Introduction
Thymoma is the most common neoplasm of the
anterosuperior mediastinum, accounting for ~20% of all mediastinal
tumors and 50% of all anterior mediastinal tumors in adults
(1). Thymomas occur at all ages, but
there is a broad peak between 35 and 70 years of age. Men and women
are approximately equally affected, although it is slightly more
common in older women (2). Numerous
thymoma cases present with systemic syndromes, such as myasthenia
gravis, or other associated syndromes, such as immune and
autoimmune diseases. Surgery remains the main treatment for
thymoma, as thymoma pathogenesis is poorly understood (3).
The Wnt signaling pathway is one of a handful of
evolutionarily conserved signal transduction pathways that are used
extensively during animal development (4). Wnt signals control multiple biological
processes, including cellular proliferation, fate specification,
polarity and migration. Activation of the Wnt signaling pathway
plays a role in a variety of human cancers (5–7), and
regulates T cell development (8). In
the thymus, Wnt ligands are expressed primarily on thymus
epithelial cells and activate a highly complex signaling network
via G-protein dependent Frizzled receptors (9).
Wnt4 is one of the Wnt proteins that can activate
non-canonical pathways. It has an important role in thymus
development and maintenance of the thymus microenvironment
(10,11). JNK signaling is necessary for planar
cell polarity (PCP)-like pathways (12), including one of the non-canonical Wnt
pathways. The present study examined the expression of Wnt4 and JNK
mRNA and protein in thymoma. Wnt4 gene expression was then blocked
in thymoma cells by short hairpin (sh)RNA interference, and changes
in JNK mRNA and protein expression and thymoma apoptosis were then
examined. The results indicate the Wnt4/JNK signaling has an
important role in thymoma development and proliferation.
Materials and methods
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of thymoma Wnt4 and
JNK mRNA expression
Thymoma and normal thymus tissue samples were
collected from 15 patients with thymoma and 6 patients with thymus
cysts at the Tianjin Medical University General Hospital (Tianjin,
China). The Tianjin Medical University General Hospital Ethics
Committee approved the present study and written informed consent
was obtained from all patients. A definite pathological diagnosis
was established in patients who underwent surgery but had not
received chemotherapy or radiotherapy between January 2013 and
December 2014. RNA was extracted by tissue homogenization in TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's instructions. Total RNA (1 µg) was
reverse transcribed into cDNA and quantified by RT-qPCR using SYBR
Premix Ex Taq™ (Takara Bio, Inc., Otsu, Japan). PCR primer pairs
for human Wnt4, JNK and GAPDH (Table
I) were designed using GeneRunner (Aoke Biological Technology,
LLC, Beijing, China). Amplification was performed at 94°C for 5 min
for predenaturation, 94°C for 40 sec for denaturation, 59°C for 30
sec for annealing and 72°C for 30 sec for extension, for a total of
45 cycles, with a final extension at 72°C for 10 min. Wnt4 and JNK
PCR products were purified by 1.2% agarose gel and the purpose
strip image was taken by GelDoc 2000 gel imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Positive bands were
measured with gray values and sequenced. The relative expression of
target genes was determined using the 2−ΔΔCq method
(13).
 | Table I.Polymerase chai reaction primer pair
sequences for human Wnt4, JNK and GAPDH. |
Table I.
Polymerase chai reaction primer pair
sequences for human Wnt4, JNK and GAPDH.
| Primer | Sequence |
|---|
| Wnt4 |
|
|
Sense |
5′-ACCTGGAAGTCATGGACTCG-3′ |
|
Antisense |
5′-TCAGAGCATCCTGACCACTG-3′ |
| JNK |
|
|
Sense |
5′-TTTGAGAAACTCTTCCCTGATG-3′ |
|
Antisense |
5′-ATTGATGTACGGGTGTTGGA-3′ |
| GAPDH |
|
|
Sense |
5′-TGGAGTCTACTGGCGTCTTC-3′ |
|
Antisense |
5′-TTCACACCCATCACAAACATG-3′ |
Immunohistochemistry of Wnt4 and JNK
protein expression in thymoma tissues
Thymoma tissue was fixed in 4% paraformaldehyde PBS
for 12 h at room temperature. Following dehydration, cleared and
paraffin embedded thymoma tissue sections (4 µm) were
deparaffinized, dehydrated and subjected to antigen retrieval with
Tris-EDTA by high temperature (117°C) and high pressure (180 kPa)
for 3 min. Tissue sections were blocked using 3% hydrogen peroxide
solution for 10 min at 37°C and non-specific binding was blocked
using 10% normal goat serum (cat. no. SP-9000, SPlink Detection
kits; ZSGB-BIO Technologies, Inc., Beijing, China) for 10 min at
room temperature. Sections were incubated with rabbit anti-Wnt4
polyclonal antibody (dilution 1:100, cat. no. ab189037; Abcam,
Cambridge, UK) and mouse anti-JNK monoclonal antibody (dilution
1:25, cat. no. ab201624; Abcam) overnight at 4°C. The sections were
incubated with 100 µl goat anti-rabbit IgG secondary antibody for
30 min at 37°C (ready to use, cat. no. SP-9000; ZSGB-BIO
Technologies, Inc.). Brown granules indicated expression. The
frequency of positive cells in five high-power fields
(magnification, ×400, Leica DMIL; Leica Microsystems GmbH, Wetzlar,
Germany) of each section was determined by counting at least 2,000
thymoma cells in a blinded manner.
Cell source and culture
A human thymoma cell line was derived in
vitro from a 50-year old Chinese man with AB-type thymoma and
myasthenia gravis. The cell line was maintained in primary culture
at Tianjin Medical University General Hospital in Dulbecco's
modified Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml
streptomycin, at 37°C in a humidified 5% CO2 atmosphere
(14).
Construction and selection of
interference plasmid
A Psi-H1 shRNA expression vector was chosen to
interfere with the plasmid hairpin loop sequence TCAAGAG. Four Wnt4
shRNA interference plasmids were constructed using the HSHTR001
plasmid, which also acted as an empty plasmid control. All plasmids
were obtained from Guangzhou Fulengen Co., Ltd. (Guangzhou, China)
(Table II). Plasmid amplification
and extraction used Trans1-T1 phage-resistant competent cells
(Wuhan Cell Marker Biotechnology Co., Ltd., Wuhan, China). Thymoma
cells were inoculated onto 12-well culture plates prior to
transfection, grown to 50% confluency, and synchronized for 12 h
with serum-free DMEM. Cells were divided into 6 transfection groups
(with 5 experimental repeats), as follows: Control, no
interference; TR001, HSHTR001 empty plasmid; Wnt4-shRNA-1,
HSH13446-1-CH1 interference plasmid; Wnt4-shRNA-2, HSH13446-2-CH2
interference plasmid; Wnt4-shRNA-3, HSH13446-3-CH3 interference
plasmid; and Wnt4-shRNA-4, HSH13446-4-CH4 interference plasmid.
