Introduction
Lung cancer is one of the most highly malignant
types of cancer; its mortality rate in the USA and China is higher
than that of other solid tumors (1).
Lung cancer is classified into two types, non-small cell lung
cancer (NSCLC) and SCLC, according to its pathological
characteristics. NSCLC accounts for 80–85% of all lung cancer cases
(2). NSCLC has a high mortality and
the 5-year survival rate is <15% despite improvements in cancer
treatment (3). The majority of
patients develop an aggressive form of NSCLC at the point of
diagnosis, which is often at a late stage of disease and which
metastasizes to other organs (4).
Thus, there is an urgent requirement for the identification of
novel biomarkers for early disease detection and therefore
improvements to treatment outcome.
MicroRNAs (miRNAs/miRs) are small (~22 nucleotides
in length), single-stranded, endogenous non-coding RNAs that
regulate gene expression by causing mRNA degradation or
translational suppression by directly binding to the
3′-untranslated regions (3′-UTRs) of target mRNAs (5–7). miRNAs
are implicated in a wide range of important physiological processes
(8–10). Evidence indicates that miRNAs act as
tumor suppressors or novel oncogenes, according to the roles of
their target genes, with aberrant miRNA expression being common in
various types of human cancer including NSCLC (11–13).
miR-20a belongs to the miR-17-92 cluster, located in
the 13q31.1 chromosomal region (14).
Previous studies have revealed that miR-20a is upregulated in
several types of cancer, suggesting its pivotal role in
tumorigenesis and progression (15,16).
However, in other tumors, including oral squamous cancer (17) and hepatic cancer (18), miR-20a acts as a tumor suppressor.
These findings suggest that the functions of miR-20a may differ
among different cell types. To date, several miRNAs have been
identified as being involved in NSCLC, including miR-30c, miR-4500,
miR-193a, miR-4782-3p and miR-138 (19–21).
However, to the best of our knowledge, the role of miR-20a in the
progression of NSCLC and its underlying mechanism has not yet been
investigated.
In the present study, the expression of miR-20a in
NSCLC samples and the impact of miR-20a on tumor biological
processes was examined. Additionally, early growth response 2
(EGR2) was identified as a direct target of miR-20a in NSCLC cells.
The present study indicates that miR-20a may be a novel target for
the treatment of NSCLC in the future.
Materials and methods
Tissue samples
A total of 35 human NSCLC tissues and 35 matched
normal tissue samples (located >5 cm away from the tumor) were
collected from patients (24 male cases and 11 female cases (mean
age, 57.68 years; range, 48–65 years) who underwent general
thoracic surgery in Hubei Cancer Hospital (Wuhan, China) between
February 2012 and July 2013. None of the patients with NSCLC had
received radio- or chemo-therapy prior to the surgery and none of
the patients had a history of having a tumor. These tissue
specimens were snap-frozen in liquid nitrogen and stored at −80°C.
The present study was approved by the Medical Ethics Committee of
Hubei Cancer Hospital and written informed consent for publication
was obtained from all patients prior to their participation in the
study.
Cell lines and culture
The NSCLCA-549, LTEP-a2, and SPC-A1 cell lines and
normal bronchial epithelial cell line 16HBE were obtained from the
Chinese Academy of Sciences (Shanghai, China). The three NSCLC cell
lines and 16HBE cells were used to determine the expression of
miR-20a, whereas A-549 was also used in further functional
analysis. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) and 1% streptomycin/penicillin at 37°C in a
humidified incubator with 5% CO2.
Establishment of A-549 cell lines with
knocked down miR-20a
Plasmids containing miR-20a siRNA or negative
control were purchased from GenePharma (Shanghai, China). A-549
cells were seeded in a 6-well plate. When cells reached 70–80%
confluency, they were transfected with 20 nM miR-20a siRNA and
negative control using riboFECT™ CP transfection reagents (RiboBio
Co., Ltd., Guangzhou, China) following the manufacturer's
instructions. miR-20a expression was upregulated by the
transfection of miR-20a mimic using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). At 24 h post
transfection, the cells were obtained and used in subsequent
analysis. Cells were then harvested and subjected to reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of miR-20a expression.
RT-qPCR
Total cellular RNA was isolated from tissues and
cell lines using TRIzol reagent (Takara Bio, Inc., Otsu, Japan),
then reverse transcribed into cDNA using PrimeScript RT Master Mix
(Takara Bio, Inc.) according to the manufacturer's instructions.
RT-qPCR was performed to determine the expression of miR-20 using a
SYBR-Green Mix kit (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol. Sequences were as
follows: miR-20a mimic sense, 5′-UAAAGUGCUUAUAGUGCAGGUAG-3′ and
antisense 5′-CUACCUGCACUAUAAGCACUUUA-3′; siRNA forward,
5′-UAAAGUGCUUAUAGUGCAGGUAGTT-3′ and reverse,
5′-CUACCUGCACUAUAAGCACUUUATT-3′; siRNA negative control forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. RT-qPCR was performed using the
Applied Biosystems 7500 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min,
followed by 40 cycles at 95°C for 10 sec, and at 62°C for 40 sec.
U6 was used as an endogenous control and relative quantification of
miR-20a expression was evaluated using the 2−ΔΔCq method
(22). All experiments were performed
in triplicate.
Cell proliferation assay
To investigate the effect of miR-20a regulation in
NSCLC cells, A-549 cells were seeded into 96-well plates at a
density of 3×103 and cultured overnight. The transfected
cells proliferation was measured using the Cell Counting Kit-8
(CCK-8) assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Briefly, 10 µl CCK-8 solution was added into each well and
the plates were incubated for another 2 h. The optical density was
then measured using a microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA) at an absorbance of 450 nm. Experiments were
performed in triplicate and repeated at least three times
independently.
