Introduction
Gastric cancer (GC) is the fourth most common cancer
worldwide and the second leading cause of cancer-related death
(1). Surgical resection and adjuvant
chemotherapy is the most recommended treatment for advanced GC;
however, recurrence rates remain high (2). Therefore, identifying biomarkers that
predict prognosis and response to chemotherapy in patients with GC
remains a high priority. Secreted protein acidic and rich in
cysteine (SPARC) is an extracellular matrix (ECM) glycoprotein and
plays essential roles in normal tissue remodeling and wound repair
(3). In cancerous tissue, SPARC has
been associated with epithelial-mesenchymal transition (EMT)
through mediating interactions with different ECM components and
growth factors, as well as regulating the properties of invasion
and metastasis (4–7).
Many studies have reported that SPARC is expressed
in various types of cancers; however, the relationship between its
expression pattern and disease prognosis is still under
investigation. Some studies concluded that overexpression of SPARC
is associated with disease progression and poor prognosis in
biliary tract cancer, esophageal squamous cell cancer, pancreatic
cancer, head and neck cancer, and breast cancer (8–12);
however, others reported that overexpression of SPARC is associated
with better prognosis in colorectal cancer (13). In GC, it remains unclear whether or
not overexpression of SPARC is associated with better prognosis.
Recently, SPARC localization was reported to be a key factor when
assessing the relationship between SPARC expression and prognosis
in various cancers (8,14). Furthermore, a relationship between
SPARC expression and chemosensitivity has been reported (8,14–17). To date, as for GC, patterns of SPARC
localization are still undetermined; moreover, few studies have
assessed the relationship between the localization of SPARC
positive cells and prognosis in terms of chemosensitivity.
In this study, we examined the localization of SPARC
in GC cells and investigated the relationship between SPARC
expression and prognosis in GC patients who underwent curative
resection. We further compared prognosis in patients who received
adjuvant chemotherapy vs. those who did not.
Materials and methods
GC resected specimens
We collected surgically resected GC specimens from
117 consecutive patients who underwent curative surgery (excluding
patients with stage IA) between 2004 and 2010 at Yamaguchi
University Hospital. Resected specimens were fixed in formalin and
embedded in paraffin prior to immunohistochemistry (IHC). The use
of resected samples was approved by the Human Ethics Committee of
the Graduate School of Medicine, Yamaguchi University. Written
informed consent was obtained from all patients included in this
study.
SPARC IHC
Three anti-SPARC antibodies, ON1-1 (Invitrogen,
Carlsbad, CA, USA), SPARCL1 (ProteinTech Group, Inc., Chicago, IL,
USA), and AON-5031 (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
were tested to select the most suitable for SPARC IHC. The AON-5031
monoclonal antibody used in the study by Inoue et al
(18), Zhao et al (19) and Zhang et al (20) was chosen because it was least prone to
nonspecific staining.
To determine the cells that express SPARC in GC, we
performed IHC using large tissue sections that contained both
noncancerous and cancerous tissues. We examined the expression of
SPARC in non-neoplastic gastric tissues, in the cytoplasm of the
primary cancer cells, and in the stromal cells surrounding the
cancer cells. Resected specimens were cut into 4-µm slices and
deparaffinized using routine techniques. Antigen retrieval was
performed in 10 mM sodium citrate buffer (pH 6.0) heated at 95°C in
a steamer for 20 min. After blocking endogenous peroxidase activity
with a 3% aqueous H2O2 solution for 5 min,
the sections were incubated with serum-free protein block (Dako,
Carpinteria, CA, USA) for 10 min and the sections were incubated
with an anti-SPARC monoclonal antibody (AON-5031) at a final
concentration of 0.1 mg/ml for 60 min. Labeling was detected with
the Envision Plus Detection kit (DAKO) following the manufacturer's
protocol, and the staining was visualized by incubating with DAB
for 5 min followed by counterstaining with hematoxylin. Sections of
human placenta were used as positive controls; for negative
controls, the primary antibody was substituted with non-immunized
immunoglobulin G (Vector Laboratories Inc. Burlingame, CA,
USA).
Evaluation of SPARC expression
Staining was analyzed by 2 certified pathologists of
Japan blinded to any knowledge of the clinicopathological
parameters. SPARC-positive cells were located only in the
peritumoral stroma; therefore, we further evaluated the various
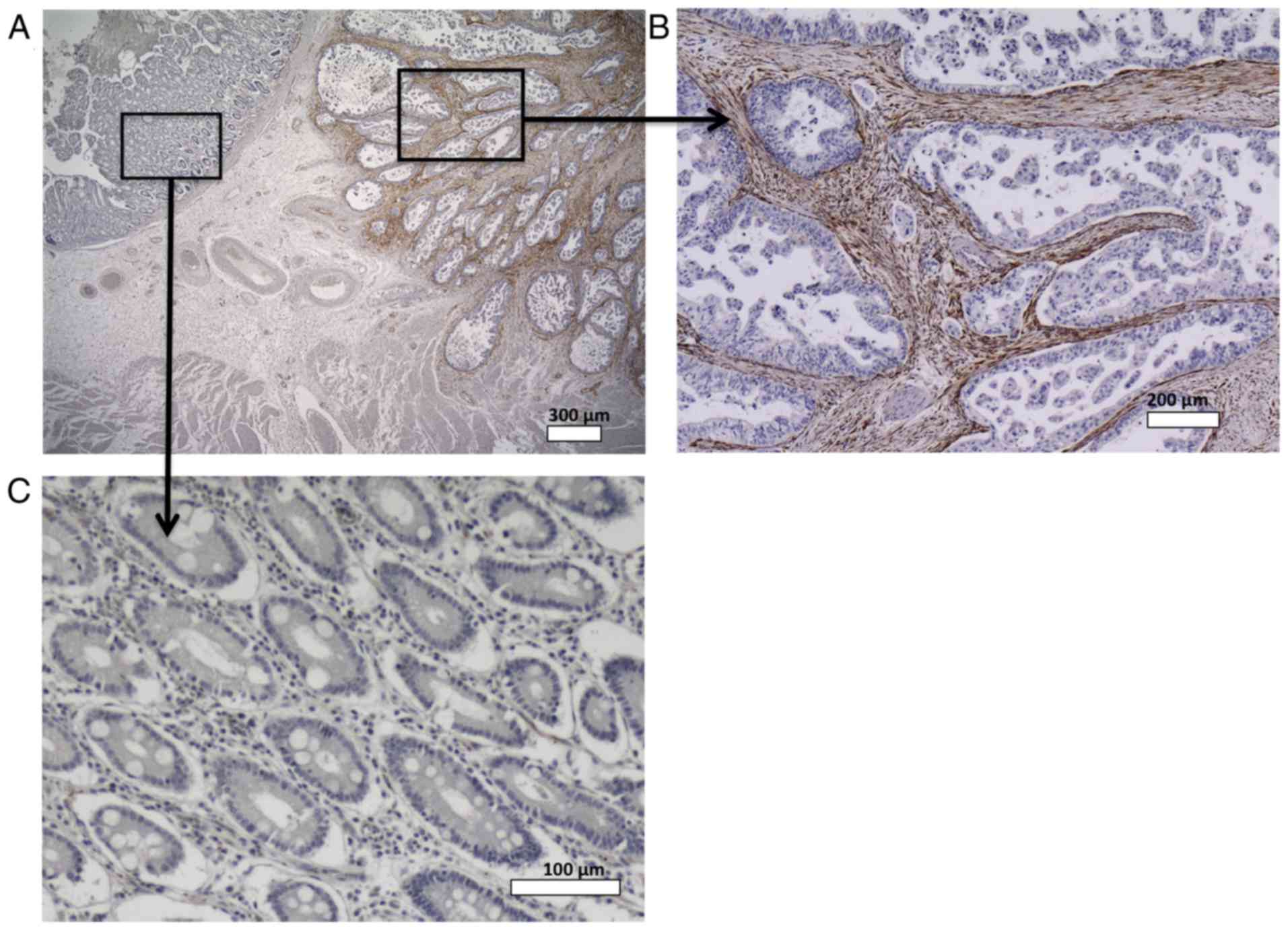
components of the cancerous lesion (Fig.
