Introduction
The number of renal cell carcinoma (RCC) patients is
increasing worldwide. Approximately 20% of RCC patients present
with advanced stage disease at the time of diagnosis, and in
patients with localized RCC, nearly 30% will experience disease
recurrence after tumor resection (1,2). Targeted
therapy using tyrosine kinase inhibitors (TKIs) is used as a
treatment for metastatic renal cell carcinoma (mRCC). TKIs inhibit
multiple receptor tyrosine kinases needed for the activation of
intracellular signaling pathways controlling cell proliferation,
survival, or angiogenesis. Among TKIs, sunitinib, an orally
available TKI, is the most commonly used molecular-targeting agent
as first-line therapy for mRCC (3).
In the sunitinib registration trial, half of the treated patients
with a favorable or intermediate risk score based on Memorial
Sloan-Kettering Cancer Center criteria achieved an objective
response, resulting in a median progression-free survival (PFS) of
11 months (4,5). However, the clinical benefit of
sunitinib in PFS is limited, and the majority of mRCC patients
treated with sunitinib ultimately experience disease progression
due to the acquisition of resistance. In such cases, progressed
patients require further sequential therapy using other TKIs or
mTOR inhibitors (mTORIs). In spite of treatment of the second
therapy, the median PFS was short; 4.8 months with axitinib, 3.4
months with sorafenib, 4.3 months with temsirolimus, 7.5 months
with pazopanib, and 4.0 months with everolimus (6–9).
Therefore, we thought that it should be necessary to lengthen PFS
in first-line therapy of sunitinib. Identifying pathways
responsible for intrinsic or acquired resistance could provide
novel directions to develop therapies that block resistance
pathways.
Intracellular drug accumulation accompanied by
increased lysosomal storage is elevated in sunitinib-resistant
cells (10). Moreover, the lysosomal
capacity is enhanced by upregulating lysosome-associated membrane
protein-1 and −2 (LAMP1/2) expression (11). Based on these data, identifying
mechanisms responsible for intrinsic or acquired sunitinib
resistance involving LAMP1/2 could provide novel directions to
develop therapies that block resistance pathways.
microRNAs (miRNAs) are noncoding single-stranded
small RNAs (~ 22 nucleotides) that regulate posttranscriptional
gene expression. miRNA levels are altered in many human diseases
including cancer (12). miRNAs play
an important role as regulators of gene expression in
tumorigenesis, tumor progression, drug resistance, and metastasis
(13,14). Thus, we previously generated a
sunitinib-resistant RCC cell line, SR-ACHN (sunitinib-resistant
ACHN), by continuous treatment with sunitinib to detect candidate
miRNAs implicated in the regulation of sunitinib resistance
(15). The aim of this study was to
identify miRNAs that suppressed sunitinib resistance via LAMP2
expression using ACHN and SR-ACHN cells.
Materials and methods
Cell lines
A human RCC cell line, ACHN, was purchased from the
American Type Culture Collection (Rockville, MD, USA) and cultured
in RPMI1640 (Thermo Fisher Scientific Inc., Waltham, MA, USA) with
10% fetal bovine serum (FBS) and 1% Penicillin-Streptomycin (Thermo
Fisher Scientific Inc.). Sunitinib-resistant ACHN (SR-ACHN) cells
were generated as previously described (15). SR-ACHN cells were maintained in the
same medium containing 10 µM sunitinib (Sigma Aldrich, St. Louis,
MO, USA).
Clinical samples
Twelve samples of advanced RCC, which had lymph node
metastasis or distant metastasis, were obtained from Tottori
University Hospital. All the materials were obtained with informed
consent, and the procedures were approved by The Ethics Committee
of Tottori University (Tottori, Japan; approval number: 1558).
Tissue samples were obtained from tumor tissues and matched normal
tissues from the same kidney specimen in RCC patients. Normal
tissues were far from tumor clearly macroscopically.
Total RNA extraction and
microarray
Total RNA from ACHN and SR-ACHN cells was extracted
using the miR-Vana™ miRNA isolation kit (Thermo Fisher
Scientific Inc.) following the manufacturer's protocol. Total RNA
quality control for quantity and purity was determined using a
NanoDrop Spectrophotometer ND-1000 (Thermo Fisher Scientific Inc.).
The RNA samples were stored at −80°C until the reverse
transcription (RT) reaction. For microarray analysis, total RNA was
labeled using a 3D-Gene miRNA labeling kit (Toray Industries;
Kamakura, Japan). Labeled RNA was hybridized to 3D-Gene human miRNA
V21 chips (Toray Industries).
Transfection with synthetic miRNAs
into SR-ACHN cells and proliferation assay
Synthetic human miRNAs (hsa-miRs) [hsa-miR-194-5p
and negative control (NC); Ambion] were transfected into SR-ACHN
cells at 60 nmol/l (final concentration) per 3×106
cell/well in a 10-cm dish, using DharmaFECT (GE Healthcare,
Pittsburg, PA). After 24 h of incubation, cells were harvested and
reseeded into a 96-well plate. miR transfected cells were plated at
5×103 cells/well in a 96-well plate. After 24 h,
sunitinib was added at different concentrations and proliferation
after 72 h was measured using the Cell Counting Kit-8 (Dojindo
Molecular Technologies, Kumamoto, Japan) according to the
manufacturer's protocol.
