Introduction
Acute promyelocytic leukemia (APL) is a subtype of
acute myeloid leukemia (AML) that is characterized by a
disease-initiating chromosome translocation of t(15;17)(q22:q21),
leading to the generationof the promyelocytic leukemia
(PML)-retinoic acid α receptor (RARα) fusion protein (1). At present, the treatment of APL involves
the use of all-trans retinoic acid (ATRA) and arsenic
trioxide (ATO). ATRA primarily induces differentiation, whereas ATO
promotes apoptosis in APL cells (2).
Despite the significant progress in therapeutic strategies, 10–30%
patients with APL are still resistant to ATRA or ATO (3). Hence, the development of new therapeutic
drugs for APL treatment is critical.
Lapatinib is a small-molecule, tyrosine kinase
inhibitor that targets the epidermal growth factor receptor and
human epidermal growth factor receptor (HER-2) (4,5).
Clinically, lapatinib is mainly used for the treatment of HER-2
positive metastatic breast cancer, and is approved for use by the
Food and Drug Administration of the USA (1,6). In
addition, since lapatinib is an oral drug and has few adverse
reactions, it has been used in the treatment of several types of
tumors, including triple-negative breast cancer, nasopharyngeal
carcinoma, head and neck cancer, pancreatic cancer, cervical
cancer, non-small cell lung cancer, and brain cancer (7–14).
Moreover, several studies have reported the use of lapatinib in the
treatment of leukemia; for example, lapatinib induces apoptosis in
cell lines derived from acute myeloid leukemia (AML) and
myelodysplastic syndrome (MDS) (15),
and it has been shown to regulate autophagy, apoptosis, and
megakaryocytic differentiation in K562 cells (16). In addition, lapatinib promotes
autophagic cell death and differentiation in AML (17). Importantly, lapatinib inhibits cell
proliferation and promotes cell apoptosis in Philadelphia
chromosome-positive acute lymphoblastic leukemia (18). However, few studies have evaluated the
effects of lapatinib in APL cells.
In the present study, we investigated the effects of
lapatinib on NB4 cells, an APL cell line. We found that lapatinib
inhibited cell proliferation and promoted apoptosis. Our
observations suggested that lapatinib treatment may represent a
novel therapeutic approach for the treatment of APL.
Materials and methods
Reagents
Lapatinib was purchased from Shanghai Selleck
Chemicals Co., Ltd., (Shanghai, China). Liu's stain was purchased
from BaSO Diagnostics Inc., (Zhuhai, China). The inhibitor of
P-38MAPK PD169316 was purchased from MedChemExpress (New Jersey,
USA). Hoechst 33258 and the JNK inhibitor SP600125 were purchased
from Beyotime Institute of Biotechnology (Shanghai, China).
Antibodies against caspase-3, caspase-9, cleaved caspase-9,
p38MAPK, JNK, p-JNK, Akt, and p-Akt were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). Antibodies against
Bax, Bcl-2, and PARP were purchased from Wanleibio (Shenyang,
China). Antibody against PML-RARα was purchased from Abcam
(Cambridge, MA, USA). Antibody against p-p38 MAPK was purchased
from EMD Millipore (Billerica, MA, USA). Goat-anti-rabbit antibody,
goat-anti-mouse antibody and anti-β-actin antibodies were purchased
from Zhongshan Golden Brige Biotechnology Co., Ltd., (Beijing,
China).
Cell culture
NB4 cells (Institutes for Biological Science,
Shanghai, China) were cultured in RPIM 1640 (Gibco Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(Gibco Life Technologies) supplemented with penicllin (100 mg/ml)
and streptomycin (100 mg/ml) at 37°C in an environment containing
5% CO2. The medium was refreshed every day.
CCK-8 assay
NB4 cells were seeded in 96-well plates at a density
of 1.0×104 cells/well. Then, cells were treated with
different concentrations of lapatinib for 24 h. After cultured 24
h, 10 µl of CCK-8 (7Sea Cell Counting Kit; Sevenseas Futai
Bio-technology Co., Ltd., Shanghai, China) was added to each well
followed by incubation for 2 h at 37°C. Cell viability was assessed
by detection of absorbance at 450 nm using a spectrophotometer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The experiment was
repeated at least three times.
Colony forming assay
NB4 cells were exposed to lapatinib or DMSO for 24
h. Then, cells were plated in 24-well plates in methylcellulose
medium in triplicate. The numbers of colonies were determined
following incubation for 14 days at 37°C and 5% CO2.
Flow cytometry analysis
NB4 cells were fixed with pre-cold 70% ethanol
overnight at 4°C and washed with PBS. Then, incubated with 50 µl
Propidium Iodide (PI) for 15 min at room temperature and cells were
analysis in flow cytometer. Each experiment was repeated at least
three times.
NB4 cells were treated of various concentrations of
lapatinib for 24 h, and cells were harvested by centrifugation at
3000 r for 5 min. After washed three times with pre-cold PBS, cells
were re-suspended in Binding Buffer (Sungene Biotech Co., Ltd.,
Tianjing, China), and stained by Annexin V-FITC and PI
(Sigma-Aldrich, St. Louis, MO, USA) for 15 min at room temperature
and cells were analysis in flow cytometer. Each experiment was
repeated at least three times.
Liu's staining
NB4 cells were treated with different concentrations
of lapatinib for 24 h. Cells in each group were collected and
plated onto the glass slides. First, Liu A was added to the sample
spot for 20 s and mixed with twice the volume of Liu B for another
30 s. Then, the slides were washed with water and air dried for
observation using a microscope.
Hoechst 33258 staining
NB4 cells were treated with different concentrations
of lapatinib for 24 h. Cells in each group were collected and
plated onto the glass slides, followed by fixative in 4%
para-formaldehyde at room temperature. After 20 min, cells were
washed twice with PBS, stained with Hoechst 33258 for 10 min and
then washed twice with PBS. Nuclear morphological changes in the
cells were observed under fluorescence microscope.
