Introduction
The Notch signaling pathway comprises Notch
transmembrane receptors, Notch ligands, DNA-binding protein
C-promotor Binding Factor 1 (CBF-1)/J κ-recombination
signal-binding protein/Suppressor of hairless (H)/lin-12 and glp-1
(CSL), several effector molecules and regulatory molecules
(1). A hallmark of Notch signaling
that distinguishes it from other conserved signaling pathways is
its mechanism of signal transduction. When Notch ligands, including
Jagged (JAG)1, JAG2, delta like canonical notch ligand (DLL)1, DLL3
and DLL4, interact with Notch transmembrane receptors, for example
Notch homolog (Notch) 1–4, on adjacent cells, this binding induces
the cleavage of Notch receptor by proteases, including a
disintegrin and metalloproteinase proteases or γ-secretase, to
release Notch1 intracellular domain (NICD) (1–3). Then,
NICD travels to the nucleus and binds to DNA binding proteins, such
as CSL, to assemble a transcription complex that activates
downstream target genes, including Hairy and enhancer of split
HES1, HES5 and Hairy/enhancer-of-split related with YRPW motif
protein 1 (1–3). This core signal transduction pathway is
used in the majority of Notch-dependent processes and is known as
the canonical CSL-NICD-Mastermind-like pathway (4,5). In
addition, depending on the cellular context, the amplitude and
duration of Notch activity may be additionally regulated at various
points in the pathway (1–3). Activated Notch signaling has been
demonstrated to serve an important role in the development and
homeostasis of tissues by regulating cell-fate decisions,
proliferation, differentiation and apoptosis (1,6). These
features confer susceptibility of Notch signaling subversion by
cancer cells.
Gastric cancer (GC) is one of the most common
malignant diseases and the third leading cause of cancer-associated
mortalities worldwide in 2012, with age-standardized incidence
rates highest in eastern Asia (7). A
previous study identified that high expression of Notch1-4 mRNA was
associated with unfavorable overall survival in 876 patients with
GC over a 20-year period (8).
Activated Notch1 was a poor prognostic factor for patients with GC
(9) and closely associated with an
advanced tumor stage, tumor metastasis and overall patient survival
(10). A previous study indicated
that Notch1 may maintain the cancer stem-like phenotype of diffuse
type GC through inducing CD133 gene expression, and that inhibiting
Notch1 may be an effective treatment for CD133-positive diffuse
type GC (11).
ββ-Dimethylacrylshikonin and Sirtuin 3 may inhibit GC cell growth
through downregulating Notch1 (12,13). In
addition, Notch1 inhibition may also impair the invasion capability
of GC cells (14). Certain previous
studies suggested that Notch1 expression may be repressed by
several microRNAs (miRNA/miR), including miR-124, miR-935 and
miR-34 during GC progression (15–17).
Concurrently, Notch1 and miR-151-5p interact with p53 in a
reciprocal regulation loop to control gastric tumorigenesis
(18). In addition, Notch2 and Notch3
receptor expression was also associated with gastric cancer
development, and Notch4 receptor promoted gastric cancer growth
(19–22). An additional study indicated that NICD
was associated with the presence of lymph node metastasis and worse
survival (9,10). However, few studies have examined the
association between NICD and differentiation of GC.
The cyclooxygenase (COX) enzyme exists in two forms:
COX-1 and COX-2. COX-1 is constitutively produced, while COX-2 is
an inducible form. Despite being previously explored as a
pro-inflammatory molecule, several data indicated the vital role of
COX-2 in GC (23), and in other types
of cancer (24,25). A previous study suggested that the
activation of Notch1 signal pathway may promote the progression of
gastric cancer through COX-2 (26).
Notch2 may also induce COX-2 expression, and the suppression of
tumor progression by Notch2 knockdown in GC cells may be reversed
by exogenous COX-2 (20).
Nevertheless, the effect of COX-2 on Notch activity in GC cells has
not yet been studied. The present study revealed that
poorly-differentiated GC expressed increased levels of NICD
compared with well-differentiated GC, indicating potential
involvement of NICD in GC differentiation. The selective COX-2
inhibitor NS-398 may enhance antitumor activity of
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester (DAPT), a non-specific inhibitor of Notch, in
poorly-differentiated GC cells via downregulating NICD level.
Materials and methods
Specimens
Human GC tissues and adjacent normal gastric tissues
(>5 cm from tumor) were obtained from 12 patients (between 40
and 80 years old, with a median age of 70; including 3 females and
9 males) who underwent gastric resection at the First Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) without
pre-operative chemotherapy or radiation between May 2012 and July
2012. Normal gastric tissue (n=1) was collected from a healthy
patient by gastroscopy. Written informed consent was obtained from
each patient and approval was obtained from the Ethical Committee
on Human Research of Wenzhou Medical University. Each tumor sample
was assigned at a histological grade based on the World Health
Organization (WHO) classification criteria of tumors of the
digestive system (27).
Robust Multi-Array mveraging (RMA)
normalized basal expression profiles
RMA normalized basal expression profiles for AGS
cell were downloaded from the Genomics of Drug Sensitivity in
Cancer Project (GDSC; version 6.1, March 2017; http://www.cancerrxgene.org/). The GDSC is a
collaboration between the Cancer Genome Project at the Wellcome
Trust Sanger Institute (Hinxton, UK) and the Center for Molecular
Therapeutics, Massachusetts General Hospital Cancer Center (Boston,
USA). The expression profiles contained the expression of 12,687
genes of 1,019 cell lines (28).
Cell culture
The human poorly differentiated GC AGS cell line
(the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences, Shanghai, China) was cultured in F12 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml-1 penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). The cells were incubated at 37°C with 5%
CO2 in humidified air.
Immunocytochemistry staining
(ICC)
1×106 AGS cells were seeded onto glass
coverslips and cultured in 37°C humidified air overnight. Cells
were fixed with 4% paraformaldehyde for 30 min. The following steps
were completed according to the manufacturer's protocol of a rabbit
polymer detection kit (cat. no. PV6001; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). The details are as
follows: Cells were incubated with endogenous peroxidase blockers
(included in the kit) for 10 min at room temperature, the slides
were probed with an rabbit anti-human NICD antibody (used for
recognizing the active form of the Notch1 receptor, exposed
following cleavage by γ-secretase; 1:100 dilution; cat. no.