Plasmids (8 µg) were transfected into each group with
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
for 6 h. Cells were then cultured in DMEM with 10% FBS for 48 h at
37°C, and total RNA was extracted for PCR using PrimeScript™ RT
reagent kit and Taq DNA Polymerase (Takara Bio, Inc.). PCR
primer pairs for human Wnt4 and GAPDH are listed in Table I. Amplification was performed at 94°C
for 3 min for pre-denaturation, followed by 35 cycles of 94°C for
30 sec for denaturation, 55°C for 30 sec for annealing, and 72°C
for 30 sec for extension, with a final extension at 72°C for 10
min. Wnt4 PCR products were purified by 1.2% agarose gel. The
relative expression of Wnt4 mRNA was calculated with gray values
using Quantity One image analysis software (version 4.4; Bio-Rad
Laboratories, Inc.) and compared with that prior to transfection in
the same cell line (15). Reactions
were performed in triplicate. The plasmid exhibiting the best
inhibition was selected for subsequent experiments.
 | Table II.Wnt4 short hairpin RNA interference
plasmids. |
Table II.
Wnt4 short hairpin RNA interference
plasmids.
| Clone name | Symbol | Location | Length | Target sequence |
|---|
|
HSH013446-1-CH1(OS241017) | WNT4 | 1475 | 19 |
GGTGGAGTAACAAGGAGTA |
|
HSH013446-2-CH1(OS241018) | WNT4 | 1845 | 19 |
GAAGAGGAAACTTAACCAC |
|
HSH013446-3-CH1(OS241019) | WNT4 | 2289 | 19 |
GCAGACAAACCAAGAATGC |
|
HSH013446-4-CH1(OS241020) | WNT4 | 2973 | 19 |
AACGTCCGAGATTCGGAAT |
Western blotting for Wnt4 and JNK
protein expression
Transfected cells were collected in one centrifuge
tube for each transfection group and centrifuged at room
temperature (110 × g, 5 min). The supernatant was removed, and the
cell pellet was washed twice with PBS (0.01 M; pH 7.2–7.3). Each
tube of cells was added into 100 µl radioimmunoprecipitation assay
lysate with phenylmethane sulfonyl fluoride (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), and protein concentration was determined
by bicinchoninic acid assay. Subsequent to quantification and
aliquoting, samples were degenerated by boiling. Equal amounts of
total protein (30 µg/sample) were subjected to SDS-PAGE (12%)
followed by electrophoretic transfer to nitrocellulose membranes.
Nitrocellulose membranes were blocked using 5% skimmed milk powder
for 1 h at room temperature. Each blot was incubated with primary
antibodies overnight at 4°C [rabbit anti-human Wnt4 polyclonal
antibody (dilution 1:100, cat. no. ab94742; Abcam), mouse
anti-human JNK polyclonal antibody (dilution 1:500, cat. no.
ab201624; Abcam) and rabbit antihuman β-actin polyclonal antibody
(dilution 1:1,000, cat. no. bs-0061R; Bioss, Beijing, China)] and a
secondary antibody for 60 min at room temperature [goat
anti-rabbit/rat monoclonal antibodies (dilution 1:8,000, cat. nos.
SA00001-1 and SA00001-2, respectively; ProteinTech Group, Inc.,
Chicago, IL, USA)]. Nitrocellulose membranes were washed with
enhanced chemiluminescence developing solution (K-12045-D20;
Advansta, Inc., Menlo Park, CA, USA), exposed and developed using
X-ray film. Grey scale analysis was performed using Quantity One
software (Bio-Rad Laboratories, Inc.).
Detection of apoptosis
Thymoma cells were divided into 4 groups, as
follows: Empty control, Lipofectamine 2000, TR001 plasmid, and
Wnt4-shRNA-3 plasmid. The aforementioned transfection steps were
used, and cell proliferation was observed using a Leica DMIL
microscope (Leica Microsystems GmbH; magnification, ×100) after 48
h. When cells were 80–90% confluent and in good condition, culture
medium (DMEM) was removed and cells were gently rinsed twice with
1X PBS. Wright-Giemsa and Hoechst-33342/propidium iodide (PI) (both
from Beijing Solarbio Science and Technology Co., Ltd., Beijing,
China) fluorescent double staining was used to stain cells.
Apoptosis was quantified by flow cytometry.
Wright-Giemsa double staining
Ethanol (1 ml; 95%) was added to each culture well
and cells were fixed for 15 min. The ethanol was removed and 1 ml
Wright-Giemsa staining solution was added. The cells were stained
for 2 min at room temperature. The staining solution was discarded,
the cells were rinsed twice in flowing water and observed using a
Leica DMIL microscope (magnification, ×400), and images were
captured. Apoptotic cells exhibited chromatin condensation, nuclear
membrane fragmentation, and appearance of apoptotic bodies. Light
staining was also observed in necrotic cells.
Hoechst-33342/PI fluorescent double
staining
Damping fluid (2 ml), Hoechst 33342 staining
solution (10 µl) and PI staining solution (10 µl) (Solarbio Science
and Technology Company, Ltd.) were added to each well and mixed
lightly. Cells were kept in the dark for 20 min at 4°C. The
staining solution was then discarded and the cells were rinsed with
1X PBS. Physiological saline (1 ml) was added, and fluorescent
images were captured using a Leica DMIL microscope (magnification,
×200). Normal cells stained blue and the nucleolus structure was
normal. Apoptotic cells stained bright blue and light red, and
necrotic cells stained light blue and bright red.
