Introduction
Among all types of female reproductive system
cancer, ovarian cancer has the second highest incidence rate and
the highest mortality rate (1). At
the time of diagnosis, 75% of patients are at an advanced stage
(III or IV) (2). The 5-year survival
rate decreases rapidly between stages I and IV (3). Currently, no effective methods exist for
the screening and early diagnosis of ovarian cancer. The majority
of patients have already reached advanced stages at diagnosis,
resulting in a poor prognosis (2).
The 5-year relative survival rate ranges from between 30 and 40% at
stage III to <10% at stage IV (2).
Nevertheless, practicing personalized medicine catering to
individual responses and a timely follow-up may achieve a
satisfactory therapeutic outcome in certain advanced-stage
patients. In the present case report, the step-by-step management
protocol followed for a patient with stage IV ovarian cancer who
survived for >9 years is described. As it is rare for a patient
with advanced-stage ovarian cancer to demonstrate such favorable
prognosis, it is hoped that the treatment protocol may be of
assistance to clinicians encountering a similar case. The patient
provided written informed consent for the publication of the
present study.
Case report
The patient was a 48-year-old female who was
admitted to the pulmonary department of Qilu Hospital of Shandong
University (Jinan, China) due to a severe irritating cough and
chest tightness in July 2004. The patient's mother had a history of
tuberculosis. Computed tomography (CT) scan results revealed
pleural effusion in the right lung and pericardial effusion with
mild ascites. The suspected diagnosis upon admission was
tuberculous pleurisy. Thoracocentesis yielded 1,000 ml yellow
serous exudate, which tested negative for Mycobacterium
tuberculosis and malignant cells. Abdominal paracentesis also
revealed similar results. Physical examination revealed a palpable
mass in the recto-uterine pouch. After 2 weeks of conservative
therapy, the patient's situation was slightly improved. However, at
the end of the third week, the patient's condition began to
deteriorate. Lumps appeared on the right arm and right
supraclavicular region. Enhanced CT scan results indicated a number
of enlarged lymph nodes surrounding the right side of the thoracic
entrance to the mediastinum, multiple swollen cervical lymph nodes
on the right side and right internal jugular vein embolus, with
bilateral pleural effusion and ascites. CT-guided pelvic mass
biopsy was then performed to clarify the diagnosis.
Histopathological examination revealed papillary adenocarcinoma
originating from the reproductive system. As the patient was not
able to tolerate surgery, neoadjuvant chemotherapy was considered,
and four courses of cisplatin and paclitaxel were administered. All
the pleural effusion, peritoneal effusion and right internal
jugular vein tumor thrombus disappeared. Mediastinal lymph nodes
and pelvic mass were also markedly decreased in size. The patient's
physical condition improved and so interval cytoreductive surgery
was performed. Routine postoperative pathological examination
identified undifferentiated carcinoma on the left ovary with right
ovary metastasis, without any metastasis to the pelvic lymph nodes.
Postoperative docetaxel plus carboplatin chemotherapy was
administered for 3 cycles and serum cancer antigen 125 (CA125)
level returned to normal. During a follow-up 19 months after
surgery, CT revealed a pelvic mass 2 cm in diameter and the serum
CA125 level increased to 41.6 U/ml. A total of 4 cycles of
liposomal doxorubicin- plus trabectedin-based second-line
chemotherapy were administered, and CA125 decreased again to normal
levels. CT scanning 7 months later revealed a mass in the left
anterior region of the sigmoid colon and the CA125 level once again
increased to 120 U/ml. Third-line docetaxel and carboplatin
chemotherapy was administered for 8 cycles and the CA125 level
subsequently decreased to 9.45 U/ml. On follow-up (1 year), the
patient's serum CA125 level was increased again to 304.6 U/ml and
ultrasonography revealed a hypoechoic solid nodule in the pelvis.
Fourth-line topotecan-based chemotherapy was administered for 2
cycles, then changed to 1 cycle of carboplatin and paclitaxel due
to marked toxicity. The patient's CA125 level gradually increased
to 452.10 U/ml during chemotherapy. The patient's condition was not
able to be controlled with the current regimen as a result of
multidrug resistance. Treatment was changed to a combination of
oxaliplatin and vinorelbine for 6 cycles, with CA125 levels
subsequently decreasing to 20.35 U/ml. The patient was admitted 5
months later due to relapse. The patient was then administered 2
cycles of gemcitabine and capecitabene and 4 cycles of gemcitabine
and cyclophosphamide. The CA125 level was 84.05 U/ml following this
round of chemotherapy. The patient relapsed again 1 month later,
and an epirubicin and vindesine regimen was administered for 3
cycles. Since January 2011, 6 cycles of a nedaplatin and
vinorelbine combination regimen were administered. Upon completion
of this course, the level of CA125 had decreased to 8.93 U/ml. The
patient then attained a stable condition and was monitored
carefully during follow-up.