07-1231; EMD Millipore, Billerica, MA, USA), at 4°C overnight and
followed by incubation with horseradish peroxidase (HRP)-labeled
goat anti rabbit IgG polymer (included in the kit) at room
temperature for 20 min. Finally, slides were stained by
diaminobenzidine (DAB; cat. no. ZLI-9017; Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 3 min and hematoxylin (cat. no.
ZLI-9610; Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 1
min. PBS was used to replace the primary antibody in for the
negative control. The slides were examined under a fluorescence
microscope (BX-50; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Whole-cell lysates were prepared from human
specimens or AGS cells using cultured cell protein extraction
reagent (cat. no. AR0103; Boster Biological Technology Co., Ltd.,
CA, USA) or mammalian tissue protein extraction reagent (cat. no.
AR0101; Boster Biological Technology Co., Ltd.). The concentration
of proteins were determined by using BCA Protein Assay kit (cat.
no. P0012; Beyotime Biotech, Nantong, China). 80 µg protein were
separated using a 10% gel and SDS-PAGE and then transferred to
polyvinylidene fluoride membranes (EMD Millipore). Subsequent to
blockage of non-specific binding sites with 5% non-fat milk at room
temperature for 90 min, the membranes were incubated with rabbit
anti-NICD antibody (1:500), rabbit anti-HES1 (1:500 dilution; cat.
no. ab71559; Abcam, Cambridge, UK), rabbit anti-HES5 (1:500
dilution; cat. no. ab194111; Abcam), rabbit anti-GAPDH antibody
(1:500 dilution; cat. no. AB-P-R 001; Hangzhou Goodhere
Biotechnology Co., Ltd., Hangzhou, China) or rabbit anti-β-actin
antibody (1:500 dilution; cat. no. Ab8227; Abcam) at 4°C overnight.
Following washing in TBST three times, membranes were incubated at
room temperature for 60 min with a horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody (1:3,000
dilution; cat. no. ZB-2301; Zhongshan Golden Bridge Biotechnology
Co., Ltd.) and detected by SuperSignal West Pico Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc.). Signal intensities were
quantified by Quantity One v4.62 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). GAPDH and β-actin were used as loading
controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from AGS cells treated with DAPT
(20 µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), NS-398 (50
µM; Sigma-Aldrich), DAPT+NS-398 or dimethyl sulfoxide for 48 h
using TRIzol® (cat. no. 15596026; Thermo Fisher
Scientific, Inc.), and then RNA was reversed to cDNA by using M-MLV
Reverse Transcriptase (cat. no. 28025021; Invitrogen; Thermo Fisher
Scientific, Inc.). qPCR was performed using iQ™ SYBR®
Green Supermix (cat. no. 170-8882; Bio-Rad Laboratories, Inc.)
according to manufacturer's protocol. The thermocycling conditions
for PCR amplification were as follows: 95°C for 2 min
(pre-denaturation), 40 cycles of 95°C for 15 sec (denaturation) and
60°C for 30 sec (annealing and elongation), using the following
primers: HES1 forward, 5′-ACACGACACCGGATAAACCAA-3′ and reverse,
5′-CGAGTGCGCACCTCGGTA-3′; and GAPDH forward,
5′-TCCCATCACCATCTTCCAGG-3′ and reverse, 5′-GATGACCCTTTTGGCTCCC-3′
(GeneCore BioTechnologies Co., Ltd., Shanghai, China). The relative
genomic copy number was calculated using the comparative Cq method
(29). GAPDH mRNA levels were
measured as a housekeeper gene for normalization of HES1 mRNA
expression values. The fold change from control group was set at
1-fold.
Cell growth assay
AGS cells (8×103 cells/well) were plated
into 96-well plates in triplicate, and then treated with DAPT (5,
10 and 20 µM) or NS-398 (25, 50 and 100 µM). Cell survival rate was
assessed at 12, 24, 48 or 72 h following treatment using a
commercial Cell Counting kit (CCK8; cat. no. C0037; Beyotime
Institute of Biotechnology, Haimen, China) according to the
protocol of the manufacturer.
Cell apoptosis assay
A cell apoptosis assay was conducted by flow
cytometry with Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit (cat. no. 556547; BD Biosciences, San Jose,
CA, USA). AGS cells (4×105 cells/well) were plated onto
6-well plates. Following attachment at 37°C overnight, cells were
treated with 50 µM NS-398, 20 µM DAPT or 50 µM NS-398 combined with
20 µM DAPT for 48 h. Subsequent to dissociation and centrifugation
at 4°C and 1,000 × g for 5 min, cells were resuspended in combined
buffer solution and double-stained with Annexin V-FITC and
propidium iodide. Apoptosis was measured by using flow cytometry
(BD Biosciences) and analyzed by using WinMDI 2.9 analysis software
(30).