Annexin V-fluorescein isothiocyanate
(FITC)/PI double staining and flow cytometry
Apoptosis was detected by flow cytometry with
Annexin V-FITC/PI kit (cat. no. FXP018-050; 4A Biotech Co., Ltd.,
Beijing, China), which distinguishes between apoptotic and dead
cells based on differential membrane staining. Different cells can
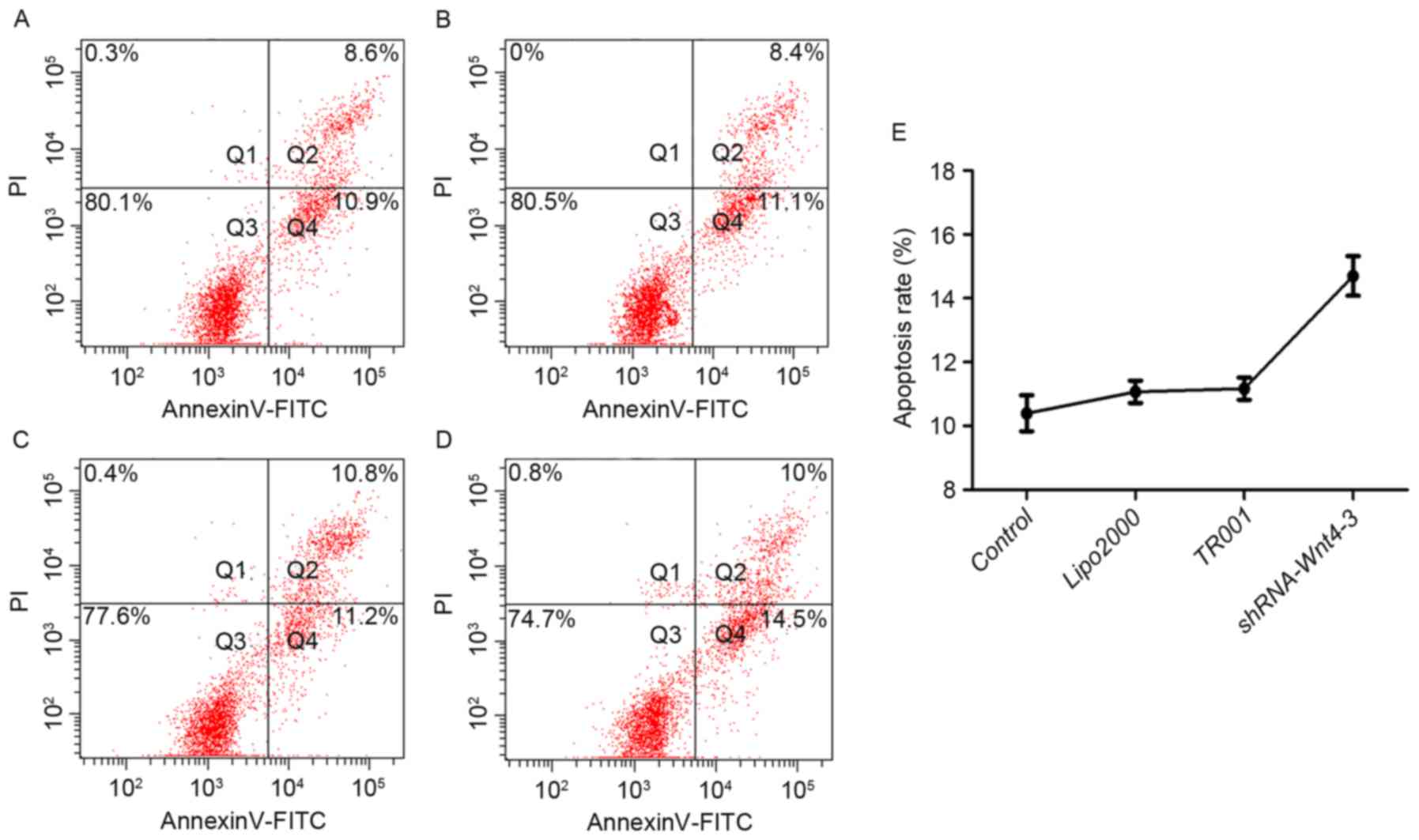
be distinguished from the four quadrant diagram: Normal live cells
are Annexin V−/PI− cells (Q3); viable
apoptotic cells are Annexin V+/PI− cells
(Q4); and advanced apoptotic cells and dead cells are Annexin
V+/PI+ cells (Q2). The percentage of
apoptosis was determined by statistical analysis of Q4 data.
Fluorescence was measured with a flow cytometer (FACSCanto II, BD
Biosciences, Franklin Lakes, NJ, USA) and analyzed using FACSDiva
version 6.1.3 (BD Biosciences).
Statistical analysis
Statistical analysis was conducted using the t-test
for paired samples, one-way analysis of variance for multi group
data, multiple comparisons between groups were performed using
least significant difference method, and Fisher's exact test for
categorical data in 2×2 contingency tables. P<0.05 was
considered to indicate a statistically significant difference. Data
were processed using SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Results
Expression of Wnt4 and JNK mRNA and
protein in thymoma
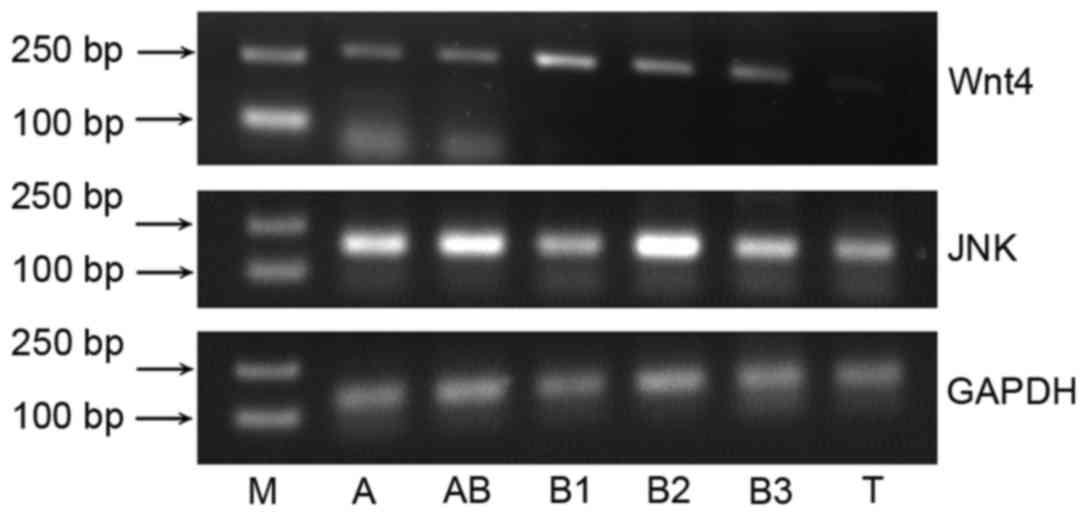
Wnt4 and JNK mRNA expression was detected in thymoma
tissues (Fig. 1). The relative
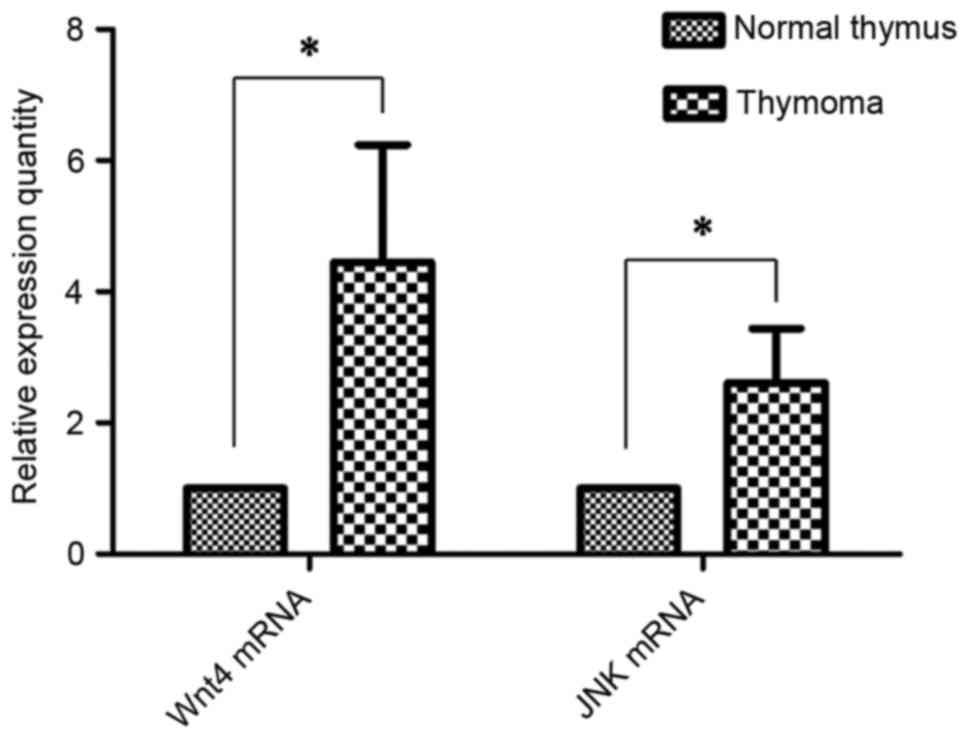
expression of Wnt4 mRNA was 4.45±1.79 (P<0.001), and JNK mRNA
was 2.61±0.83, compared with normal thymus tissues (Fig. 2; P<0.001).