Western blot analysis
NB4 cells were lysed in radioimmunoprecipitation
solution containing protease inhibitor phenylme-thanesulfonyl
fluoride (PMSF), phosphatase inhibitor Na F and Na3VO3. Protein
concentration was determined with BCA method. A total of 50 µg of
proteins were separated by 10% SDS-PAGE and transferred to
polyvinylidence difluoride membrane. Membranes were blocked with 5%
non-fat milk for 2 h, and incubated with specific antibodies
(1:1,000) over night at 4°C. Then, membranes were incubated with
goat-anti-rabbit and goat-anti-mouse secondary antibodies (1:4,000)
for 1 h at 37°C. After washing with Tris-buffered saline containing
Tween-20 (TBST), the immunoreactive complexes were visualized using
an enhanced chemiluminescence system. Protein bands were analyzed
by Quantity One Software (Bio-Rad Laboratories, Inc.). Each
experiment was repeated at least three times.
Statistical analysis
Data were expressed as means ± standard deviation
(SD). Statistical analysis was performed with SPSS version 17.0. An
independent samples t-test was used to compare the results of two
groups. Statistical analysis of western blot results was performed
using analysis of variance (ANOVA). P<0.05 was considered to
indicate a statistically significant difference. Each experiment
was repeated at least three times.
Results
Lapatinib inhibits the proliferation
of NB4 cells
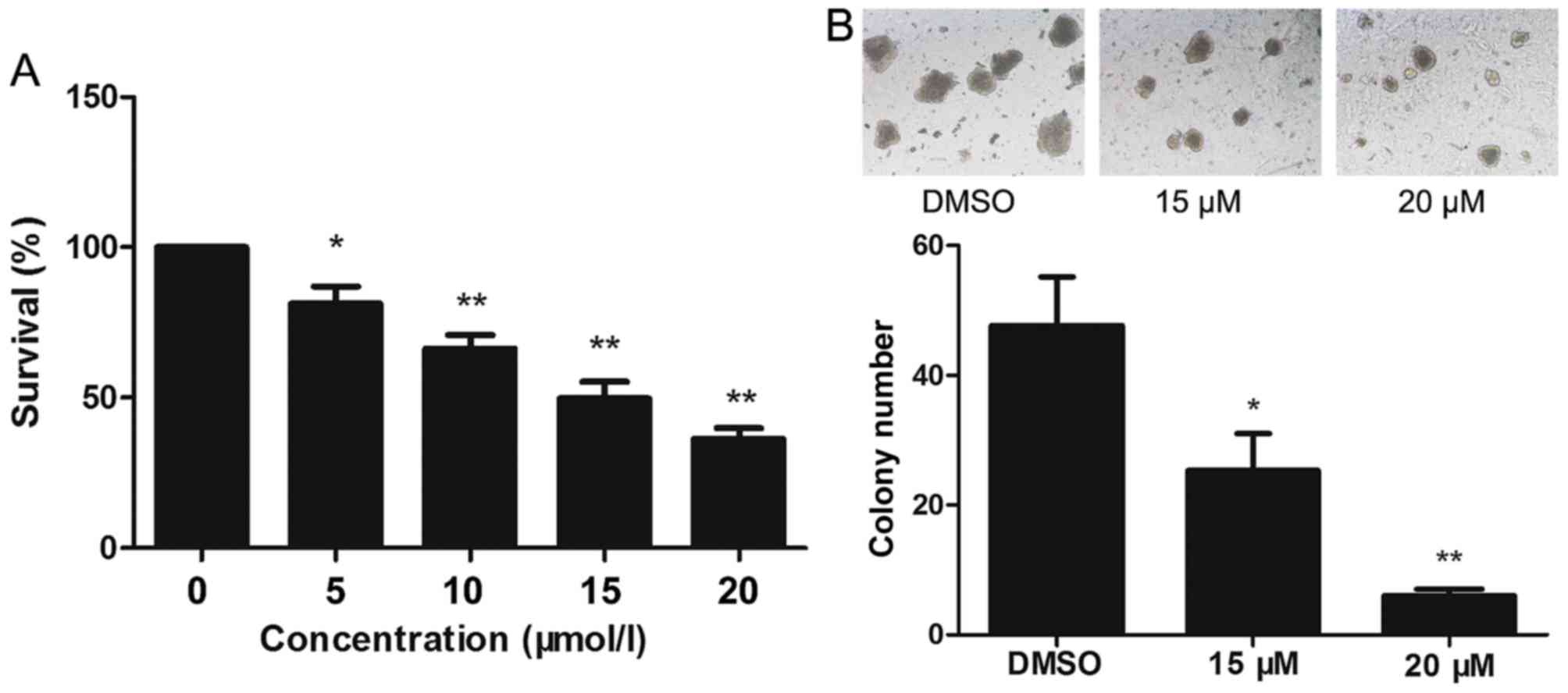
The effect of lapatinib on the viability of NB4
cells was evaluated using CCK-8 assays. Treatment of NB4 cells with
0–20 µM lapatinib for 24 h led to a concentration-dependent
reduction in cell viability (Fig.
1A). Based on these observations, 15 and 20 µM lapatinib were
selected for our study. Exposure to 15 and 20 µM lapatinib visibly
inhibited the colony-forming capacity of NB4 cells compared to the
cells of the dimethyl sulfoxide (DMSO) group (Fig. 1B).
