Introduction
Epigenetic regulation includes histone modification
and DNA methylation, which are involved in the regulation of cell
growth and development in mammals (1,2). The
methylation of cytosine occurs via DNA methyltransferases (DNMTs),
which use S-adenosylmethionine as the donor for the methyl group.
In mammalian cells, DNMT genes are classified into de novo
(DNMT3A and DNMT3B) and maintenance (DNMT1) methyltransferases;
these genes serve various functions in setting the methylation maps
of the mammalian genome (3). During
carcinogenesis, the DNA methylation level gradually decreases in
the DNA repetitive region, leading to genomic DNA instability
(4–7).
In general, high methylation of a gene promoter is associated with
gene silencing. Numerous tumor suppressors have been identified
with a hypermethylated promoter that suppresses their transcription
potential in multiple types of human cancer (8–11).
Therefore, establishing and maintaining DNA methylation status is
essential in human types of cancer.
Previous studies have reported that a sixth base,
5-hydroxymethylcytosine (5hmC), is present in a number of tissues,
including the muscle, lung, kidney and heart, and is highly
expressed in the brain and embryonic cells (2,12–15). Furthermore, 5hmC is generated by the
oxidation of 5-methylcytosine (5mC) by ten-eleven translocation
protein (TET), and 5hmC serves critical roles in various tissues
(2,13,15–22).
Furthermore, 5hmC has been suggested to serve a crucial role in
gene regulation via the demethylation process (2,13,15–22). The
two following theories suggest the involvement of 5hmC in the DNA
demethylation process: i) Passive DNA demethylation, where DNMT
protein does not recognize the 5hmC-rich region, thus preventing
maintenance methylation during DNA replication; ii) active DNA
demethylation, where the 5hmC-rich region is recognized by DNA
glycosylase protein, which converts 5hmC to cytosine (2,13,15–22).
Previous studies have reported that hyperhydroxymethylation of the
promoter region of metalloproteinase and homeobox A9 genes
increases the expression levels of these genes (3,23).
Therefore, high 5hmC expression levels in the promoter region may
activate gene transcription via the promotion of DNA demethylation
(2,24).
Although 5mC is the upstream substrate for
generating 5hmC via the catalysis of TET proteins, the expression
level and distribution of 5hmC is not associated with that of 5mC
in human types of cancer (25).
Therefore, global DNA hypomethylation in human types of cancer only
partially explain the low 5hmC expression levels in human tumors.
Multiple previous studies have indicated that the dysfunction of
isocitrate dehydrogenase [NAPD(+))1/2, activation-induced deaminase
and TET genes are associated with aberrant expression of 5hmC in
human cancer cells (16,26–28).
However, the mechanism underlying the depletion of 5hmC expression
levels in gastric cancer cells remains unclear. The present study
investigated the status of DNA modification and TET1 and TET2
protein expression levels in gastric cancer tissue, and provided
another potential explanation for 5hmC expression level depletion
in gastric cancer.
Materials and methods
Clinical samples and DNA/RNA
extraction
A total of 16 gastric cancer and corresponding
adjacent normal tissue samples were collected from patients with
gastric cancer who underwent surgery at the Department of Surgery,
Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan) between
July 2013 and August 2014. They did not receive any treatment prior
to surgery. These patients included 10 males and 6 females, whose
age ranged between 55 and 70 years old. The study protocol was
independently reviewed and approved by the Institutional Review
Board of Kaohsiung Veterans General Hospital (approval no.
VGHKS12-CT3-10). The methods performed were in accordance with
approved guidelines, and written informed consent was obtained from
all patients prior to enrolment in the present study.
Immunohistochemical (IHC) analysis and
scoring
Human gastric carcinoma tissue microarrays,
including adjacent normal tissues (cat. no. IMH-341) and tumors
(cat. no. IMH-316) from 59 patients with gastric cancer were
obtained from Imgenex; Novus Biologicals, LLC (Littleton, CO, USA).
IHC analyses were performed using the Novolink Max Polymer
Detection System (Leica Microsystems, Ltd., Milton Keynes, UK). The
tissue slides were sectioned at a thickness of 5 µm, deparaffinized
in xylene and rehydrated in 100, 95 and 75% ethanol for 3 min each.
Antigen retrieval was performed by immersing the slides in
Tris-EDTA (10 mM; pH 9.0) for 10 min at 125°C in a pressure boiler.
Endogenous peroxidase activity was blocked by incubating the slides
for 30 min with 3% hydrogen peroxide in methanol. The slides were
then blocked with protein block buffer (0.4% casein in PBS, with
stabilizers, surfactant and 0.2% bronidox L as a preservative).
Following blocking at room temperature (RT) for 30 min, primary
antibodies were immediately applied and the slides were incubated
overnight at 4°C in a wet chamber. The primary antibodies used were
mouse monoclonal anti-5mC (1:500; cat. no. ab10805; Abcam,
Cambridge, UK); rabbit polyclonal anti-5hmC (1:1,000; cat. no.
39769; Active Motif, Carlsbad, CA, USA), rabbit polyclonal
anti-TET1 (1:150; cat. no. TA309902; OriGene Technologies, Inc.,
Rockville, MD, USA) and goat polyclonal anti-TET2 (1:50; cat. no.