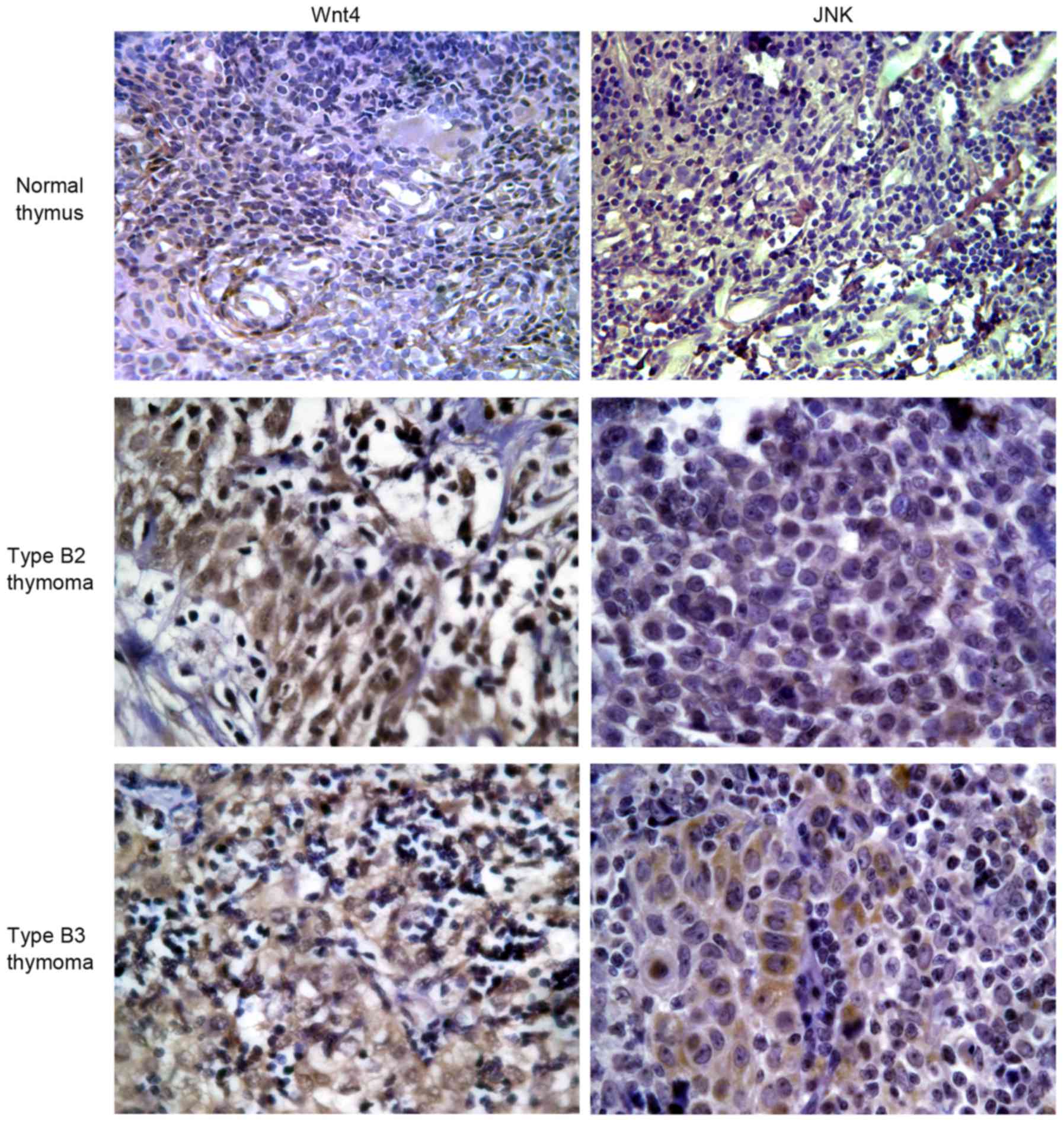
The positive staining of Wnt4 protein in normal
thymus and thymoma tissues was 0.0 and 93.3%, respectively
(P<0.001), and positive staining of JNK protein in normal thymus
tissue and thymoma was 16.7 and 80.0%, respectively (P=0.014)
(Table III; Fig. 3).
 | Table III.Cases of positive expression of Wnt4
and JNK protein in normal thymus tissue and thymoma. |
Table III.
Cases of positive expression of Wnt4
and JNK protein in normal thymus tissue and thymoma.
|
| Wnt4 expression,
n | JNK expression,
n |
|---|
|
|
|
|
|---|
| Tissue | + | − | P-value | + | − | P-value |
|---|
| Thymus | 0 | 6 | 0.000 | 1 | 5 | 0.014 |
| Thymoma | 14 | 1 |
| 12 | 3 |
|
Screening of psi-H1-shRNA-Wnt4
interference plasmid and its inhibitory effect on Wnt4
The plasmids of positive electrophoresis strips were
selected and sent to sequence analysis (Aoke Biotechnology Co.,
Ltd.). Results showed that the complete target gene inserted, and
that the sequence was identical with the sequence designed.
