Introduction
The telomere, located at the ends of eukaryotic
chromosomes, is a structure composed of tandem repeats of a DNA
sequence (5′-TTAGGG-3′)n together with specific binding
proteins that function in telomere formation, protection and length
maintenance (1,2). The telomere repeat sequence becomes
shortened at each cell division, or this may occur through DNA
damage resulting from oxidative stress, or changes in
telomere-associated proteins (3,4). Telomeres
play a central role in cell fate and aging by adjusting the
cellular response to stress and growth stimulation that results
from previous cell divisions and DNA damage; specifically, when
telomeres have sufficient length, they are able to prevent DNA
modification and chromosomal instability through DNA repair
mechanisms at chromosome ends (1,2). In fact,
the presence of telomere repeats of a few hundred nucleotides at
each chromosome end is needed to avoid activation of DNA repair
pathways (1,2). Somatic non-malignant cells with limited
expression of human telomerase reverse transcriptase (hTERT) have a
decreased capacity for repair, once telomeres have become
critically shortened or lost, thus triggering apoptosis or cellular
senescence (1,2,5). It has
been reported that telomeres and/or telomerase status are
intimately involved in the pathogenesis of various cancers; cancer
cells with impaired DNA damage responses (e.g., through loss of
functional p53) continue to divide in the presence of dysfunctional
telomeres, resulting in genome instability via chromosome fusions,
chromosome breaks, and repetitive break-fusion bridge events
(1,6).
Moreover, in cancer cells with hTERT expression, only a limited
number of short ends can be elongated by hTERT, representing
so-called ‘telomere salvage pathways’. Telomere shortening and
hTERT expression have been reported in various cancers and their
precursor lesions including gastric cancer, thyroid cancer, bladder
cancer, colon cancer, and ulcerative colitis (7–11). As a
biological marker, telomere length reflects malignant potential,
and might also be associated with genetic instability and the
degree of malignancy risk (10).
Non-melanoma skin cancers including basal cell
carcinoma (BCC), squamous cell carcinoma (SCC), Bowen's disease
(BD) and actinic keratosis (AK) are the most common malignant skin
tumors occurring world-wide (12).
SCC is an epidermis-derived invasive malignancy characterized
histopathologically by infiltration of atypical keratinocytes with
occasional by some level of keratinization, that invade through the
basement membrane into the dermis (13). BD is regarded as SCC in situ,
being characterized histopathologically by atypical cellular
pleomorphism including cell clumping, irregular mitosis and
individual cell keratinization within the epidermis, and having the
potential to transform into invasive SCC (14). AK is also regarded as an SCC in
situ, characterized histopathologically by atypia or dysplasia
of keratinocytes in the epidermal basal layers accompanied by
dermal solar elastosis, having the potential to transform into
invasive SCC (15). BCC is a
non-keratinizing basal epidermal cell neoplasm and is characterized
histopathologically by nests of basophilic epidermal basal
cell-like cells with a peripheral palisading arrangement. BCC is
regarded as a lineage that differs from SCC, BD or AK in that BD
and AK do not develop into BCC, and BCC does not develop into SCC,
except for a specific form of ‘basosquamous cell carcinoma’. The
metastatic potential of AK, BD and BCC is almost zero, whereas the
risk of SCC metastasis is estimated to be 0.1–13.7% (16). BCC and SCC, but not BD and AK, have
invasion potential (13).
Several reports have described the telomere and
telomerase status of non-melanoma skin cancers (17–20), as
well as for various other malignancies. Wainwright et al
reported that telomeres in BCC were not shortened in comparison to
control epidermis (17). Parris et
al reported that telomerase activity was increased in BD and AK
but only minimally increased in SCC (18). Despite these studies, the association
between telomeres/telomerase and the malignant potential of
non-melanoma skin cancers has not been well documented.
The purpose of the present study was to determine
whether telomere length has a relationship to the malignant
potential of non-melanoma skin cancers. Our analysis demonstrated
clear associations between the telomere lengths of various
non-melanoma skin cancers and their malignant potential.
Materials and methods
Tissue specimens
Fresh tissue samples were obtained by trepan or
scalpel from patients at the time of excisional surgery, and then
immediately frozen. The samples comprised 36 non-melanoma skin
cancers including 12 BCCs (patient age range, 48–92 year), 9 SCCs
(41–96 year), 9 BDs (56–91 year) and 6 AKs (62–81 year), and 26
samples of epidermal tissue, each surrounding a corresponding
tumor, including 9 BCCs (patient age range, 48–92 year), 6 SCCs
(41–92 year), 6 BDs (56–91 year) and 5 AKs (62–81 year). The
diagnoses were made histopathologically by more than one
experienced board-certified dermatopathologist. All samples were
collected at the Department of Dermatology, The Jikei University
School of Medicine. For use of the clinical specimens, written
informed consent was obtained from all reachable donors, as allowed
by the ethics committee of The Jikei University School of
Medicine.