Colony formation assay
A total of 24 h after transfection with miR-20a
siRNA or siRNA negative control, A549 cells were treated with 0.25%
trypsin plus 0.5 mM ethylenediaminetetraacetic acid solution at
37°C for 3 min and seeded in 6-well plates (500 cells per well) and
cultured for 2 weeks. Then, cell colonies were stained with 0.5%
crystal violet for 30 min at room temperature. The number of
colony-forming cells were calculated using an AID iSpot Reader
(Autoimmun Diagnostika GmbH, Strassberg, Germany). Three
independent experiments were performed.
Cell migration and invasion assay
Transwell chambers with 8-µm pores were obtained
from Corning Incorporated (Corning, NY, USA). For the migration
assay, the transfected A549 cells were harvested and resuspended in
100 µl serum-free medium and then transferred to the upper chambers
(2×104 cells per well). A total of 600 µl medium
supplemented with 10% FBS was added to the lower chamber. After
incubation for 24 h, cells that had migrated to the bottom chamber
were fixed with methanol for 30 min at room temperature, stained
with crystal violet for 30 min at 37°C, and then counted under a
light microscope. For the invasion assay, the Transwell membrane
was pre-coated with 30 µl Matrigel [1:3 mixed with phosphate
buffered saline (PBS); BD Biosciences, Franklin Lakes, NJ, USA] and
incubated for 48 h, with the remaining experimental procedures
identical to the migration assay.
Flow cytometry apoptosis assay
Following transfection, A-549 cells were seeded in
25 cm2 culture flasks at a density of
20×104/well in DMEM, and harvested when they reached 70%
confluence. Then, cells were stained with 5 µl Annexin V-FITC (BD
Biosciences) for 15 min and 5 µl propidium iodide (PI) for another
5 min at room temperature. Cell cycle was determined using a
FACScanto Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA) and the data was analyzed using CFlow Plus 1.0.264.15
software (BD Biosciences).
Western blot analysis
Following transfection, A-549 cells were harvested
and lysed using RIPA Lysis buffer (Beyotime Institute of
Biotechnology Co., Ltd., Haimen, China). Protein concentrations
were determined using a Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology, Beijing, China). Equal
amounts (40 µg) of protein were separated by 10% SDS-PAGE and
transferred onto a polyvinylidenedifluoride membrane with a pore
size of 0.45 µm (Millipore, Billerica, MA, USA). Following blocking
in 5% skimmed milk in Tris-buffered saline-Tween-20 (TBST) for 1 h
at room temperature, the membranes were incubated with the
following rabbit anti-human antibodies: Anti-EGR2 antibody (cat.
no. ab108399; Abcam, Cambridge, UK) and anti-β-actin antibody (cat.
no. ab16039; Abcam) at the recommended dilution (1:1,000) overnight
at 4°C. Subsequent to being washed with TBST, the membranes were
further incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (1:2,000; cat. no. ab150077;
Abcam) for 1 h. Enhanced Chemiluminescence Plus kit (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) was used to visualized the
protein bands according to manufacturer's protocol and the protein
expression was quantified using Laboratory Work Image Acquisition
and Analysis software version 4.0 (UVP, Inc., Upland, CA, USA).
β-actin was used as a loading control.
Dual luciferase reporter assay
To construct a luciferase reporter vector, the EGR2
3′-UTR fragment containing putative binding sites for miR-20a was
amplified using PCR and cloned in the psiCHECK-2 vector (Promega
Corporation). Site-directed mutagenesis of the miR-20a seed
sequence in the 3′-UTR of EGR2 (Mut) was performed using the
QuikChange™ Site-Directed Mutagenesis kit (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's protocol. Next, 1 µg of constructs were
co-transfected with 1 µg miR-20a precursor or control inA-549
cells. Transfection was performed using Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.). At 48 h after transfection,
luciferase activity was measured using the
Dual-Luciferase® Reporter Assay system (Promega
Corporation) following the manufacturer's instructions. Signals
were normalized using the Renilla luminescence of the
controls.
Animal work
Female nude (BALB/c-nu) mice (n=9, 4–5 weeks old,
17–20 g) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The mice were maintained at
a temperature of 18–22°C and a humidity of 50–60% under a 12:12 h
light-dark cycle. A total of 5×106 A549 cells in 0.2 ml
PBS were transfected with miR-20a siRNA and siRNA negative control.
The cells at exponential stage were harvested, and were then mixed
and injected into the left flanks of the mice. with free access to
food and water. Tumor size was measured by caliper every 2 days.
Both length (L) and width (W) of the tumor were measured and the
tumor size was calculated as ½LW2. After 20 days, the
mice were sacrificed and photographed. Tumors were dissected and
weighed. Three animals were included in each group.