Lapatinib induces cell cycle arrest at
the S phase
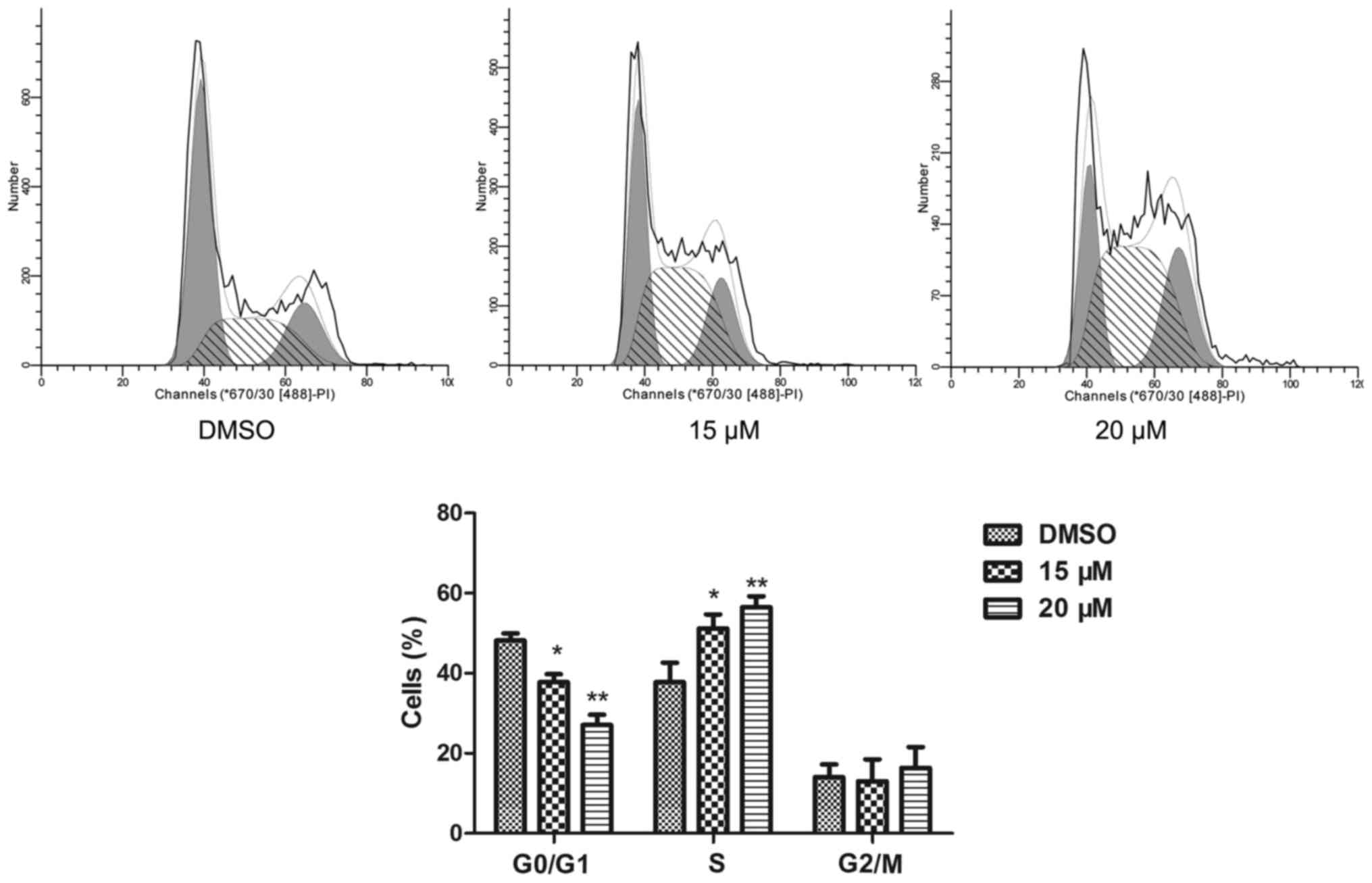
The cell cycle of NB4 cells was detected by a flow
cytometric assay. Compared to the cell cycle profile of the DMSO
treated group, 15 and 20 µM lapatinib treatment dramatically
increased the percentage of cells in the S phase (Fig. 2).
Lapatinib promotes apoptosis in NB4
cells
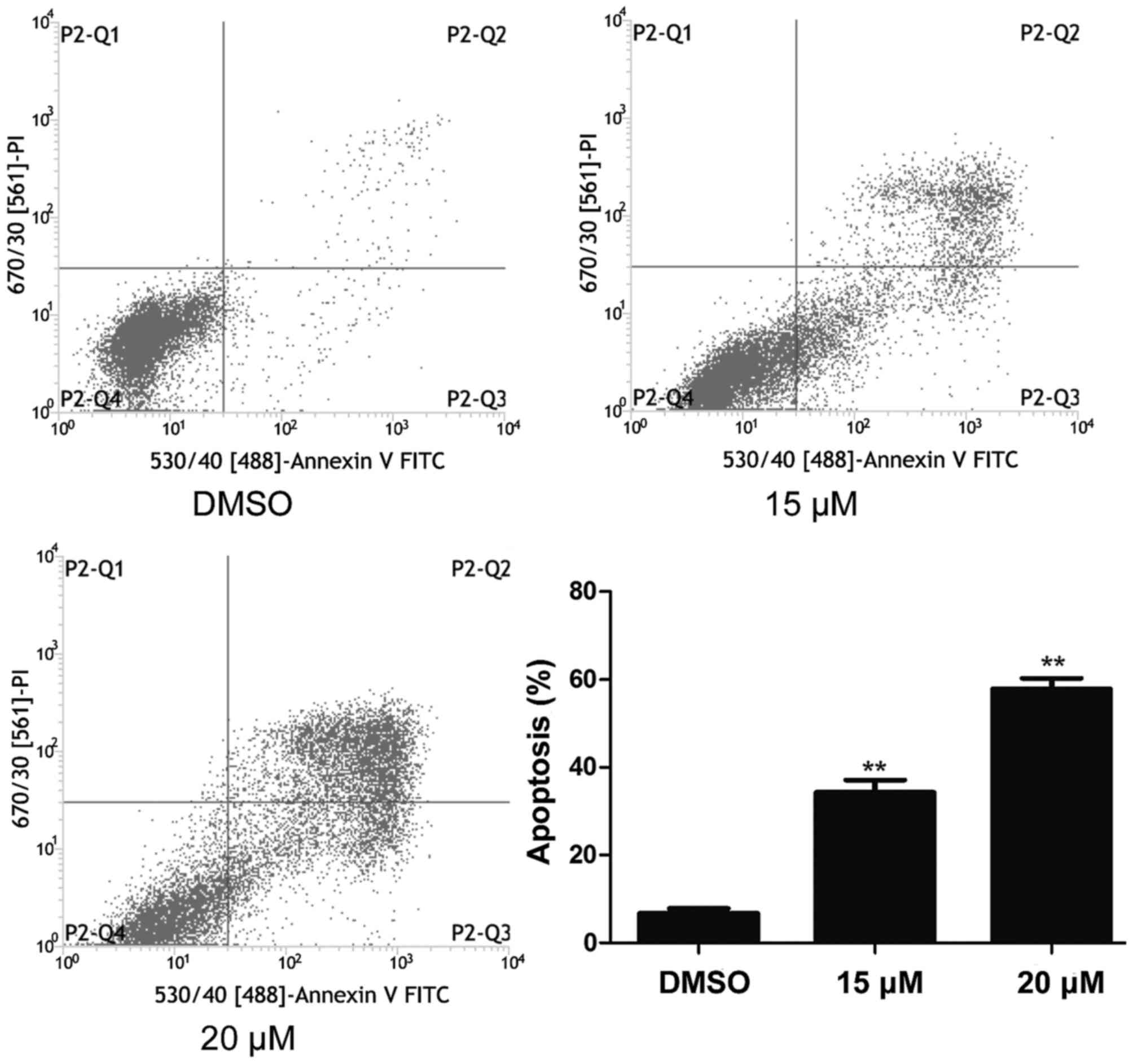
NB4 cells were treated with 15 and 20 µM lapatinib
for 24 h and cell apoptosis was detected using flow cytometry. As
shown in Fig. 3, cell apoptosis
increased in the lapatinib treated groups compared to that of the
DMSO-treated group (Fig. 3).