RNA extraction and quantitative
real-time PCR of miRNAs
Total RNA from cell lines and clinical samples was
extracted using a miR-Vana™ miRNA Isolation Kit (Thermo
Fisher Scientific Inc.,) according to the manufacturer's protocol.
miR-194-5p-specific complementary DNA was generated from 20 ng
total RNA, using the TaqMan MicroRNA RT kit (Applied Biosystems,
Foster City, CA) and the miR-194-5p-specific RT-primer from the
TaqMan Micro RNA Assay (Applied Biosystems). miR-194-5p levels were
also measured using the miR-194-5p-specific probe included with the
TaqMan Micro RNA Assay on an ABI 7900HT System and SDS software
(Applied Biosystems).
Western blotting
The cells were lysed with cell lysis buffer {1
mmol/l Na3VO4, 100 mmol/l
phenylmethylsulfonyl fluoride (PMSF), 500 mmol/l NaF, 500 mmol/l
ethylenediaminetetraacetic acid (EDTA), 10% NP-40, 2 mg/ml
aprotinin, 2 mg/ml leuptin, 1 mol/l Tris pH 7.6, 5 mol/l NaCl and
distilled water}. Protein concentrations were determined by the
Micro BCA protein assay (Thermo Fisher Scientific Inc.). Samples
containing 20 µg protein underwent electrophoresis on 10% SDS
polyacrylamide gels and were subsequently transferred to PVDF
membranes. The membranes were blotted with a mouse monoclonal
antibody against LAMP-2 (1:250; ab119124; Abcam, Cambridge, MA), or
with a monoclonal antibody against β-actin (1:2,000; AC-15; Sigma,
Aldrich).
Signals were visualized using an enhanced
chemiluminescence system (ECL Detection System; Amersham Pharmacia
Biotech, Piscataway, NJ).
Immunohistochemistry
Clinical tissues were fixed in 10% formalin and
embedded in paraffin. Sections 3-µm thick were examined by
immunohistochemistry. The sections were deparaffinized and antigens
were retrieved using an autoclave in 10 mmol/l citrate buffer (pH
6.0) at 121°C for 10 min. Endogenous peroxidase activity was
blocked by immersing the slides in 0.6% hydrogen peroxide in
methanol for 20 min. The sections were immunostained using a
Histofine rabbit stain kit (Nichirei, Tokyo, Japan). The primary
antibody was a mouse monoclonal antibody against LAMP-2 (1:10;
ab25631; Abcam) followed by incubation with secondary antibodies.
Immunoreactions were visualized with diaminobenzidine and the
sections were counterstained with hematoxylin.
Statistical analysis
Statistical significance was determined by the
two-tailed unpaired Student's t-test and using the Pearson
correlation coefficient. Differences were considered to be
statistically significant when P<0.05.
Results
miRNA microarray analysis and
validation of the array data by real-time RT-qPCR
A pair of cell lines (ACHN and SR-ACHN) was used to
identify miRNA candidates involved in regulating sunitinib
resistance, based on the premise that sunitinib resistance-related
miRNAs are identifiable by comparing the miRNA expression patterns
in these cells. miRNA microarray analysis was performed by
comparing ACHN and SR-ACHN cells to evaluate the miRNA profiles of
each cell type. The expression levels of many miRNAs were different
between the two cell lines. Fifteen miRNAs were significantly
upregulated over 8-fold (sunitinib-resistant miRNAs) whereas
thirty-one miRNAs, including miR-194-5p, were significantly
down-regulated over 4-fold in SR-ACHN compared with that in ACHN
cells (Table I).
 | Table I.microRNAs in sunitinib-resistant ACHN
cells increase 8-fold and decrease 4-fold when compared with ACHN
cells. |
Table I.
microRNAs in sunitinib-resistant ACHN
cells increase 8-fold and decrease 4-fold when compared with ACHN
cells.
| Name | Ratio of ACHN/SR-ACHN
cells |
|---|
| A, Upregulated
miRNA |
|
|
| miR-575 | 16.71 |
| miR-4459 | 15.30 |
| miR-6088 | 15.05 |
| miR-4430 | 13.35 |
| miR-642b-3p | 12.40 |
| miR-4294 | 11.46 |
| miR-6808-5p | 11.13 |
| miR-6769b-5p | 10.24 |
| miR-675-5p | 9.37 |
| miR-6076 | 9.10 |
| miR-671-5p | 8.68 |
| miR-6501-3p | 8.47 |
| miR-4651 | 8.32 |
| miR-4467 | 8.15 |
| miR-4433b-3p | 8.05 |
|
| B, Downregulated
miRNA |
|
|
| miR-7-5p | 0.05 |
| miR-29b-1-5p | 0.06 |
| miR-155-5p | 0.10 |
| miR-4521 | 0.12 |
| miR-29a-5p | 0.13 |
| miR-652-3p | 0.13 |
| miR-192-5p | 0.14 |
| miR-194-5p | 0.14 |
| miR-16-2-3p | 0.14 |
| miR-215-5p | 0.16 |
| miR-222-5p | 0.16 |
| miR-518b | 0.17 |
| miR-3194-3p | 0.17 |
| miR-21-3p | 0.18 |
| miR-18a-5p | 0.18 |
| miR-376c-3p | 0.18 |
| miR-20a-3p | 0.20 |
| miR-495-3p | 0.20 |
| miR-3200-3p | 0.20 |
| miR-3175 | 0.21 |
| miR-18b-5p | 0.22 |
| miR-20b-5p | 0.23 |
| miR-431-3p | 0.23 |
| miR-454-3p | 0.24 |
| miR-130b-3p | 0.24 |
| miR-590-5p | 0.24 |
| miR-301a-3p | 0.24 |
| miR-106a-5p | 0.24 |
| miR-4284 | 0.25 |
| miR-4259 | 0.25 |
On the basis of the microarray results, we further
examined the expression level of miR-194-5p by real-time RT-qPCR.