Target prediction
Three publicly available databases, including
TargetScan (23), microRNA (24), miRDB (25), were used to predict the candidate
targets of miR-20a. The common gene, which was simultaneously
predicted by these three algorithms, was selected for further
analysis.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences between two groups were assessed using
Fisher's exact test or Student's t-test, while differences among
multiple groups were analyzed using one-way analysis of variance
followed by Bonferroni's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-20a is upregulated in patients
with NSCLC and in NSCLC cell lines
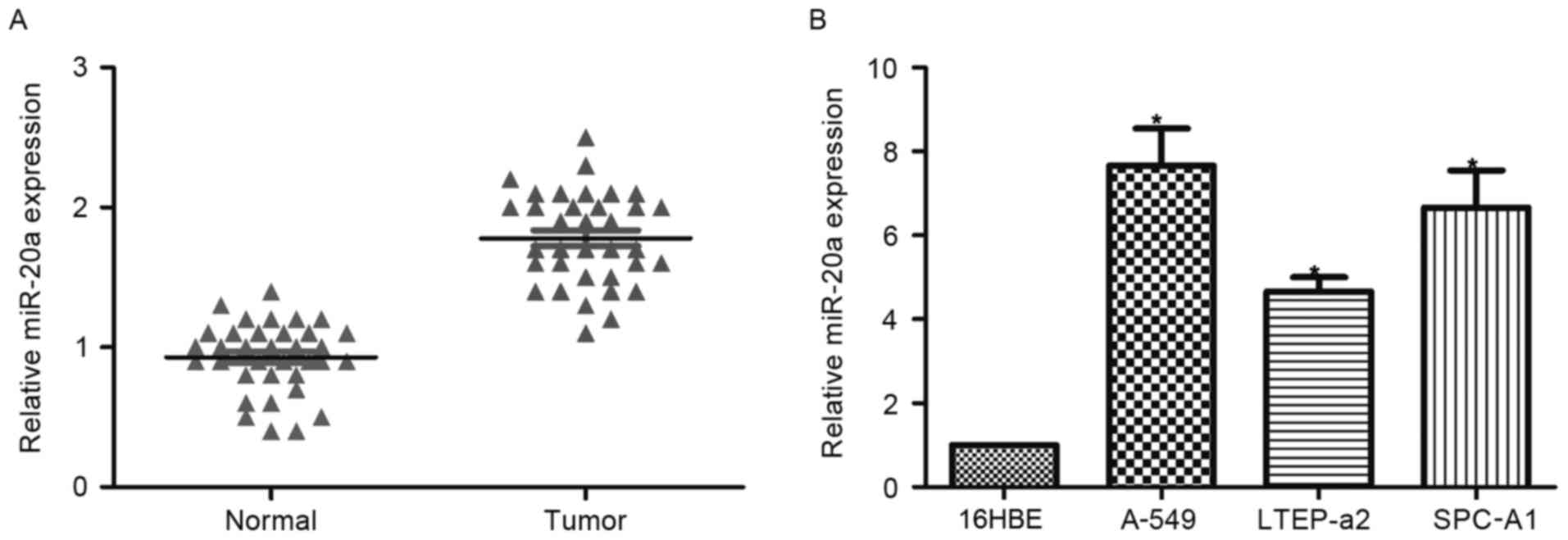
The expression of miR-20a was detected by RT-qPCR in
tumor and adjacent normal tissues of 35 patients. Expression of
miR-20a in tumor tissues was significantly higher than that in
normal tissues (Fig. 1A). Similarly,
all three tested NSCLC cell lines exhibited significantly
upregulated miR-20a levels compared with the normal control 16HBE
cell line. These data suggested that miR-20a may serve as a
prognostic marker for patients with NSCLC.
Levels of miR-20a in NSCLC cell lines
with knocked down miR-20a
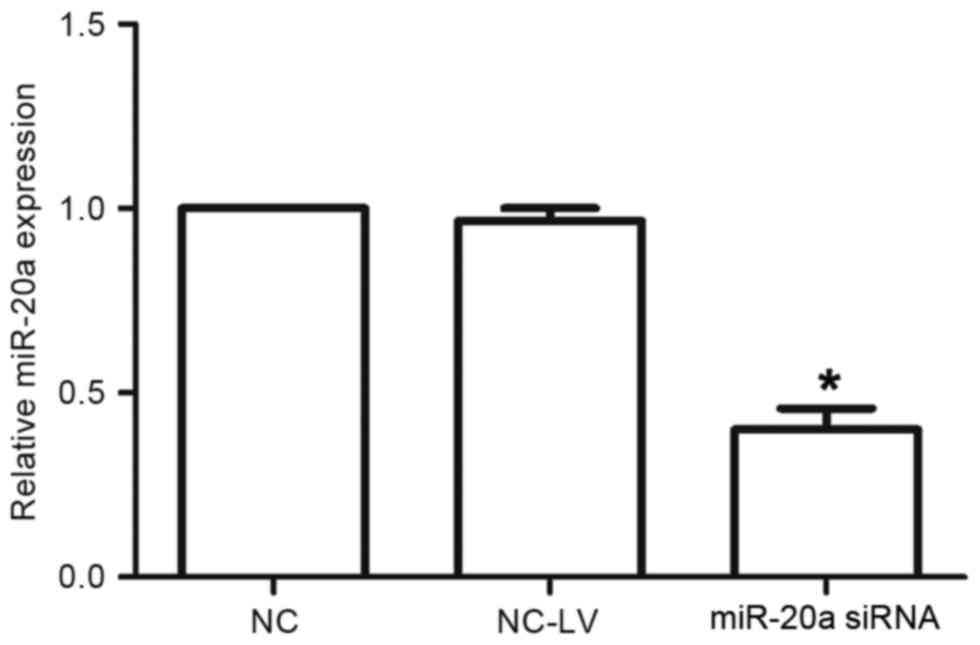
A-549 cells were transfected with miR-20a-inhibitor
or negative-control plasmids (termed miR-20a siRNA and NC-LV cells,
respectively). The expression levels of miR-20a were assessed by
RT-qPCR. The expression of miR-20a in the miR-20a siRNA cells was
decreased compared with that in the NC-LV cells (P<0.05;
Fig. 2).