Tissue quantitative florescence in
situ hybridization (Q-FISH)
Tissue Q-FISH was performed in accordance with
previously described processing procedures (21–23). In
brief, the samples were fixed for 2 h in 10% buffered formalin
solution and then embedded in paraffin according to standard
processing procedures. They were then cut into 5 µm-thick sections
for tissue Q-FISH analysis. The tissue sections were deparaffinized
in xylene, pre-treated with 0.2 N HCl and 1.0 M sodium thiocyanate
at 80°C, and then with 1.0% pepsin at 37°C. They were also treated
with 0.5 mg/ml RNase at 37°C to remove RNA. A peptide nucleic acid
(PNA) telomere probe conjugated to Cy3 (Telo C-Cy3 probe:
5′-CCCTAACCCTAACCCTAA−3′, Fasmac, Atsugi, Japan) and a PNA
centromere probe conjugated to fluorescein isothiocyanate (FITC)
(Cenp 1 probe: 5′-CTTCGTTGGAAACGGGGT-3′, Fasmac) were applied to
each section. The nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
FISH images were captured by a charge-coupled device
camera attached to an epifluorescence microscope (Eclipse 90i;
Nikon, Tokyo, Japan) equipped with a triple band-pass filter set
for DAPI/FITC/Cy3 (61000v2 m; Chroma Technology Corp., Rockingham,
VT, USA) and a ×40 objective lens (Plan Fluor ×40/0.75; Nikon).
Microscope control and image recording were performed using
Image-Pro Plus software (version 7.01; Media Cybernetics, Bethesda,
MD, USA). The recorded images were analyzed using an original
software package, ‘Tissue Telo Version 3.2’, which allows manual
identification of nuclear regions from the composite color image:
DAPI (blue channel), FITC (green), and Cy3 (red). Fluorescence
intensities of telomere signals (Cy3) and centromere signals (FITC)
for each nucleus were measured. The telomere-centromere ratio (TCR)
was then calculated, because there is no guarantee that all
information regarding telomere signals will be acquired within any
given tissue section.
Statistical analysis
Statistical analysis was performed with the ‘SPSS
statistics 23’ software package. Normality of each mean TCR value
was confirmed by the Kolmogorov-Smirnov test. Equality of variance
was examined by Levene's test. The association between TCR and
clinical information was examined by multiple linear regression
analysis. The significance of differences among mean TCR values was
examined by one-way analysis of variance and Bonferroni's multiple
comparisons test when both normality and equality of variance were
confirmed, and by the Kruskal-Wallis test when either normality or