OAEB00839; AVIVA Systems Biology, San Diego, CA, USA) in primary
antibody diluent (ScyTek Laboratories, Logan, UT, USA). Following
washing with PBS, the slides were incubated with a rabbit
anti-mouse poly-horseradish peroxidase (HRP)-immunoglobulin (Ig)G
(cat. no. RE7159; Leica Microsystems, Ltd., Milton Keynes, UK) and
goat anti-rabbit poly-HRP-IgG (cat. no. RE7161; Leica Microsystems,
Ltd., Milton Keynes, UK) for 10 min at RT. Finally, the color was
developed using a 0.03% diaminobenzidine solution (ScyTek
Laboratories) for 2 min at room temperature. Subsequently the
tissue sections were counterstained with hematoxylin for 10 min at
room temperature.
The expression scores of individual candidates for
nuclear or cytoplasmic staining were determined on the basis of
staining intensity (0, no signal; 1, mild; 2, moderate; 3, strong).
The proportion of positively stained tumor cells (scored as 0–100%)
was evaluated in the whole field of each core. The score of
individual candidates was calculated using the following formula:
Intensity × percentage of positively stained cells.
Gene expression data
The microarray data of TET1 and TET2 in 311 gastric
cancer tissues and 57 adjacent normal tissues were obtained from
the Gene Expression Across Normal and Tumor Tissue (GENT) database
(http://medicalgenome.kribb.re.kr/GENT/search/search.php)
(29).
Western blot analysis
The tissue samples were lysed using a lysis buffer
(50 mM Tris-HCl at pH 8.0, 150 mM NaCl, 1% NP-40, 0.02% sodium
azide, 1 µg/ml aproteinin and 1 mM PMSF) at 4°C for 30 min. The
lysates were collected and centrifuged at 16,000 × g at 4°C for 10
min to remove cell debris. Protein assays were performed using the
Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) based on the Bradford dye-binding procedure (30). Protein samples (60 µg) were separated
by 8–10% SDS-PAGE. The separated proteins were then
electrotransferred onto nitrocellulose membranes (GE Healthcare,
Chicago, IL, USA). Following blocking overnight at 4°C using 0.1%
of Tween in PBS supplemented with 5% skimmed milk, the membranes
were incubated with mouse monoclonal anti-TET1 (1:500; cat. no.
GTX627420; GeneTex Inc., Irvine, CA, USA), mouse monoclonal
anti-green fluorescent protein (GFP; 1:2,000; cat. no. sc-9996;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse
monoclonal anti-actin (1:3,000; cat. no. MAB1501; EMD Millipore,
Billerica, MA, USA) for 1 h in PBS-Tween supplemented with 5%
skimmed milk at room temperature. The membranes were then incubated
with HRP-conjugated goat-anti-rabbit-IgG (1:5,000; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or
goat-anti-mouse IgG secondary antibodies (1:5,000; cat. no.
sc-2005; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h
at RT. Following washing three times with PBS-Tween, immunoreactive
bands were detected using an enhanced chemiluminescence kit (cat.
no. K-12045-D50; Advansta, Inc., Menlo Park, CA, USA).
Gastric cancer cell lines and
transfection assay
Human gastric cancer AGS cells were obtained from
American Type Culture Collection (Manassas, VA, USA), and cultured
in Dulbecco's modified Eagle's medium (Biological Industries Israel
Beit-Haemek Ltd., Kibbutz Beit-Haemek, Israel) supplemented with
10% fetal bovine serum (GE Healthcare Life Sciences, Logan, UT,
USA) and 1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA). In
the present study, transfection assay was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
DNA dot-blot assay
DNA samples were added to the denaturation buffer
(0.4 mM NaOH and 10 mM EDTA) and denatured at 100°C for 10 min.
Furthermore, the samples were chilled on ice for 5 min and applied
on a positive-charged nylon membrane (Roche, Basel, Switzerland).
The membrane was UV cross-linked and dried for 1 h at 70°C. The
membranes were probed with rabbit polyclonal anti-5hmC (1:5,000;
cat. no. 39769; Active Motif, Carlsbad, CA, USA) at 4°C overnight.
The membranes were then incubated with HRP-conjugated goat
anti-rabbit IgG (1:5,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h at RT. The target bands were
visualized using WesternBright enhanced chemiluminescence reagent
(Advansta, Inc., Menlo Park, CA, USA) and the results of the
immunoreactions were analyzed with a BioSpectrum® 500
Imaging System (Ultra-Violet Products Ltd., Cambridge, UK). The
membranes were stained with methylene blue (Sigma-Aldrich; Merck
KGaA) as a loading control.
Predict nuclear export signals (NESs)
by bioinformatic approach
A useful web server (NetNES 1.1) for predicting
potential leucine-rich NESs within protein sequences is available
at http://www.cbs.dtu.dk/services/NetNES/ (31). The TET1 protein sequences
(NP_085128.2) were downloaded from The National Center for
Biotechnology Information databases (NCBI; https://www.ncbi.nlm.nih.gov/). TET1 protein sequences
in FASTA format was uploaded to NetNES 1.1 website. According to
the characteristics and the homology of the NESs (Nuclear export
signals), NetNES 1.1 could predict putative NESs within TET1
protein.