miR-20a promotes growth and inhibits
apoptosis in NSCLC cells
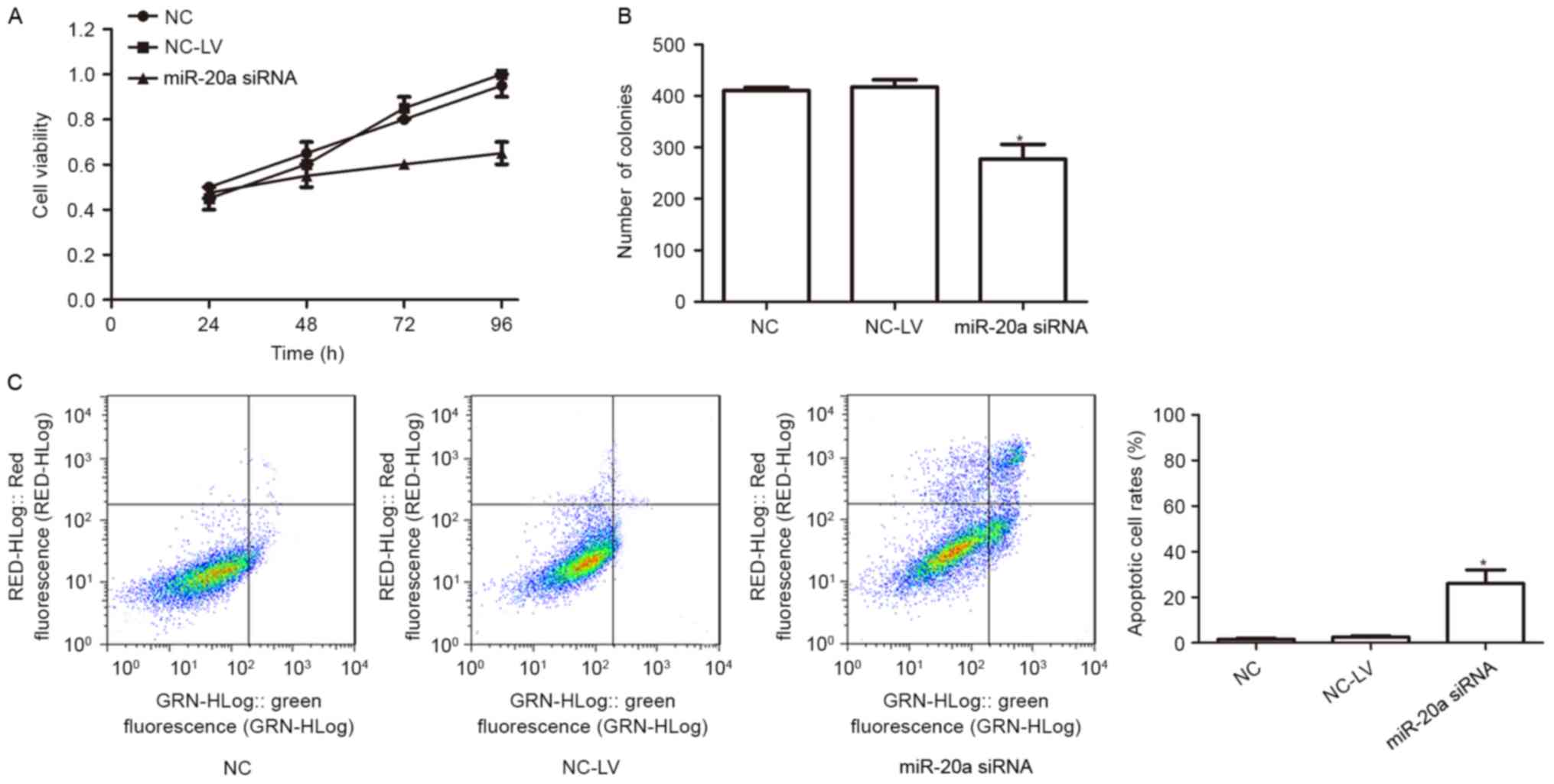
To assess the biological role of miR-20a in the
A-549 cell line, a series of experiments were performed. CCK8 was
used to determine the effect of miR-20a on A-549 miR-20a siRNA and
NC-LV cells. It was revealed that the proliferation of A-549
decreased significantly in the miR-20a siRNA group (Fig. 3A). To determine the long-term effect
of miR-20a on the growth of A-549 cells, a colony formation assay
was performed. Cells transfected with miR-20a inhibitor exhibited
lower colony formation than cells transfected with control
(Fig. 3B).
To further elucidate the mechanism of
miR-20a-mediated cell growth in A-549 cells, flow cytometry
analysis of apoptosis was performed. The results revealed that,
when compared with the control group, miR-20a-knockdown promoted
the apoptosis of A-549 (Fig. 3C).