equality was not confirmed. P<0.05 was considered to indicate a
statistically significant difference.
Results
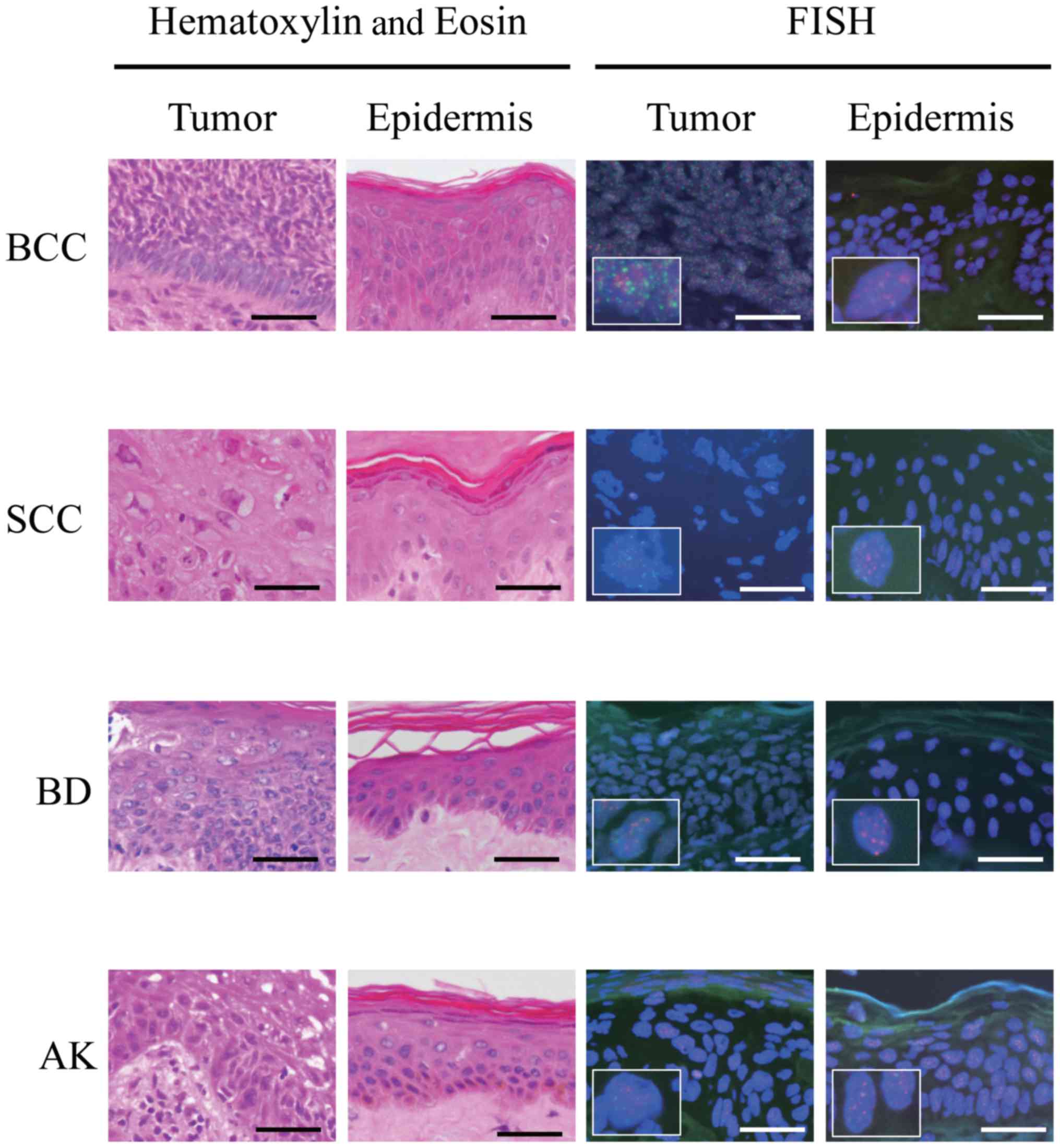
FISH analyses detected telomeres and centromeres as
small red and green dots, respectively, within nuclei (Fig. 1). Telomere signals of BCC, SCC, BD and
AK tumor cells appeared to be weaker than those of the epidermal
cells located close to the respective tumors in most samples.
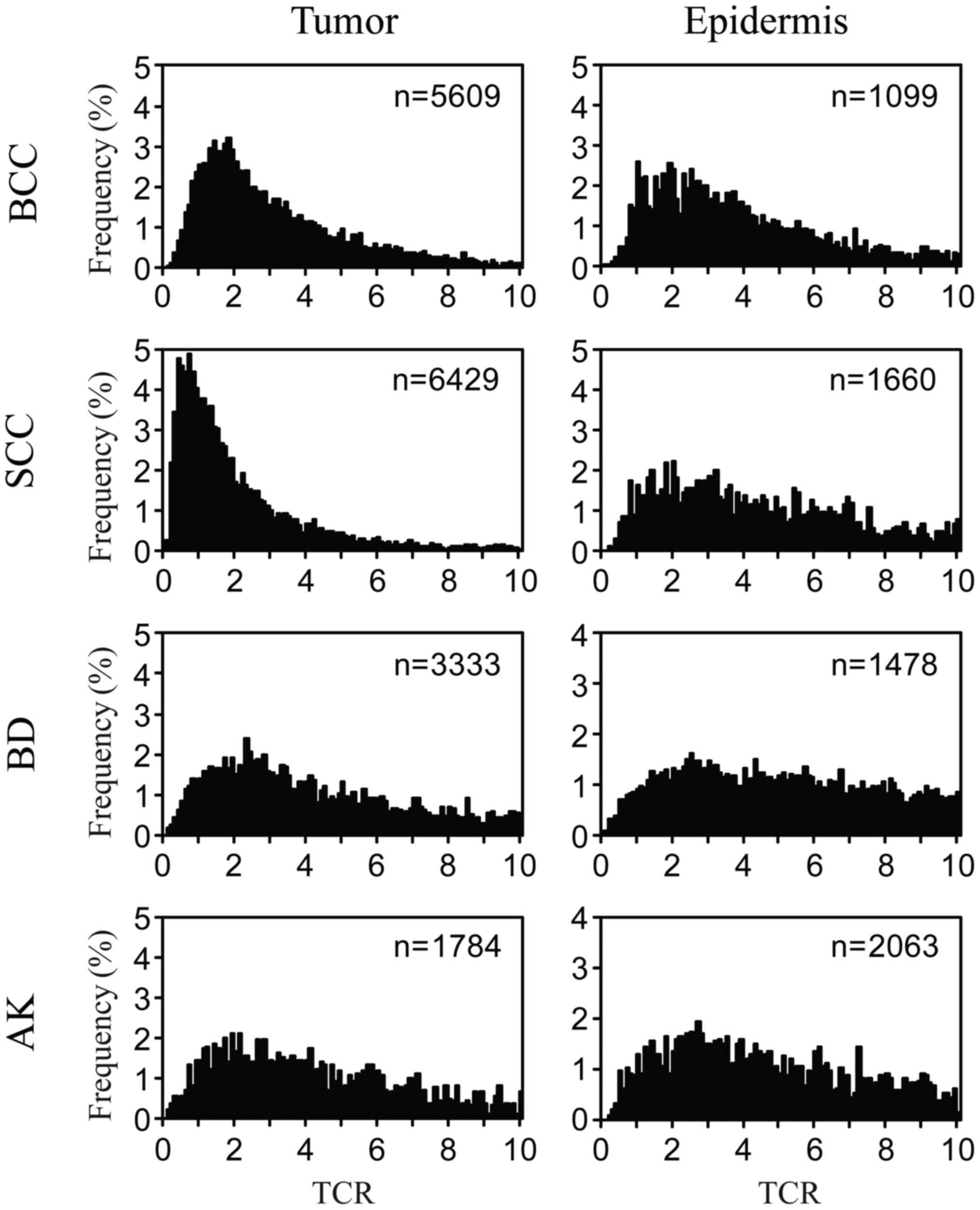
Captured FISH images were quantified (Table I), yielding in a mean TCR value ±
standard deviation for tumor cells of BCC, SCC, BD and AK of
3.02±2.05, 2.01±1.86, 3.97±2.51 and 4.04±2.47, respectively, and
corresponding values for epidermal cells located close to the
tumors of 3.87±2.39, 4.20±2.51, 4.86±2.63 and 4.48±2.55,
respectively. Histograms showing the distribution of TCR values for
each type of tumor cell and the corresponding closely located
epidermal cells were constructed based on the quantified FISH
images (Fig. 2). These histograms
showed that the peak frequency of TCR in BCC cells was closed to
1–2, whereas that in SCC cells was <1. It was also evident that
TCR values for SCC and BCC cells were distributed within a lower
range than those for BD and AK cells. TCR values for epidermal
cells located close to each tumor type had a very wide
distribution, and the peak frequency was ≥2. TCR values for each
type of tumor cell appeared to be distributed within a lower range
than those of the corresponding closely located epidermal
cells.
 | Table I.Clinical data for patients who donated
samples. |
Table I.
Clinical data for patients who donated
samples.
|
| Average TCR
value |
|---|
|
|
|
|---|
| ID | Age | Sex | Samplea | Diagnosis | TNMb | F/Uc | Rec.d | Tumor |
Epidermise |
|---|
| No. 1 | 48 | M | Face | BCC | pT2N0M0 | 5 | + | 2.54±1.58 | 4.39±2.34 |
| No. 2 | 83 | F | Face | BCC | pT1N0M0 | 65 | − | 4.22±2.32 | 4.54±2.28 |
| No. 3 | 92 | M | Face | BCC | pT1N0M0 | 8 | − | 4.81±2.29 | 5.24±2.58 |
| No. 4 | 73 | F | Face | BCC | pT1N0M0 | 48 | − | 3.18±2.18 | 4.50±2.61 |
| No. 5 | 77 | M | Face | BCC | pT2N0M0 | 40 | − | 3.42±2.03 | nd |
| No. 6 | 73 | M | Face | BCC | pT1N0M0 | 0 | − | 3.56±2.27 | nd |
| No. 7 | 84 | M | Face | BCC | pT2N0M0 | 49 | − | 2.19±1.32 | 2.93±2.01 |
| No. 8 | 77 | M | Face | BCC | pT2N0M0 | 35 | − | 2.21±1.63 | 2.88±2.04 |
| No. 9 | 79 | M | Face | BCC | pT1N0M0 | 8 | − | 2.52±1.85 | nd |
| No. 10 | 80 | M | Face | BCC | pT1N0M0 | 0 | − | 2.78±2.04 | 3.34±2.07 |