In vitro DNA demethylation assay
pEGFP plasmids (Clontech, Mountain View, CA, USA)
were completely methylated in vitro using M.SssI
methylase enzymes (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Subsequently, methylated reporter vectors were
co-transfected with TET1-FL or TET1-delNLS into AGS gastric cancer
cells. Following transfection for 24–48 h, demethylation activity
in cells was examined by western blotting as aforementioned.
Statistical analysis
The expression levels of 5mC and 5hmC in paired
gastric cancer were analyzed using a paired Student's t-test. The
expression levels of TET1 and TET2 from the TCGA database and the
association between various localizations of TET1 protein and 5hmC
expression levels in human gastric cancer was analyzed using
Student's t-tests. The TET1 activity assays were performed in
triplicate. The intensity of GFP was quantified using ImageJ
Software 1.45s (National Institutes of Health, Bethesda, MD, USA)
and presented graphically. The data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference and P<0.01 was considered
to indicate a highly significant difference.
Results
5hmC expression level depletion in
gastric cancer cells
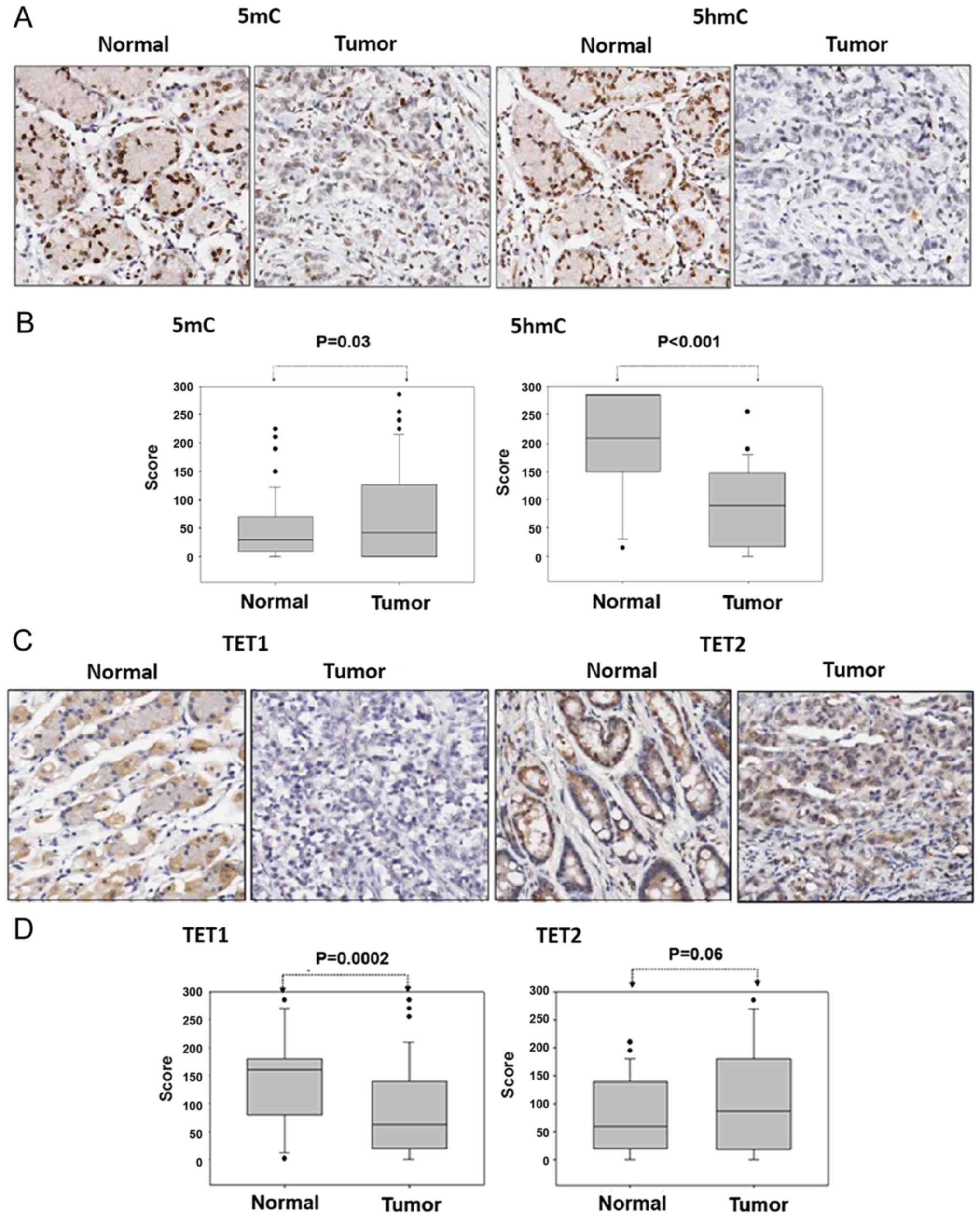
The IHC staining revealed that compared with the
corresponding adjacent normal tissues, the expression level of 5mC
was slightly increased and that of 5hmC was significantly depleted
in gastric cancer tissues (5mC, P=0.03 and 5hmC, P<0.001;
Fig. 1A and B). These results
indicated that 5hmC expression level depletion was not due to low
5mC expression levels in gastric cancer tissues. The potential
mechanism underlying 5hmC expression level depletion may therefore
be the dysfunction of metabolic enzymes involved in the DNA
demethylation signaling pathway.
