of G-CSF in tumor tissues (3,4).
At initial examination, the patient described in
this report was found to have marked leukocytosis, predominantly
consisting of neutrophils, and increased serum G-CSF levels.
Immunohistochemical staining of a tongue biopsy specimen with
monoclonal anti-G-CSF antibody yielded negative results, similar to
findings in many other patients with G-CSF producing tumors
(5,6).
Negative staining of tumor tissue may be caused by the rapid
secretion of G-CSF from tumor cells (7). Although this tumor was not resected,
oral chemotherapy, which reduced the size of the tumor, reduced his
WBC count and serum G-CSF concentration to near normal levels. S1
can cause hematotoxicity, resulting in decreased WBC count.
Therefore, the reductions of WBC count and serum G-CSF
concentration in our patient may have been related to the side
effects caused by S1. However, subsequent regrowth of the tumor on
the left node was accompanied by increases in WBC count and serum
G-CSF concentration. The correlation of these two parameters with
shrinkage and enlargement of the tumor suggested that this tumor
produced G-CSF.
FDG-PET, which assesses glucose uptake and
metabolism, is broadly recognized as a useful modality for tumor
imaging. FDG-PET imaging of patients with G-CSF-producing tumors
has shown diffuse uptake of FDG throughout the bone marrow and
markedly elevated uptake of FDG by the primary tumor (2). Moreover, the bone marrow uptake of FDG
was found to correlate with peripheral WBC count, especially
neutrophils, and to reflect the increased metabolic activity of
bone marrow (8). Treatment with
recombinant G-CSF has been reported to increase the metabolism and
cellularity of bone marrow, resulting in increased bone marrow
uptake of FDG (9). Thus, G-CSF
producing tumors are thought to enhance bone marrow metabolism,
resulting in FDG uptake in the bone marrow.
Cancer patients with FDG-uptake in the bone marrow
require differential diagnosis of bone metastases. In general, bone
metastases from oral squamous cell carcinoma, a solid tumor type,
are present as solid tumors with focal uptake of FDG. In contrast,
the diffuse uptake of FDG in the bone marrow has also been reported
in patients with leukemia, lymphoma, histiocytosis, myeloma, and
myeloid hyperplasia, as well as after treatment with cytokines and
erythropoietin (10). In other words,
diffuse uptake of FDG in bone marrow does not indicate metastases
from oral cancer, in general. In our patient, uptake of FDG in the
bone marrow was not focal but diffuse. Therefore, we deemed that
there was no metastasis to the bone marrow and that diffuse uptake
of FDG in the bone marrow was founded to reflect the increased
metabolic activity of bone marrow by G-CSF. Moreover, our patient
had no symptoms or laboratory findings suggesting that bone marrow
disease or hematopoietic malignancy was the cause of diffuse uptake
of FDG in bone marrow. Therefore, we did not perform a bone marrow
examination in this patient.
G-CSF produced by a tumor may also contribute to
marked tumor infiltration by inflammatory cells, a process that
which may enhance tumor uptake of FDG (2). Our patient showed marked FDG uptake by
both the tongue tumor and the metastatic nodes. In addition, the
regrown tumor in the left node showed marked FDG uptake, with an
SUVmax of 17.52, despite the absence of uptake by the primary
tongue tumor and the other metastatic nodes. WBC count, CRP
concentration and FDG uptake in bone marrow were also increased at
the same time. These findings suggested that the tumor in the left
node, rather than the primary tongue tumor and the other metastatic
nodes, may have the potential to produce G-CSF.
G-CSF producing tumors are associated with
aggressive tumor behavior and poor patient prognosis, regardless of
the primary site and pathological type of the tumor. The lungs are
the most frequent site of G-CSF producing tumors (2), followed by sites such as the liver
(11), stomach (5) and bladder (1). However, G-CSF producing tumors are
rarely detected in the oral regions (4,7,12,13). The
survival time of patients with G-CSF-producing tumors has been
reported to be approximately 6 months (5). To our knowledge, 7 of 8 patients with
these tumors in the head and neck region died within 1 year of
diagnosis (average 8 months), as did our patient (4,7,12–17).
Several hypotheses may explain the causes poor
prognosis of patients with G-CSF-producing tumors. G-CSF is
frequently produced by poorly differentiated and undifferentiated
carcinomas (5,6), and many of these G-CSF producing tumors
are initially detected at advanced stage (1,5–7,12,13). In addition, the G-CSF produced by
these tumors may contribute to tumor progression by autocrine and
paracrine mechanisms (18,19), as well as promoting angiogenesis and
tumor growth (20). No standard
therapy has yet been established for G-CSF producing tumors, but
multimodal treatment should be initiated rapidly to improve
prognosis.
In conclusion, although oral G-CSF producing tumors
are rare, the combination of diffuse uptake of FDG in the bone
marrow and leukocytosis, especially of neutrophils, may suggest
this tumor type. Alterations in these parameters may be useful as
markers of tumor status.
Acknowledgements
This study was partially supported by JSPS KAKENHI
(Grant no. 6K20433).
References
|
1
|
Kumar AK, Satyan MT, Holzbeierlein J,
Mirza M and Van Veldhuizen P: Leukemoid reaction and autocrine
growth of bladder cancer induced by paraneoplastic production of
granulocyte colony-stimulating factor-a potential neoplastic
marker: A case report and review of the literature. J Med Case Rep.