| No. 11 | 73 | F | Trunk | BCC | pT2N0M0 | 12 | − | 2.43±2.05 | 5.00±2.48 |
| No. 12 | 68 | F | Face | BCC | pT1N0M0 | 53 | − | 2.89±1.98 | 3.40±2.10 |
| No. 13 | 92 | F | Face | SCC | pT1N0M0 | 16 | + | 1.47±1.16 | 2.47±1.62 |
| No. 14 | 87 | F | Face | SCC | pT1N0M0 | 63 | − | 0.91±0.91 | 5.69±2.42 |
| No. 15 | 96 | F | Hand | SCC | pT1N0M0 | 14 | + | 3.62±2.31 | 4.75±2.58 |
| No. 16 | 52 | M | Genitalia | SCC | pT1N0M0 | 51 | − | 2.52±1.90 | 5.09±2.49 |
| No. 17 | 41 | M | Hand | SCC | pT1N0M0 | 25 | − | 2.15±1.72 | 4.65±2.37 |
| No. 18 | 92 | M | Thigh | SCC | pT1N0M0 | 12 | − | 2.07±1.96 | 4.10±2.32 |
| No. 19 | 96 | F | Hand | SCC | pT2N0M0 | 11 | − | 0.80±0.59 | nd |
| No. 20 | 94 | F | Foot | SCC | pT2N0M0 | 20 | − | 1.06±0.87 | nd |
| No. 21 | 89 | M | Face | SCC | pT1N0M0 | 8 | + | 0.67±0.70 | nd |
| No. 22 | 88 | F | Genitalia | BD | pTisN0M0 | 0 | − | 3.78±2.33 | nd |
| No. 23 | 89 | F | Thigh | BD | pTisN0M0 | 11 | − | 5.26±2.42 | 4.79±2.69 |
| No. 24 | 56 | F | Forearm | BD | pTisN0M0 | 35 | − | 4.89±3.05 | 5.22±2.66 |
| No. 25 | 66 | F | Knee | BD | pTisN0M0 | 45 | − | 3.22±2.40 | nd |
| No. 26 | 72 | M | Trunk | BD | pTisN0M0 | 1 | − | 3.07±1.96 | nd |
| No. 27 | 91 | M | Face | BD | pTisN0M0 | 7 | − | 4.85±2.37 | 5.04±2.29 |
| No. 28 | 70 | M | Thigh | BD | pTisN0M0 | 12 | − | 4.02±2.66 | 4.61±2.78 |
| No. 29 | 58 | F | Trunk | BD | pTisN0M0 | 0 | − | 4.45±2.52 | 4.84±2.68 |
| No. 30 | 57 | F | Thigh | BD | pTisN0M0 | 2 | − | 4.53±2.49 | 4.92±2.71 |
| No. 31 | 67 | M | Face | AK | pTisN0M0 | 0 | − | 3.69±2.26 | 4.72±2.42 |
| No. 32 | 71 | M | Face | AK | pTisN0M0 | 28 | − | 4.75±2.45 | 5.00±2.38 |
| No. 33 | 62 | M | Face | AK | pTisN0M0 | 11 | − | 4.93±2.53 | 4.78±2.66 |
| No. 34 | 81 | M | Face | AK | pTisN0M0 | 45 | + | 4.49±2.75 | 3.57±2.50 |
| No. 35 | 80 | M | Face | AK | pTisN0M0 | 13 | − | 1.76±2.19 | nd |
| No. 36 | 73 | M | Face | AK | pTisN0M0 | 1 | − | 4.66±2.76 | 4.49±2.49 |
To analyze the association between the TCR values
for the various types of tumor cells and clinical parameters
including patient age, sex, sampling site (sites chronically or
non-chronically exposed to the sun) and the TCR values for
epidermal cells located close to each tumor type, multiple linear
regression analyses were performed using the TCR value for each
type of tumor cell as the dependent variable (Table II). These statistical analyses
indicated that the TCR value for each type of tumor cell was not
associated with patient age, sex, sampling site or the TCR value of