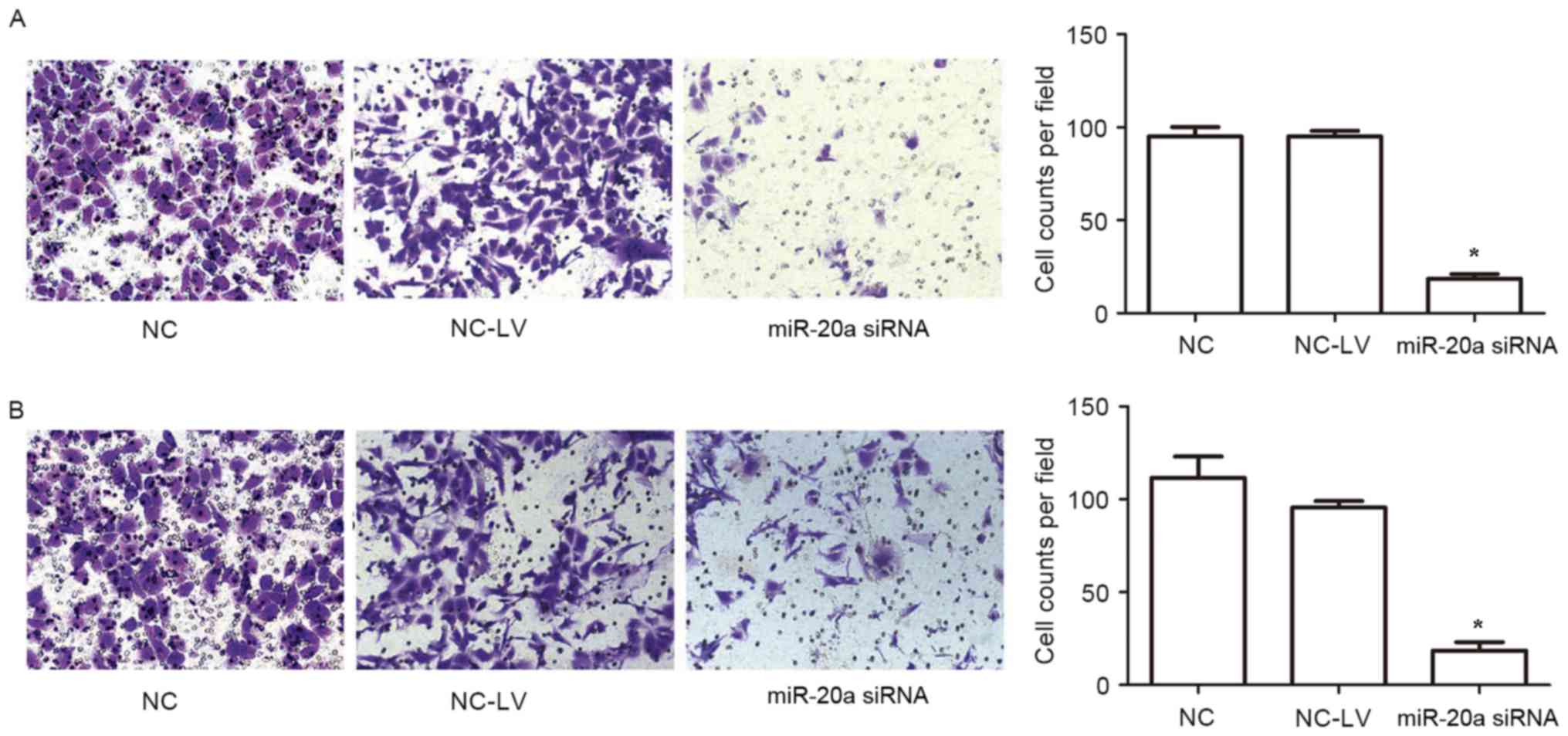
miR-20a promotes migration and
invasion of NSCLC cells in vitro
To further assess the effects of miR-20a on
malignant tumor progression and metastasis, migration and invasion
assays were used to determine the role served by miR-20a. As shown
in Fig. 4, knockdown of miR-20a
significantly suppressed the migration and invasion of A-549 cells.
Collectively, these results suggested that miR-20a increases the
migratory and invasive abilities of A-549 cells.
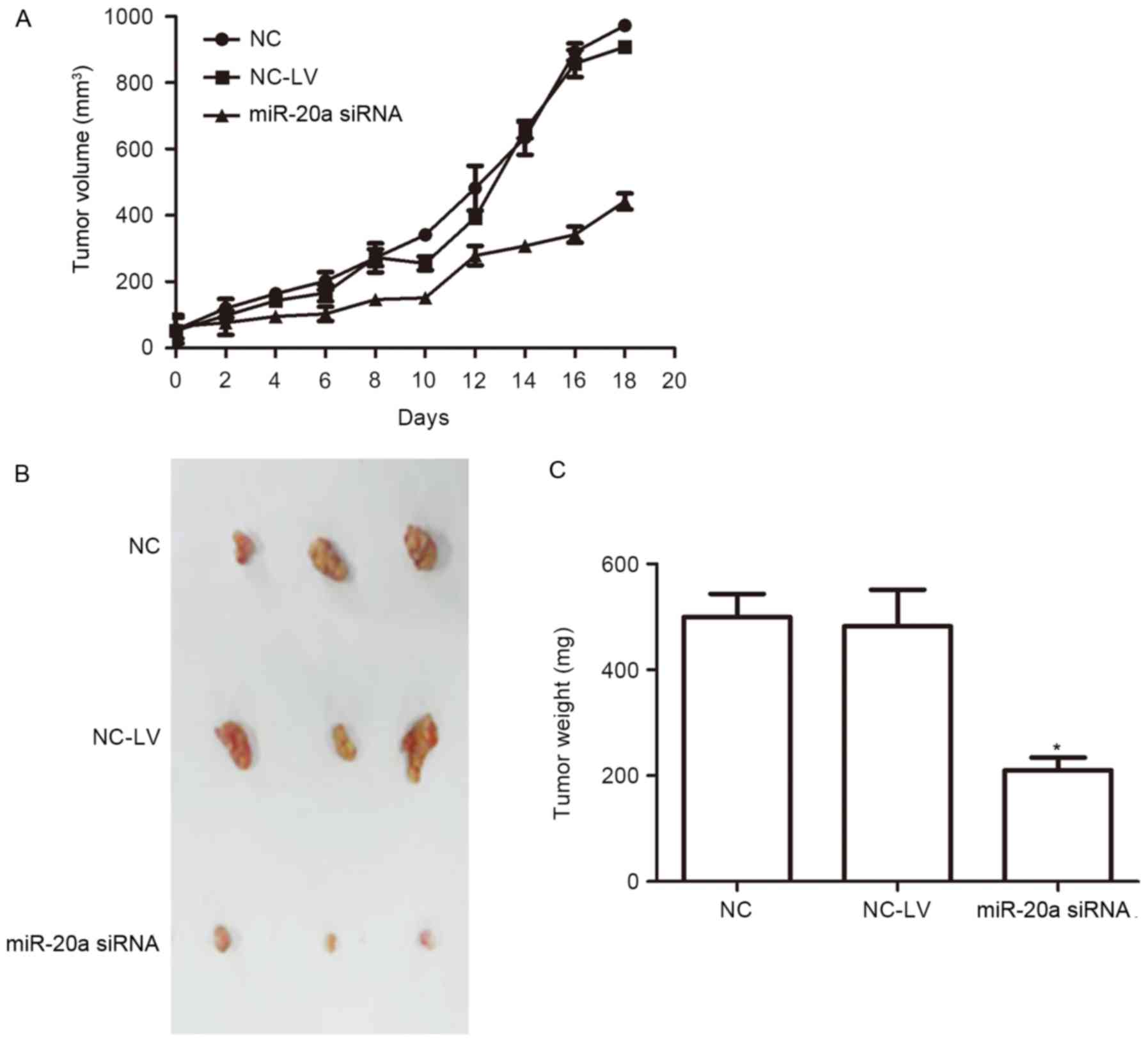
miR-20a promotes tumorigenicity in
vivo
To further demonstrate the role of miR-20a, A-549
cells infected with miR-20a inhibitor and inhibitor negative
control plasmids were injected into female nude mice. Tumors
derived from miR-20a siRNA A-549 cells grew much more slowly than
those of the NC group, and the tumor weight was also significantly
less than in the NC group (Fig.