Statistical analysis
All experiments were repeated in triplicate. The
data were processed by the SPSS 16.0 statistical software (SPSS,
Inc., Chicago, IL, USA), and presented as means ± standard
deviation. A one-way analysis of variance with post hoc contrasts
using Dunnett's test was used to assess statistical significance of
difference between treatment groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
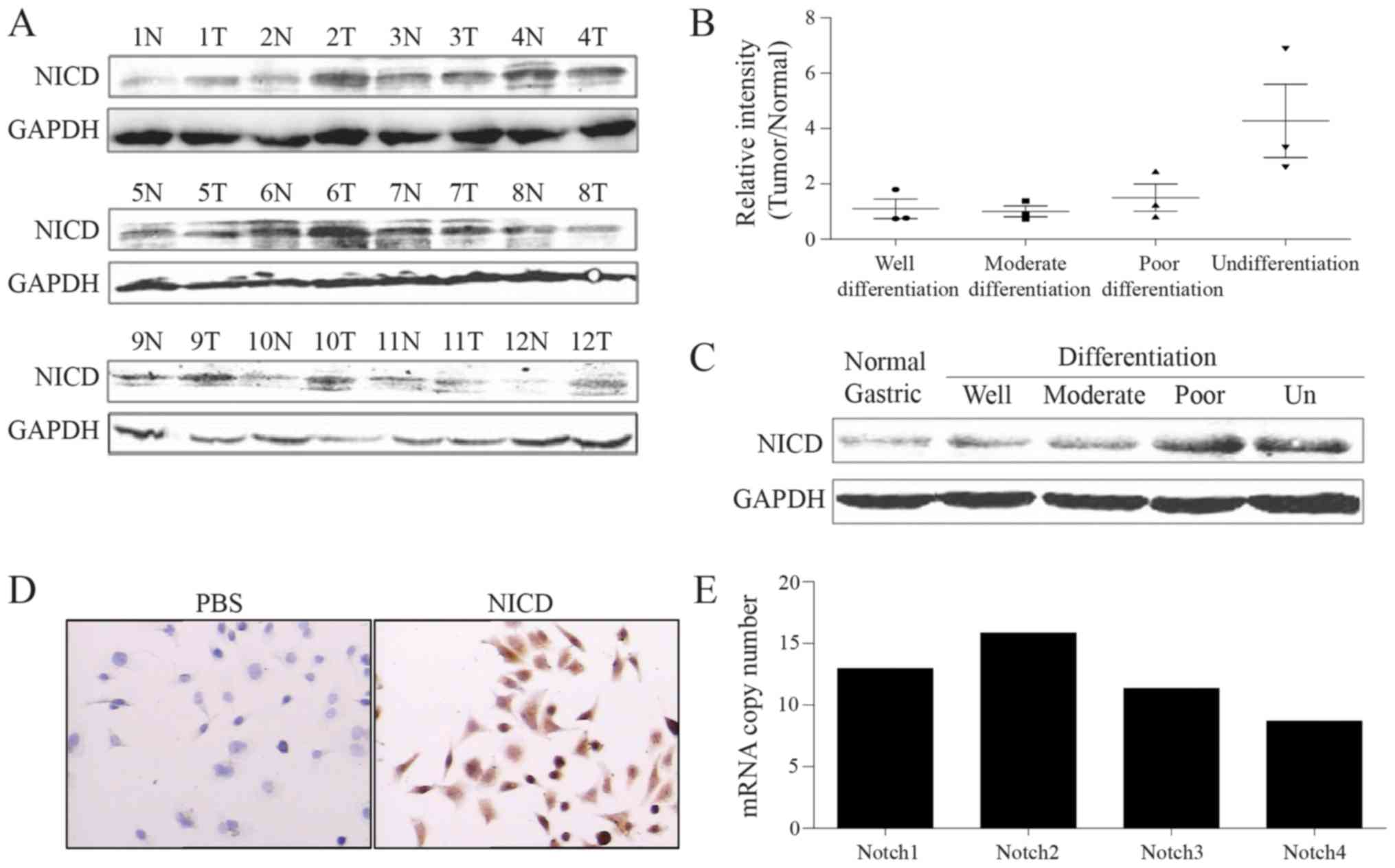
Poorly differentiated GC expresses
increased levels of NICD
Previous study has indicated that Notch signaling
was closely associated with GC (8–22). Western
blot analysis was used to detect NICD protein levels in 12 GC
specimens. The data indicated that the NICD amplification rate in
poor differentiation GC was increased compared with
well-differentiated GC (Fig. 1A and
B; Table I). The level of NICD
proteins in GC specimens of different differentiation levels and
normal gastric epithelial tissues was additionally analyzed through
western blot analysis. The data demonstrated that the poorer the
level of differentiation of the GC tissue, the higher the level of
NICD proteins it possessed (Fig. 1C).
In the poorly differentiated GC AGS cell line, NICD proteins were
also expressed in a high level (Fig.
1D). GDSC database was employed, and it was identified that
Notch1 was also expressed in AGS cells (Fig. 1E). All the aforementioned results