Effects of lapatinib on NB4 cell
morphology
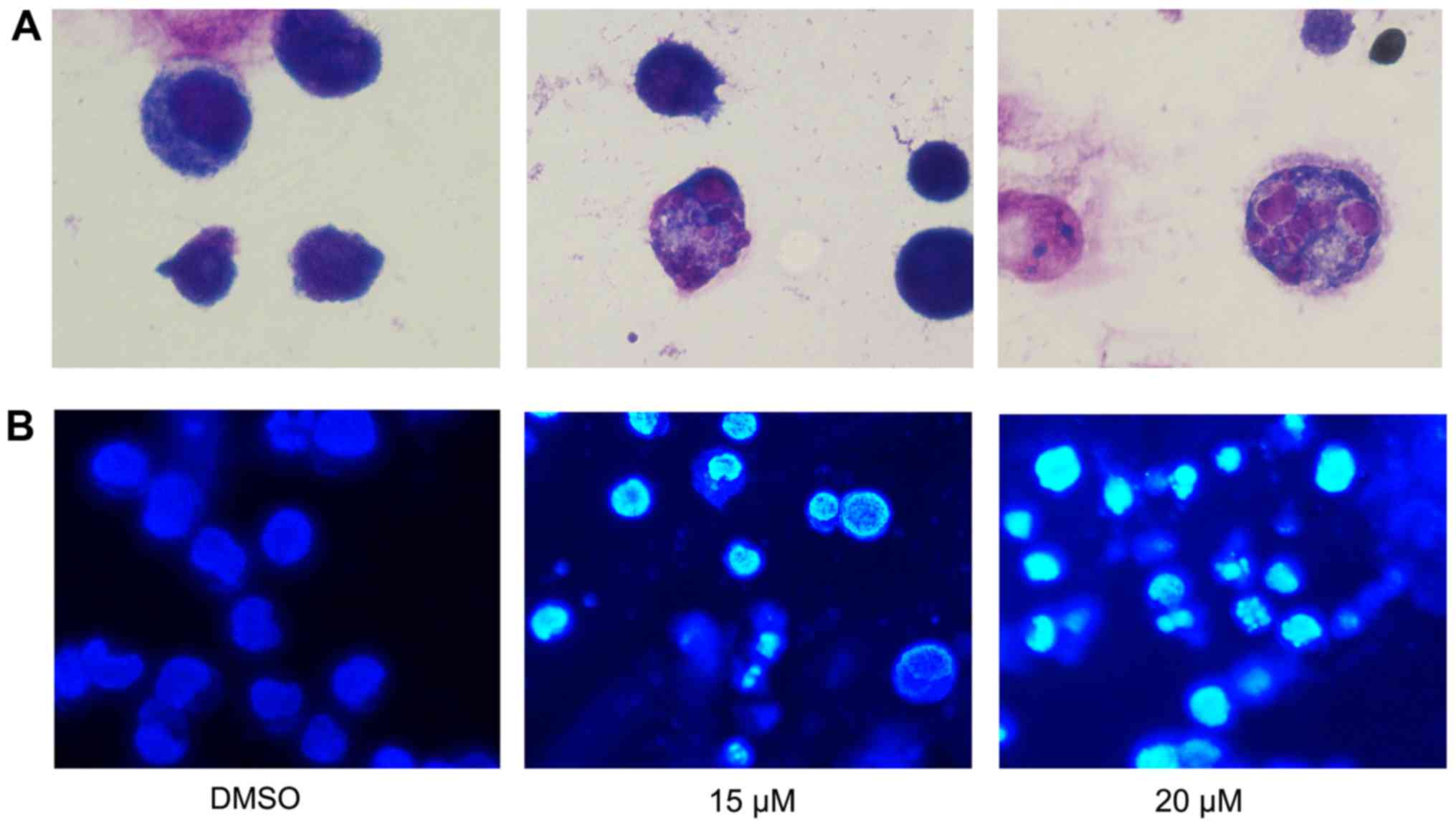
To further assess the effect of lapatinib treatment
on NB4 cells' morphology, cells were stained with Liu's stain after
treatment with 15 and 20 µM lapatinib. Results showed that nuclear
fragmentation was observed in the lapatinib-treated group (Fig. 4A). In addition, after incubation with
15 and 20 µM lapatinib, cells were harvested and stained with
Hoechst 33258. The nuclear fluorescence intensities of the
lapatinib treated groups were stronger than that of the negative
control group (Fig. 4B), and nuclear
fragmentation and condensation were observed in the lapatinib-
treated group (Fig. 4).