5).
EGR2 is a direct target of
miR-20a
It is generally accepted that miRNAs regulate the
expression of mRNAs by targeting the mRNA 3′UTR (26). All of these algorithms (TargetScan,
microRNA, miRDB) were indicated that EGR2, a member of a multi-gene
family encoding zinc finger proteins that is involved in the
regulation of cellular proliferation and the cell cycle, was a
putative target of miR-20a. Thus, EGR2 was selected as a target for
further analysis (Fig. 6A).
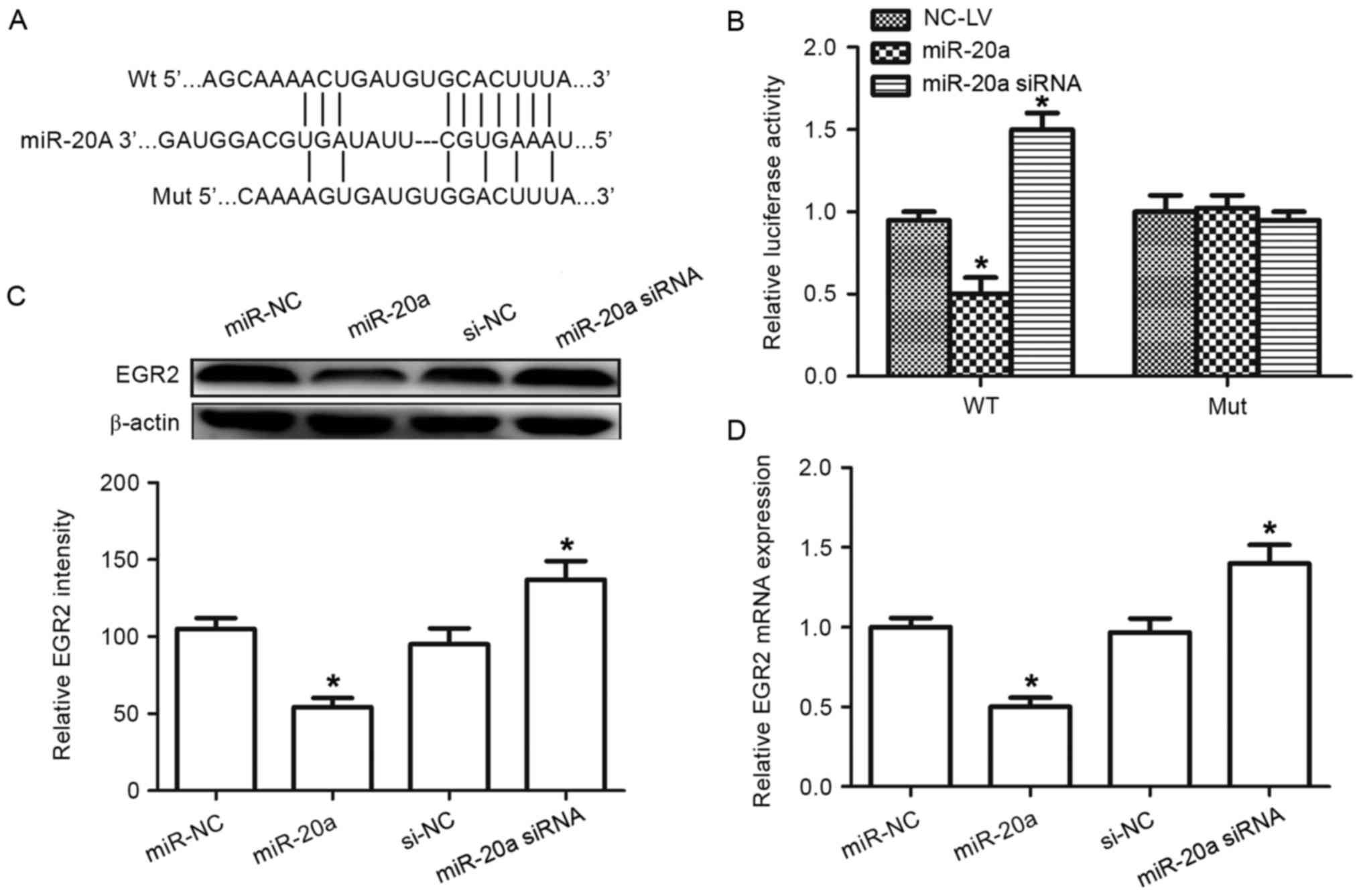 | Figure 6.Identification of EGR2 as a target of
miR-20a. (A) Wild-type and mutated sequences of the EGR2 3′-UTR
(nucleotides 458–465). (B) Luciferase activity was detected once
A-549 cells had been co-transfected with negative control, miR-20a
precursor or miR-20a siRNA with EGR2 3′-UTR fragment containing
either the miR-20a target sequence (WT) or a mutant. (C) Western
blot analysis was used to detect EGR2 protein level in cells
transfected with miR-20a precursor, siRNA or the corresponding
control. (D) Reverse transcription-quantitative polymerase chain
reaction was used to detect expression of EGR2 mRNA in cells
transfected with miR-20a precursor, siRNA or the corresponding
control. β-actin was used as an internal control. *P<0.05
compared with control. EGR2, early growth response 2; miR-20a,
microRNA-20a; WT, wild-type; Mut, mutant; siRNA, small interfering
RNA; 3′-UTR, 3′-untranslated region; NC, negative control. |
To investigate whether EGR2 was a functional target
of miR-20a, a dual-luciferase reporter assay was performed
(Fig. 6B). The EGR2 3′-UTR containing
the target sequences, or the mutants, was cloned into a luciferase
reporter vector. miR-20a suppressed the luciferase activity of the
wild-type EGR2 3′ UTR, whereas mutation of the miR-20a binding
sites blocked this suppression in the A-549 cells. Western blot
analysis demonstrated that EGR2 expression was inhibited following
transfection of miR-20a precursor in A-549 cells, whereas
transfection with the miR-20a inhibitor elevated EGR2 protein
levels (Fig. 6C). Similar results
were also observed in RT-qPCR analysis, in which the miR-20a
precursor decreasedEGR2 mRNA expression, whereas transfection with
the miR-20a inhibitor increased expression of EGR2 mRNA (Fig. 6D). Taken together, these results
indicate that miR-20a can directly target EGR2 in A-549 cells.
Discussion
Although numerous miRNAs are involved in NSCLC
carcinogenesis, their underlying molecular mechanisms in NSCLC
development remain poorly understood. Hence, it is of great value
to investigate the function of miRNAs involved in NSCLC
carcinogenesis and to screen for novel targets for diagnosis and
therapy. miR-20a and miR-17 (also known as miR-17-5p) are located
together in the miR-17-92 cluster, sharing the same seed sequence,
AAAGUG (14). Aberrant expression of
miR-20a has been observed in colorectal, cervical and prostate