suggested that the level of NICD may be associated with the
differentiation of GC.
 | Table I.Rate of high NICD expression in
gastric cancer tissues. |
Table I.
Rate of high NICD expression in
gastric cancer tissues.
| Gastric cancer
subtype | Rate of high NICD
expression, % |
|---|
|
Undifferentiated | 100.00 (3/3) |
| Low
differentiation | 66.70 (2/3) |
| Moderate
differentiation | 33.30 (1/3) |
|
Well-differentiated | 33.30 (1/3) |
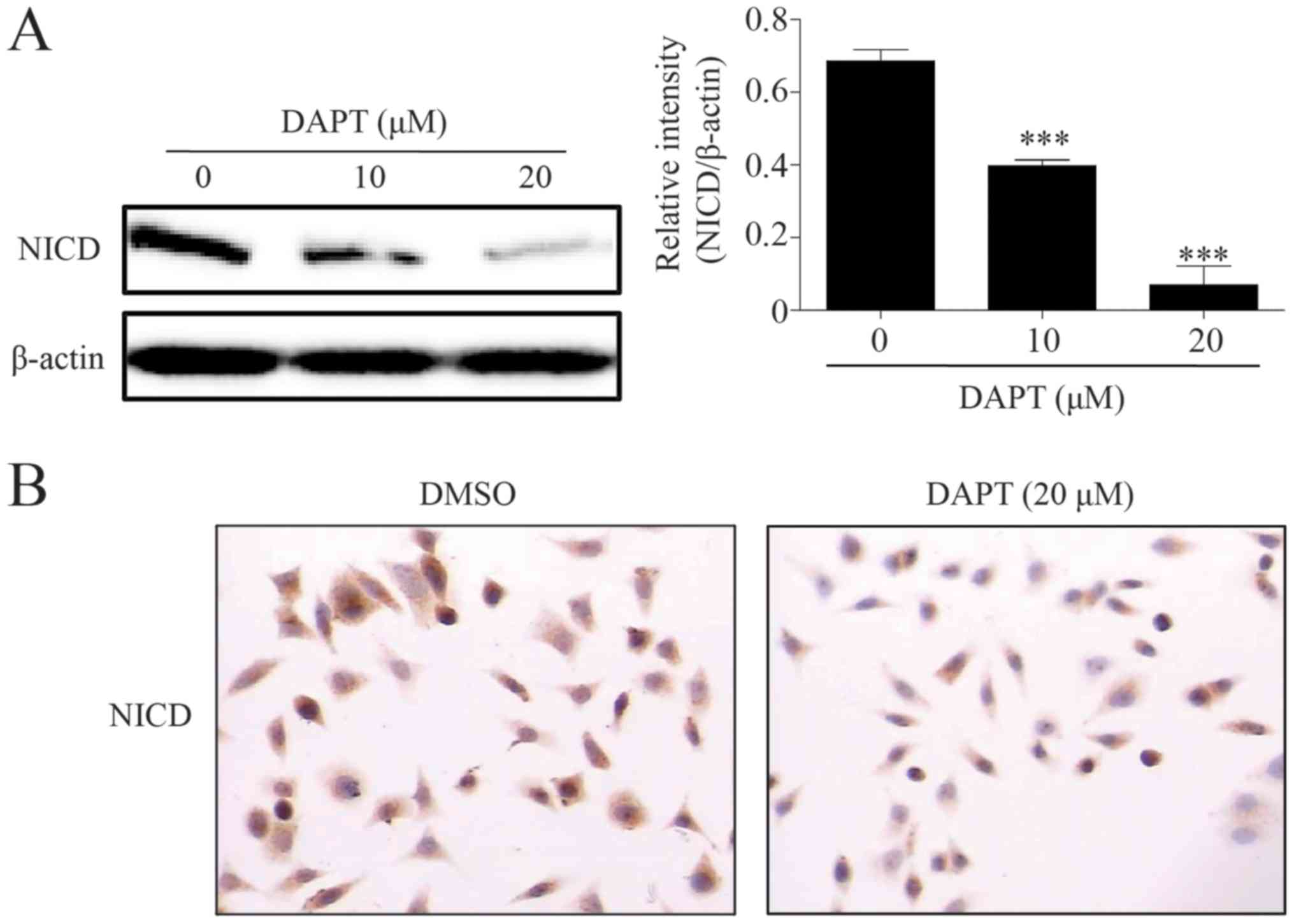
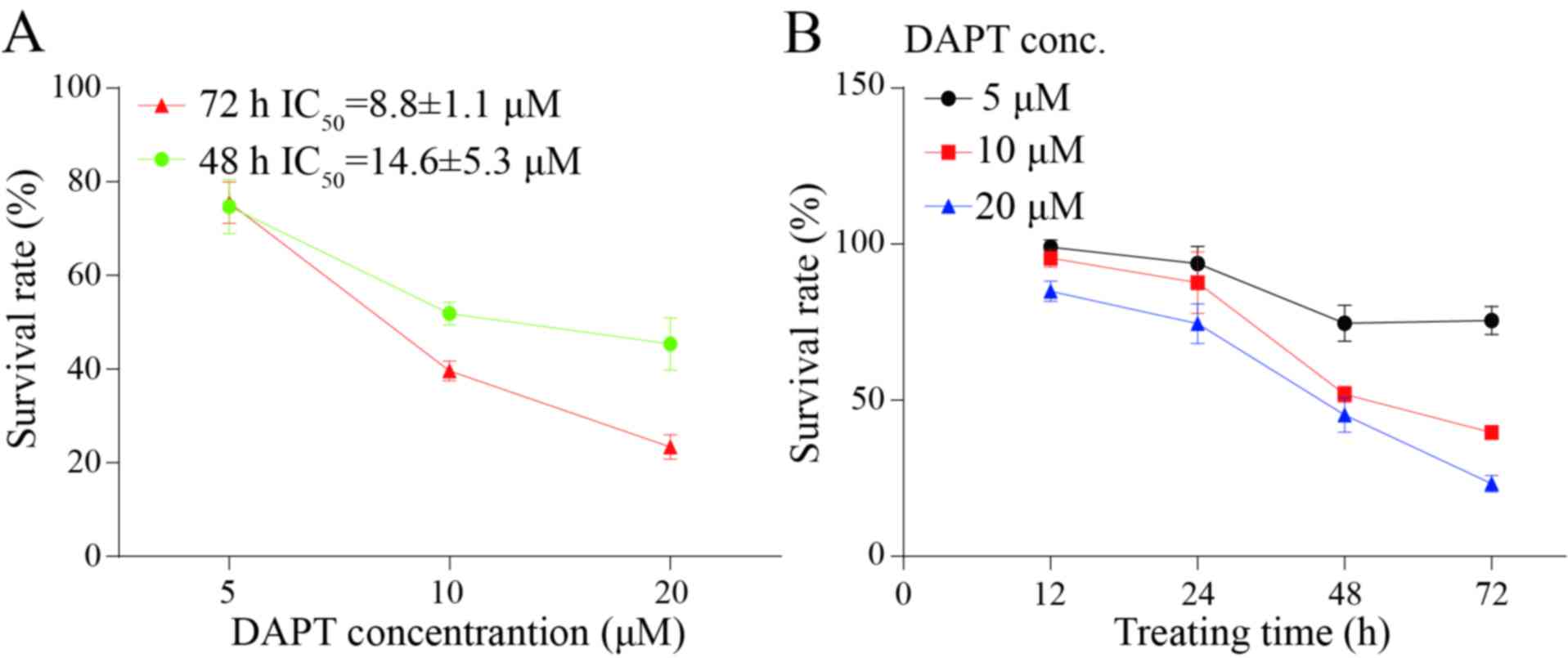
γ-secretase inhibitor DAPT inhibits
growth of poorly differentiated GC AGS cell line
γ-secretase serves a key function in the Notch
signal pathway: γ-secretase blockage may suppress the cleavage of
Notch receptor and block signaling transduction (1–3). The data
indicated that the γ-secretase inhibitor DAPT downregulated the
level of NICD in a dose-dependent manner (Fig. 2A). The ICC results indicated that DAPT
may also decrease the level of nuclear NICD (Fig. 2B). Considering the biological function
of NICD and Notch1 signaling, a CCK8 kit was used to detect the
growth inhibition of DAPT in the poorly differentiated GC AGS cell
line. Results suggested that DAPT may decrease the growth of AGS in
a dose- and time-dependent manner. Following treatment with DAPT
for 48 and 72 h, the half-maximal inhibitory concentration was
8.8±1.1 and 14.6±5.3 µM, respectively (Fig. 3A and B).