We selected miR-194-5p based on a previous study, which reported
that higher miR-194 expression correlated with significantly longer
disease-free survival and overall survival compared to those with
lower expression in patients with RCC (16). RNA pools from the same RNA samples
used for the microarray experiments were prepared. We used
RNU6B as a reference gene for normalization of the miRNA
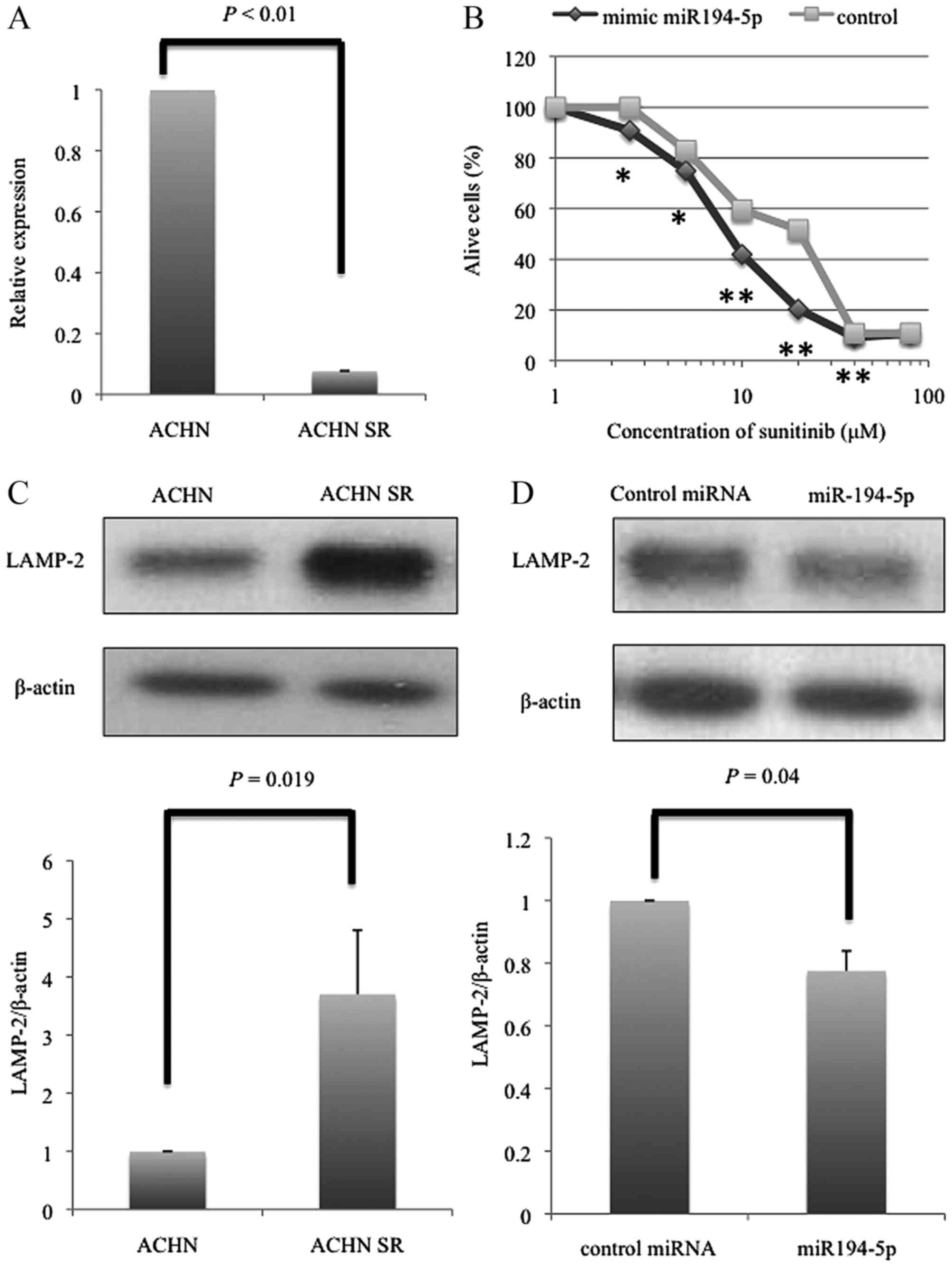
data. The CT values of miR-194-5p were significantly decreased in
SR-ACHN cells compared with that in ACHN cells (P<0.01)
(Fig. 1A). The PCR result was
consistent with the microarray data.
miRNA mimic oligonucleotide transfer
restores sunitinib resistance in ACHN cell lines
The IC50 concentration of sunitinib for ACHN and
SR-ACHN cells was 10 and 21 µM, respectively, showing that SR-ACHN
cells exhibited significantly higher resistance to sunitinib
treatment compared with ACHN cells, as previously reported by
Yamaguchi et al (15). When
SR-ACHN cells transfected with miR-194-5p or negative control miR
were treated with sunitinib, the number of live cells was
significantly decreased in miR-194-5p-transfected cells than in
negative control miR-transfected SR-ACHN cells at a sunitinib
concentration range of 2.5 to 40 µM (Fig.