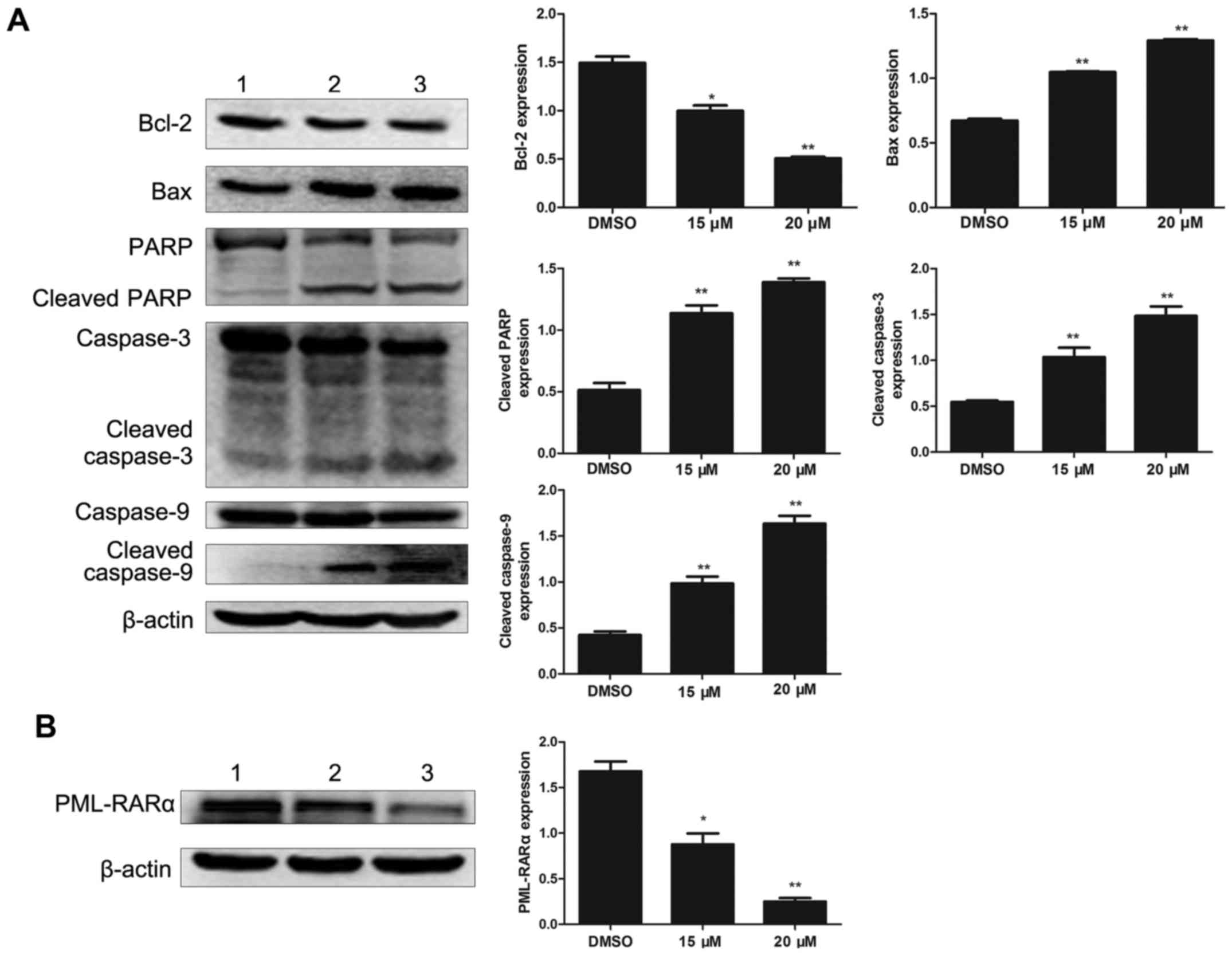
Effects of lapatinib on
apoptosis-related protein in NB4 cells
To investigate the effect of lapatinib on apoptosis
in NB4 cells, the levels of apoptosis-related proteins were
detected by western blotting. Bcl-2 was notably down-regulated with
lapatinib treatment. In addition, the expression of Bax, cleaved
caspase-3, cleaved caspase-9, and cleaved PARP were up-regulated in
the lapatinib group but not in the DMSO group (Fig. 5A). The level of the fusion protein
PML-RARα was down-regulated with lapatinib treatment (Fig. 5B).
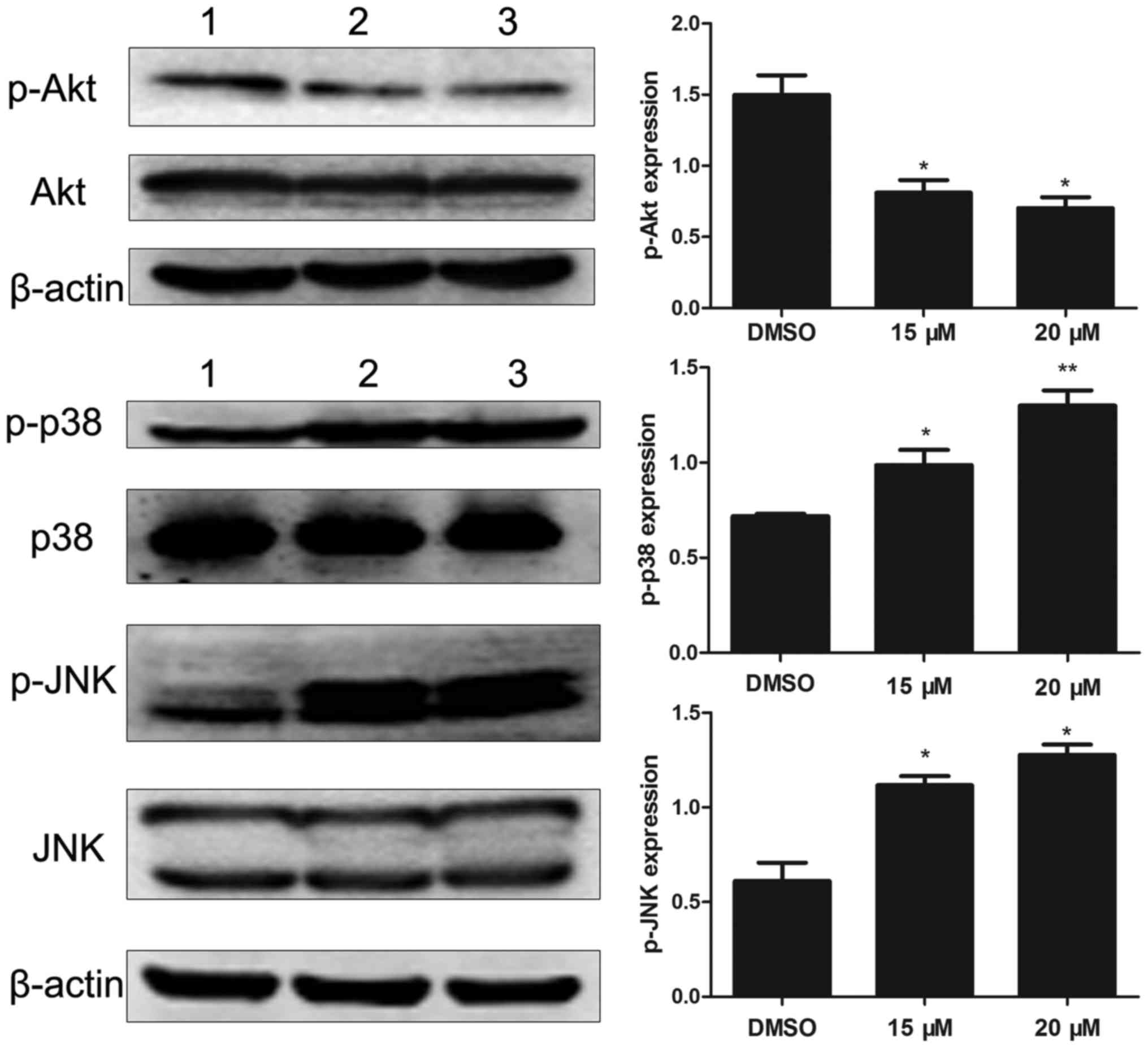
Effects of lapatinib on MAPKs and AKT
signaling pathways
To investigate the molecular mechanisms of lapatinib
action on NB4 cells, we analyzed the levels of Akt, p-Akt, p38MAPK,
p-p38MAPK, JNK, and p-JNK. Results showed that lapatinib notably
down-regulated the expression of p-Akt and up-regulated the
expression of p-p38MAPK and p-JNK (Fig.