1). The IHC for SPARC was scored and categorized as previously
reported by Zhao et al (19);
briefly, the proportions of cells with SPARC expression were rated
as follows: 0, ≤5% positive cells; 1, 6–25% positive cells; 2,
26–50% positive cells; and 3, ≥51% positive cells. The intensity of
staining varied from weak to strong and was classified on a scale
of 0 (no staining); 1 (weak staining, light yellow); 2 (moderate
staining, yellowish brown), and 3 (strong staining, brown). The
staining index (SI) was calculated as the product of the staining
intensity score and the proportion of positive cells; we obtained
SI scores of 0, 1, 2, 3, 4, 6 or 9. An SI score ≥4 was defined as
high SPARC expression, while a score ≤3 was defined as low SPARC
expression. The heterogeneity of SPARC expression was defined as
previously reported by Lee et al (21). Briefly, samples with >5% and ≤50%
stromal cells with a SPARC IHC intensity of 2 or 3 were considered
to be heterogeneous, whereas the others were considered to be
homogeneous SPARC expression in the large section.
Double staining
Some tissue sections were double-stained with
anti-α-smooth muscle actin (α-SMA) antibody (1:125; ab5694; Abcam,
Cambridge, MA, USA) and anti-SPARC antibody, in the same way
described above, to analyze the relationship between SPARC
expression and α-SMA-positive fibroblasts.
Statistical analysis
All data were expressed as medians with
interquartile ranges. Baseline patient characteristics were
compared by using the Wilcoxon-Mann-Whitney test for continuous
variables and Fisher's exact test for non-continuous variables.
Overall survival (OS) and recurrence free survival (RFS) rates were
analyzed using the Kaplan-Meier method with log-rank tests. The
independent significance of each factor was determined by the Cox
proportional hazards model, following inclusion of prognostic
variables showing a significant P-value on univariate analysis. On
multivariate analyses of all patients, all variables
(differentiation, pT stage, pN stage, vascular invasion, UICC
stage, adjuvant chemotherapy, and stromal SPARC expression) were
included to predict significant risk factors for OS, RFS, and
invasion to vascular systems.
To adjust for significant differences in the
baseline characteristics of patients, propensity score matching was
used. Propensity scores were calculated accounting for all factors
significantly associated with the staining index through a logistic
regression model based on the following 9 covariates: Age, sex,
differentiation, depth of wall invasion, invasion into the venous
system, invasion into the lymphatic system, nodal status, TNM
stages, and adjuvant chemotherapy. Propensity scores represented
the likelihood of a patient expressing high SPARC relative to low
SPARC; patients of these 2 groups were then paired 1:1 based on
propensity scores using the ‘greedy’ nearest neighbor matching
algorithm without replacement. A caliper size of 0.2xlog (standard
deviation of the propensity score) was utilized. Standardized
differences were estimated before and after matching to evaluate
the balance of covariates. Following 1:1 propensity score matching,
OS and RFS between the matched 2 groups were examined by
Kaplan-Meier estimates using the log rank test.
In general, all statistical analyses were 2-tailed,
and P<0.05 was considered to indicate a statistically
significant difference.. All statistical analyses were performed
with JMP Pro 11.0 software (SAS Institute, Cary, NC, USA).
Results
SPARC expression and localization in
the resected GC specimens
Fig. 1 shows SPARC
expression in GC and normal gastric tissues. In normal tissues,
SPARC staining was observed in the nerve bundle and endothelial
cells; however, SPARC staining was rare in the gastric mucosa and
stromal cells. In cancer tissues, SPARC was observed in the
cytoplasm of fibroblasts surrounding the cancer cells, but was rare
in the cytoplasm of cancer cells themselves.
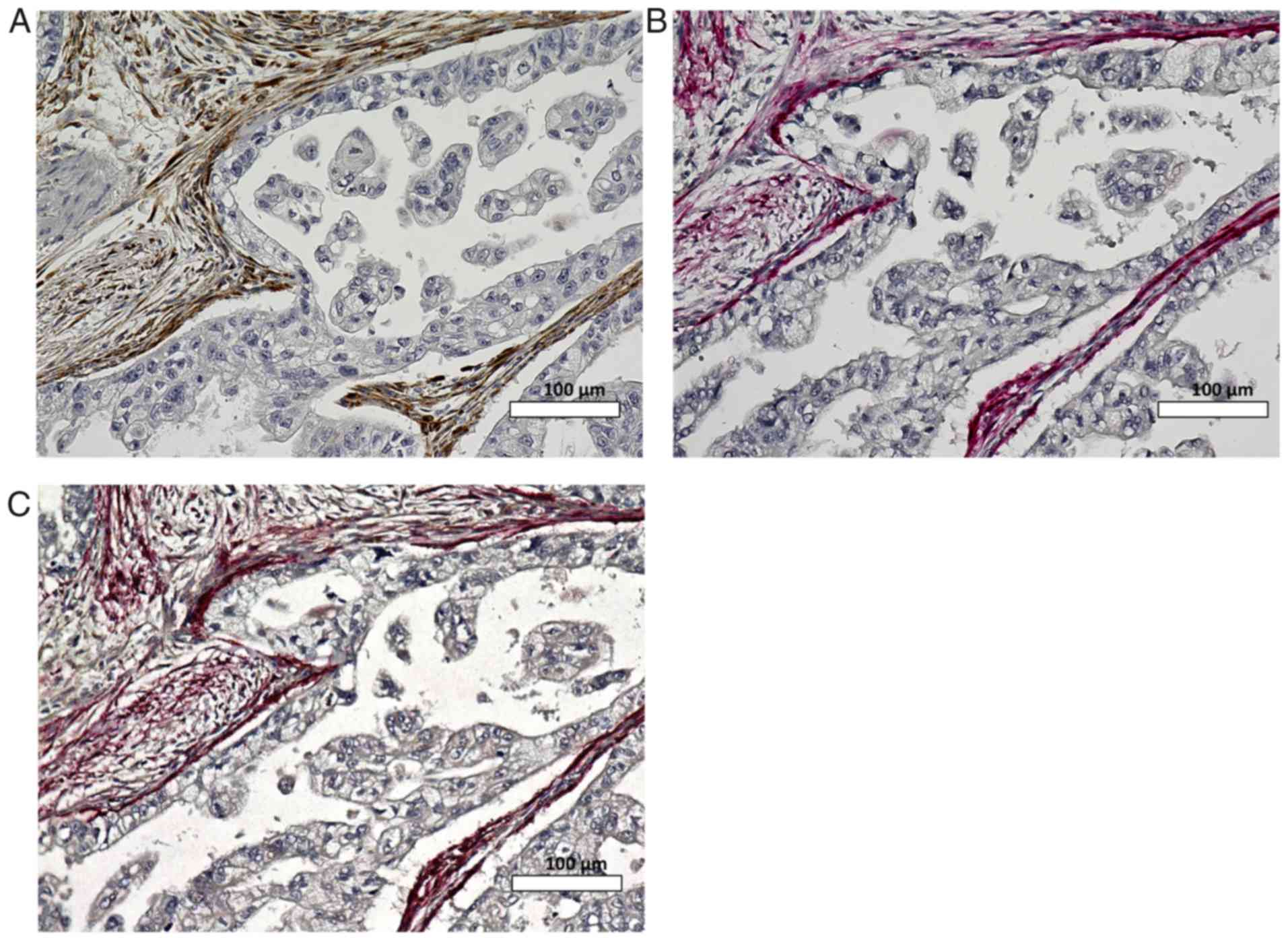
In certain SPARC-positive sections, cancer cells
were surrounded by anti-α-SMA antibody-stained fibroblasts that
were strongly positive for SPARC. Fig.