1B).
Detection and identification of
miR-194-5p target genes
To elucidate sunitinib resistance-related miR-194-5p
target genes in SR-ACHN cells, candidate target genes were selected
using miRDB 5.0. Out of the many miR-194-5p target genes, we
focused on LAMP2, which is known to contribute to sunitinib
resistance in renal cell cancer cells (17). In fact, western blot analysis revealed
that LAMP2 protein expression was markedly higher in SR-ACHN cells
than in ACHN cells (P=0.019, Fig.
1C). These data motivated us to examine whether miR-194-5p
could suppress the expression of LAMP2 in SR-ACHN cells. As shown
in Fig. 1D, the expression of LAMP-2
was significantly decreased by miR-194-5p transfection compared
with that by miR-NC transfection (P=0.04).
Expression of miR-194-5p and LAMP-2 in
clinical samples
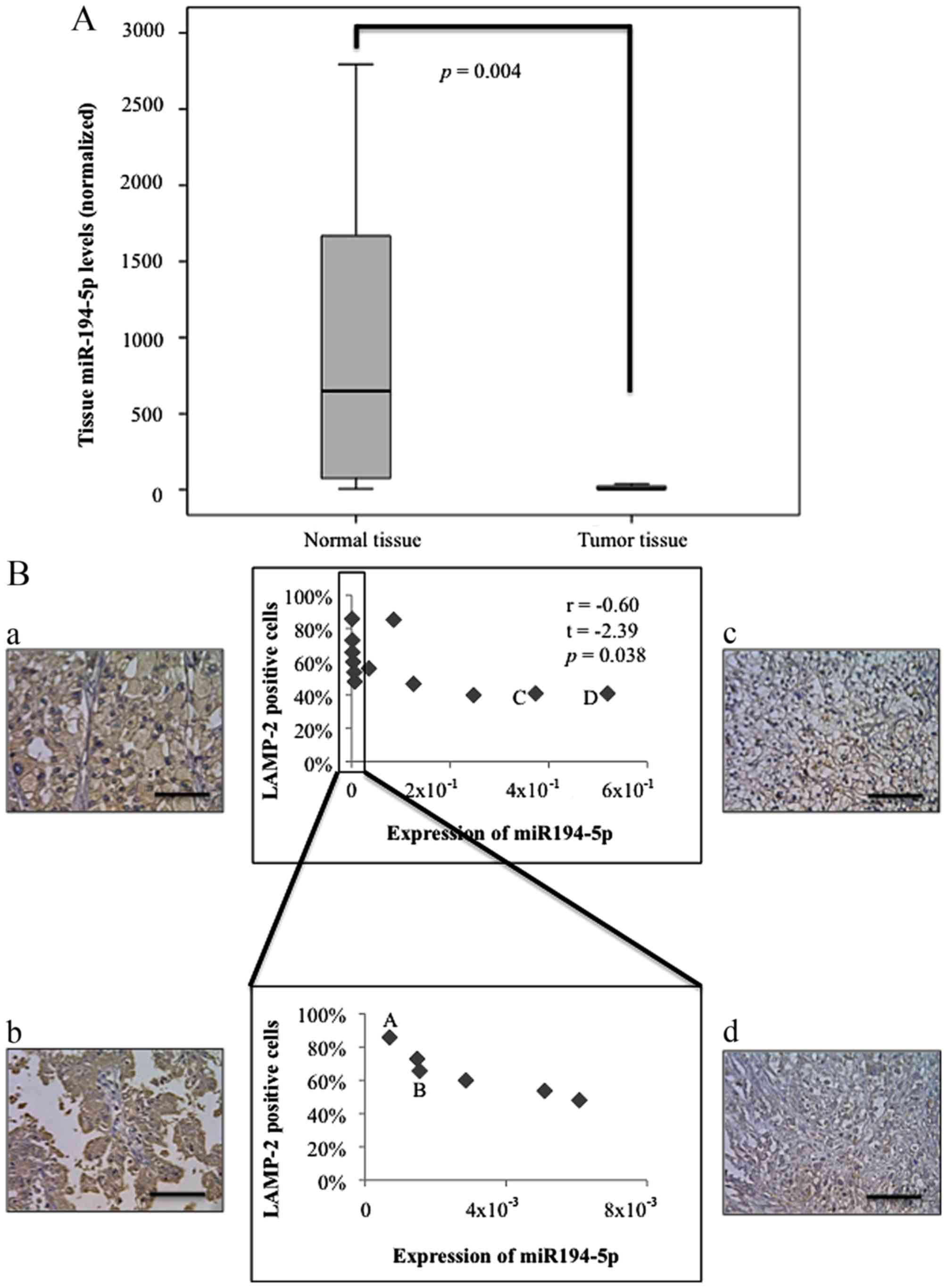
Finally, we evaluated the expression of miR-194-5p
in human advanced RCC samples from radical nephrectomies. The
characteristics of clinical samples are shown in Table II. Twelve samples of advanced RCC
were analyzed for the expression of miR-194-5p by RT-qPCR. The
miR-194-5p expression data were normalized to that of RNU6B.