the epidermal cells located close to each tumor type. To analyze
the association between the TCR value of the epidermal cells close
to each type of tumor and clinical parameters including patient
age, sex, sampling site (chronically or non-chronically exposed to
the sun) and the TCR value for each type of tumor cell, multiple
linear regression analyses were performed using the TCR value of
the closely located epidermal cells as the dependent variable
(Table II). These statistical
analyses indicated that the TCR value for the epidermal cells
located close to each type of tumor were not associated with
patient age, sex, sampling site or the TCR value for each type of
tumor cell.
 | Table II.Results of multiple regression
analysis with TCR as the dependent variable. |
Table II.
Results of multiple regression
analysis with TCR as the dependent variable.
|
| Tumor |
Epidermisc |
|---|
|
|
|
|
|---|
| Independent
variable |
β-valueb | P-value | β-valueb | P-value |
|---|
| Age | 0.162 | 0.208 | −0.264 | 0.194 |
| Sex | 0.002 | 0.988 | −0.125 | 0.561 |
| Chronic
sun-exposurea | 0.076 | 0.600 | −0.133 | 0.564 |
| TCR values for
epidermis | 0.277 | 0.053 |
|
|
| TCR values for
tumor |
|
| 0.695 | 0.053 |
To compare TCR values among BCC, SCC, BD and AK
tumors, one-way analysis of variance followed by Bonferroni's
multiple comparison test was performed for each type of tumor cell
whose distribution showed both normality and equality of variance
(Table III). These analyses showed
that the TCR values for SCC were significantly lower than those for
BCC, BD or AK, and that the TCR values for BCC were significantly
lower than those for BD. To compare the TCR values for epidermal
cells located close to BCC, SCC, BD and AK tumors, the
Kruskal-Wallis test was performed for cases where the distribution
did not show equality of variance. These analyses showed that the
TCR values for epidermal cells located close to each tumor were not
associated with the tumor type. To confirm these results, multiple
linear regression analyses were performed (Table IV), and this demonstrated that i) the
TCR values for SCC or BCC were significantly lower than those for
BD or AK; ii) the TCR values for BCC, BD or AK were significantly
higher than those for SCC; and iii) the TCR values for epidermal
cells located close to each tumor type were not associated with the
type of tumor cell. These results were compatible with those of
one-way analysis of variance or Kruskal-Wallis test.