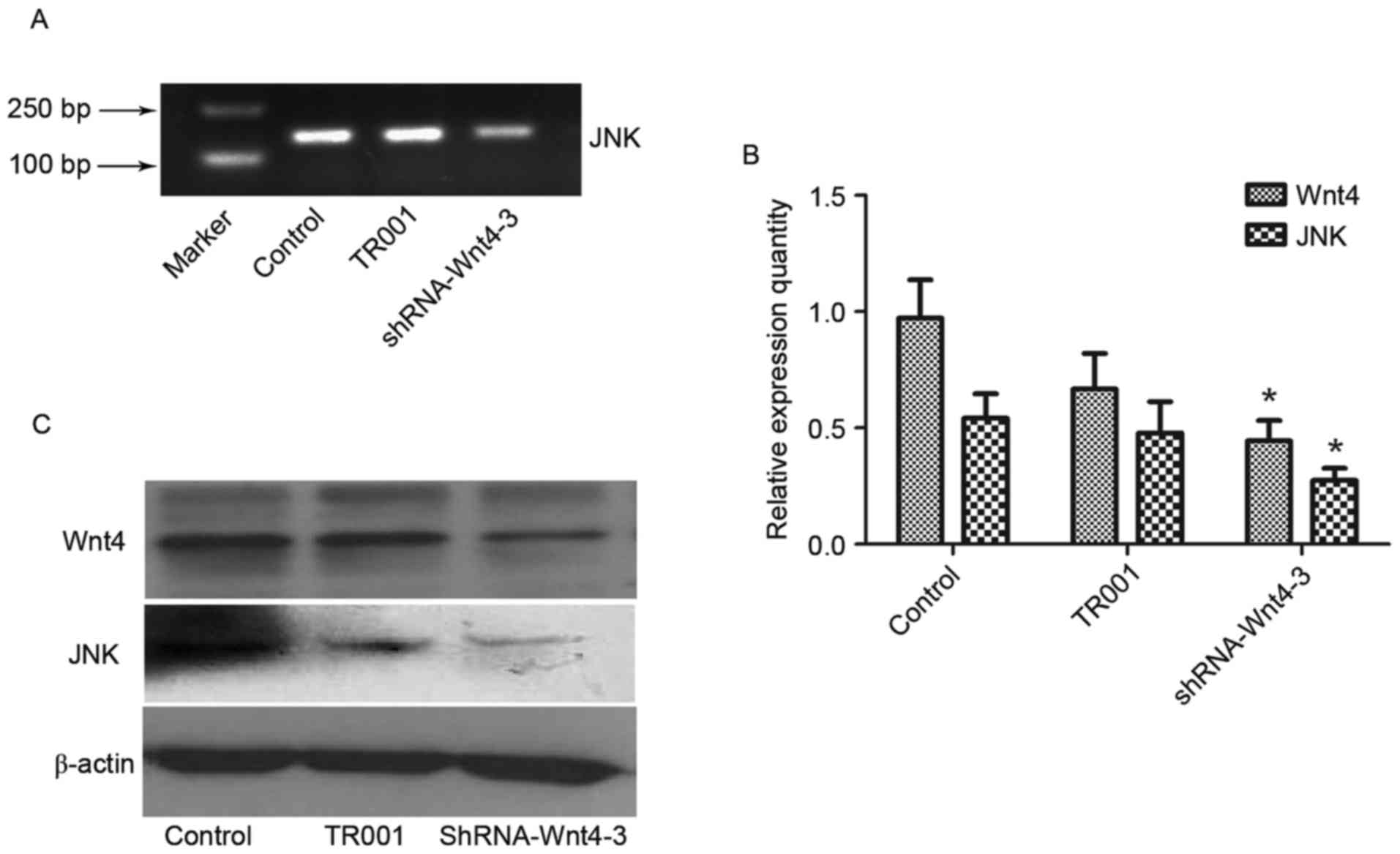
Wnt4 expression was determined by semi-quantitative
RT-PCR (Fig. 4). The results showed
that Wnt4 expression in each experimental group was decreased
compared with the controls. However, Wnt4-1 expression in the
shRNA-group was elevated compared with the TR001 group, indicating
that this plasmid did not successfully inhibit expression.
Expression in the other experimental groups was lower than in the
TR001 group, indicating that the shRNA-Wnt4 interference plasmid
was constructed successfully, and effectively inhibits Wnt4
expression. The relative inhibitions of the four candidate plasmids
were 15.50, 40.87, 52.37 and 34.89% respectively. Expression
following transfection of shRNA-Wnt4-3 was the lowest (P<0.01).
Therefore, shRNA-Wnt4-3 was selected as the best silencing plasmid
and used in subsequent experiments.
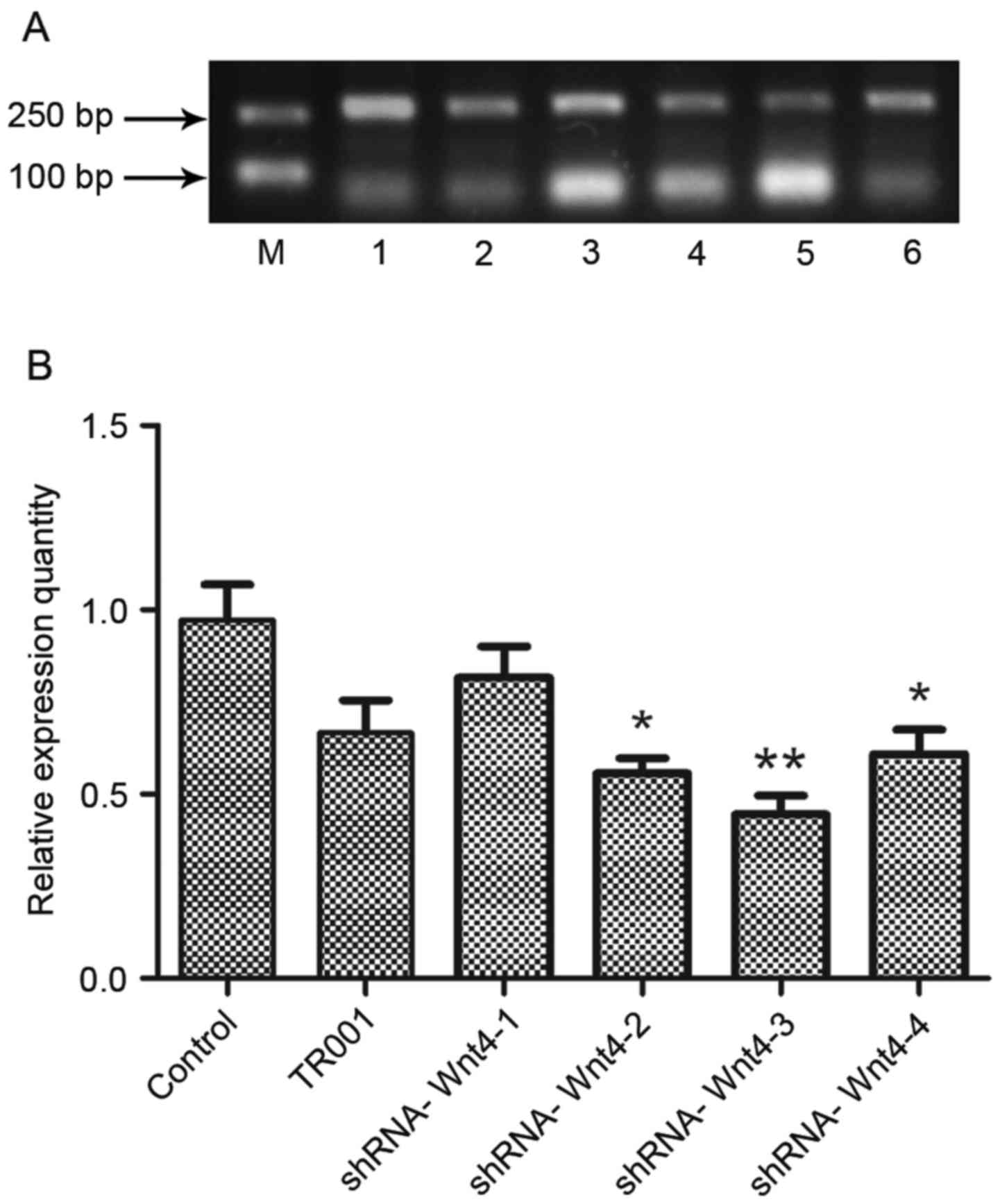 | Figure 4.Wnt4 mRNA expression following
candidate plasmid transfection. (A) Agarose gel electropherogram of
Wnt4 mRNA expression following transfection. (B) Evaluation of mRNA
expression levels showed that plasmid shRNA-Wnt4 effectively
silences Wnt4 expression in the latter three groups, but
shRNA-Wnt4-3 was most effective. Therefore, shRNA-Wnt4-3 was
selected as the best interference plasmid and used in subsequent
experiments. *P<0.05 and **P<0.01 vs. control group. shRNA,
short hairpin RNA; Lane M, marker; lane 1, control; lane 2, TR001;