Tissue miR-194-5p levels were significantly lower in tumor tissues
than in normal tissues (P=0.004, Fig.
2A). LAMP-2 expression was evaluated by immunohistopathology.
As shown in Fig. 2B, LAMP2
immunoreactivity was observed in tumor cell cytoplasm. The
percentage of LAMP-2-positive tumor cells was inversely correlated
to miR-194-5p expression levels (r=−0.60, t=−2.39, P=0.038).
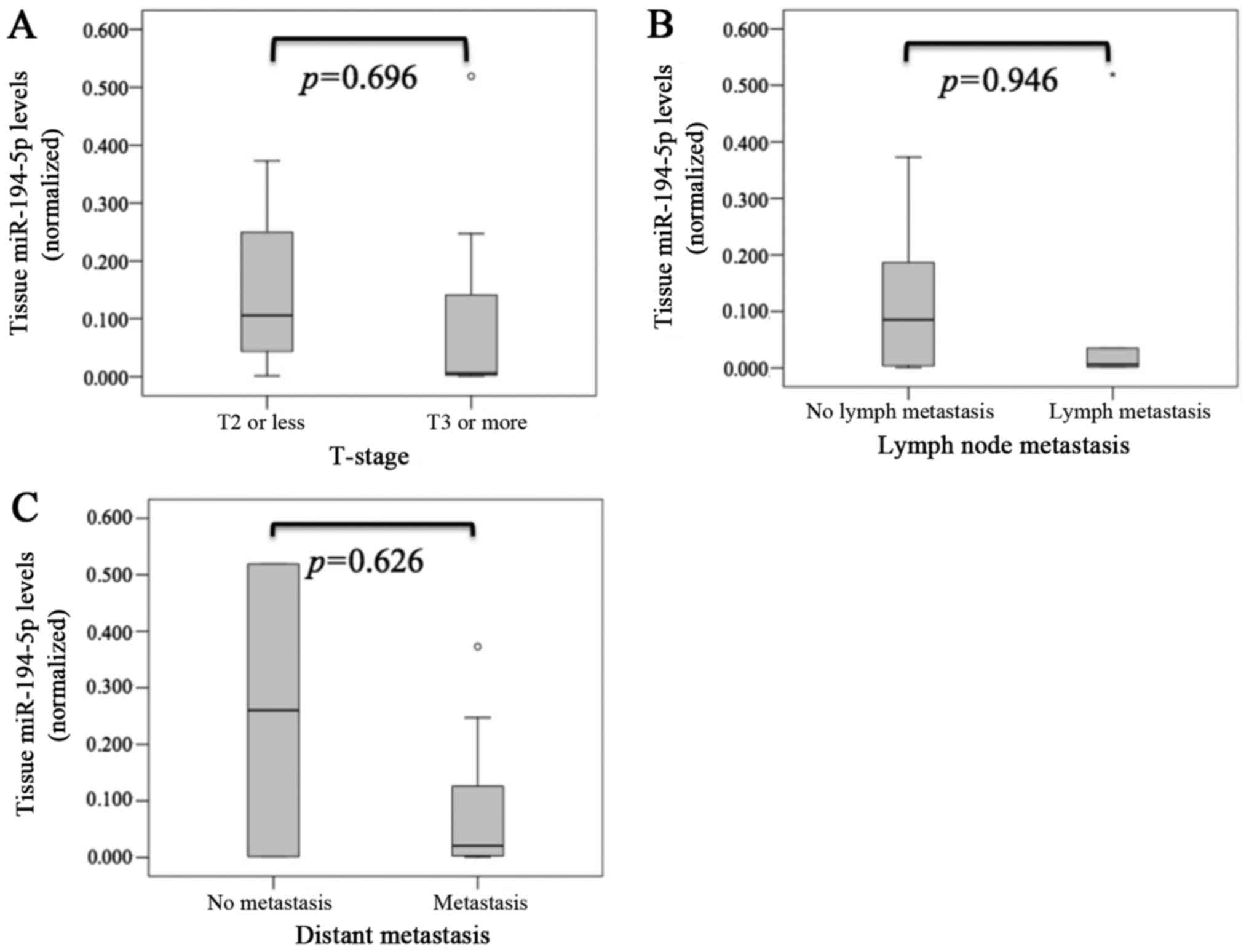
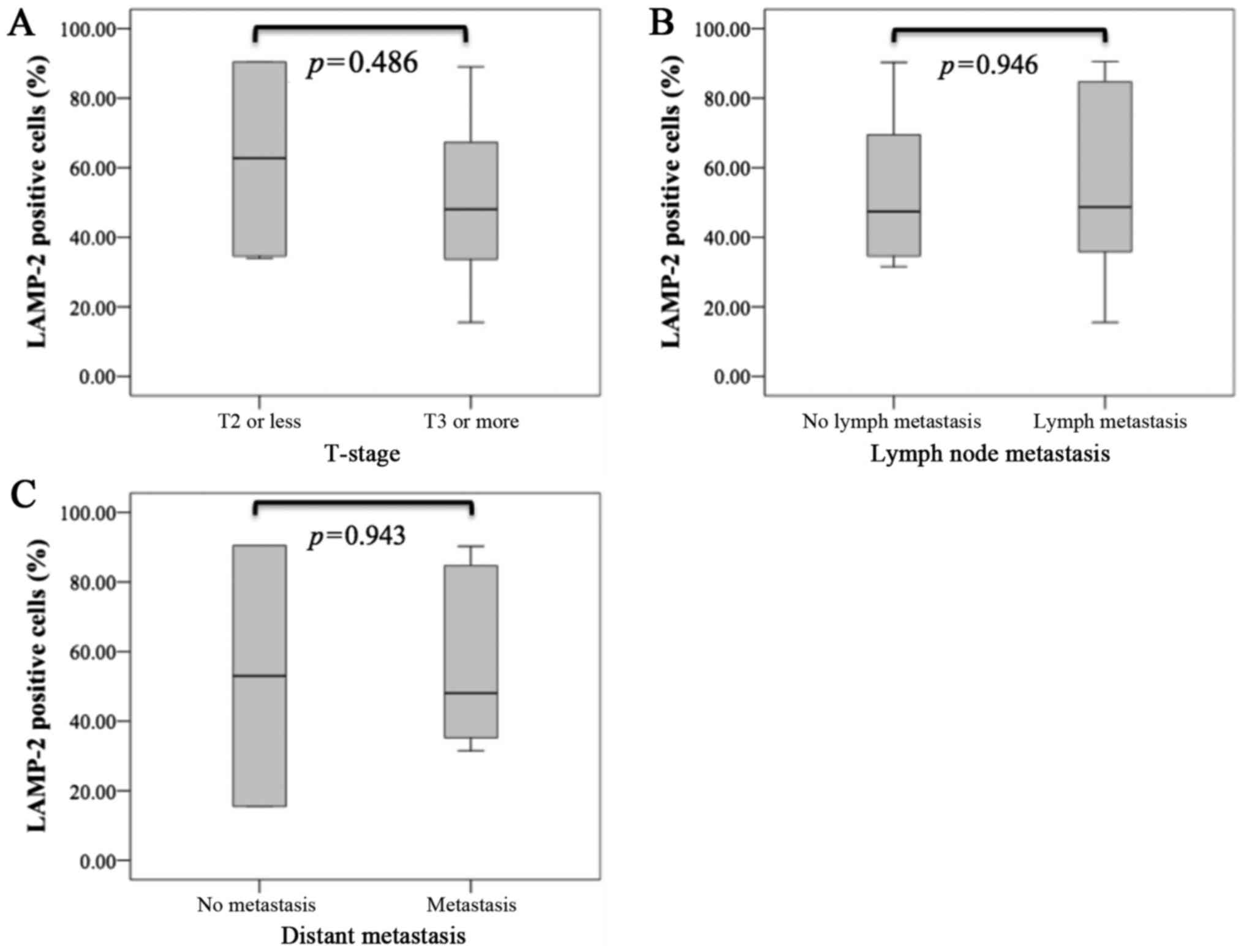
Although we analyzed the relationship between miR-194-5p or LAMP-2
expression and clinicopathological parameters, there were no
significant differences in T stage, lymph node metastasis, and
distant metastasis for miR-194-5p (Fig.
3) or LAMP-2 (Fig. 4).
 | Table II.Patient characteristics (n=12). |
Table II.
Patient characteristics (n=12).
| Characteristic | Number of patients
(n) |
|---|
| Age (years) |
|
|
Mean | 60 |
|
Range | 26–70 |
| Sex |
|
|
Male | 6 |
|
Female | 6 |
| Histopathology |
|
| Clear
cell | 6 |
|
Papillary | 2 |
|
Chromophobe | 2 |
|
Spindle | 2 |
| Pathological
stage |
|
|
pT3 | 8 |
|
pT2 | 1 |
|
pT1 | 3 |
| Lymph
nodes metastasis | 5 |
| Distant
metastasis | 10 |
| Grade |
|
| G3 | 9 |
| G2 | 3 |
Discussion
Although sunitinib is a TKI indicated as a
first-line treatment for mRCC, the clinical benefit of sunitinib in
PFS is limited, and the majority of mRCC patients treated with
sunitinib ultimately experience disease progression due to the
acquisition of resistance (3–5). miRNAs play a crucial role in modulating
the sensitivity to chemotherapeutic agents in multiple tumors
(18,19), indicating that miRNAs are promising
therapeutic targets in cancers. Therefore, identifying miRNAs that
could eliminate resistance to sunitinib in RCC cells may help
elucidate the mechanism of sunitinib resistance and clinically
benefit RCC patients. The present study revealed that restoring
miR-194-5p expression in SR-ACHN cells sensitized to sunitinib via
down-regulating LAMP2, a possible target of miR-194-5p in RCC
cells, increased sunitinib sensitivity. It is strongly suggested
that over-expression of LAMP2 by inhibiting miR-194-5p may lead to
sunitinib resistance acquisition.