 | Table III.Results of Bonferroni's multiple
comparison test. |
Table III.
Results of Bonferroni's multiple
comparison test.
| Comparison of TCR
values | P-value |
|---|
| BCC vs. AK | 0.236 |
| BCC vs. BD | 0.041a |
| BCC vs. SCC | 0.011a |
| SCC vs. AK |
<0.001a |
| SCC vs. BD |
<0.001a |
| BD vs. AK | 1.000 |
 | Table IV.Comparison among TCR values by
multiple regression analysis. |
Table IV.
Comparison among TCR values by
multiple regression analysis.
|
| Tumor |
Epidermise |
|---|
|
|
|
|
|---|
| Independent
variable | β-valuef | P-value |
β-valuef | P-value |
|---|
| SCC vs.
BCCa | −0.362 | 0.018g | 0.418 | 0.098 |
| BD vs.
BCCa | 0.498 | 0.007g | −0.062 | 0.864 |
| AK vs.
BCCa | 0.418 | 0.010g | −0.064 | 0.820 |
| BCC vs.
SCCb | 0.409 | 0.018g | −0.472 | 0.098 |
| BD vs.
SCCb | 0.860 |
<0.001g | −0.480 | 0.233 |
| AK vs.
SCCb | 0.757 |
<0.001g | −0.455 | 0.227 |
| BCC vs.
BDc | −0.562 | 0.007g | 0.070 | 0.846 |
| SCC vs.
BDc | −0.860 |
<0.001g | 0.480 | 0.233 |
| AK vs.
BDc | −0.048 | 0.793 | −0.006 | 0.984 |
| BCC vs.
AKd | −0.504 | 0.010g | 0.078 | 0.820 |
| SCC vs.
AKd | −0.809 |
<0.001g | 0.487 | 0.227 |
| BD vs.
AKd | 0.051 | 0.793 | 0.006 | 0.984 |
Discussion
In the present study, we employed the tissue Q-FISH
method for the determination of telomere length. This approach has
some merits for evaluation of telomeres because it independently
measures the fluorescence intensity of the telomere and that of the
centromere, the latter being used as a standard whose reliability
as an accurate internal control parameter has been confirmed by
numerous previous studies (21–23). This
allowed us to obtain the telomere-centromere ratio (TCR), which has
been shown to reflect telomere length more accurately than
procedures that lack an internal control (21–23). Data
on telomere length can be obtained in this way by performing in
situ hybridization on histological sections, allowing specific
evaluation of cancer cells or the cells surrounding the tumor on
tissue sections, which commonly include a number of other cell
types such as inflammatory cells. Several previous studies have
investigated telomeres in esophageal, breast, and thyroid cancers
using tissue Q-FISH, and the results obtained were very similar to
ours in terms of the TCR distributions of tumor cells and the cells
surrounding tumors. As was seen in our present study, the TCR
distribution shown in previous studies peaked at a value of <1.0
for cancer cells and >1.0 for tumor-surrounding cells.
Additionally, the TCR distributions of cells with normal appearance
surrounding the tumor cells showed a wide distribution similar to
that of the epidermal cells close to each tumor in our present
study (22–25). These findings suggest that tissue
Q-FISH is a reliable procedure for determining the telomere length
of solid cancers including skin cancers.
The present study represents the first comprehensive
examination of telomere length in non-melanoma skin cancers,
including the lineages of epidermis-derived cancers, i.e., AK-SCC
and BD-SCC. Our findings suggest that telomere length in
non-melanoma skin cancer cells is intrinsically related to aspects
of biological behavior including the potential for metastasis and
invasion. Use of two different statistical analyses, i.e., multiple
linear regression analysis and Bonferroni's multiple comparison
test, yielded similar results in terms of the TCR values for the
various types of tumor cells; i.e., these TCR values showed the
order SCC<BCC<BD or AK. These TCR values were completely
consistent with the metastatic potential of the tumors; SCC, BCC
and BD/AK have some, minimal and no metastatic potential,
respectively. In this context, the metastatic potential of
non-melanoma skin cancers could be associated with telomere length,
and thus evaluable by the TCR value, although we did not
investigate variation in metastatic potential within a single type
of tumor. In addition, BCC has a distinct tendency to invade to the
deeper levels including the dermis, whereas BD/AK has no invasion
potential, suggesting that telomere length could also be associated
with the invasion potential of non-melanoma skin cancers, and thus
evaluable by the TCR value, although we did not investigate
difference in invasion potential within a single tumor type.