2 shows SPARC and α-SMA expression using the double staining
method. α-SMA strong-positive fibroblasts were localized to
peritumoral cells; these cells expressed SPARC more intensely than
α-SMA negative fibroblasts.
Intensity and heterogeneity of SPARC
expression of the resected GC specimens
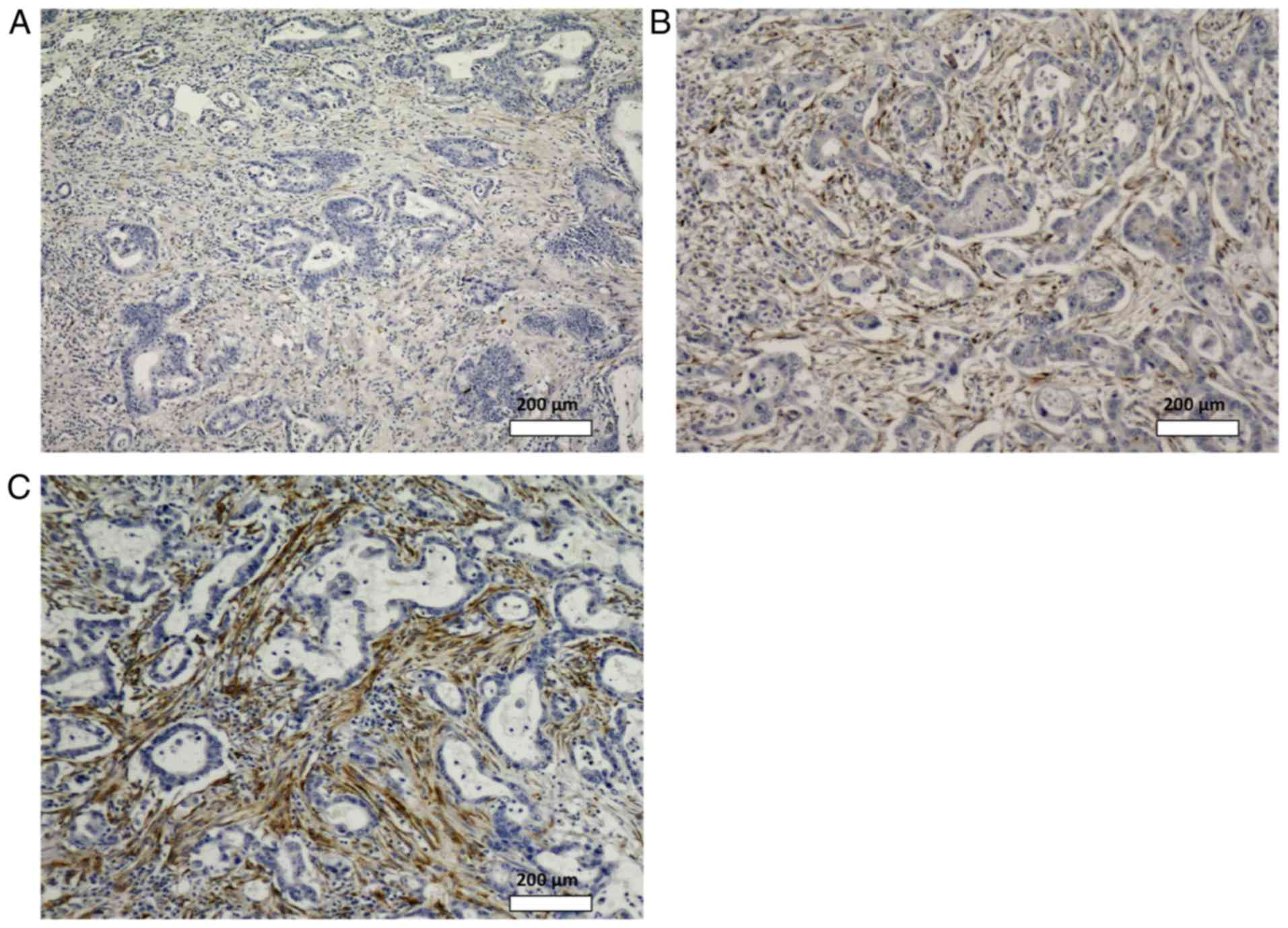
Among the 117 patients we evaluated, the number of
cases with SPARC SI scores of 0, 1, 2, 3, 4, 6, and 9 were 24, 9,
21, 16, 11, 29, and 7, respectively. High SPARC expression (SI ≥4)
was observed in 47 cases (40%). Fig.
3 shows examples of stromal SPARC expression with SI scores of
0, 4, and 9, respectively. Heterogeneity of staining was observed
in 52 cases (44%), among which 23 showed high SPARC expression.