The patient's CA125 levels once again increased to
166.70 U/ml 1 month later. The patient was therefore administered 6
cycles of lobaplatin and vinorelbine. The patient's CA125 level was
measured at 25.69 U/ml following this course of chemotherapy, until
relapse, increasing to 262.50 U/ml after 3 months. After 18 days of
treatment with 1 cycle of lobaplatin (50 mg) and irinotecan (280
mg), the patient developed severe bone marrow suppression [white
blood cells (WBC), 1.72×109/l; platelets (PLT),
7×109/l], high fever and diarrhea. However, the CA125
levels returned to normal at 28.51 U/ml. Dosage was decreased to 40
mg lobaplatin and 200 mg irinotecan owing to the potential side
effects. The CA125 levels were unsatisfactory after 1 month of
treatment (54.49 U/ml), thus irinotecan (240 mg) was added for a
further 2 cycles. However, the CA125 levels increased continuously
to 86.93 U/ml. CA125 levels were consistently increased after 1
month, therefore treatment was changed to nedaplatin and irinotecan
which yielded poor results. The level of CA125 even increased
further to 321.2 U/ml. At this stage, an alternative drug regimen
was advised, which the patient declined due to high expense. Thus,
1 cycle of lobaplatin was added without any marked alteration in
the CA125 levels. Furthermore, the patient presented with severe
bone marrow suppression (WBC, 0.4×109/l; PLT,
2×109/l) and hemorrhage of the digestive tract, which
were managed symptomatically. In June 2013, the patient's CA125
level was revealed to be 2,222 U/ml. The patient was hospitalized 2
days later due to severe gastrointestinal bleeding. Positron
emission tomography-CT revealed a recurrent pelvic lesion measuring
9.2×7.2×5.5 cm, involving the rectum. CT revealed bilateral pleural
effusion. B-mode ultrasonography revealed multiple solid masses in
the liver. Considering the poor outcome and relapse of tumor with
metastasis, palliative treatment was offered. The patient was
hospitalized again due to severe gastrointestinal hemorrhage in
August 2015. The patient's family members declined any further
treatment and the patient was discharged having received
symptomatic treatment. The patient succumbed 2 months later. All
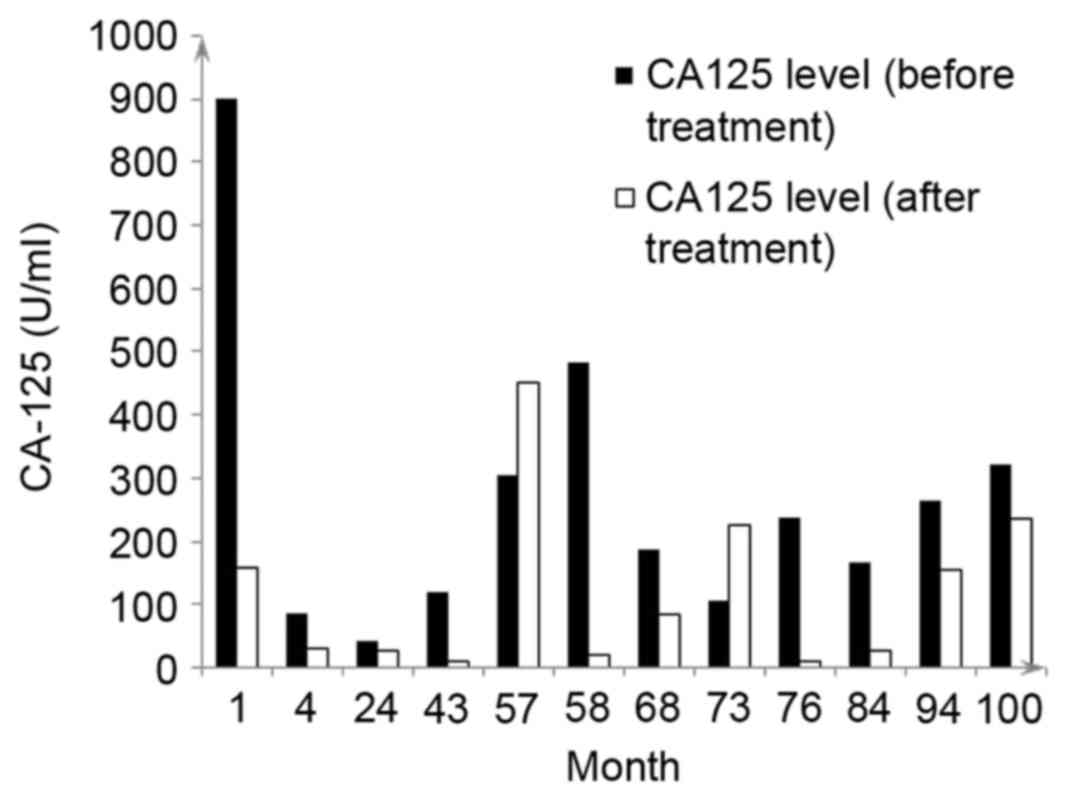
chemotherapy treatments received are summarized in Table I. The CA125 levels of the patient
prior to and following treatment are presented in Fig. 1.