lane 3, shRNA-Wnt4-1; lane 4, shRNA-Wnt4-2; lane 5, shRNA-Wnt4-3;
and lane 6, shRNA-Wnt4-4. |
Wnt4 shRNA inhibits JNK
JNK expression decreased after Wnt4 shRNA, as
detected by semi-quantitative RT-PCR, consistent with Wnt4
expression level (P<0.01), indicating that transfection of
shRNA-Wnt4-3 inhibits JNK expression (Fig. 5A and B).
Western blot analysis demonstrated JNK and Wnt4
protein expression in the control and TR001 groups was
significantly increased compared with the shRNA-Wnt4-3 group
(P<0.05), although β-actin levels had not changed (Fig. 5C). This indicated that the shRNA-Wnt4
plasmid inhibits Wnt4 protein expression, which suppresses JNK
protein expression.
Detection of apoptosis subsequent to
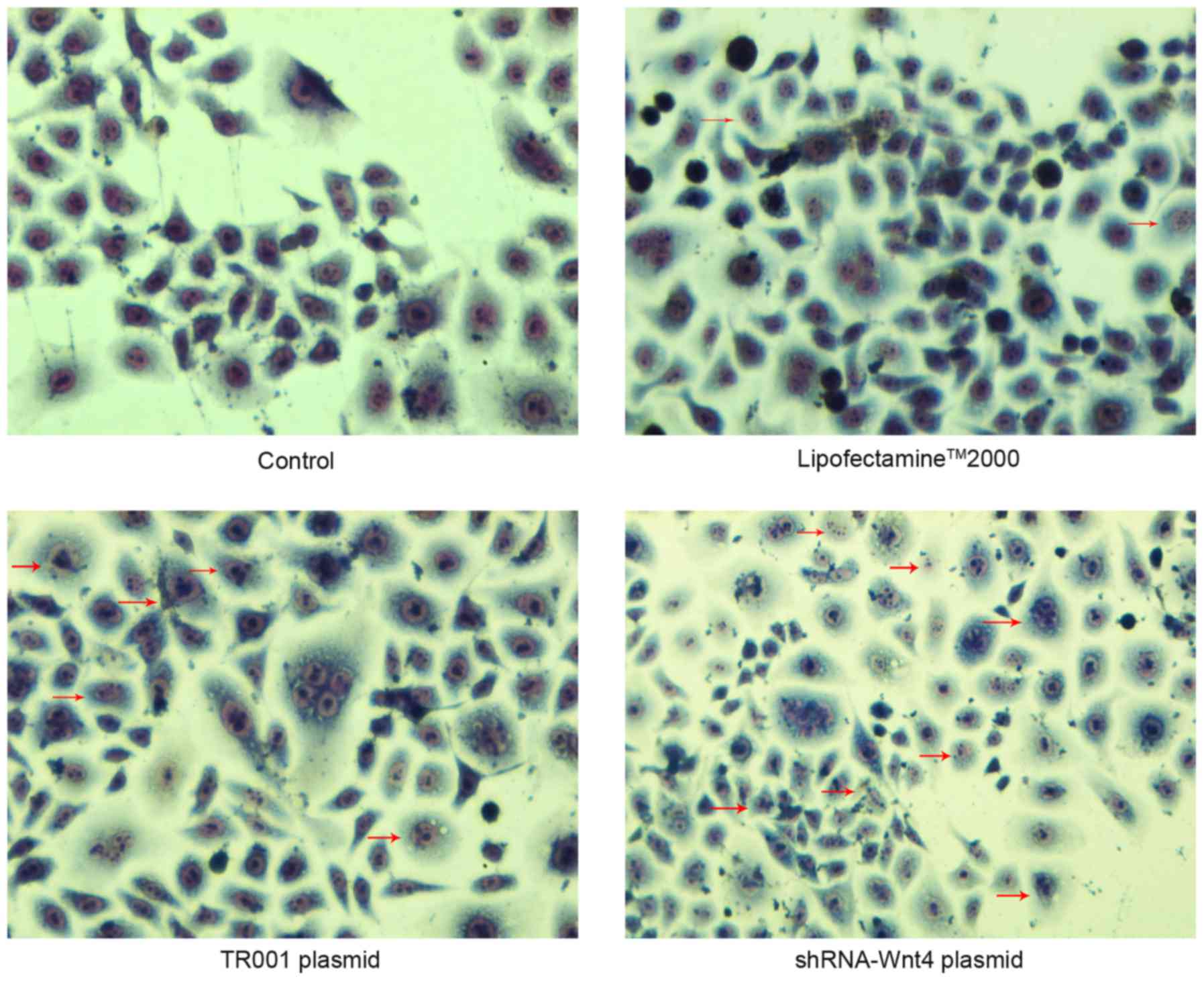
transfection Wright-Giemsa staining
The cellular morphology of control and Lipofectamine
2000 groups was polygonal. The cytoplasmic staining was light and
uniform, with hyperchromatic nuclei. Nuclear morphology indicated
the cellular division phase, but no apoptotic bodies or cytoplasmic
vacuoles were observed. These observations indicated that thymomas
in the control and Lipofectamine 2000 groups were not undergoing
apoptosis. However, characteristic apoptotic morphological changes,
such as nuclear chromatin agglutination, invagination of the
cellular membrane, and apoptotic body formation were observed in
the TR001 and shRNA transfection groups. These morphological
changes were more prevalent in the shRNA-transfected groups
(Fig. 6).