Expression levels of TET1/2 in gastric
cancer cells
The present study investigated the expression levels
of TET1 and TET2 in a gastric cancer tissue array (n=59 cases)
using IHC. TET1 protein expression was markedly decreased and TET2
protein expression was slightly increased in the gastric cancer
tissues compared with the adjacent normal tissues (TET1, P=0.0002
and TET2, P=0.06; Fig. 1C and D).
This result was further confirmed by mRNA and protein expression
level analysis using the GENT database and western blotting,
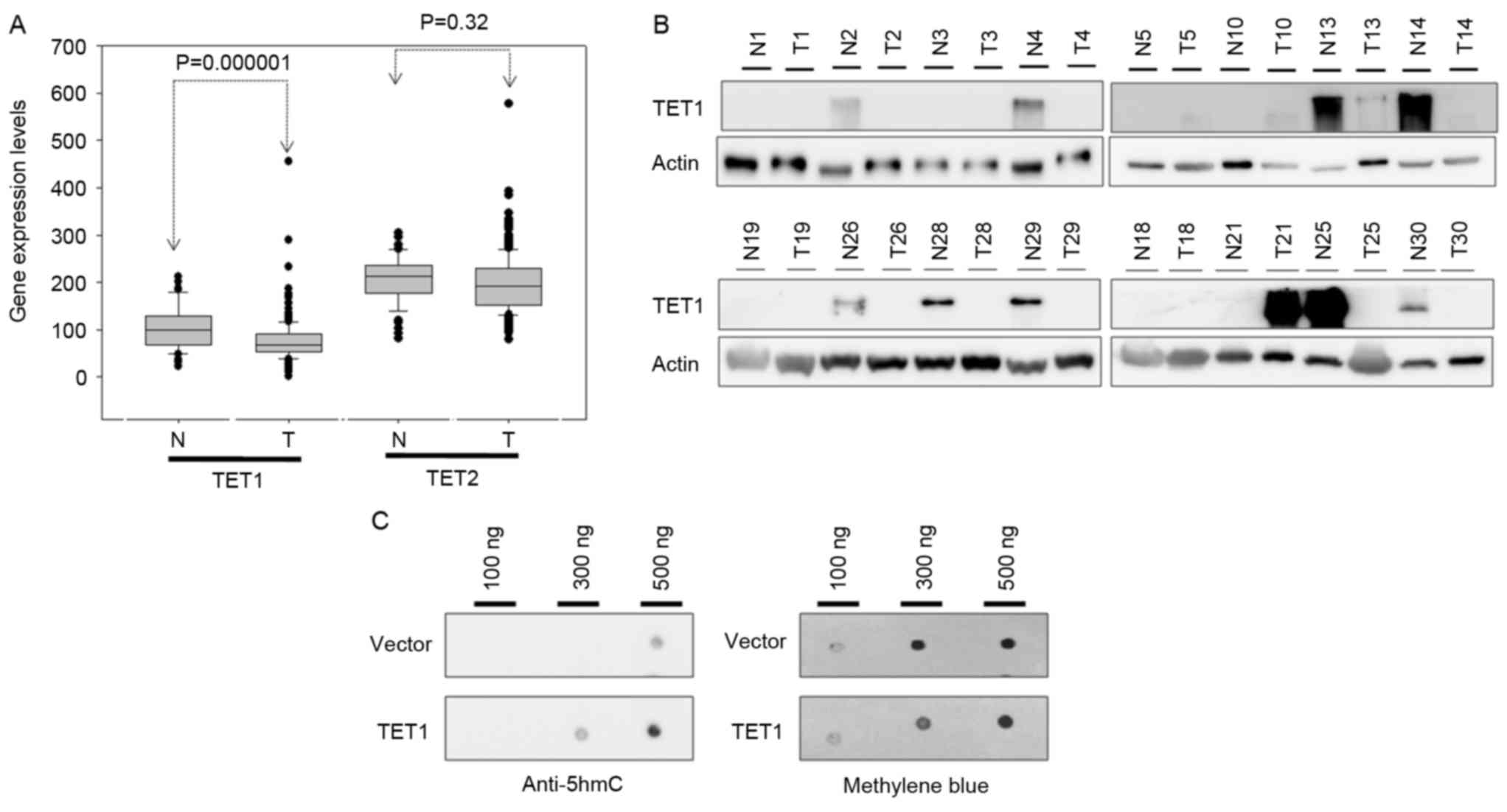
respectively. By analyzing the GENT database, the present study
revealed that the transcriptional expression levels of TET1 were
substantially decreased in the gastric cancer tissues compared with
normal adjacent tissues (P<0.001; Fig.
2A). This result was consistent with that of IHC analyses.
Furthermore, as presented in Fig. 2B,
compared with the corresponding adjacent normal tissues, the
protein expression levels of TET1 were frequently decreased in the
tumor tissues (in 8 out of 16 cases). The transfection of TET1 into
AGS cells for 24 h resulted in an increase in 5hmC expression
levels (Fig. 2C). These results
indicated that the decrease in 5hmC expression levels in gastric
cancer may be induced by low TET1 protein expression levels.