6).
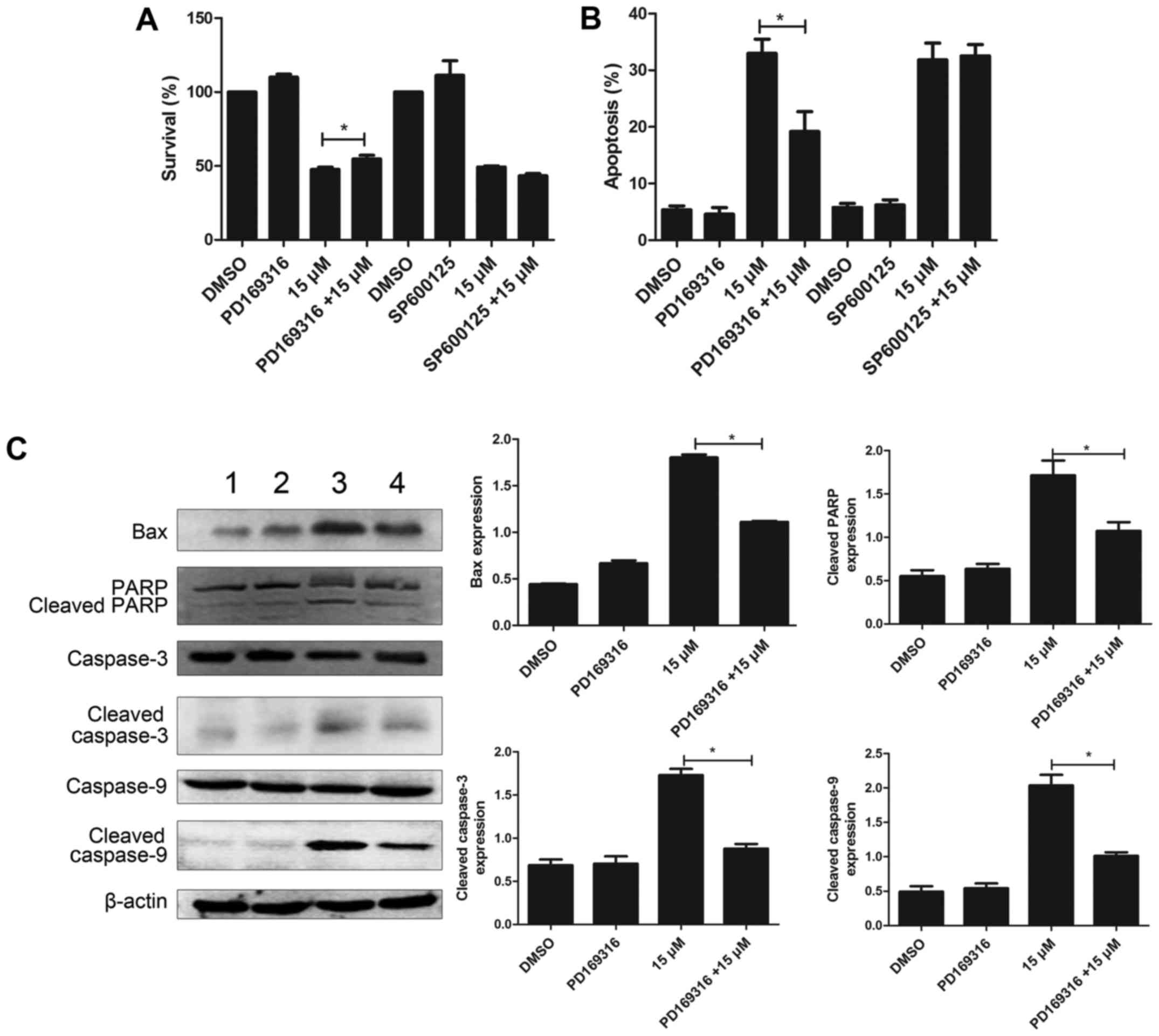
PD169316 weakens the effect of
lapatinib in NB4 cells
To further study the involvement of p38MAPK and JNK
signaling pathways, in the mode of action of lapatinib, we treated
the cells with 10 µM each of PD169316 or SP600125 for 30 min before
incubating with 15 µM lapatinib. The results of the CCK-8 assay
showed that PD169316 could partially reverse the growth inhibition
induced by lapatinib treatment. FCM detections demonstrated that
PD169316 partially reduced lapatinib-induced apoptosis. However,
the JNK inhibitor, SP600125, had no such effect (Fig. 7A and B). In addition, the levels of
Bax, cleaved caspase-3, cleaved caspase-9, and cleaved PARP were
significantly reduced upon treatment with a combination of
lapatinib and PD169316 than with lapatinib treatment alone
(Fig. 7C).