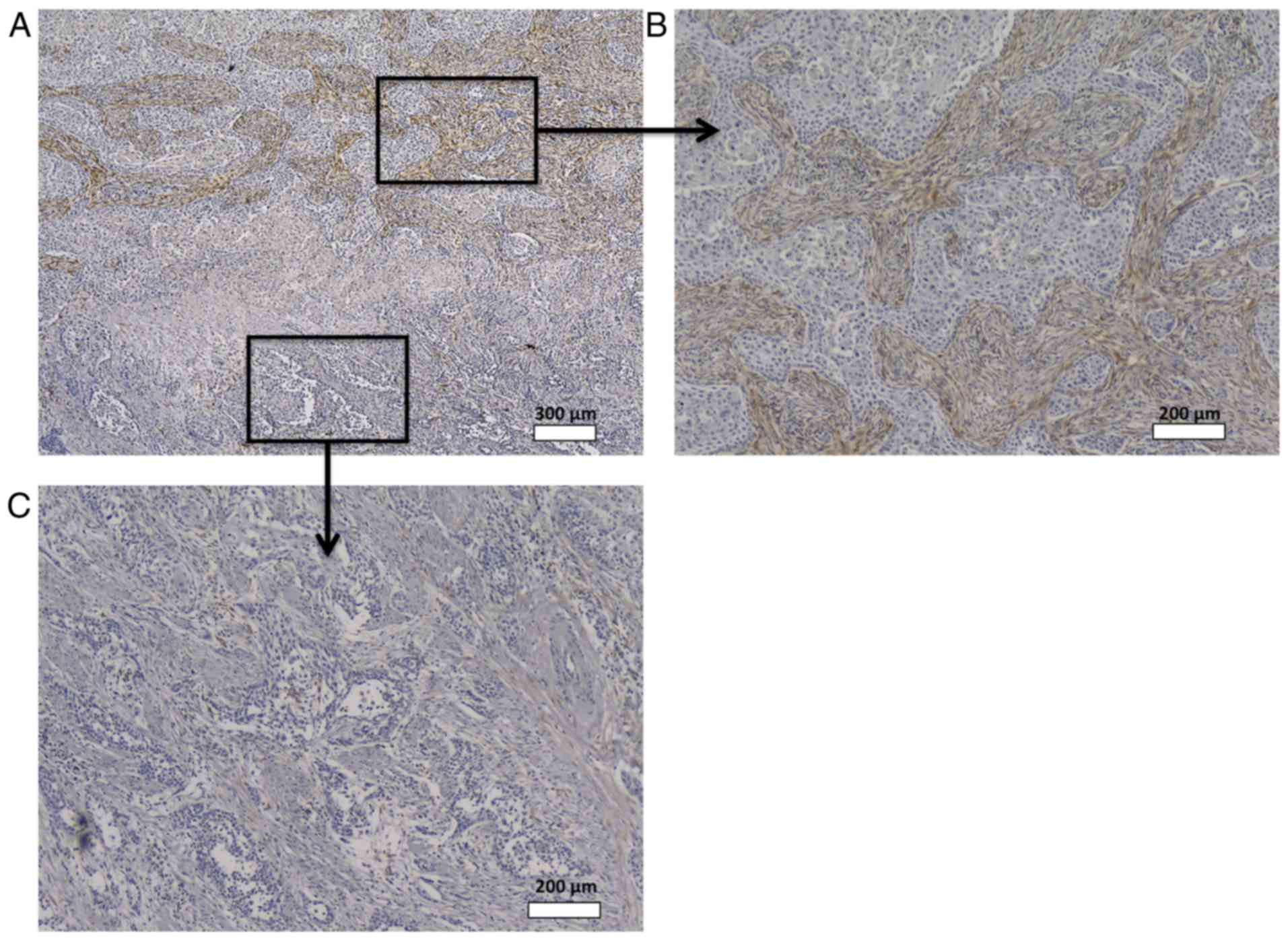
Fig. 4 shows a representative case of
heterogeneous SPARC expression.
Relationship between stromal SPARC expression and
clinicopathological factors of cohorts before and after propensity
score matching. The patients' characteristics are summarized in
Table I. All patients underwent R0
resection, and 76 (66%) had pathological lymph node metastases.
Seventy-three patients received adjuvant chemotherapy (60%),
including 49 with S-1, 20 with tegafur-uracil, and 24 with
paclitaxel (22 patients received more than 1 regimen). Age and
histology were significantly different between the high- and
low-SPARC groups before propensity matching; however, 36 couples
were matched after propensity score-matched analysis, and all their
variables were balanced (Table
I).
 | Table I.Clinicopathological characterristics
of study group and association with SPARC expression before and
after propensity matching. |
Table I.
Clinicopathological characterristics
of study group and association with SPARC expression before and
after propensity matching.
|
| Before propensity
matching | After propensity
matching |
|---|
|
|
|
|
|---|
|
| SPARC Scoring
index |
| SPARC Scoring
index |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | ≦3 (N=70) | ≧4 (N=47) | P-value | ≦3 (N=36) | ≧4 (N=36) | P-value |
|---|
| Age (median) | 66 | 63 | 0.046 | 65 | 64 | 0.959 |
| Gender |
|
| 0.546 |
|
| 1.000 |
|
Male | 50 | 31 |
| 23 | 23 |
|
|
Female | 20 | 16 |
| 13 | 13 |
|
| Histology |
|
| 0.013 |
|
| 1.000 |
|
Intestinal | 22 | 26 |
| 16 | 16 |
|
|
Diffuse | 48 | 21 |
| 20 | 20 |
|
| Depth of wall
invasion |
|
| 0.696 |
|
| 0.799 |
| T1,
T2 | 51 | 20 |
| 10 | 12 |
|
| T3,
T4 | 45 | 32 |
| 26 | 24 |
|
| Lymph node
metastasis |
|
| 0.331 |
|
| 1.000 |
|
Negative | 22 | 19 |
| 11 | 12 |
|
|
Positive | 48 | 28 |
| 25 | 24 |
|
| TNM stages |
|
| 0.703 |
|
| 0.813 |
| I,
II | 40 | 29 |
| 18 | 20 |
|
| III,
IV | 30 | 18 |
| 18 | 16 |
|
| Venous
invasion |
|
| 0.839 |
|
| 0.786 |
|
Negative | 23 | 14 |
| 8 | 10 |
|
|
Positive | 47 | 33 |
| 28 | 26 |
|
| Lymphatic
invasion |
|
| 0.761 |
|
| 0.735 |
|
Negative | 8 | 4 |
| 6 | 4 |
|
|
Positive | 62 | 43 |
| 30 | 32 |
|
| Adjuvant
chemotherapy |
|
| 0.437 |
|
| 1.000 |
|
Negative | 24 | 20 |
| 15 | 14 |
|
|
Positive | 46 | 27 |
| 21 | 22 |
|
Relationship between SPARC expression
and survival after surgery
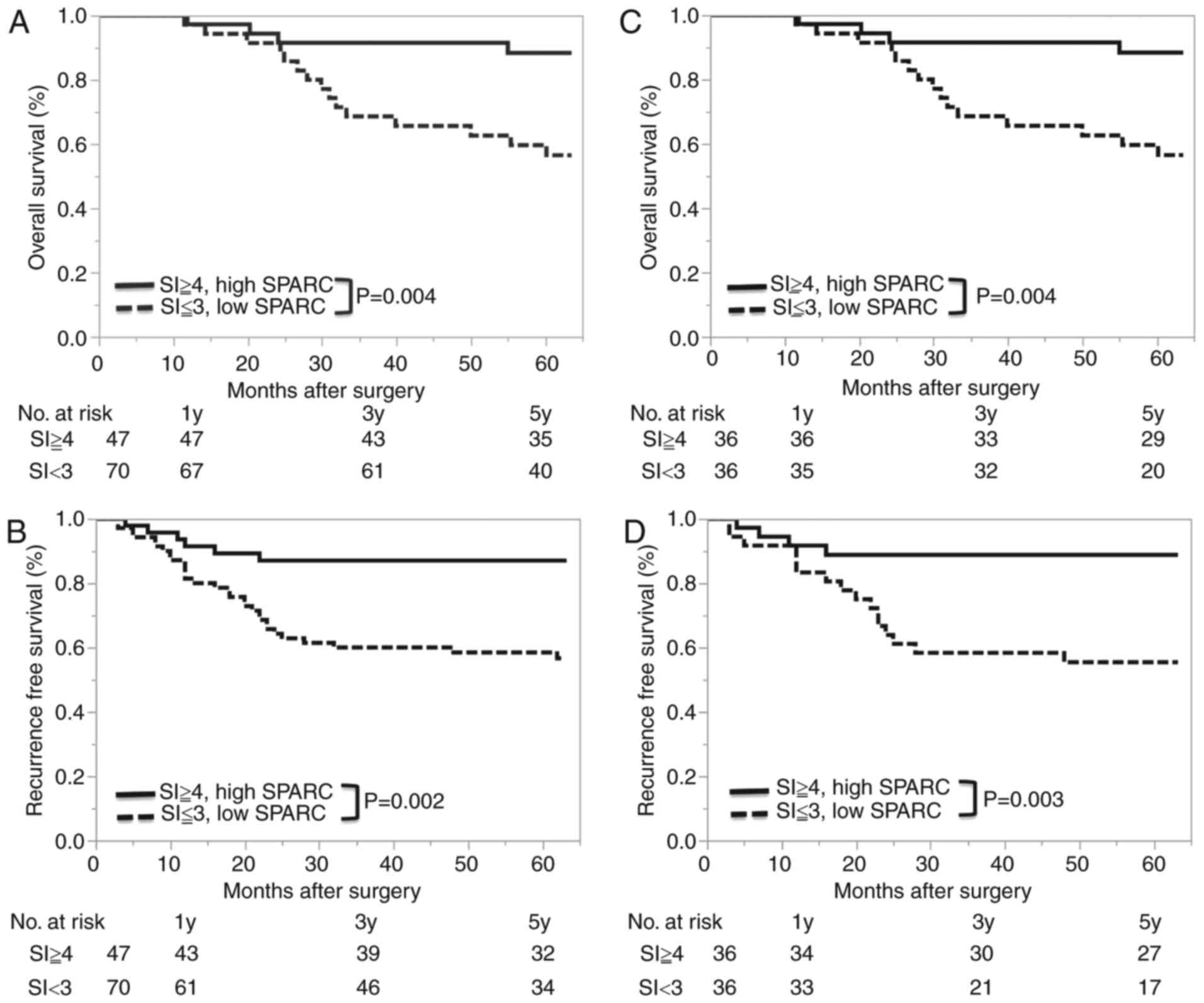
The 5-year OS rate was 87% (95% confidence interval
(CI), 73–94%) in the high SPARC group, and 62% (95% CI, 50–71%) in
the low SPARC group. The hazard ratio (HR) for death in the high
SPARC group compared to the low SPARC group was 0.294 (95% CI,
0.109–0.666, log-rank P=0.004) (Fig.
5). The 5-year RFS rate was 87% (95% CI, 74–97%) in the high