Nuclear exclusion of TET1 is
associated with 5hmC expression level depletion in gastric
cancer
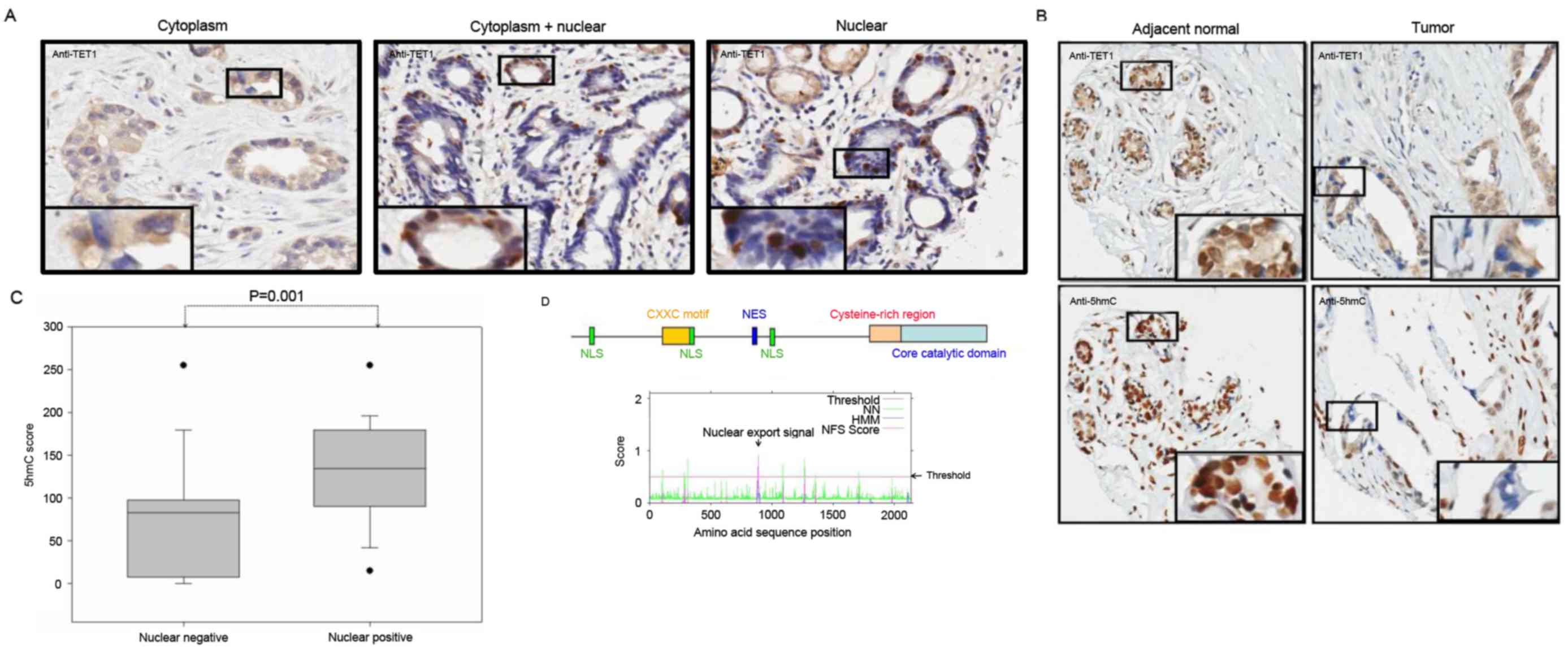
The present study not only observed the depletion of
TET1 protein expression levels in gastric cancer tissues, but also
observed marked differences in its subcellular localization. As
presented in Fig. 3A, TET1 protein
was detected in three subcellular localizations in the gastric
cancer tissues: Cytoplasm (left panel); cytoplasm and nucleus
(middle panel); nuclear accumulation (right panel). Furthermore,
the present study detected TET1 expression level in gastric cancer
tissue samples, except in 3 samples where no TET1 protein
expression was observed. Of the 59 tumor samples, the protein
expression level was restricted to the cytoplasm without nuclear
staining in 36 (61%) samples, in the cytoplasm and nucleus in 9
(15.3%) samples and predominantly in the nucleus in 11 (18.6%)
samples. Furthermore, as presented in Fig. 3B, TET1 nuclear localization was
associated with 5hmC expression levels. The comparison of the 5hmC
expression levels with TET1 cellular localization revealed that
TET1 nuclear staining had a significant association with high 5hmC
expression levels (P=0.001) in gastric cancer (Fig. 3C). These results implied that TET1
protein may shuttle between the nucleus and the cytoplasm. Using
bioinformatics, the present study identified three nuclear
localization signals (NLS; NLS1:aa20-50, NLS2:aa620-653 and
NLS3:1158-1162) and one nuclear export signal (NES; aa877-889) in
the N-terminal region of TET1 protein (Fig. 3D). However, this result requires
further confirmation by future studies.