cancer (17,27,28).
miR-20a overexpression has been observed to inhibit cellular
proliferation, invasion and tumor metastasis in certain cancer cell
lines (18,29), suggesting a tumor suppressor role.
However, certain studies have shown that miR-20a may promote
cellular proliferation in colon adenocarcinoma and glioma (30,31),
suggesting that it functions as an onco-miRNA. This difference
indicates that the effect of miR-20a dysregulation in different
types of cancer depends on the specific cell type and extracellular
factors. However, few reports exist concerning the potential
function of this miRNA in human NSCLC progression. The present
study is concerned with the role of miR-20a in the malignant
progression of NSCLC cells.
The present study detected miR-20a expression in 35
pairs of human NSCLC and matched normal tissues, as well as in
different cell lines, as determined by RT-qPCR. miR-20a was
frequently upregulated in tissue specimens and malignant cells.
Prior to the current study, however, the role of miR-20a and its
target genes in NSCLC was unclear. miR-20a may be a novel tumor
oncogene and its deregulation may be associated with the
progression of human NSCLC. Therefore, the functions and molecular
mechanisms of miR-20a in human NSCLC were the focal point of the
present study.
Previous studies have indicated that miR-20a is
associated with cellular proliferation (29) and apoptosis (32). In the present study, miR-20a was found
to be associated with cell growth, and its knockdown could inhibit
the growth of A-549 cells. A soft-agar colony formation assay
revealed that miR-20a could promote the growth of A-549 cells. An
invasion assay showed that miR-20a was associated with invasion
activity in NSCLC cells. To investigate the role of miR-20a in
NSCLC in vivo, anmiR-20a-knockdown model of NSCLC xenografts
in nude mice was generated. Growth curve results demonstrated that
miR-20a-knockdown can significantly suppress the growth of NSCLC
xenografts in nude mice.
To determine how miR-20a acts as an oncogene
further, potential targets were identified using bioinformatic
analytic tools. The tumor suppressor EGR2 was downregulated in
NSCLC. Luciferase activity assay suggested direct targeting of EGR2
by miR-20a. mRNA and protein levels of EGR2 were decreased in A-549
cells transfected with miR-20a, but were increased in
miR-20a-knockdown A-549 cells. These results indicated that EGR2 is
a target of miR-20a in NSCLC cells. EGR2 belongs to a multi-gene
family that encodes C2H2-type zinc-finger proteins (33). EGR2 is a key regulator of cellular
proliferation and the cell cycle (34). EGR2 is a target of the tumor protein
p53 family, and overexpression of EGR2 leads to apoptosis, whereas
downregulation of EGR2 attenuates p53-mediated apoptosis (35). EGR2 can be also regulated by other
miRNAs (36). The present study sheds
fresh light on the regulation of EGR2.