SPARC group, and 59% (95% CI, 47–70%) in the low SPARC group. The
HR for recurrence in the high SPARC group compared to the low SPARC
low group was 0.305 (95% CI, 0.113–0.689, log-rank P=0.004)
(Fig. 5). There was no relationship
between the heterogeneity of the SPARC expression and
prognosis.
Tables II and
III show the univariate and
multivariate analyses of OS and RFS, respectively, using Cox
regression. Univariate analysis revealed that low stromal SPARC
expression, UICC stage, invasion into the lymphatic system,
invasion into the venous system, and the depth of wall invasion
were associated with poor prognosis. Multivariate logistic
regression analysis demonstrated that only low stromal SPARC
expression remained a poor prognostic factor for OS (HR, 3.884; 95%
CI, 1.691–10.514, P=0.0009); furthermore, low stromal SPARC
expression and invasion into the lymphatic system were poor
prognostic factors for RFS independent of other clinicopathological
factors. Multivariate logistic regression analysis revealed that
high SPARC expression had no significant association with venous or
lymphatic invasion (OR, 0.439; 95% CI, 0.065–2.380, P=0.439).
 | Table II.The univariate and multivariate
analysis for overall survival using Cox regression analysis. |
Table II.
The univariate and multivariate
analysis for overall survival using Cox regression analysis.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Covariates | N | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
Differentiation |
|
|
| 0.893 |
|
| 0.377 |
|
Intestinal | 69 | 1 |
|
| 1 |
|
|
|
Diffuse | 45 | 0.954 | 0.481–1.939 |
| 0.721 | 0.353–1.507 |
|
| Depth of wall
invasion |
|
|
| 0.015 |
|
| 0.718 |
| T1,
T2 | 40 | 1 |
|
| 1 |
|
|
| T3,
T4 | 77 | 2.727 | 1.204–7.309 |
| 1.217 | 0.433–3.808 |
|
| Lymph node
metastasis |
|
|
| 0.060 |
|
| 0.998 |
|
Negative | 41 | 1 |
|
| 1 |
|
|
|
Positive | 76 | 2.117 | 0.970–5.290 |
| 0.998 | 0.259–3.548 |
|
| TNM stages |
|
|
| 0.001 |
|
| 0.08 |
|
≦II | 69 | 1 |
|
| 1 |
|
|
|
≧III | 48 | 3.063 | 1.523–6.422 |
| 2.572 | 0.9–8.99 |
|
| Venous
invasion |
|
|
| 0.007 |
|
| 0.113 |
|
Negative | 37 | 1 |
|
| 1 |
|
|
|
Positive | 80 | 3.167 | 1.331–9.331 |
| 2.184 | 0.842–6.896 |
|
| Lymphatic
invasion |
|
|
| 0.002 |
|
| 0.054 |
|
Negative | 12 | 1 |
|
| 1 |
|
|
|
Positive | 105 | 1.656 | 1.632–5.363 |
| 3.718 | 0.912–9.325 |
|
| SPARC expression
(H/L) |
|
|
| 0.002 |
|
| <0.001 |
|
Low | 70 | 1 |
|
| 1 |
|
|
|
High | 47 | 0.294 | 0.109–0.666 |
| 0.258 | 0.095–0.592 |
|
| Adjuvant
chemotherapy |
|
|
| 0.169 |
|
| 0.385 |
|
Negative | 44 | 1 |
|
| 1 |
|
|
|
Positive | 73 | 1.679 | 0.808–3.817 |
| 0.615 | 0.213–1.884 |
|
 | Table III.The univariate and multivariate
analysis for relapse free survival using Cox regression
analysis. |
Table III.