TET-1 localization may contribute to
the active DNA demethylation signaling pathway
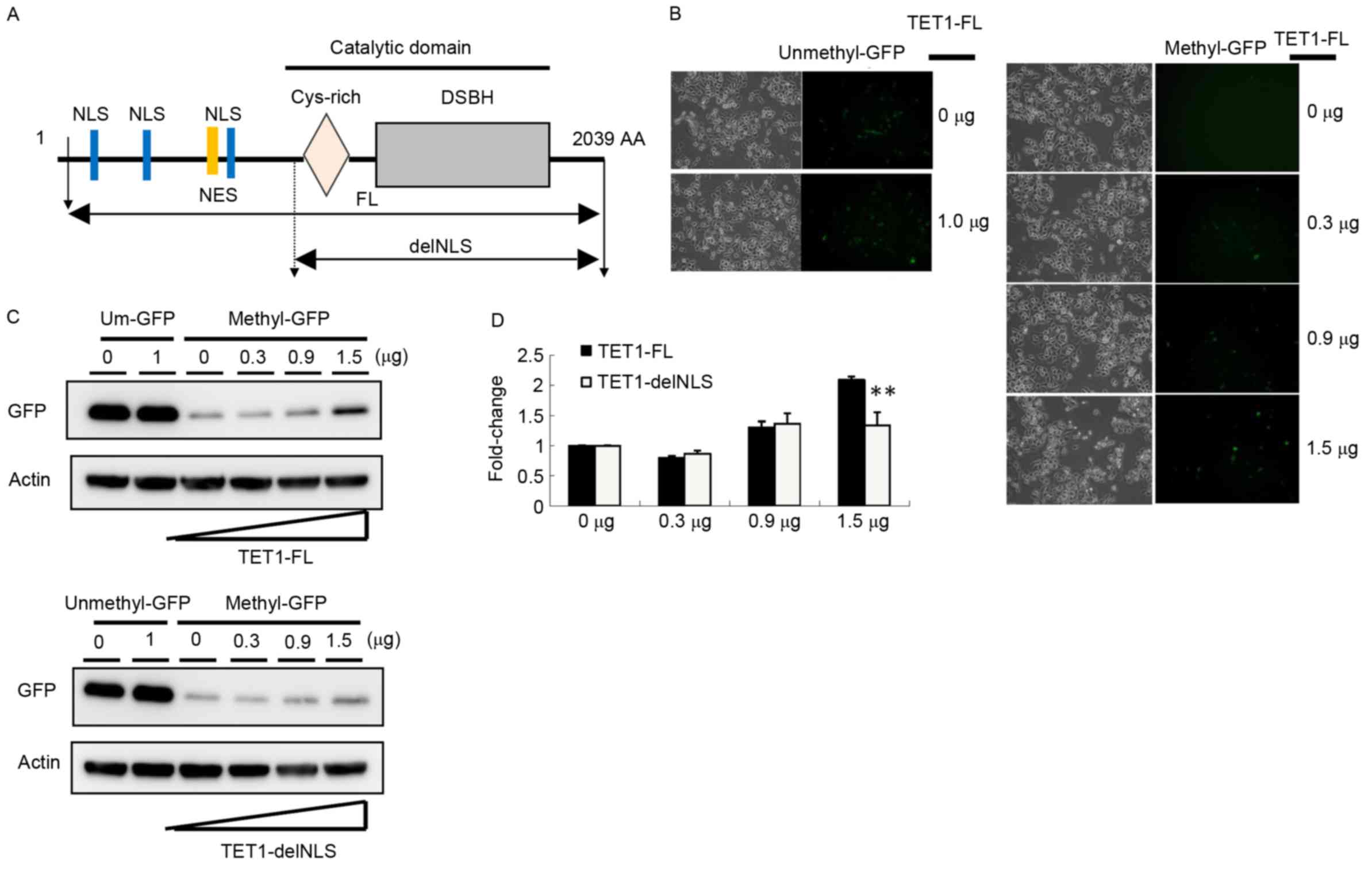
TET1 nuclear exclusion was associated with 5hmC
depletion in gastric cancer tissues according to the IHC data, so
the present study evaluated whether different cellular
localizations of TET1 protein affected its demethylation activity
by constructing two TET1 expression vectors, TET1-FL and
TET1-delNLS. TET1 contains a critical catalytic domain and three
NLSs, whereas TET1-delNLS contained only a catalytic domain
(Fig. 4A). A reporter assay was used
to assess DNA demethylation activity. The GFP plasmid was
methylated in vitro with CpG methyltransferase
(M.SssI). Furthermore, the completely methylated pEGFP
plasmids were co-transfected into AGS cells with TET1-FL or
TET1-delNLS. As presented in Fig. 4B,
GFP expression was completely silenced in AGS cells transfected
with the methylated GFP expression vector. Following
co-transfection with the TET1-FL expression vector for 24 h, the
number of GFP-positive cells markedly increased (Fig. 4B). By performing western blot
analysis, the present study observed that the GFP expression level
increased 2.2-fold following transfection with 1.5 µg TET1
(Fig. 4C and D). Compared with cells
transfected with TET1-delNLS, the GFP expression level was slightly
increased in cells transfected with 1.5 µg of TET1-delNLS
(~1.3-fold). These results indicated that the nuclear localization
of TET1 may serve a crucial role in the modulation of gene
expression, particularly in gastric cancer.