Our statistical analyses suggested that the TCR
values for epidermal cells located close to each tumor were not
associated with patient age, sex, tumor type or sampling site
(i.e., chronically or non-chronically exposed to solar radiation).
It has been reported previously that TCR values for epidermal cells
are reduced with increasing age and chronic sun-exposure (26,27). There
are a number of possible explanations for the discrepancy between
the present results and the previous ones. The epidermal cells
examined in the present study were located very close to each
tumor, and therefore were likely to have been sampled from areas
with field defects, which can be regarded as precursors of
malignancy even though they often appear to be histopathologically
normal. In contrast, the ‘control’ epidermal cells examined in
previous studies were probably sampled from areas without field
defects. Any subtle differences in TCR values resulting from age or
chronic sun exposure might be masked by the effects of field
defects, which might be associated with low TCR (28,29).
Several previous studies have suggested that
invasive SCC develops through stepwise progression, i.e., some
invasive SCCs develop from AK (30–32).
However, on the basis of DNA microarray profiling of over 47,000
genes, Ra et al have concluded that AK and SCC are distinct
entities, since each has its own unique molecular signature
(33). Our present finding that
telomere length in SCC was significantly shorter than that in BD or
AK suggests that these entities may be biologically distinct from
one another. Our present finding also suggests that telomere
shortening is correlated with invasive progression, thus being a
useful parameter for distinguishing SCC from other tumors.
The limitation of the present study was the small
number of samples employed. In order to examine more accurately the
difference in telomere length between BD and AK, a larger number of
samples might be needed. Another limitation was that none of the
epidermal samples were taken from areas that were distant from the
tumor. Comparison of the TCRs of epidermal cells in areas close to
the tumor with those of cells located distant from the tumor would
likely show that causable field defects in non-melanoma skin cancer
are caused, at least in part, by telomere shortening.
In conclusion, our comprehensive examination
suggests that the telomere length of non-melanoma skin cancer cells
is closely related to biological behavior including the potential
for metastasis and invasion.
Acknowledgements
We thank Dr. Yoshiyuki Sugishita (Department of
Laboratory, Kanaji Thyroid Hospital), Dr. Makoto Kammori, Dr.
Ryoichi Kamide (Hihuno Clinic), and Dr Mariko Honda (Dr. Mariko
Skin and Dermatology Clinic) for providing technical support or
expert advice.
References
|
1
|
Aubert G and Lansdorp PM: Telomeres and
aging. Physiol Rev. 88:557–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Zglinicki T: Oxidative stress shortens
telomeres. Trends Biochem Sci. 27:339–344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Artandi SE and DePinho RA: Telomeres and
telomerase in cancer. Carcinogenesis. 31:9–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blasco MA: Telomeres and human disease:
Ageing, cancer and beyond. Nat Rev Genet. 6:611–622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Furugori E, Hirayama R, Nakamura KI,
Kammori M, Esaki Y and Takubo K: Telomere shortening in gastric
carcinoma with aging despite telomerase activation. J Cancer Res
Clin Oncol. 126:481–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kammori M, Takubo K, Nakamura K, Furugouri
E, Endo H, Kanauchi H, Mimura Y and Kaminishi M: Telomerase
activity and telomere length in benign and malignant human thyroid
tissues. Cancer Lett. 159:175–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broberg K, Björk J, Paulsson K, Höglund M
and Albin M: Constitutional short telomeres are strong genetic
susceptibility markers for bladder cancer. Carcinogenesis.
26:1263–1271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura K, Furugori E, Esaki Y, Arai T,
Sawabe M, Okayasu I, Fujiwara M, Kammori M, Mafune K, Kato M, et
al: Correlation of telomere lengths in normal and cancers tissue in
the large bowel. Cancer Lett. 158:179–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Sullivan JN, Bronner MP, Brentnall TA,
Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH,
Crispin DA, et al: Chromosomal instability in ulcerative colitis is
related to telomere shortening. Nat Genet. 32:280–284. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Firnhaber JM: Diagnosis and treatment of
Basal cell and squamous cell carcinoma. Am Fam Physician.
86:161–168. 2012.PubMed/NCBI
|
|
13
|
Yanofsky VR, Mercer SE and Phelps RG:
Histopathological variants of cutaneous squamous cell carcinoma: A
review. J Skin Cancer. 2011:2108132011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Röwert-Huber J, Patel MJ, Forschner T,
Ulrich C, Eberle J, Kerl H, Sterry W and Stockfleth E: Actinic
keratosis is an early in situ squamous cell carcinoma: A proposal
for reclassification. Br J Dermatol. 3(156 Suppl): S8–S12. 2007.