 | Table I.Treatments administered to the patient
with Stage IV ovarian cancer. |
Table I.
Treatments administered to the patient
with Stage IV ovarian cancer.
| Time following
diagnosis, months | CA125 level prior to
treatment, U/ml | Treatment | CA125 level following
treatment, U/ml |
|---|
|
1 | >900.0 | Cisplatin +
paclitaxel, 4 cycles | 157.00 |
|
4 | 157.0 | Interval
cytoreductive surgery |
86.00 |
|
4 | 86.0 | Docetaxel +
carboplatin, 3 cycles |
35.00 |
| 24 | 41.6 | Liposomal doxorubicin
+ trabectedin, 4 cycles |
35.00 |
| 43 | 120.0 | Docetaxel +
carboplatin, 8 cycles |
35.00 |
| 57 | 304.6 | Topotecan, 2 cycles;
carboplatin + Taxol, 1 cycle | 452.10 |
| 58 | 482.5 | Oxaliplatin +
vinorelbine, 6 cycles |
20.35 |
| 68 | 186.5 | Gemcitabine +
campecitabene, 2 cycles; gemcitabine + cyclophosphamide, 4
cycles |
84.04 |
| 73 | 105.0 | Epirubicin +
vindesine, 3 cycles | 225.30 |
| 76 | 238.1 | Nedaplatin +
vinorelbine, 6 cycles |
35.00 |
| 84 | 166.7 | Lobaplatin +
vinorelbine, 7 cycles |
35.00 |
| 94 | 262.5 | Lobaplatin +
irinotecan, 4 cycles | 154.70 |
| 100 | 321.2 | Nedaplatin +
irinotecan, 2 cycles Lobaplatin + nedaplatin + irinotecan, 1
cycle | 234.20 |
| 105 | 2,222.0 | Symptomatic
treatment |
|
| 109 |
| Patient
succumbed |
|
Discussion
Among all types of female reproductive system
cancer, ovarian cancer exhibits the second highest incidence rate
and the highest mortality rate (1,3). Ovarian
cancer in 75% of patients has already reached an advanced stage at
the time of diagnosis (2). In the
present case, the patient initially presented with pleural effusion
and ascites. Supraclavicular lymph node metastasis indicated that
the disease had already progressed to stage IV.
The patient's poor physical condition precluded
surgery directly, although a concurrent pelvic mass existed.
Neoadjuvant chemotherapy was selected as the first-line therapy
based on histological evidence. Histopathology confirmed the
diagnosis of pelvic malignancy.
The role of neoadjuvant chemotherapy in overall
survival rates of patients with ovarian cancer is controversial.
The European Organization for Research and Treatment of Cancer
(EORTC) attained clinical trial results (4) which support neoadjuvant chemotherapy in
patients with ovarian cancer and demonstrated improvements in
survival rates. However, the US GOG152 study and a small-scale
British randomized controlled trial (RCT) (4,5)
demonstrated no benefit in survival rates. Previous joint study
efforts of the EORTC and National Cancer Institute of Canada
identified that neoadjuvant chemotherapy is no less effective than
standard first-line treatment (6,7).
Nevertheless, in the present case neoadjuvant chemotherapy resulted
in a favorable outcome. The metastatic lesions disappeared
following 4 cycles of neoadjuvant chemotherapy. The patient's
general condition improved significantly, which made interval
cytoreductive surgery possible. An additional 3 courses of
postoperative chemotherapy achieved complete clinical remission.
Although relapse occurred 19 months after surgery, the disease was
sensitive to platinum-based chemotherapy with a favorable
prognosis. At this stage, the patient was enrolled in an
international Phase III RCT for platinum-sensitive chemotherapy for
recurrent cancer, where 4 cycles of combined liposomal doxorubicin
and trabectidin second-line chemotherapy were administered. Once
again, the disease was effectively controlled. Subsequently, the
disease relapsed on multiple occasions and began developing
resistance to chemotherapy. However, the patient's disease was
controlled effectively for a long time with appropriate
modifications to the drug regime. Eventually the patient succumbed
9 years after the primary diagnosis.