Discussion
Previous studies showed that lapatinib decreased the
viability of MOLM-13 and HL-60 cells (15), head and neck squamous carcinoma cells
(19), gastric cancer cells (20), and breast cancer cells (21). In this study, the CCK-8 assay showed
that proliferation of NB4 cells was inhibited by 5–20 µM lapatinib
in a concentration-dependent manner. Colony-forming assays showed
that 15 and 20 µM lapatinib dramatically reduced colony formation,
indicating that lapatinib could suppress NB4 cell proliferation.
Moreover, some recent studies have reported that lapatinib promotes
cell cycle arrest in the G0/G1 phase
(19–21). However, our results showed that
lapatinib reduced the number of cells in the G1 phase
and increased the number of cells in the S phase. Based on these
results, we hypothesize that lapatinib inhibits cell proliferation
by inducing S phase arrest.
Apoptosis is a process of programmed cell death and
involves balancing of pro-survival and pro-cell death functions in
the cell (22). Apoptosis is
characterized by nuclear condensation, nuclear fragmentation, and
formation of apoptotic bodies. Additionally, caspase-3 and PARP are
cleaved during activation of apoptosis (23–25). In
our study, flow cytometry analysis showed that lapatinib promoted
cell apoptosis; indeed, nuclear fragmentation, nuclear
condensation, and the presence of few apoptotic bodies were
observed in NB4 cells after lapatinib treatment, and lapatinib
induced cleavage of caspase-3 and PARP in a dose-dependent manner.
Two apoptotic pathways exist: the extrinsic apoptotic pathway
involving the death receptors is regulated by caspase-8, whereas
the intrinsic apoptotic pathway involving the mitochondria is
related to activation of caspase-3 and caspase-9 (26). Our data showed that treatment with
lapatinib induced the activation of caspase-9 rather than caspase-8
(data not shown); thus, we assumed that lapatinib may induce
apoptosis via the intrinsic apoptotic pathway in NB4 cells. Indeed,
our analysis showed that lapatinib decreased Bcl-2 and increased
Bax levels, thereby demonstrating that lapatinib induces apoptosis
through the intrinsic pathway mediated by the Bcl-2 family, which
involves Bcl-2 and Bax. Interestingly, lapatinib also reduce the
level of PML-RARα, which blocks granulocyte cell maturation. This
result showed that lapatinib-induced apoptosis was associated with
the degradation of PML-RARα. Taken together, our results suggested
that lapatinib treatment may represent a therapeutic option for
APL, although further studies are required to fully elucidate the
mechanisms involved in this process.
MAPK family members can be activated by different
factors and have important roles in apoptosis. MAPK signaling
pathways, including ERK, p38 MAPK, and JNK, have been identified as
chemotherapeutic targets for sensitizing cancer cells for apoptosis
(27). Typically, p38 kinase and JNK
are known as cell death signals, whereas ERK is a survival signal
(28). Previous studies have shown
that lapatinib induces apoptosis in breast cancer cells through the
STAT5 and JNK signaling pathways (29). Indeed, after treatment with lapatinib,
cells showed increased phosphorylation of p38 MAPK and JNK in a
concentration-dependent manner. In addition, the p38 inhibitor
PD169316 reduced the lapatinib-induced inhibition of cell viability
and apoptosis, whereas the JNK inhibitor SP600125 did not affect
these processes. Moreover, co-treatment with lapatinib and a p38
inhibitor significantly decreased the levels of Bax, cleaved PARP,
cleaved caspase-3 and cleaved caspase-9 compared to those in cells
treated with lapatinib alone. These results suggested that the p38
MAPK pathway was involved in lapatinib-induced apoptosis. In
addition to the MAPK pathway, the PI3K/AKT signal transduction
pathway plays a vital role in cell survival and prevents cancer
cells from undergoing apoptosis during tumorigenesis (30). Several studies have reported that
lapatinib induces apoptosis in triple-negative breast cancer cells
by inhibiting the CIP2A/PP2A/P-AKT signaling pathways (11). Additionally, lapatinib can potentiate
radiation-induced cell death in HER-2-overexpressing breast cancer
cells via reduction of p-AKT levels (31). Consistent with this observation, our
results showed that lapatinib exerted significant inhibitory
effects on p-AKT levels. These results suggested that the AKT
pathway was involved in lapatinib-induced apoptosis in NB4
cells.
Previous studies have reported that lapatinib
induces autophagic cell death in acute myeloblastic
leukemia-derived cell lines (17);
however, we did not detect any changes in the levels of autophagy
markers ATG5, ATG7, and LC3II following lapatinib treatment. These
contradictory results could be attributed to variations in the
concentrations of lapatinib, differences in the time points used in
the experiments, cell status, and variations in other experimental
factors.
In conclusion, our present study indicated that
lapatinib inhibited NB4 cell growth and caused cell cycle arrest,
and promoted apoptosis potentially through the p38MAPK and AKT
signaling pathways. These results suggested that treatment with
lapatinib may be an effective strategy for APL therapy. Further
investigations are required for developing novel therapeutic
approaches for the treatment of leukemia.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171658) and the
Natural Science Foundation Project of CQ CSTC (grant no.
2011BA5037).
References
|
1
|
Ma H and Yang J: Insights into the
all-trans-retinoic acid and arsenic trioxide combination treatment
for acute promyelocytic leukemia: A meta-analysis. Acta Haematol.
134:101–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown G and Hughes P: Retinoid
differentiation therapy for common types of acute myeloid leukemia.