The univariate and multivariate
analysis for relapse free survival using Cox regression
analysis.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Covariates | N | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
Differentiation |
|
|
| 0.926 |
|
| 0.265 |
|
Intestinal | 69 | 1 |
|
| 1 |
|
|
|
Diffuse | 45 | 0.969 | 0.502–1.915 |
| 0.672 | 0.339–1.363 |
|
| Depth of wall
invasion |
|
|
| 0.029 |
|
| 0.754 |
| T1,
T2 | 40 | 1 |
|
| 1 |
|
|
| T3,
T4 | 77 | 2.369 | 1.085–5.925 |
| 1.178 | 0.434–3.449 |
|
| Lymph node
metastasis |
|
|
| 0.051 |
|
| 0.871 |
|
Negative | 41 | 1 |
|
| 1 |
|
|
|
Positive | 76 | 2.087 | 0.997–4.907 |
| 0.904 | 0.255–3.008 |
|
| TNM stages |
|
|
| 0.004 |
|
| 0.219 |
|
≦II | 69 | 1 |
|
| 1 |
|
|
|
≧III | 48 | 2.770 | 1.391–5.808 |
| 1.873 | 0.703–5.84 |
|
| Venous
invasion |
|
|
| 0.007 |
|
| 0.094 |
|
Negative | 37 | 1 |
|
| 1 |
|
|
|
Positive | 80 | 3.167 | 1.331–9.331 |
| 2.204 | 0.883–6.749 |
|
| Lymphatic
invasion |
|
|
| 0.002 |
|
| 0.039 |
|
Negative | 12 | 1 |
|
| 1 |
|
|
|
Positive | 105 | 1.656 | 1.475–4.912 |
| 4.223 | 1.078–4.398 |
|
| SPARC expression
(H/L) |
|
|
| 0.003 |
|
| <0.001 |
|
Low | 70 | 1 |
|
| 1 |
|
|
|
High | 47 | 0.305 | 0.113–0.689 |
| 0.232 | 0.086–0.527 |
|
| Adjuvant
chemotherapy |
|
|
| 0.056 |
|
| 0.994 |
|
Negative | 44 | 1 |
|
| 1 |
|
|
|
Positive | 73 | 2.009 | 0.982–4.528 |
| 0.996 | 0.367–2.939 |
|
Chemotherapy sensitivity and SPARC
expression
Among the 117 patients who underwent R0 surgery, 73
patients (62%) received adjuvant chemotherapy. Metastatic lymph
nodes, higher TNM stage, deeper wall invasion, and venous and
lymphatic invasion were more common in patients treated with
adjuvant chemotherapy than in patients without adjuvant
chemotherapy (P<0.05 respectively, data not shown); however,
there was no difference in SI scores between the groups.
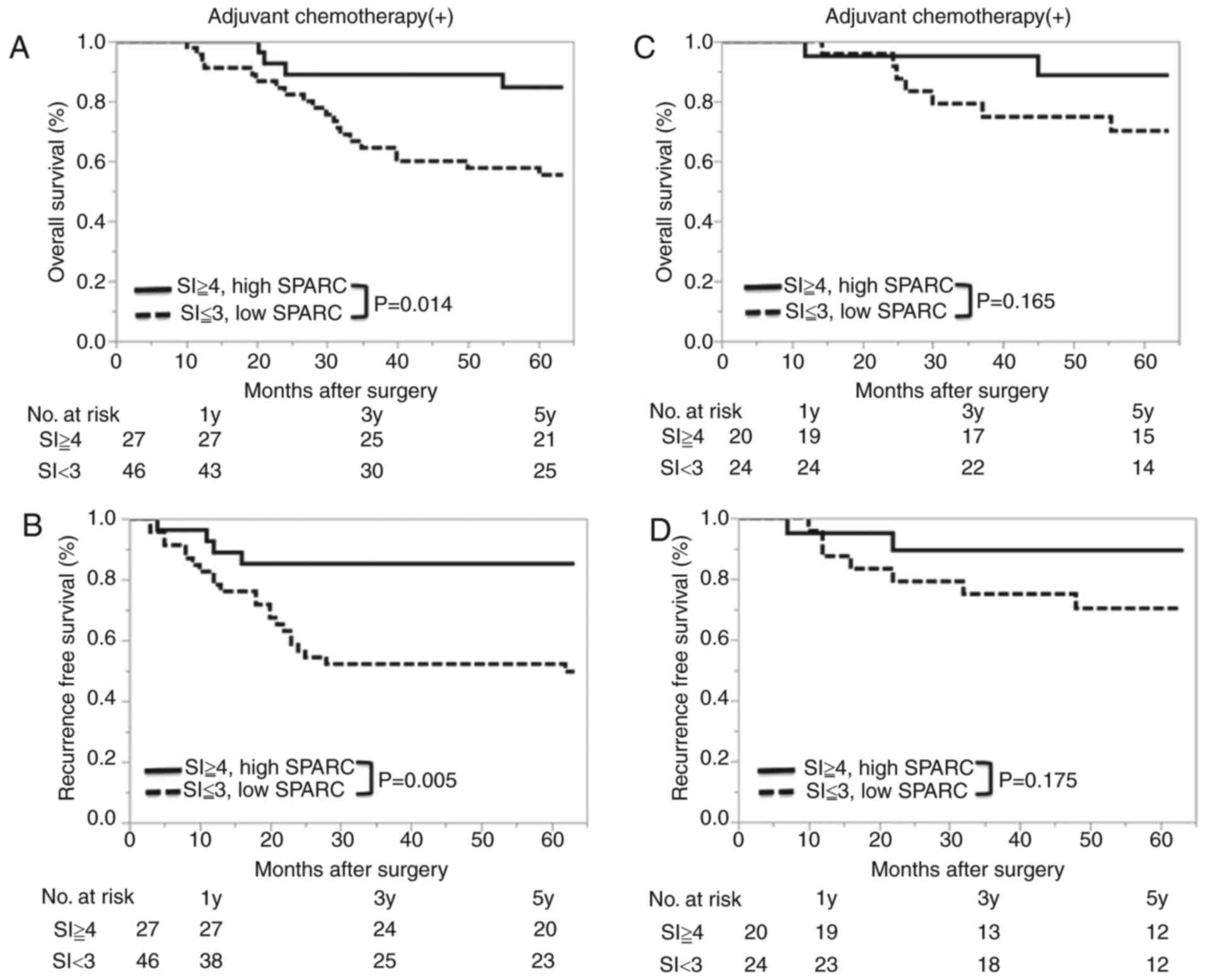
Fig. 6 shows the
relationship between SPARC expression and prognosis according to
adjuvant chemotherapy. In patients who received adjuvant
chemotherapy, better prognosis was observed in the high SPARC group
(for OS: HR, 3.54; 95% CI, 1.34–12.2, P=0.0091; for RFS: HR, 4.09;
95% CI, 1.57–13.9, P=0.0026). However, in patients without adjuvant
chemotherapy, there was no relationship between prognosis and SPARC
expression. Regarding the type of chemotherapy regimen, only in the
patients with the taxane-containing chemotherapy regimen, there was
significant difference about RFS between the two groups.