Once ovarian cancer relapses, the therapeutic
modality changes from curative to palliative. Recurrent ovarian
cancer, in the majority of instances, cannot be cured (3). However, palliative treatment may be
effective in prolonging the survival rate and improving quality of
life. In the past, platinum-sensitive chemotherapy for relapse
involved the use of a single platinum-based agent. However, more
recently, evidence-based medicine has revealed that a
platinum-based combination therapy led to an improved outcome over
monotherapy (8). On the basis of
OVA-301 Phase III RCT results, platinum-containing combination
agents including liposomal doxorubicin and trabectedin may
significantly improve survival rates (8). The patient was enrolled in the OVA-301
study and benefited markedly with prolonged progression-free
survival (>1 year). Therefore, patients with ovarian cancer
relapse may be encouraged to participate in clinical trials
(9,10). CA125 testing served a key role in
disease follow-up. A previous Phase III RCT has confirmed that the
initiation of chemotherapy at the onset of CA125 increase does not
improve patients' long-term survival rates (8). Thus, a patient's CA125 level has a
limited diagnostic role in the context of relapse and should always
be supported by further imaging studies. Treatment should only be
initiated upon confirmation of the diagnosis using imaging and
physical examination (11).
In the present case of a patient with stage IV
ovarian cancer, neoadjuvant chemotherapy, interval cytoreductive
surgery and long-term multiple chemotherapy following surgery
controlled the disease effectively. Multiple surgeries should not
be considered as the standard treatment for recurrent ovarian
cancer. Instead, multiple sessions of chemotherapy may be useful in
the management of relapsed disease. Appropriate treatment should be
selected on the basis of the patient's condition in order to
maximize the therapeutic efficacy.
References
|
1
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narod S: Can advanced-stage ovarian cancer
be cured? Nat Rev Clin Oncol. 13:255–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson B and Tropé CG: Ovarian cancer:
Diagnostic, biological and prognostic aspects. Womens Health
(Lond). 10:519–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Burg ME, van Lent M, Buyse M,
Kobierska A, Colombo N, Favalli G, Lacave AJ, Nardi M, Renard J and
Pecorelli S: The effect of debulking surgery after induction
chemotherapy on the prognosis in advanced epithelial ovarian
cancer. Gynecological cancer cooperative group of the European
organization for research and treatment of cancer. N Engl J Med.
332:629–634. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Redman CW, Warwick J, Luesley DM, Varma R,
Lawton FG and Blackledge GR: Intervention debulking surgery in
advanced epithelial ovarian cancer. Br J Obstet Gynaecol.
101:142–146. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose PG, Nerenstone S, Brady MF,
Clarke-Pearson D, Olt G, Rubin SC, Moore DH and Small JM;
Gynecologic Oncology Group, : Secondary surgical cytoreduction for
advanced ovarian carcinoma. N Engl J Med. 351:2489–2497. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ,
Panici PB, et al: Neoadjuvant chemotherapy or primary surgery in
stage IIIC or IV ovarian cancer. N Engl J Med. 363:943–953. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colombo N: Efficacy of trabectedin in
platinum-sensitive-relapsed ovarian cancer: New data from the
randomized OVA-301 study. Int J Gynecol Cancer. 21 Suppl 1:S12–S16.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaye SB, Colombo N, Monk BJ, Tjulandin S,
Kong B, Roy M, Chan S, Filipczyk-Cisarz E, Hagberg H, Vergote I, et
al: Trabectedin plus pegylated liposomal doxorubicin in relapsed
ovarian cancer delays third-line chemotherapy and prolongs the
platinum-free interval. Ann Oncol. 22:49–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poveda A, Vergote I, Tjulandin S, Kong B,
Roy M, Chan S, Filipczyk-Cisarz E, Hagberg H, Kaye SB, Colombo N,
et al: Trabectedin plus pegylated liposomal doxorubicin in relapsed
ovarian cancer: Outcomes in the partially platinum-sensitive
(platinum-free interval 6–12 months) subpopulation of OVA-301 phase
III randomized trial. Ann Oncol. 22:39–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rustin GJ: Follow-up with CA125 after
primary therapy of advanced ovarian cancer has major implications
for treatment outcome and trial performances and should not be
routinely performed. Ann Oncol. 22 Suppl 8:viii45–viii48. 2011.
View Article : Google Scholar : PubMed/NCBI
|