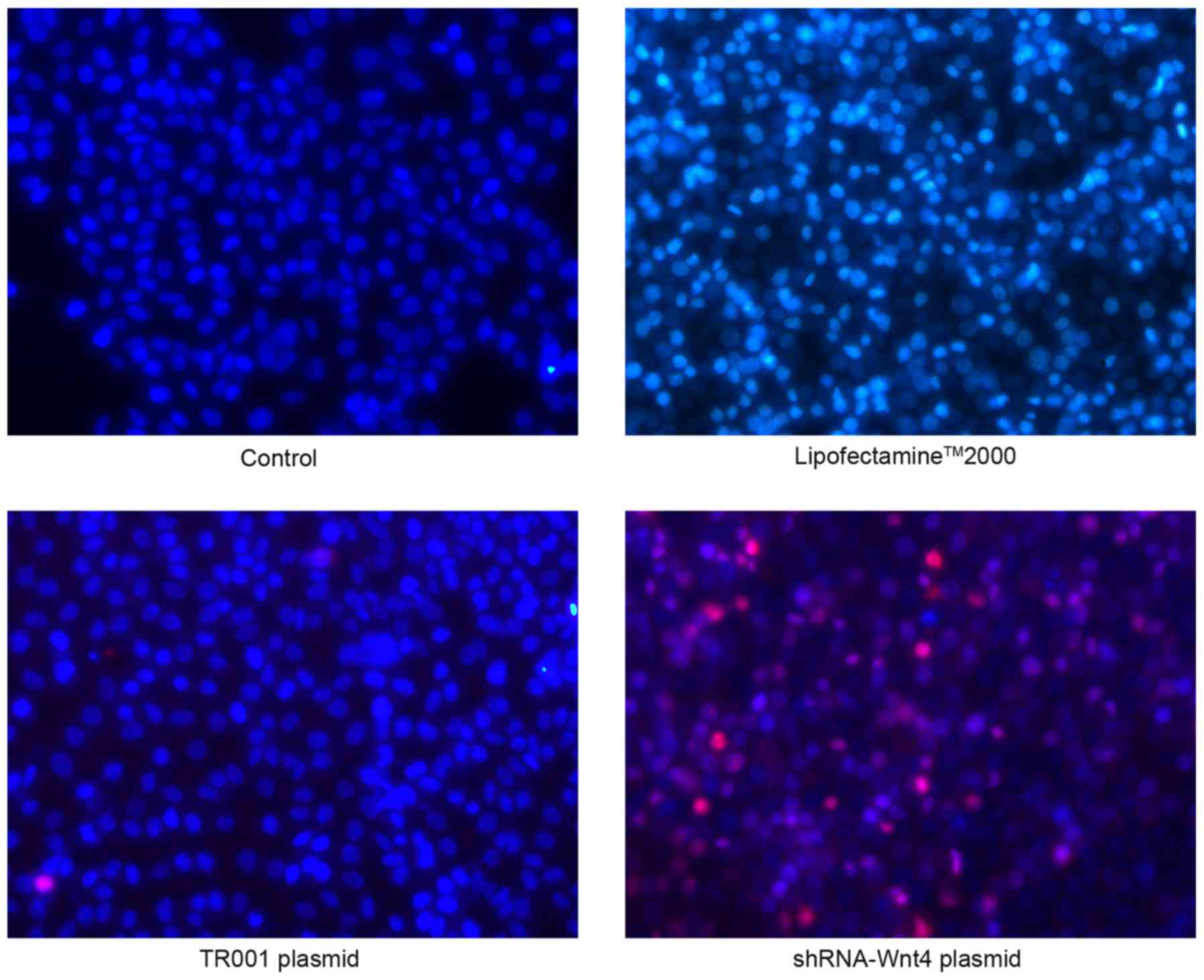
Hoechst-33342/PI fluorescent double
staining
Normal cells stain low bright blue and no red
(Hoechst 33342+/PI−), with control cells in
the visual field having a typical polygon shape. In the
Lipofectamine 2000 group, a small number of bright blue (Hoechst
33342++/PI−) cells were normal or undergoing
early stage apoptosis, but there were no red-stained cells.
Transfection of plasmid TR001 resulted in a small number of blue
and red double stained apoptotic cells (Hoechst
33342++/PI+). Notably, shRNA transfection
produced a large number of apoptotic (Hoechst
33342++/PI+) and necrotic (Hoechst
33342+/PI++) cells (Fig. 7).
Flow cytometry
Apoptosis in the Lipofectamine 2000 and TR001 groups
was 11.07±0.35% and 11.17±0.35%, respectively, although they did
not differ compared with controls (10.40±0.56%) (P>0.05). The
apoptosis rate following shRNA transfection was 14.70±0.62%, which
was significant compared with controls (Fig. 8; P<0.001).
Discussion
Few studies have investigated thymoma signaling
pathways. Notably, Wnt receptors are present in all thymocyte
subsets (16), and Wnt pathways are
associated with malignant tumors. Wnt-4 activates the JNK-dependent
noncanonical signaling pathway (17).
Ma et al (18) demonstrated
that the overexpression of c-Jun in thymus epithelial tumors was
closely associated with the pathogenesis and biological behavior of
the neoplasm (18). Thus, the present
study aimed to explore the role that Wnt4 signaling may play in
promoting thymoma cell proliferation via a non-classical
JNK-mediated pathway. The present results showed that the thymoma
Wnt4 and JNK gene and protein expression was elevated relative to
that in the normal thymus. In addition, Wnt4 and JNK expression and
apoptosis changes were observed in thymoma cells subsequent to Wnt4
gene silencing.
Since Napoli et al (19) identified botanic co-suppression in
1990, RNAi technology has been widely used. In the present study,
the Wnt4 shRNA sequence was designed and a shRNA plasmid vector was
constructed to silence Wnt4. Sequence analysis showed that Wnt4
shRNA was successfully inserted into the expression vector and
effectively inhibited Wnt4 expression, indicating that the
interference plasmid was constructed successfully. The shRNA-Wnt4-3
inhibition was 52.37%, which was the best of four constructed
plasmids (P<0.01), prompting us to chose this plasmid for
subsequent experiments. Although certain studies have shown that
the development of thymus epithelial cells requires activation of
the β-catenin-mediated canonical Wnt signaling pathway (11,20,21),
elevated expression of β-catenin was not observed in the present
study. However, expression of the JNK mRNA and protein were
significantly decreased following transfection of the shRNA-Wnt4
plasmid compared with the controls. This result may indicate that
the Wnt4-activated pathway is a JNK-mediated PCP-like pathway in
thymoma.