Propensity score matching
analysis
As previously mentioned, 36 couples were matched
after propensity score-matched analysis. All of the baseline
clinicopathological characteristics between the high and low SPARC
groups were balanced, and OS and RFS remained longer in the high
SPARC group (Fig. 5).
Discussion
The present study revealed 3 important clinical
findings. First, SPARC is expressed in the cytoplasm of fibroblasts
(especially α-SMA-positive fibroblasts) surrounding the cancer
cells; however, SPARC was rarely expressed in the cytoplasm of
cancer cells themselves or in the stromal cells of normal tissue in
GC patients. Second, high SPARC expression was observed in 40% of
GC samples, and heterogeneity of SPARC expression was observed in
44%. Third, high stromal SPARC expression was found to be an
independent prognostic factor for more favorable OS and RFS in GC
patients who underwent curative resection; this relationship was
significant in patients who received adjuvant chemotherapy.
Previously, various studies found that SPARC is
upregulated in GC; however, there are discrepancies regarding the
reported localization of SPARC in the stomach. Some papers reported
a differential expression of SPARC, mainly in the cytoplasm of GC
cells (19,22,23);
whereas, others found that SPARC exists mainly in the stromal cells
surroundings the GC cells (24–26). These
discrepancies may be attributed to the method of IHC (choice of
primary antibody, antibody dilutions and staining protocols) and/or
the selection of tissue specimens. We established a precise IHC
method to determine SPARC levels in gastric tissue while reducing
nonspecific staining, and demonstrated that SPARC is expressed in
the fibroblasts surrounding the cancer cells. Previous studies in
other cancer types (13,24–26) are
consistent with our results; this provides strong evidence that our
methods are reliable. Moreover, SPARC was mainly expressed in
α-SMA-positive peritumoral fibroblasts. A proportion of
α-SMA-positive fibroblasts are reported to be cancer-associated
fibroblasts derived from bone marrow (27), signifying a possible relationship
between these types of fibroblasts and SPARC; however, this
requires further study.
Although high stromal SPARC expression was a
significant prognostic factor in patients who received adjuvant
chemotherapy, no such relationship was observed in those without
adjuvant chemotherapy. Our findings are inconsistent with those
described in previous reports (19,22–24). This
may be explained by the localization and heterogeneity of SPARC
expression as they relate to chemosensitivity. Recent reports
suggest that only stromal SPARC expression plays an important role
in prognosis (8,14). Moreover, our study was the first to
describe heterogeneity of SPARC expression, which in our case was
found in 44% of our samples. Zhao et al (19) evaluated SPARC expression using tissue
microarray sections, and reported that cases with high SPARC
expression in tumoral and peritumoral cells showed poor prognosis;
however, they never evaluated the heterogeneity and location of
SPARC expression (24). Therefore, it
is very important to use large tissue sections to be able to assess
the localization and heterogeneity of SPARC expression in the tumor
milieu. To our knowledge, our study is the largest series that used
large tissue sections to evaluate ‘stromal’ SPARC expression in GC
patients. Moreover, our study is the first to use
propensity-matched analysis for evaluating the relationship between
SPARC expression and prognosis. Therefore, we propose that our
study is more reliable compared to those previously reported.
Hence, high SPARC expression in peritumoral fibroblasts is likely
associated with better prognosis.
The next challenge is to explain why SPARC affects
prognosis. There are two possibilities; one is association with EMT
and the other is chemosensitivity. Previous reports indicated that
high SPARC expression is correlated with a lower potential for EMT
in experimental models (4,5,28,29). Our study showed that high stromal
SPARC expression was associated with better RFS for the first time;
however, we could not demonstrate a relationship between the SPARC
expression and vascular invasion by multivariate analysis. On the
other hand, high stromal SPARC expression was found to be
associated with better prognosis in patients with adjuvant
chemotherapy but not in those without. Therefore, we hypothesized
that SPARC improves prognosis because of an association with
improved chemosensitivity. Previous studies reported the
possibility of SPARC being associated with chemosensitivity in
various cancers (8,14–17,30). As
for GC, there was only 1 study that evaluated the relationship
between SPARC expression and prognosis from the viewpoint of
chemosensitivity (17); however, that
study included patients with preoperative chemotherapy and
evaluated the association between SPARC expression in cancer cells
per se and OS. Our findings raise the possibility that
stromal SPARC increases chemosensitivity in patients with GC. It
has been reported that the antitumor activity of nab-paclitaxel, an
albumin-bound paclitaxel, may be enhanced by binding to
SPARC-positive stroma surrounding the cancer cells (14,31–33). Like
nab-paclitaxel, other cytotoxic drugs may accumulate in the high
SPARC-expressing stroma surroundings GC cells (17). Further study is required to explore
this notion and its associated mechanisms.
Our study is limited by the fact that it was small
and retrospective. To overcome this limitation, prospective
multicenter studies with standardized IHC methods are required to
evaluate stromal SPARC expression using large tissue sections.
In conclusion, our study revealed that high stromal
SPARC expression is an independent and favorable prognostic factor,
in terms of OS and RFS, in GC patients who undergo curative
resection. High SPARC expression may increase the tumor's
sensitivity to adjuvant chemotherapy through accumulating the
anti-cancer drugs in the SPARC positive fibroblasts surrounding the
cancer cells. Hence, evaluating stromal SPARC expression may help
develop individualized therapy in GC patients.
Acknowledgements
The authors would like to thank Ms. Akiko Sano, Ms.
Kaori Kaneyasu, Ms. Madoka Kajiwara and Ms. Yuko Takenouchi for
their technical assistance with this study.
References
|
1
|
Lordick F, Allum W, Carneiro F, Mitry E,
Tabernero J, Tan P, Van Cutsem E, van de Velde C and Cervantes A:
Unmet needs and challenges in gastric cancer: The way forward.
Cancer Treat Rev. 40:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noh SH, Park SR, Yang HK, Chung HC, Chung
IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, et al: Adjuvant
capecitabine plus oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): 5-year follow-up of an open-label,
randomised phase 3 trial. Lancet Oncol. 15:1389–1396. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradshaw AD and Sage EH: SPARC, a
matricellular protein that functions in cellular differentiation
and tissue response to injury. J Clin Invest. 107:1049–1054. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin J, Chen G, Liu Y, Liu S, Wang P, Wan
Y, Wang X, Zhu J and Gao H: Downregulation of SPARC expression
decreases gastric cancer cellular invasion and survival. J Exp Clin
Cancer Res. 29:592010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Wang P, Zhu J, Wang W, Yin J,
Zhang C, Chen Z, Sun L, Wan Y, Wang X, et al: SPARC expression is
negatively correlated with clinicopathological factors of gastric
cancer and inhibits malignancy of gastric cancer cells. Oncol Rep.