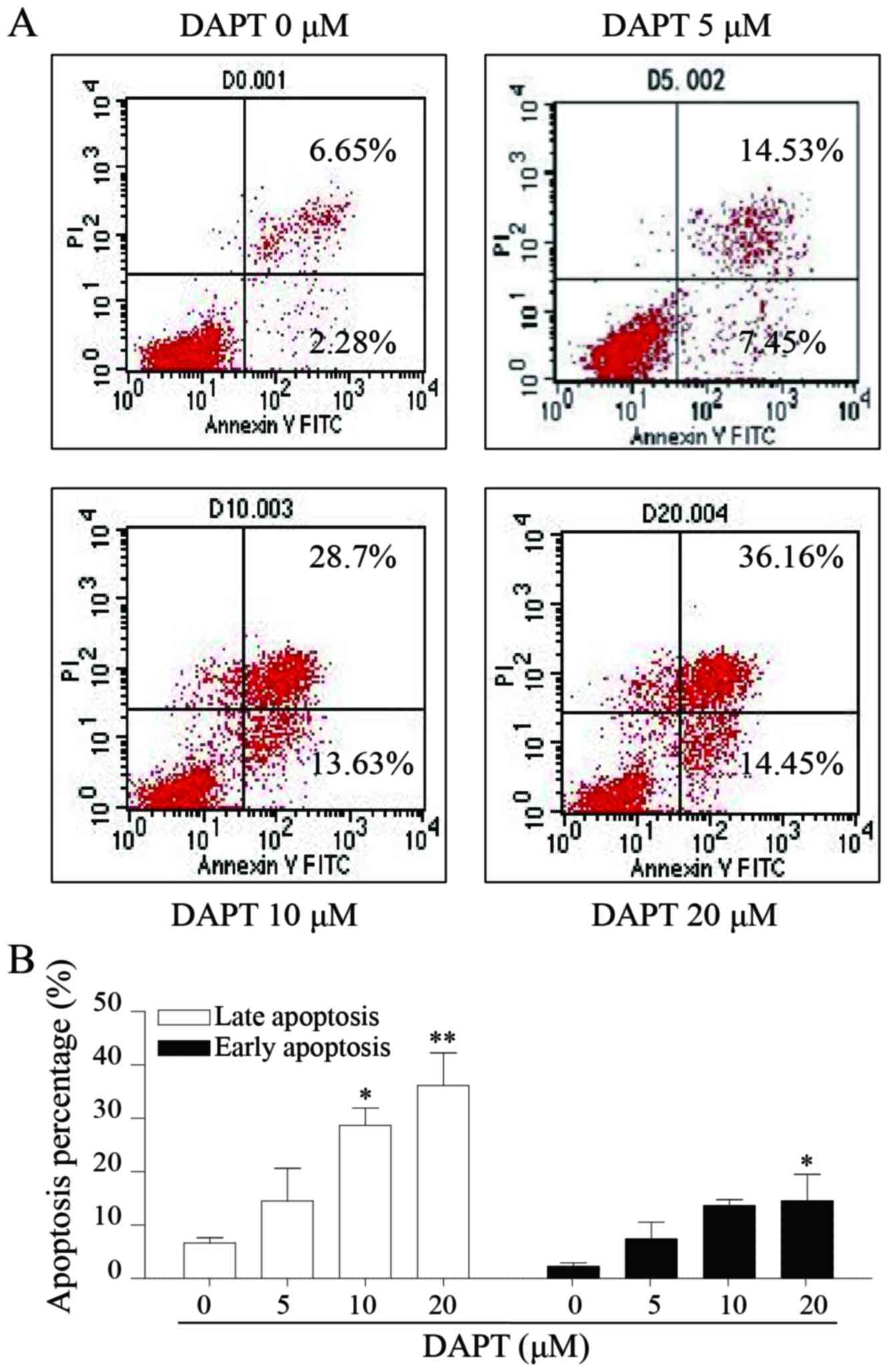
DAPT induces apoptosis of AGS
Notch signal pathway may also regulate cell
apoptosis. In the present study, flow cytometry was applied for the
investigation of the effect of DAPT on apoptosis in AGS cells. The
data suggested that DAPT may significantly induce cell apoptosis in
AGS cells in a dose-dependent manner. A total of 20 µM DAPT caused
levels of early and late apoptosis of ~14.55 and 36.16%,
respectively (Fig. 4A and B).
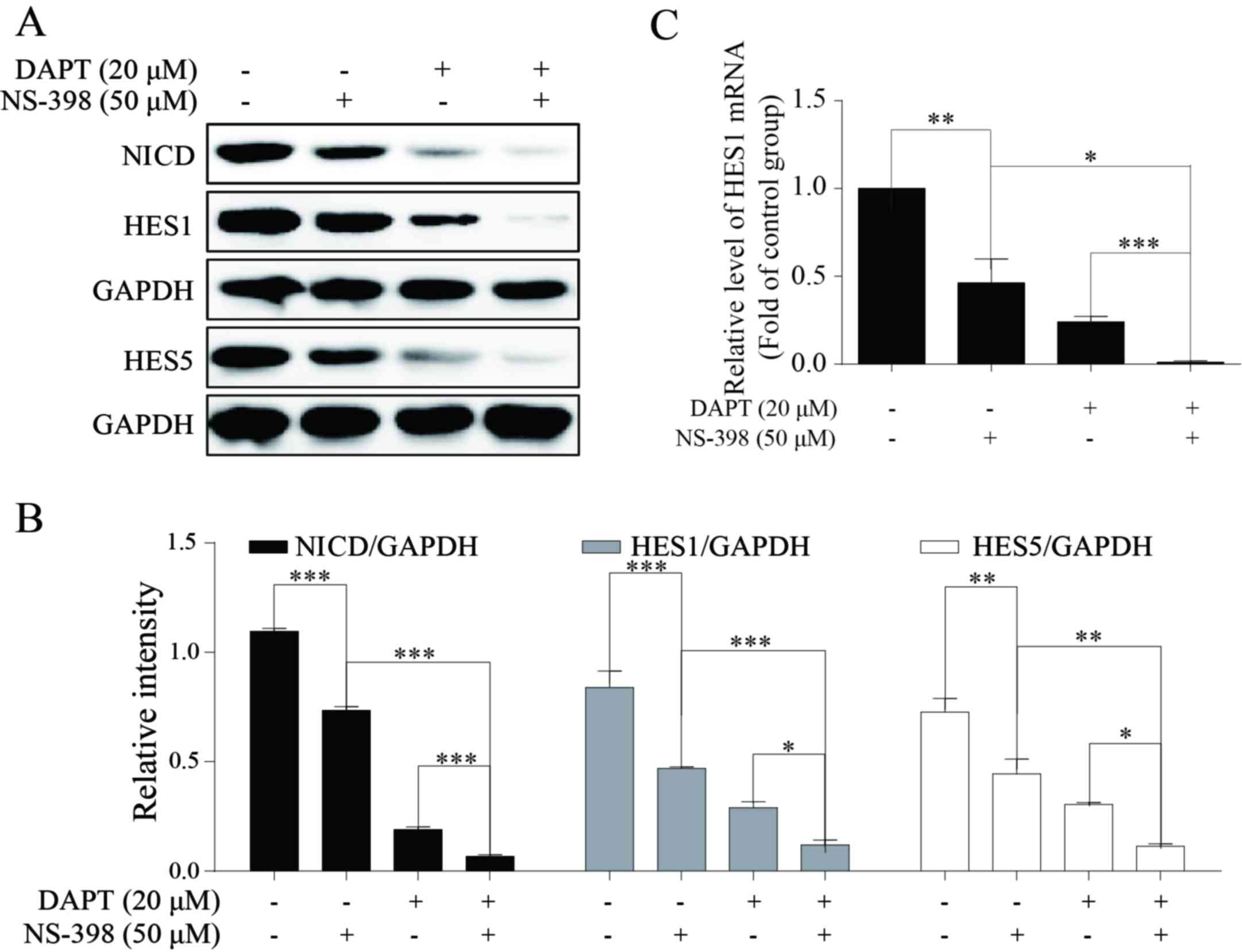
COX-2 inhibitor NS-398 may enhance the
inhibitory effect of DAPT to Notch1 signaling
A number of previous studies have demonstrated the
association between COX-2 and Notch signaling: It has been
suggested that Notch signal may upregulate the expression of COX-2
(20,26). A small number of studies have focused
on the effect of COX-2 on Notch signaling: The downregulation of
NICD, HES1 and HES5 indicated the inhibition of Notch1 signaling
(31–33). HES1 and HES5 proteins were employed to
reveal the effect of the treatment in the present study to Notch1
signaling. The present study identified that 50 µM NS-398 may
significantly downregulate the expression levels of NICD and its
target genes HES1 and HES5 (Fig. 5A and
B). Additional data indicated that combination treatment of