Leuk Res Treatment. 2012:9390212012.PubMed/NCBI
|
|
3
|
Kawasaki K, Akaike H, Miyauchi A and Ouchi
K: Sivelestat relieves respiratory distress refractory to
dexamethasone in all-trans retinoic acid syndrome: A report of two
cases. Eur J Haematol. 77:448–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito Y, Tokudome N, Sugihara T, Takahashi S
and Hatake K: Does lapatinib, a small-molecule tyrosine kinase
inhibitor, constitute a breakthrough in the treatment of breast
cancer? Breast cancer. 14:156–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kopper L: Lapatinib: A sword with two
edges. Pathol Oncol Res. 14:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konecny GE, Pegram MD, Venkatesan N, Finn
R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beck TN, Georgopoulos R, Shagisultanova
EI, Sarcu D, Handorf EA, Dubyk C, Lango MN, Ridge JA, Astsaturov I,
Serebriiskii IG, et al: EGFR and RB1 as dual biomarkers in
HPV-negative head and neck cancer. Mol Cancer Ther. 15:2486–2497.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gore J, Imasuen-Williams IE, Conteh AM,
Craven KE, Cheng M and Korc M: Combined targeting of TGF-β, EGFR
and HER2 suppresses lymphangiogenesis and metastasis in a
pancreatic cancer model. Cancer Lett. 379:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao YC, Yeh MH, Chen YJ, Liu JF, Tang CH
and Huang WC: Lapatinib increases motility of triple-negative
breast cancer cells by decreasing miRNA-7 and inducing
Raf-1/MAPK-dependent interleukin-6. Oncotarget. 6:37965–37978.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin HO, Hong SE, Kim CS, Park JA, Kim JH,
Kim JY, Kim B, Chang YH, Hong SI, Hong YJ, et al: Combined effects
of EGFR tyrosine kinase inhibitors and vATPase inhibitors in NSCLC
cells. Toxicol Appl Pharmacol. 287:17–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CY, Hu MH, Hsu CJ, Huang CT, Wang DS,
Tsai WC, Chen YT, Lee CH, Chu PY, Hsu CC, et al: Correction:
Lapatinib inhibits CIP2A/PP2A/p-Akt signaling and induces apoptosis
in triple negative breast cancer cells. Oncotarget.
8:107602017.PubMed/NCBI
|
|
12
|
Liu L, Huang P, Wang Z, Chen N, Tang C,
Lin Z and Peng P: Inhibition of eEF-2 kinase sensitizes human
nasopharyngeal carcinoma cells to lapatinib-induced apoptosis
through the Src and Erk pathways. BMC Cancer. 16:8132016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh DY, Kim S, Choi YL, Cho YJ, Oh E, Choi
JJ, Jung K, Song JY, Ahn SE, Kim BG, et al: HER2 as a novel
therapeutic target for cervical cancer. Oncotarget. 6:36219–36230.
2015.PubMed/NCBI
|
|
14
|
Booth L, Cruickshanks N, Ridder T, Chen
CS, Grant S and Dent P: OSU-03012 interacts with lapatinib to kill
brain cancer cells. Cancer Biol Ther. 13:1501–1511. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lainey E, Thépot S, Bouteloup C, Sébert M,
Adès L, Tailler M, Gardin C, de Botton S, Baruchel A, Fenaux P, et
al: Tyrosine kinase inhibitors for the treatment of acute myeloid
leukemia: delineation of anti-leukemic mechanisms of action.
Biochem Pharmacol. 82:1457–1466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang HL, Chen YC, Huang YC, Yang KC, Pan
Hy, Shih SP and Chen YJ: Lapatinib induces autophagy, apoptosis and
megakaryocytic differentiation in chronic myelogenous leukemia K562
cells. PLoS One. 6:e290142011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YJ, Fang LW, Su WC, Hsu WY, Yang KC
and Huang HL: Lapatinib induces autophagic cell death and
differentiation in acute myeloblastic leukemia. Onco Targets Ther.
9:4453–4464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irwin ME, Nelson LD, Santiago-O'Farrill
JM, Knouse PD, Miller CP, Palla SL, Siwak DR, Mills GB, Estrov Z,
Li S, et al: Small molecule ErbB inhibitors decrease proliferative
signaling and promote apoptosis in philadelphia chromosome-positive
acute lymphoblastic leukemia. PLoS One. 8:e706082013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fumagalli I, Dugue D, Bibault JE,
Clémenson C, Vozenin MC, Mondini M and Deutsch E: Cytotoxic effect
of lapatinib is restricted to human papillomavirus-positive head
and neck squamous cell carcinoma cell lines. Onco Targets Ther.
8:335–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oshima Y, Tanaka H, Murakami H, Ito Y,
Furuya T, Kondo E, Kodera Y and Nakanishi H: Lapatinib
sensitivities of two novel trastuzumab-resistant HER2
gene-amplified gastric cancer cell lines. Gastric Cancer.
17:450–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang L, Wang Y, Strom A, Gustafsson JA and
Guan X: Lapatinib induces p27(Kip1)-dependent G1 arrest through
both transcriptional and post-translational mechanisms. Cell Cycle.
12:2665–2674. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motomura M, Kwon KM, Suh SJ, Lee YC, Kim
YK, Lee IS, Kim MS, Kwon DY, Suzuki I and Kim CH: Propolis induces
cell cycle arrest and apoptosis in human leukemic U937 cells
through Bcl-2/Bax regulation. Environ Toxicol Pharmacol. 26:61–67.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 7:pii: a0267162015. View Article : Google Scholar
|
|
27
|
Su CC, Chen JY, Din ZH, Su JH, Yang ZY,
Chen YJ, Wang RY and Wu YJ: 13-acetoxysarcocrassolide induces
apoptosis on human gastric carcinoma cells through
mitochondria-related apoptotic pathways: p38/JNK activation and
PI3K/AKT suppression. Mar Drugs. 12:5295–5315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osaki LH and Gama P: MAPKs and signal
transduction in the control of gastrointestinal epithelial cell
proliferation and differentiation. Int J Mol Sci. 14:10143–10161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gschwantler-Kaulich D, Grunt TW, Muhr D,
Wagner R, Kolbl H and Singer CF: HER specific TKIs exert their
antineoplastic effects on breast cancer cell lines through the
involvement of STAT5 and JNK. PLoS one. 11:e01463112016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castaneda CA, Cortes-Funes H, Gomez HL and
Ciruelos EM: The phosphatidyl inositol 3-kinase/AKT signaling
pathway in breast cancer. Cancer Metastasis Rev. 29:751–759. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu T, Cho BJ, Choi EJ, Park JM, Kim DH and
Kim IA: Radiosensitizing effect of lapatinib in human epidermal
growth factor receptor 2-positive breast cancer cells. Oncotarget.
7:79089–79100. 2016.PubMed/NCBI
|