31:2312–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brekken RA and Sage EH: SPARC, a
matricellular protein: At the crossroads of cell-matrix
communication. Matrix Biol. 19:816–827. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rivera LB, Bradshaw AD and Brekken RA: The
regulatory function of SPARC in vascular biology. Cell Mol Life
Sci. 68:3165–3173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakashima S, Kobayashi S, Sakai D,
Tomokuni A, Tomimaru Y, Hama N, Wada H, Kawamoto K, Marubashi S,
Eguchi H, et al: Prognostic impact of tumoral and/or peri-tumoral
stromal SPARC expressions after surgery in patients with biliary
tract cancer. J Surg Oncol. 110:1016–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagai MA, Gerhard R, Fregnani JH, Nonogaki
S, Rierger RB, Netto MM and Soares FA: Prognostic value of NDRG1
and SPARC protein expression in breast cancer patients. Breast
Cancer Res Treat. 126:1–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantoni TS, Schendel RR, Rödel F,
Niedobitek G, Al-Assar O, Masamune A and Brunner TB: Stromal SPARC
expression and patient survival after chemoradiation for
non-resectable pancreatic adenocarcinoma. Cancer Biol Ther.
7:1806–1815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamashita K, Upadhay S, Mimori K, Inoue H
and Mori M: Clinical significance of secreted protein acidic and
rich in cystein in esophageal carcinoma and its relation to
carcinoma progression. Cancer. 97:2412–2419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HY, Li YY, Shao Q, Hou JH, Wang F,
Cai MB, Zeng YX and Shao JY: Secreted protein acidic and rich in
cysteine (SPARC) is associated with nasopharyngeal carcinoma
metastasis and poor prognosis. J Transl Med. 10:272012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chew A, Salama P, Robbshaw A, Klopcic B,
Zeps N, Platell C and Lawrance IC: SPARC, FOXP3, CD8 and CD45
correlation with disease recurrence and long-term disease-free
survival in colorectal cancer. PLoS One. 6:e220472011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Von Hoff DD, Ramanathan RK, Borad MJ,
Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias
JL, et al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: A phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeno A, Takemasa I, Doki Y, Yamasaki M,
Miyata H, Takiguchi S, Fujiwara Y, Matsubara K and Monden M:
Integrative approach for differentially overexpressed genes in
gastric cancer by combining large-scale gene expression profiling
and network analysis. Br J Cancer. 99:1307–1315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai IT, Dai M, Owen DA and Chen LB:
Genome-wide expression analysis of therapy-resistant tumors reveals
SPARC as a novel target for cancer therapy. J Clin Invest.
115:1492–1502. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao YY, Han RB, Wang X, Ge SH, Li HL, Deng
T, Liu R, Bai M, Zhou LK, Zhang XY, et al: Change of SPARC
expression after chemotherapy in gastric cancer. Cancer Biol Med.
12:33–40. 2015.PubMed/NCBI
|
|
18
|
Inoue M, Senju S, Hirata S, Ikuta Y,
Hayashida Y, Irie A, Harao M, Imai K, Tomita Y, Tsunoda T, et al:
Identification of SPARC as a candidate target antigen for
immunotherapy of various cancers. Int J Cancer. 127:1393–1403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang JL, Chen GW, Liu YC, Wang PY, Wang
X, Wan YL, Zhu J, Gao HQ, Yin J, Wang W and Tian ML: Secreted
protein acidic and rich in cysteine (SPARC) suppresses angiogenesis
by down-regulating the expression of VEGF and MMP-7 in gastric
cancer. PLoS One. 7:e446182012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HE, Park KU, Yoo SB, Nam SK, Park DJ,
Kim HH and Lee HS: Clinical significance of intratumoral HER2
heterogeneity in gastric cancer. Eur J Cancer. 49:1448–1457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeung HC, Rha SY, Im CK, Shin SJ, Ahn JB,
Yang WI, Roh JK, Noh SH and Chung HC: A randomized phase 2 study of
docetaxel and S-1 versus docetaxel and cisplatin in advanced
gastric cancer with an evaluation of SPARC expression for
personalized therapy. Cancer. 117:2050–2057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang CS, Lin KH, Chen SL, Chan YF and
Hsueh S: Overexpression of SPARC gene in human gastric carcinoma
and its clinic-pathologic significance. Br J Cancer. 91:1924–1930.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franke K, Carl-McGrath S, Röhl FW,
Lendeckel U, Ebert MP, Tänzer M, Pross M and Röcken C: Differential
expression of SPARC in intestinal-type gastric cancer correlates
with tumor progression and nodal spread. Transl Oncol. 2:310–320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeng HY, Song SB, Choi DK, Kim KE, Jeong
HY, Sakaki Y and Furihata C: Osteonectin-expressing cells in human
stomach cancer and their possible clinical significance. Cancer
Lett. 184:117–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Yang M, Shan L, Qi L, Chai C, Zhou
Q, Yao K, Wu H and Sun W: The role of SPARC protein expression in
the progress of gastric cancer. Pathol Oncol Res. 18:697–702. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Sah B, Ma J, Shang C, Huang Z and
Chen Y: A prospective, randomized, controlled, trial comparing
occult-scar incision laparoscopic cholecystectomy and classic
three-port laparoscopic cholecystectomy. Surg Endosc. 28:1131–1135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puolakkainen PA, Brekken RA, Muneer S and
Sage EH: Enhanced growth of pancreatic tumors in SPARC-null mice is
associated with decreased deposition of extracellular matrix and
reduced tumor cell apoptosis. Mol Cancer Res. 2:215–224.
2004.PubMed/NCBI
|
|
30
|
Sinn M, Sinn BV, Striefler JK, Lindner JL,
Stieler JM, Lohneis P, Bischoff S, Bläker H, Pelzer U, Bahra M, et
al: SPARC expression in resected pancreatic cancer patients treated
with gemcitabine: Results from the CONKO-001 study. Ann Oncol.
25:1025–1032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hidalgo M, Plaza C, Musteanu M, Illei P,
Brachmann CB, Heise C, Pierce D, Lopez-Casas PP, Menendez C,
Tabernero J, et al: SPARC expression did not predict efficacy of
nab-paclitaxel plus gemcitabine or gemcitabine alone for metastatic
pancreatic cancer in an exploratory analysis of the phase III MPACT
trial. Clin Cancer Res. 21:4811–4818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schneeweiss A, Seitz J, Smetanay K,
Schuetz F, Jaeger D, Bachinger A, Zorn M, Sinn HP and Marmé F:
Efficacy of nab-paclitaxel does not seem to be associated with
SPARC expression in metastatic breast cancer. Anticancer Res.
34:6609–6615. 2014.PubMed/NCBI
|