NS-398 and DAPT may result in decreased expression levels of NICD,
HES1 and HES5 compared with DAPT treatment alone (Fig. 5A and B). In addition, HES1 was
selected and detected by RT-qPCR. The results of this analysis
suggested that combination treatment may also inhibit Notch1
signaling to a greater extent compared with single-agent treatment
with DAPT or NS-398 (Fig. 5C).
Therefore, reduced NICD, HES1 and HES5 protein expression suggests
that the inhibition of growth may be attributed to Notch1 signal
blockage. These outcomes suggested that COX-2 inhibition may
significantly increase the Notch1 blockage level in AGS cells, and
that patients with GC may benefit from this combination
treatment.
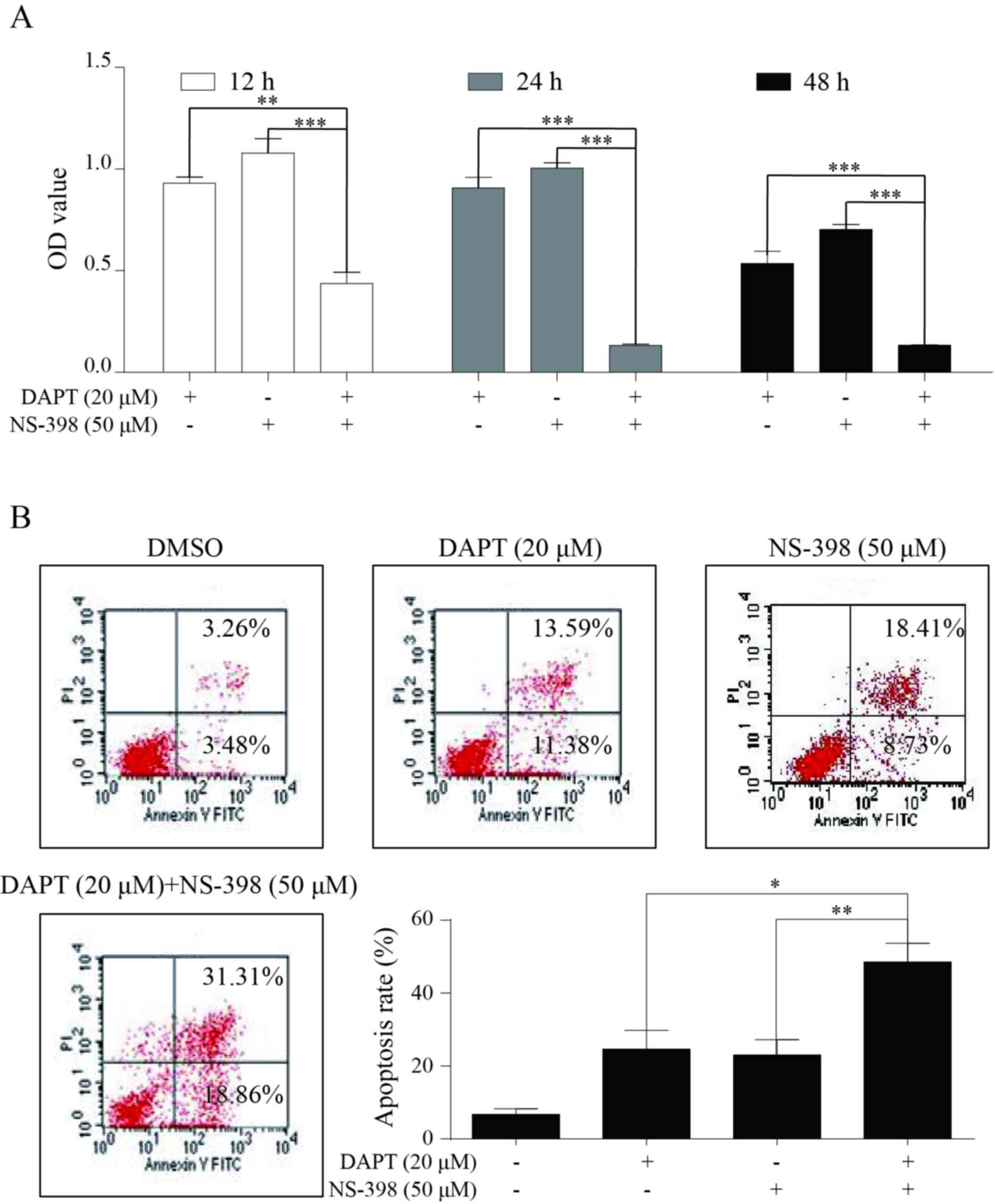
Combination treatment of DAPT and
NS-398 notably suppresses growth in AGS cells
Finally, the antitumor activity of this combination
plan in AGS cells was assessed by employing CCK8 and flow
cytometry. Compared with single drug treatment, combination
treatment induced an increased level of growth inhibition in AGS
cells following treatment for 12, 24 and 48 h (Fig. 6A). Data from the flow cytometry
analysis indicated a similar synergistic effect, as the rate of
cell apoptosis caused by the combination treatment group was
increased compared with the monotherapy (Fig. 6B).