There is only one study showing that miR-194-5p is
associated with drug resistance in human cancers. Zhu et al
reported that miR-194-5p is down-regulated in the
cisplatin-resisted human non-small cell lung cancer cell
line-A549/DDP and over-expression of miR-194-5p increases cisplatin
sensitivity via down-regulating FOXA1, a target of miR-194-5p
(20). However, the paper reported
that down-regulation of miR-194-5p contributed to drug resistance
against cisplatin, but not sunitinib. So far, various miRNAs have
been reported to contribute to sunitinib resistance in RCCs
(12,21–23). For
example, Merhautova et al reported that decreased tissue
levels of miR-155 and miR-484 are significantly associated with
prolonged time to progression in RCC patients treated with
sunitinib (21). In addition, Berkers
et al reported that miR-141 down-regulation-driven
epithelial-to-mesenchymal transition (EMT) in clear cell carcinoma
is associated with an unfavorable response to sunitinib, indicating
that low miR-141 expression results in poor prognosis (22). Goto et al reported that miR-101
is markedly suppressed in sunitinib-treated RCC tissues and
restoration of miR-101 significantly inhibits migration and
invasion in Caki-1 and 786-O cells (23). A comprehensive analysis screened 673
miRNAs in tumor tissues from mRCC patients and revealed that higher
miR-942 expression is an independent predictor of inadequate
sunitinib efficacy (12). Although
such miRNAs related to sunitinib resistance might be useful as
prognostic markers, no data was shown regarding whether the altered
expression of such miRNAs directly gave rise to sunitinib-resistant
RCCs. Our data showed that not only low-expression of miR-194-5p
was associated with resistance against sunitinib, but also
susceptibility to sunitinib was ameliorated by miR-194-5p
restoration in RCC cells via LAMP-2 down-regulation.
LAMP-2 is present at the lysosomal membrane and
contributes to lysosome function. In recent studies, the lysosome
was associated with the sunitinib resistance mechanism. Giuliano
et al reported that sequestration of sunitinib in lysosomes
and the subsequent inhibition of the autophagy flux participate in
sunitinib resistance (24). The
incomplete autophagic flux is caused by suppression of the
lysosomal protease cathepsin B activity. In addition, Gotink et
al reported fluorescent microscopy data revealing intracellular
sunitinib distribution mainly in acidic lysosomes, which are also
significantly increased in sunitinib-resistant renal cancer cells
compared to that in parental cells (25). These data indicate that sunitinib
resistance is dependent on the lysosomal capacity, which is
reflected by the LAMP-2 expression level. Therefore, expansion of
sunitinib accumulation in lysosomes may be induced by increasing
LAMP-2 expression, contributing to sunitinib resistance in mRCC
cells.
We investigated the relationship between miR-194-5p
or LAMP-2 expression and T-stage, lymph node metastasis, and
distant metastasis, and found there were no significant
differences. In the previous report, Lee et al reported that
miR-194-5p might be used as diagnostic biomarkers in adenocarcinoma
in uterine cervix, but there were no significant differences
between miR-194-5p and T-stage, lymph node metastasis, and distant
metastasis (26). In addition, LAMP-1
might influence local tumor progression rather than the formation
of tumor metastasis in pancreatic carcinoma, but no relation was
found between LAMP-2 and the tumor stage or lymph node metastasis
(27). In lung cancer, Giatromanolaki
et al reported that LAMP-2 was related to high histology
grade, and was not related tumor stage (28). However, many reports described that
LAMP2 was related to drug resistance (24,25). These
data showed that these two molecules contributed to drug resistance
acquisition by sunitinib uptake by lysosomes, and suggested that
down-regulation of LAMP-2 by miR-194-5p was independent of cell
proliferation, cell death resistance, invasion, and metastasis.
Therefore, we thought that clarifying the intracellular signaling
transmission pathways regulated by miR-194-5p could identify
mechanisms responsible for intrinsic or acquired sunitinib
resistance.
In conclusion, we have identified miR-194-5p as a
sunitinib-resistant suppressive miRNA that down-regulates LAMP2 in
human RCC cells. Targeting miR-194-5p could contribute to a new
therapy against sunitinib resistance and improve PFS for patients
with mRCC.
References
|
1
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eggener SE, Yossepowitch O, Pettus JA,
Snyder ME, Motzer RJ and Russo P: Renal cell carcinoma recurrence
after nephrectomy for localized disease: Predicting survival from
time of recurrence. J Clin Oncol. 24:3101–3106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmidinger M, Larkin J and Ravaud A:
Experience with sunitinib in the treatment of metastatic renal cell
carcinoma. Ther Adv Urol. 4:253–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Bacik J and Mazumdar M:
Prognostic factors for survival of patients with stage IV renal
cell carcinoma: Memorial sloan-kettering cancer center experience.
Clin Cancer Res. 10:6302S–6303S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutson TE, Escudier B, Esteban E,
Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR,
Hariharan S and Motzer RJ: Randomized phase III trial of
temsirolimus versus sorafenib as second-line therapy after
sunitinib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 32:760–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hainsworth JD, Rubin MS, Arrowsmith ER,
Khatcheressian J, Crane EJ and Franco LA: Pazopanib as second-line
treatment after sunitinib or bevacizumab in patients with advanced
renal cell carcinoma: A Sarah Cannon Oncology Research Consortium
Phase II trial. Clin Genitourin Cancer. 11:270–275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gotink KJ, Rovithi M, de Haas RR,
Honeywell RJ, Dekker H, Poel D, Azijli K, Peters GJ, Broxterman HJ
and Verheul HM: Cross-resistance to clinically used tyrosine kinase
inhibitors sunitinib, sorafenib and pazopanib. Cell Oncol (Dordr).