In conclusion, the present study demonstrated that
miR-20a expression is upregulated in NSCLC tissues and cells.
miR-20a promotes cell growth, migration and invasion by targeting
EGR2 in human NSCLC cells. These results suggest that miR-20a
serves a critical role in NSCLC growth by regulating EGR2 and
indicate that miR-20a could represent a biomarker and therapeutic
target in NSCLC therapy.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laskin JJ and Sandler AB: State of the art
in therapy for non-small cell lung cancer. Cancer Invest.
23:427–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferguson MK: Diagnosing and staging of
non-small cell lung cancer. Hematol Oncol Clin North Am.
4:1053–1068. 1990.PubMed/NCBI
|
|
5
|
Del Vescovo V, Grasso M, Barbareschi M and
Denti MA: MicroRNAs as lung cancer biomarkers. World J Clin Oncol.
5:604–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang T, Thakur A, Chen T, Yang L, Lei G,
Liang Y, Zhang S, Ren H and Chen M: MicroRNA-15a induces cell
apoptosis and inhibits metastasis by targeting BCL2L2 in non-small
cell lung cancer. Tumour Biol. 36:4357–4365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CZ and Lodish HF: MicroRNAs as
regulators of mammalian hematopoiesis. Semin Immunol. 17:155–165.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nature Rev Cancer. 6:857–866. 2006. View Article : Google Scholar
|
|
11
|
Li J, Wang Y, Song Y, Fu Z and Yu W:
miR-27a regulates cisplatin resistance and metastasis by targeting
RKIP in human lung adenocarcinoma cells. Mol Cancer. 13:1932014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A-549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan P, Yin Z, Li X, Wu W and Zhou B:
Meta-analysis of human lung cancer microRNA expression profiling
studies comparing cancer tissues with normal tissues. J Exp Clin
Cancer Res. 31:542012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuchida A, Ohno S, Wu W, Borjigin N,
Fujita K, Aoki T, Ueda S, Takanashi M and Kuroda M: miR-92 is a key
oncogenic component of the miR-17-92 cluster in colon cancer.
Cancer Sci. 102:2264–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Pan JH, Song B, Xiong EQ, Chen ZW,
Zhou ZS and Su YP: Suppression of CX43 expression by miR-20a in the
progression of human prostate cancer. Cancer Biol Ther. 13:890–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang CC, Yang YJ, Li YJ, Chen ST, Lin BR,
Wu TS, Lin SK, Kuo MY and Tan CT: MicroRNA-17/20a functions to
inhibit cell migration and can be used a prognostic marker in oral
squamous cell carcinoma. Oral Oncol. 49:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan MQ, Huang CB, Gu Y, Xiao Y, Sheng JX
and Zhong L: Decrease expression of microRNA-20a promotes cancer
cell proliferation and predicts poor survival of hepatocellular
carcinoma. J Exp Clin Cancer Res. 32:212013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia Y, Chen Q, Zhong Z, Xu C, Wu C, Liu B
and Chen Y: Down-regulation of miR-30c promotes the invasion of
non-small cell lung cancer by targeting MTA1. Cell Physiol Biochem.
32:476–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Zhang H, Zhao M, Lv Z, Zhang X,
Qin X, Wang H, Wang S, Su J, Lv X, et al: MiR-138 inhibits tumor
growth through repression of EZH2 in non-small cell lung cancer.
Cell Physiol Biochem. 31:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu N, Zhang C, Bai C, Han YP and Li Q:
MiR-4782-3p inhibited non-small cell lung cancer growth via USP14.
Cell Physiol Biochem. 33:457–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
ELife. 4:102015. View Article : Google Scholar
|
|
24
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36:D149–D153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43(Database issue): D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang Y, Liu C, Yang J, Liu G, Feng F,
Tang J, Hu L, Li L, Jiang F, Chen C, et al: MiR-20a triggers
metastasis of gallbladder carcinoma. J Hepatol. 59:518–527. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J, Liu R, Luo C, Zhou X, Xia K, Chen
X, Zhou M, Zou Q, Cao P and Cao K: MiR-20a inhibits cutaneous
squamous cell carcinoma metastasis and proliferation by directly
targeting LIMK1. Cancer Biol Ther. 15:1340–1349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malzkorn B, Wolter M, Liesenberg F,
Grzendowski M, Stühler K, Meyer HE and Reifenberger G:
Identification and functional characterization of microRNAs
involved in the malignant progression of gliomas. Brain Pathol.
20:539–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang G, Liu X, Wang W, Cai Y, Li S, Chen
Q, Liao M, Zhang M, Zeng G, Zhou B, et al: Down-regulation of
miR-20a-5p triggers cell apoptosis to facilitate mycobacterial
clearance through targeting JNK2 in human macrophages. Cell Cycle.
15:2527–2538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joseph LJ, le Beau MM, Jamieson GA Jr,
Acharya S, Shows TB, Rowley JD and Sukhatme VP: Molecular cloning,
sequencing and mapping of EGR2, a human early growth response gene
encoding a protein with ‘zinc-binding finger’ structure. Proc Natl
Acad Sci USA. 85:pp. 7164–7168. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dzialo-Hatton R, Milbrandt J, Hockett RD
Jr and Weaver CT: Differential expression of Fas ligand in Th1 and
Th2 cells is regulated by early growth response gene and NF-AT
family members. J Immunol. 166:4534–4542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Unoki M and Nakamura Y: Growth-suppressive
effects of BPOZ and EGR2, two genes involved in the PTEN signaling
pathway. Oncogene. 20:4457–4465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K,
Ren G, Su T, Pan Y, Feng B, et al: miR-150 promotes gastric cancer
proliferation by negatively regulating the pro-apoptotic gene EGR2.
Biochem Biophys Res Commun. 392:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|