Discussion
Despite a decline in the overall incidence, gastric
carcinoma remains a critical global health problem (7). Previous data have indicated that the
tumor cellular differentiation degree has a close association with
the prognosis of patients with GC (34–37).
Certain studies indicated that the poorly differentiated
adenocarcinoma subtype progressed to lymph node metastasis more
easily, and was demonstrated to be poor prognostic factors in
patients with gastric cancer with bone metastases (35,36). The
rate of poor differentiation was significantly increased in younger
cases compared with older patients, particularly in young female
patients (37). The degree of cell
differentiation is also an important predictor of survival in
advanced GC (38). Therefore,
exploration of the molecular mechanisms and identification of the
phenotype of GC differentiation will facilitate the identification
of novel targets and the development of personalized therapies in
GC.
A previous study indicated that the differentiation
induced by modulating Musashi/Numb/Notch signaling in cancer cells
may be a novel therapeutic target for advanced leukemia and other
solid carcinomas (39). Multiple
studies have demonstrated that Notch signaling is likely to serve
an oncogenic role in several cancer cell types, as it favors
development and differentiation in various cell types, including
myeloid cells or secretory cells (40,41).
Notch1 and Notch2 inhibited by hypoxia may induce neuroendocrine
differentiation in prostate cancer (42). In addition, Notch signaling may induce
aberrant differentiation in several types of cancer, including
pancreatic cancer, medulloblastoma and mucoepidermoid carcinoma
(43). In the present study, a poorly
differentiated GC cell line was employed to reveal the potential
association between Notch1 signaling and GC differentiation.
Convincing evidence indicated that Notch1 and NICD was highly
expressed in the AGS cell line (10,44). An
additional study indicated that there was no significant difference
between the levels of Notch2 in the normal gastric epithelial AGS
and GES-1 cell lines (45). For
Notch3 and Notch4, there have been no studies that have observed a
difference in Notch3 or Notch4 expression between the AGS cell line
and normal gastric cells. However, Ji et al (17) also detected the expression of Notch3
and Notch4 in AGS cell lines. The present study examined the GDSC
database (28) and it was confirmed
that all 4 Notch receptors were expressed in AGS cells It was also
observed that NICD was expressed in gastric cancer tissues.
Furthermore, the present study identified that the high level of
NICD was significantly associated with poor differentiation. In
addition, the poorly differentiated human gastric adenocarcinoma
AGS cell line also harbored amplified NICD protein (Fig. 1). These results suggested that the
therapeutic potential of NICD to treat patients with poorly
differentiated GC.
Tumors with aberrantly activated oncogenes were
frequently dependent on oncogene-associated signaling pathways
(46). In the present study, when
cells were treated with DAPT, their growth and survival were
markedly inhibited. These data suggested that the Notch1 pathway
may be a major signaling pathway for survival of AGS cells, and a
potential target for poorly differentiated GC. Nevertheless,
specific targeted therapies often require specific patient
conditions in order to be effective. For example, human epidermal
growth factor receptor 2 (HER2) expression is a validated
predictive biomarker for anti-HER2 target therapy, and patients
with lung cancer who possessed the FGFR1 gene amplification
benefitted more from FGFR inhibitors than those not exhibiting the
FGFR1 gene amplification (47,48).
Consequently, a precise genotype classification of patients with GC
may improve the success rate of Notch-inhibiting treatment.
Amplified COX-2 was an independent prognostic factor
of GC (25). Previous studies have
primarily focused on the effect of Notch signaling on COX-2
(20,26). In the present study, NS-398 treatment
was used to block COX-2 activity, and NS-398 could significantly
inhibit the growth of GC cells. It was also identified that NS-398
also markedly downregulated the level of NICD and HES1. However,
the underlying mechanism for the crosslinking of these two pathways
is not clear, and merits additional study. Concomitant with the
volume of evidence that associates the activation of Notch
signaling with oncogenesis, there is much supporting evidence for a
tumor-suppressive role of Notch in certain situations: Guo
et al (49) indicated that
Notch2 may negatively regulate cell invasion by inhibiting the
Phosphoinositide 3-kinase/Protein kinase B signaling pathway in
gastric cancer. Zhou et al (50) also suggested that Notch1 regulated
Phosphatase and tensin homolog expression through CBF-1, and served
a pro-apoptotic role in gastric cancer cells (50). The present study only identified that
combination therapy of NS-398 and DAPT demonstrated a more improved
antitumor activity in poorly differentiated GC cells than
monotherapy of NS-398 or DAPT; this observation was not verified in
other GC cell lines, for example in MKN7, a well differentiated GC
cell line (51). Therefore, the
effect of DAPT and NS-398 in well-differentiated GC cell lines
requires additional study. Taken together, COX-2 inhibition in turn
suppressed Notch1 signal pathway transduction, and the combination
treatment of γ-secretase and COX-2 inhibitors may have therapeutic
potential in patients with poorly-differentiated GC.