38:119–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saftig P and Klumperman J: Lysosome
biogenesis and lysosomal membrane proteins: Trafficking meets
function. Nat Rev Mol Cell Biol. 10:623–635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slaby O, Jancovicova J, Lakomy R, Svoboda
M, Poprach A, Fabian P, Kren L, Michalek J and Vyzula R: Expression
of miRNA-106b in conventional renal cell carcinoma is a potential
marker for prediction of early metastasis after nephrectomy. J Exp
Clin Cancer Res. 29:902010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwamoto H, Kanda Y, Sejima T, Osaki M,
Okada F and Takenaka A: Serum miR-210 as a potential biomarker of
early clear cell renal cell carcinoma. Int J Oncol. 44:53–58. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi N, Osaki M, Onuma K, Yumioka T,
Iwamoto H, Sejima T, Kugoh H, Takenaka A and Okada F:
Identification of MicroRNAs involved in resistance to sunitinib in
renal cell carcinoma cells. Anticancer Res. 37:2985–2992.
2017.PubMed/NCBI
|
|
16
|
Nofech-Mozes R, Khella HW, Scorilas A,
Youssef L, Krylov SN, Lianidou E, Sidiropoulos KG, Gabril M, Evans
A and Yousef GM: MicroRNA-194 is a marker for good prognosis in
clear cell renal cell carcinoma. Cancer Med. 5:656–664. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gotink KJ, Broxterman HJ, Honeywell RJ,
Dekker H, de Haas RR, Miles KM, Adelaiye R, Griffioen AW, Peters
GJ, Pili R and Verheul HM: Acquired tumor cell resistance to
sunitinib causes resistance in a HT-29 human colon cancer xenograft
mouse model without affecting sunitinib biodistribution or the
tumor microvasculature. Oncoscience. 1:844–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ayers D and Vandesompele J: Influence of
microRNAs and long non-coding RNAs in cancer chemoresistance. Genes
(Basel). 8:pii: E952017. View Article : Google Scholar
|
|
19
|
An X, Sarmiento C, Tan T and Zhu H:
Regulation of multidrug resistance by microRNAs in anti-cancer
therapy. Acta Pharm Sin B. 7:38–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Li D, Yu F, Jia C, Xie J, Ma Y, Fan
S, Cai H, Luo Q, Lv Z and Fan L: miR-194 inhibits the
proliferation, invasion, migration, and enhances the
chemosensitivity of non-small cell lung cancer cells by targeting
forkhead box A1 protein. Oncotarget. 7:13139–13152. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Merhautova J, Hezova R, Poprach A,
Kovarikova A, Radova L, Svoboda M, Vyzula R, Demlova R and Slaby O:
miR-155 and miR-484 are associated with time to progression in
metastatic renal cell carcinoma treated with sunitinib. Biomed Res
Int. 2015:9419802015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berkers J, Govaere O, Wolter P, Beuselinck
B, Schöffski P, van Kempen LC, Albersen M, van den Oord J, Roskams
T, Swinnen J, et al: A possible role for microRNA-141
down-regulation in sunitinib resistant metastatic clear cell renal
cell carcinoma through induction of epithelial-to-mesenchymal
transition and hypoxia resistance. J Urol. 189:1930–1938. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goto Y, Kurozumi A, Nohata N, Kojima S,
Matsushita R, Yoshino H, Yamazaki K, Ishida Y, Ichikawa T, Naya Y
and Seki N: The microRNA signature of patients with sunitinib
failure: Regulation of UHRF1 pathways by microRNA-101 in renal cell
carcinoma. Oncotarget. 7:59070–59086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giuliano S, Cormerais Y, Dufies M, Grépin
R, Colosetti P, Belaid A, Parola J, Martin A, Lacas-Gervais S,
Mazure NM, et al: Resistance to sunitinib in renal clear cell
carcinoma results from sequestration in lysosomes and inhibition of
the autophagic flux. Autophagy. 11:1891–1904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gotink KJ, Broxterman HJ, Labots M, de
Haas RR, Dekker H, Honeywell RJ, Rudek MA, Beerepoot LV, Musters
RJ, Jansen G, et al: Lysosomal sequestration of sunitinib: A novel
mechanism of drug resistance. Clin Cancer Res. 17:7337–7346. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee H, Kim KR, Cho NH, Hong SR, Jeong H,
Kwon SY, Park KH, An HJ, Kim TH, Kim I, et al: MicroRNA expression
profiling and Notch1 and Notch2 expression in minimal deviation
adenocarcinoma of uterine cervix. World J Surg Oncol. 12:3342014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Künzli BM, Berberat PO, Zhu ZW, Martignoni
M, Kleeff J, Tempia-Caliera AA, Fukuda M, Zimmermann A, Friess H
and Büchler MW: Influences of the lysosomal associated membrane
proteins (Lamp-1, Lamp-2) and Mac-2 binding protein (Mac-2-BP) on
the prognosis of pancreatic carcinoma. Cancer. 94:228–239. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giatromanolaki A, Kalamida D, Sivridis E,
Karagounis IV, Gatter KC, Harris AL and Koukourakis MI: Increased
expression of transcription factor EB (TFEB) is associated with
autophagy, migratory phenotype and poor prognosis in non-small cell
lung cancer. Lung Cancer. 90:98–105. 2015. View Article : Google Scholar : PubMed/NCBI
|