Cancer cells escape apoptosis by manipulating the
expression level of anti-apoptotic molecules or inactivating
apoptotic elements (22). Studies
have shown that inhibition of Wnt signaling induces apoptosis and
suppresses cell proliferation (23,24). Age
associated changes in thymus cell number and function were
accompanied by a decrease in the transcription levels of Wnt4, and
activation of the Wnt4 signaling pathway promoted thymus epithelial
cell proliferation and alleviated apoptosis (25). Therefore, the present study
qualitatively (Wright-Giemsa and Hoechst-33342/PI staining) and
quantitatively (Annexin V-FITC/PI staining) analyzed thymoma
apoptosis subsequent to downregulation of Wnt4 by transfection with
the shRNA-Wnt4-3 plasmid. The results indicated that cells
exhibited increased cellular apoptosis and characteristic apoptotic
morphological changes, including nuclear chromatin agglutination,
and apoptotic body formation, compared with controls. Collectively,
these results indicate that downregulation of Wnt4 promotes thymoma
apoptosis, and that Wnt4 activation may be one of the causes of
thymoma development.
The present results suggest that thymomas exhibit
elevated Wnt4 and JNK mRNA and protein expression. Thymomas also
show increased apoptosis subsequent to RNAi inhibition of Wnt4.
Thus, Wnt4 may have an important role in promoting thymoma
development, and may be activated through a JNK mediated PCP-like
pathway.
Acknowledgements
This study was supported by the Natural Sciences
Foundation of Tianjin (grant no. 14JCZDJC57100) and the Special
Fund for Clinical Research of Wu Jieping Medical Foundation (grant
no. 320.6750.14318). English language editing was provided by Edanz
China (Beijing, China).
References
|
1
|
Singh G, Rumende CM and Amin Z: Thymoma:
Diagnosis and treatment. Acta Med Indones. 43:74–78.
2011.PubMed/NCBI
|
|
2
|
Detterbeck FC: Evaluation and treatment of
stage I and II thymoma. J Thorac Oncol. 5 Suppl 4:S318–S322. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marx A, Willcox N, Leite MI, Chuang WY,
Schalke B, Nix W and Ströbel P: Thymoma and paraneoplastic
myasthenia gravis. Autoimmunity. 43:413–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis - a look outside the nucleus.
Science. 287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, Li
H, Guo Y and Qi D: Wnt2 promotes non-small cell lung cancer
progression by activating WNT/β-catenin pathway. Am J Cancer Res.
5:1032–1046. 2015.PubMed/NCBI
|
|
6
|
Chiurillo MA: Role of the Wnt/β-catenin
pathway in gastric cancer: An in-depth literature review. World J
Exp Med. 5:84–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacMillan CD, Leong HS, Dales DW,
Robertson AE, Lewis JD, Chambers AF and Tuck AB: Stage of breast
cancer progression influences cellular response to activation of
the WNT/planar cell polarity pathway. Sci Rep. 4:63152014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Loosdregt J, Fleskens V, Tiemessen MM,
Mokry M, van Boxtel R, Meerding J, Pals CE, Kurek D, Baert MR,
Delemarre EM, et al: Canonical Wnt signaling negatively modulates
regulatory T cell function. Immunity. 39:298–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kvell K, Varecza Z, Bartis D, Hesse S,
Parnell S, Anderson G, Jenkinson EJ and Pongracz JE: Wnt4 and
LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One.
5:e107012010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinonen KM, Vanegas JR, Brochu S, Shan J,
Vainio SJ and Perreault C: Wnt4 regulates thymic cellularity
through the expansion of thymic epithelial cells and early thymic
progenitors. Blood. 118:5163–5173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kvell K, Fejes AV, Parnell SM and Pongracz
JE: Active Wnt/beta-catenin signaling is required for embryonic
thymic epithelial development and functionality ex vivo.
Immunobiology. 219:644–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tejada-Romero B, Carter JM, Mihaylova Y,
Neumann B and Aboobaker AA: JNK signalling is necessary for a Wnt-
and stem cell-dependent regeneration programme. Development.
142:2413–2424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Wang Y, Zhang P, Chen Y, Liu Y,
Guo F and Zhang H: Establishment and characterization of a novel
cell line derived from thymoma with myasthenia gravis patients.
Thoracic Cancer. 6:194–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kojima S and Borisy GG: An image-based,
dual fluorescence reporter assay to evaluate the efficacy of shRNA
for gene silencing at the single-cell level. F1000Res.
3:602014.PubMed/NCBI
|
|
16
|
Weerkamp F, Baert MR, Naber BA, Koster EE,
de Haas EF, Atkuri KR, van Dongen JJ, Herzenberg LA and Staal FJ:
Wnt signaling in the thymus is regulated by differential expression
of intracellular signaling molecules. Proc Natl Acad Sci USA.
103:pp. 3322–3326. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heinonen KM, Vanegas JR, Lew D, Krosl J
and Perreault C: Wnt4 enhances murine hematopoietic progenitor cell
expansion through a planar cell polarity-like pathway. PLoS One.
6:e192792011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Y, Li Q, Cui W, Miao N, Liu X, Zhang W,
Zhang C and Wang J: Expression of c-Jun, p73, Casp9, and N-ras in
thymic epithelial tumors: Relationship with the current WHO
classification systems. Diagn Pathol. 7:1202012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Napoli C, Lemieux C and Jorgensen R:
Introduction of a chimeric chalcone synthase gene into petunia
results in reversible co-suppression of homologous genes in trans.
Plant Cell. 2:279–289. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang CC, You LR, Yen JJ, Liao NS,
Yang-Yen HF and Chen CM: Thymic epithelial β-catenin is required
for adult thymic homeostasis and function. Immunol Cell Biol.
91:511–523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zuklys S, Gill J, Keller MP, Hauri-Hohl M,
Zhanybekova S, Balciunaite G, Na KJ, Jeker LT, Hafen K, Tsukamoto
N, et al: Stabilized beta-catenin in thymic epithelial cells blocks
thymus development and function. J Immunol. 182:2997–3007. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang KS, Zhou Q, Wang YF and Liang LJ:
Inhibition of Wnt signaling induces cell apoptosis and suppresses
cell proliferation in cholangiocarcinoma cells. Oncol Rep.
30:1430–1438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bilir B, Kucuk O and Moreno CS: Wnt
signaling blockage inhibits cell proliferation and migration, and
induces apoptosis in triple-negative breast cancer cells. J Transl
Med. 11:2802013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei T, Zhang N, Guo Z, Chi F, Song Y and
Zhu X: Wnt4 signaling is associated with the decrease of
proliferation and increase of apoptosis during age-related thymic
involution. Mol Med Rep. 12:7568–76. 2015. View Article : Google Scholar : PubMed/NCBI
|