Discussion
Previous studies have reported that 5hmC expression
levels are significantly lower in gastric cancer tissues compared
with in adjacent normal tissues (32–34). In
addition, Yang et al (33)
reported that a low 5hmC expression level is an independent factor
for poor prognosis in patients with gastric cancer. They also
reported that a low 5hmC expression level was associated with the
majority of recorded clinicopathological characteristics, including
the tumor size, stage, lymph node metastasis and overall survival
rate. These results indicated that the mechanism underlying 5hmC
depletion served a crucial role in gastric cancer progression. The
results of the present study revealed that the potential mechanism
underlying 5hmC expression level depletion in gastric cancer cells
may be a low TET1 protein expression level. Furthermore, Yang et
al (33) reported a strong
association between the decrease in 5hmC expression levels and
decrease in TET1 mRNA expression levels; however, this link was not
observed for TET2 and TET3 proteins. Frycz et al (35) demonstrated that TET1 transcript and
protein expression levels were associated with the metastasis stage
in patients with gastric cancer. The results of the present study
revealed that TET1 transcript and protein expression levels were
significantly decreased in gastric cancer tissues compared with
adjacent normal tissues. In addition, the present study observed a
marked difference in subcellular localization. The localization of
TET1 protein expression level in the nucleus was associated with
5hmC expression levels and contributed to the active demethylation
process. Our previous study reported a similar phenomenon and
revealed that the cytoplasmic mislocalization of TET1 reduced the
expression level of 5hmC in breast cancer (25). A low 5hmC expression level is an
effective independent prognostic biomarker for breast ductal
carcinoma, particularly in patients with an endocrine
receptor/progesterone receptor-negative subtype (25). Similarly, Müller et al
(36) reported that a decrease in
5hmC expression levels is attributable to the nuclear exclusion of
TET1 from the nuclei of glioma cells. Using the bioinformatics
approach, the present study identified three NLSs and one NES in
the N-terminal region of TET1 protein (Fig. 3D). This result suggested that TET1
protein shuttles between the nucleus and the cytoplasm. In
conclusion, these results revealed that the nuclear-to-cytoplasm
shuttling of TET proteins in tumor cells may be a critical event
contributing to human cancer progression.
Acknowledgements
The present study was supported by the Kaohsiung
Veterans General Hospital (grant no. VGHKS-105-016), the Zuoying
Branch of Kaohsiung Armed Forces General Hospital (grant no.
ZBH-103-27) and Yen Tjing Ling Medical Foundation (grant nos.
CI-102-13 and CI-103-18).
References
|
1
|
Esteller M: Cancer epigenetics for the
21st Century: What's next? Genes Cancer. 2:604–606. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dahl C, Grønbæk K and Guldberg P: Advances
in DNA methylation: 5-hydroxymethylcytosine revisited. Clin Chim
Acta. 412:831–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun M, Song CX, Huang H, Frankenberger CA,
Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C and Rosner
MR: HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer
growth and metastasis. Proc Natl Acad Sci USA. 110:pp. 9920–9925.
2013; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim B, Kim JH, Kim M and Kim SY: Genomic
and epigenomic heterogeneity in molecular subtypes of gastric
cancer. World J Gastroenterol. 22:1190–1201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto H and Imai K: Microsatellite
instability: An update. Arch Toxicol. 89:899–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KJ, Lee TH, Cho NY, Yang HK, Kim WH
and Kang GH: Differential clinicopathologic features in
microsatellite-unstable gastric cancers with and without MLH1
methylation. Hum Pathol. 44:1055–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling ZQ, Tanaka A, Li P, Nakayama T,
Fujiyama Y, Hattori T and Sugihara H: Microsatellite instability
with promoter methylation and silencing of hMLH1 can regionally
occur during progression of gastric carcinoma. Cancer Lett.
297:244–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai KW, Hu LY, Wu CW, Li SC, Lai CH, Kao
HW, Fang WL and Lin WC: Epigenetic regulation of miR-196b
expression in gastric cancer. Genes Chromosomes Cancer. 49:969–980.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai KW, Liao YL, Wu CW, Hu LY, Li SC,
Chan WC, Ho MR, Lai CH, Kao HW, Fang WL, et al: Aberrant
hypermethylation of miR-9 genes in gastric cancer. Epigenetics.
6:1189–1197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai
CH, Kao HW, Fang WL, Huang KH, Chan WC and Lin WC: Epigenetic
regulation of miR-34b and miR-129 expression in gastric cancer. Int
J Cancer. 129:2600–2610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ehrlich M, Gama-Sosa MA, Huang LH, Midgett
RM, Kuo KC, McCune RA and Gehrke C: Amount and distribution of
5-methylcytosine in human DNA from different types of tissues of
cells. Nucleic Acids Res. 10:2709–2721. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin SG, Wu X, Li AX and Pfeifer GP:
Genomic mapping of 5-hydroxymethylcytosine in the human brain.
Nucleic Acids Res. 39:5015–5024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koh KP, Yabuuchi A, Rao S, Huang Y,
Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky
G, et al: Tet1 and Tet2 regulate 5-hydroxymethylcytosine production
and cell lineage specification in mouse embryonic stem cells. Cell
Stem Cell. 8:200–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pastor WA, Pape UJ, Huang Y, Henderson HR,
Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P,
et al: Genome-wide mapping of 5-hydroxymethylcytosine in embryonic
stem cells. Nature. 473:394–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cimmino L, Abdel-Wahab O, Levine RL and
Aifantis I: TET family proteins and their role in stem cell
differentiation and transformation. Cell Stem Cell. 9:193–204.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ficz G, Branco MR, Seisenberger S, Santos
F, Krueger F, Hore TA, Marques CJ, Andrews S and Reik W: Dynamic
regulation of 5-hydroxymethylcytosine in mouse ES cells and during
differentiation. Nature. 473:398–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W,
Xie ZG, Shi L, He X, Jin SG, et al: The role of Tet3 DNA
dioxygenase in epigenetic reprogramming by oocytes. Nature.