8:1472014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morooka M, Kubota K, Murata Y, Shibuya H,
Ito K, Mochizuki M, Akashi T, Chiba T, Nomura T, Ito H and Morita
T: (18)F-FDG-PET/CT findings of granulocyte colony stimulating
factor (G-CSF)-producing lung tumors. Ann Nucl Med. 22:635–639.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asano S, Urabe A, Okabe T, Sato N and
Kondo Y: Demonstration of granulopoietic factor(s) in the plasma of
nude mice transplanted with a human lung cancer and in the tumor
tissue. Blood. 49:845–852. 1977.PubMed/NCBI
|
|
4
|
Kobayashi J, Miyazaki A, Yamamot T,
Nakamori K, Suzuki R, Kaneko T, Suzuki N and Hiratsuka H:
Granulocyte colony-stimulating factor-producing squamous cell
carcinoma of the lower gingiva: A case report. Head Neck Oncol.
4:352012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawaguchi M, Asada Y, Terada T, Takehara
A, Munemoto Y, Fujisawa K, Mitsui T, Iida Y, Miura S and Sudo Y:
Aggressive recurrence of gastric cancer as a
granulocyte-colony-stimulating factor-producing tumor. Int J Clin
Oncol. 15:191–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vinzens S, Zindel J, Zweifel M, Rau T,
Gloor B and Wochner A: Granulocyte colony-stimulating factor
producing anaplastic carcinoma of the pancreas: Case report and
review of the literature. Anticancer Res. 37:223–228. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horii A, Shimamura K, Honjo Y, Mitani K,
Miki T, Takashima S and Yoshida J: Granulocyte colony stimulating
factor-producing tongue carcinoma. Head Neck. 19:351–356. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murata Y, Kubota K, Yukihiro M, Ito K,
Watanabe H and Shibuya H: Correlations between 18F-FDG uptake by
bone marrow and hematological parameters: Measurements by PET/CT.
Nucl Med Biol. 33:999–1004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugawara Y, Zasadny KR, Kison PV, Baker LH
and Wahl RL: Splenic fluorodeoxyglucose uptake increased by
granulocyte colony-stimulating factor therapy: PET imaging results.
J Nucl Med. 40:1456–1462. 1999.PubMed/NCBI
|
|
10
|
Goshen E, Davidson T, Yeshurun M and Zwas
ST: Combined increased and decreased skeletal uptake of F-18 FDG.
Clin Nucl Med. 31:520–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagata H, Komatsu S, Takaki W, Okayama T,
Sawabe Y, Ishii M, Kishimoto M, Otsuji E and Konosu H: Granulocyte
colony-stimulating factor-producing hepatocellular carcinoma with
abrupt changes. World J Clin Oncol. 7:380–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneko N, Kawano S, Matsubara R, Goto Y,
Jinno T, Maruse Y, Sakamoto T, Hashiguchi Y, Iida M and Nakamura S:
Tongue squamous cell carcinoma producing both parathyroid
hormone-related protein and granulocyte colony-stimulating factor:
A case report and literature review. World J Surg Oncol.
14:1612016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishigami T, Yoshida H, Yusa H and Yanagawa
T: Gingival cancer suspected of producing granulocyte
colony-stimulating factor: Report of a case. J Oral Maxillofac
Surg. 59:804–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yazawa S, Toshimori H, Nakatsuru K,
Katakami H, Takemura J and Matsukura S: Thyroid anaplastic
carcinoma producing granulocyte-colony-stimulating factor and
parathyroid hormone-related protein. Intern Med. 34:584–588. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoneda T, Nishimura R, Kato I, Ohmae M,
Takita M and Sakuda M: Frequency of the hypercalcemia-leukocytosis
syndrome in oral malignancies. Cancer. 68:617–622. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka K and Nibu K: Laryngeal squamous
cell carcinoma with ectopic production of granulocyte
colony-stimulating factor and parathyroid hormone-related protein.
Int J Clin Oncol. 10:195–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tamura K, Yoshinaga T, Tanino M, Kimura T,
Yamada N, Nishimura M, Fukuda S, Nishihara H, Shindoh M and Tanaka
S: Hypopharyngeal squamous cell carcinoma producing both
granulocyte colony-stimulating factor and parathyroid
hormone-related protein. Pathol Int. 58:652–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue M, Minami M, Fujii Y, Matsuda H,
Shirakura R and Kido T: Granulocyte colony-stimulating factor and
interleukin-6-producing lung cancer cell line, LCAM. J Surg Oncol.
64:347–350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ichiishi E, Yoshikawa T, Kogawa T, Yoshida
N and Kondo M: Possible paracrine growth of adenocarcinoma of the
stomach induced by granulocyte colony stimulating factor produced
by squamous cell carcinoma of the oesophagus. Gut. 46:432–434.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Natori T, Sata M, Washida M, Hirata Y,
Nagai R and Makuuchi M: G-CSF stimulates angiogenesis and promotes
tumor growth: Potential contribution of bone marrow-derived
endothelial progenitor cells. Biochem Biophys Res Commun.
297:1058–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|