View Article : Google Scholar
|
|
15
|
Glogau RG: The risk of progression to
invasive disease. J Am Acad Dermatol. 42:23–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim RH and Armstrong AW: Nonmelanoma skin
cancer. Dermatol Clin. 30:125–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wainwright LJ, Middleton PG and Rees JL:
Changes in mean telomere length in basal cell carcinomas of the
skin. Genes Chromosomes Cancer. 12:45–49. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parris CN, Jezzard S, Silver A, MacKie R,
McGregor JM and Newbold RF: Telomerase activity in melanoma and
non-melanoma skin cancer. Br J Cancer. 79:47–53. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han J, Qureshi AA, Prescott J, Guo Q, Ye
L, Hunter DJ and De Vivo I: A prospective study of telomere length
and the risk of skin cancer. J Invest Dermatol. 129:415–421. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leufke C, Leykauf J, Krunic D, Jauch A,
Holtgreve-Grez H, Böhm-Steuer B, Bröcker EB, Mauch C, Utikal J,
Hartschuh W, et al: The telomere profile distinguishes two classes
of genetically distinct cutaneous squamous cell carcinomas.
Oncogene. 33:3506–3518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aida J, Izumiyama-Shimomura N, Nakamura K,
Ishii A, Ishikawa N, Honma N, Kurabayashi R, Kammori M, Poon SS and
Arai T: Telomere length variations in 6 mucosal cell types of
gastric tissue observed using a novel quantitative fluorescence in
situ hybridization method. Hum Pathol. 38:1192–1200. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kammori M, Izumiyama N, Nakamura K,
Kurabayashi R, Kashio M, Aida J, Poon SS and Kaminishi M: Telomere
metabolism and diagnostic demonstration of telomere measurement in
the human esophagus for distinguishing benign from malignant tissue
by tissue quantitative fluorescence in situ hybridization.
Oncology. 71:430–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugishita Y, Kammori M, Yamada O, Yamazaki
K, Ito K, Fukumori T, Yoshikawa K and Yamada T: Biological
differential diagnosis of follicular thyroid tumor and Hurthle cell
tumor on the basis of telomere length and hTERT expression. Ann
Surg Oncol. 21:2318–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurabayashi R, Takubo K, Aida J, Honma N,
Poon SS, Kammori M, Izumiyama-Shimomura N, Nakamura K, Tsuji E,
Matsuura M, et al: Luminal and cancer cells in the breast show more
rapid telomere shortening than myoepithelial cells and fibroblasts.
Hum Pathol. 39:1647–1655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugishita Y, Kammori M, Yamada O, Poon SS,
Kobayashi M, Onoda N, Yamazaki K, Fukumori T, Yoshikawa K, Onose H,
et al: Amplification of the human epidermal growth factor receptor
2 gene in differentiated thyroid cancer correlates with telomere
shortening. Int J Oncol. 42:1589–1596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikeda H, Aida J, Hatamochi A, Hamasaki Y,
Izumiyama-Shimomura N, Nakamura K, Ishikawa N, Poon SS, Fujiwara M,
Tomita K, et al: Quantitative fluorescence in situ hybridization
measurement of telomere length in skin with/without sun exposure or
actinic keratosis. Hum Pathol. 45:473–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buckingham EM and Klingelhutz AJ: The role
of telomeres in the ageing of human skin. Exp Dermatol. 20:297–302.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aida J, Izumo T, Shimomura N, Nakamura K,
Ishikawa N, Matsuura M, Poon SS, Fujiwara M, Sawabe M, Arai T and
Takubo K: Telomere lengths in the oral epithelia with and without
carcinoma. Eur J Cancer. 46:430–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuhn E, Meeker A, Wang TL, Sehdev AS,
Kurman RJ and Ie M Shih: Shortened telomeres in serous tubal
intraepithelial carcinoma: An early event in ovarian high-grade
serous carcinogenesis. Am J Surg Pathol. 34:829–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Czarnecki D, Meehan CJ, Bruce F and Culjak
G: The majority of cutaneous squamous cell carcinomas arise in
actinic keratoses. J Cutan Med Surg. 6:207–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu W and Cockerell CJ: The actinic (solar)
keratosis: A 21st-century perspective. Arch Dermatol. 139:66–70.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cassarino DS, Derienzo DP and Barr RJ:
Cutaneous squamous cell carcinoma: A comprehensive
clinicopathologic classification-part two. J Cutan Pathol.
33:261–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ra SH, Li X and Binder S: Molecular
discrimination of cutaneous squamous cell carcinoma from actinic
keratosis and normal skin. Mod Pathol. 24:963–973. 2011.PubMed/NCBI
|