477:606–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo JU, Su Y, Zhong C, Ming GL and Song H:
Emerging roles of TET proteins and 5-hydroxymethylcytosines in
active DNA demethylation and beyond. Cell Cycle. 10:2662–2668.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q,
Ding J, Jia Y, Chen Z, Li L, et al: Tet-mediated formation of
5-carboxylcytosine and its excision by TDG in mammalian DNA.
Science. 333:1303–1307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito S, D'Alessio AC, Taranova OV, Hong K,
Sowers LC and Zhang Y: Role of Tet proteins in 5mC to 5hmC
conversion, ES-cell self-renewal and inner cell mass specification.
Nature. 466:1129–1133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito S, Shen L, Dai Q, Wu SC, Collins LB,
Swenberg JA, He C and Zhang Y: Tet proteins can convert
5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine.
Science. 333:1300–1303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu CH, Peng KL, Kang ML, Chen YR, Yang
YC, Tsai CH, Chu CS, Jeng YM, Chen YT, Lin FM, et al: TET1
suppresses cancer invasion by activating the tissue inhibitors of
metalloproteinases. Cell Rep. 2:568–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams K, Christensen J, Pedersen MT,
Johansen JV, Cloos PA, Rappsilber J and Helin K: TET1 and
hydroxymethylcytosine in transcription and DNA methylation
fidelity. Nature. 473:343–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai KW, Li GC, Chen CH, Yeh MH, Huang JS,
Tseng HH, Fu TY, Liou HH, Pan HW, Huang SF, et al: Reduction of
global 5-hydroxymethylcytosine is a poor prognostic factor in
breast cancer patients, especially for an ER/PR-negative subtype.
Breast Cancer Res Treat. 153:219–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kinney SR and Pradhan S: Ten eleven
translocation enzymes and 5-hydroxymethylation in mammalian
development and cancer. Adv Exp Med Biol. 754:57–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pérez C, Martínez-Calle N, Martín-Subero
JI, Segura V, Delabesse E, Fernandez-Mercado M, Garate L, Alvarez
S, Rifon J, Varea S, et al: TET2 mutations are associated with
specific 5-methylcytosine and 5-hydroxymethylcytosine profiles in
patients with chronic myelomonocytic leukemia. PLoS One.
7:e316052012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu
J, Xu ZD, Zhu HG, Ling ZQ, Ye D, et al: Tumor development is
associated with decrease of TET gene expression and
5-methylcytosine hydroxylation. Oncogene. 32:663–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin G, Kang TW, Yang S, Baek SJ, Jeong YS
and Kim SY: GENT: Gene expression database of normal and tumor
tissues. Cancer Inform. 10:149–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duhamel RC, Meezan E and Brendel K: The
addition of SDS to the Bradford dye-binding protein assay, a
modification with increased sensitivity to collagen. J Biochem
Biophys Methods. 5:67–74. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
la Cour T, Kiemer L, Mølgaard A, Gupta R,
Skriver K and Brunak S: Analysis and prediction of leucine-rich
nuclear export signals. Protein Eng Des Sel. 17:527–536. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JL, Kim HJ, Seo EH, Kwon OH, Lim B,
Kim M, Kim SY, Song KS, Kang GH, Kim HJ, et al: Decrease of 5hmC in
gastric cancers is associated with TET1 silencing due to with DNA
methylation and bivalent histone marks at TET1 CpG island 3′-shore.
Oncotarget. 6:37647–37662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Q, Wu K, Ji M, Jin W, He N, Shi B and
Hou P: Decreased 5-hydroxymethylcytosine (5-hmC) is an independent
poor prognostic factor in gastric cancer patients. J Biomed
Nanotechnol. 9:1607–1616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kudo Y, Tateishi K, Yamamoto K, Yamamoto
S, Asaoka Y, Ijichi H, Nagae G, Yoshida H, Aburatani H and Koike K:
Loss of 5-hydroxymethylcytosine is accompanied with malignant
cellular transformation. Cancer Sci. 103:670–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frycz BA, Murawa D, Borejsza-Wysocki M,
Marciniak R, Murawa P, Drews M, Kołodziejczak A, Tomela K and
Jagodziński PP: Decreased expression of ten-eleven translocation 1
protein is associated with some clinicopathological features in
gastric cancer. Biomed Pharmacother. 68:209–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Müller T, Gessi M, Waha A, Isselstein LJ,
Luxen D, Freihoff D, Freihoff J, Becker A, Simon M, Hammes J, et
al: Nuclear exclusion of TET1 is associated with loss of
5-hydroxymethylcytosine in IDH1 wild-type gliomas. Am J Pathol.
181:675–683. 2012. View Article : Google Scholar : PubMed/NCBI
|