In summary, the expression of NICD was associated
with the differentiation of GC. A limitation of the present study
was the small sample size, therefore, additional studies concerning
the association between NICD and GC are required. In addition,
combined with NS-398, treatment of DAPT supported the novel
strategies for cancer therapy in patients with
poorly-differentiated GC.
Acknowledgements
The present study was supported by the Zhejiang
Province Natural Science Fund of China (grant nos. Y2101458,
LY14H160044 and LY14H030001).
References
|
1
|
Kopan R and Ilagan MX: The canonical notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roy M, Pear WS and Aster JC: The
multifaceted role of Notch in cancer. Curr Opin Genet Dev.
17:52–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katoh M: Dysregulation of stem cell
signaling network due to germline mutation, SNP, Helicobacter
pylori infection, epigenetic change and genetic alteration in
gastric cancer. Cancer Biol Ther. 6:832–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katoh M and Katoh M: Notch signaling in
gastrointestinal tract. Int J Oncol. 30:247–251. 2007.PubMed/NCBI
|
|
6
|
Dontu G, Jackson KW, McNicholas E,
Kawamura MJ, Abdallah WM and Wicha MS: Role of Notch signaling in
cell-fate determination of human mammary stem/progenitor cells.
Breast Cancer Res. 6:R605–R615. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Liu W, Tang D, Xiao H, Wu Z, Chen C,
Yao X, Liu F and Li G: Prognostic values of four Notch receptor
mRNA expression in gastric cancer. Sci Rep. 6:280442016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Wang X, Xu J and Sun Y: Notch1
activation is a poor prognostic factor in patients with gastric
cancer. Br J Cancer. 110:2283–2290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo DH, Zhou Q, Hu SK, Xia YQ, Xu CC, Lin
TS, Pan YT, Wu JS and Jin R: Differential expression of Notch1
intracellular domain and p21 proteins and their clinical
significance in gastric cancer. Oncol Lett. 7:471–478. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Konishi H, Asano N, Imatani A, Kimura O,
Kondo Y, Jin X, Kanno T, Hatta W, Ara N, Asanuma K, et al: Notch1
directly induced CD133 expression in human diffuse type gastric
cancers. Oncotarget. 7:56598–56607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhen-Jun S1, Yuan-Yuan Z, Ying-Ying F,
Shao-Ju J, Jiao Y, Xiao-Wei Z, Jian C, Yao X and Li-Ming Z: β,
β-Dimethylacrylshikonin exerts antitumor activity via Notch-1
signaling pathway in vitro and in vivo. Biochem Pharmacol.
84:507–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Wang WY and Cao LP: SIRT3 inhibits
cell proliferation in human gastric cancer through down-regulation
of Notch-1. Int J Clin Exp Med. 8:5263–5271. 2015.PubMed/NCBI
|
|
14
|
Li LC, Wang DL, Wu YZ, Nian WQ, Wu ZJ, Li
Y, Ma HW and Shao JH: Gastric tumor-initiating CD44+ cells and
epithelial-mesenchymal transition are inhibited by gamma-secretase
inhibitor DAPT. Oncol Lett. 10:3293–3299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang L, Lin T, Xu C, Hu S, Pan Y and Jin
R: miR-124 interacts with the Notch1 signalling pathway and has
therapeutic potential against gastric cancer. J Cell Mol Med.
20:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan C, Yu J, Kang W, Liu Y, Ma Z and Zhou
L: miR-935 suppresses gastric signet ring cell carcinoma
tumorigenesis by targeting Notch1 expression. Biochem Biophys Res
Commun. 470:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji Q, Hao X, Meng Y, Zhang M, Desano J,
Fan D and Xu L: Restoration of tumor suppressor miR-34 inhibits
human p53-mutant gastric cancer tumorspheres. BMC Cancer.
8:2662008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu KW, Fang WL, Huang KH, Huang TT, Lee
HC, Hsieh RH, Chi CW and Yeh TS: Notch1 pathway-mediated
microRNA-151-5p promotes gastric cancer progression. Oncotarget.
7:38036–38051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Gao X, Liu J, Kong QY, Wang XW,
Chen XY, Wang Q, Cheng YF, Qu XX and Li H: Differential Notch1 and
Notch2 expression and frequent activation of Notch signaling in
gastric cancers. Arch Pathol Lab Med. 135:451–458. 2011.PubMed/NCBI
|
|
20
|
Tseng YC, Tsai YH, Tseng MJ, Hsu KW, Yang
MC, Huang KH, Li AF, Chi CW, Hsieh RH, Ku HH and Yeh TS:
Notch2-induced COX-2 expression enhancing gastric cancer
progression. Mol Carcinog. 51:939–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang H, An HJ, Song JY, Kim TH, Heo JH,
Ahn DH and Kim G: Notch3 and Jagged2 contribute to gastric cancer
development and to glandular differentiation associated with MUC2
and MUC5AC expression. Histopathology. 61:576–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian C, Liu F, Ye B, Zhang X, Liang Y and
Yao J: Notch4 promotes gastric cancer growth through activation of
Wnt1/β-catenin signaling. Mol Cell Biochem. 401:165–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams CS, Tsujii M, Reese J, Dey SK and
DuBois RN: Host cyclooxygenase-2 modulates carcinoma growth. J Clin
Invest. 105:1589–1594. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta S, Srivastava M, Ahmad N, Bostwick
DG and Mukhtar H: Over-expression of cyclooxygenase-2 in human
prostate adenocarcinoma. Prostate. 42:73–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cervello M, Bachvarov D, Cusimano A,
Sardina F, Azzolina A, Lampiasi N, Giannitrapani L, McCubrey JA and
Montalto G: COX-2-dependent and COX-2-independent mode of action of
celecoxib in human liver cancer cells. OMICS. 15:383–392. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: World Health Organization and International Agency for
Research on Cancer: WHO Classification of Tumours of the Digestive
System. IARC Press; Lyon: 2010
|
|
28
|
Yang W, Soares J, Greninger P, Edelman EJ,
Lightfoot H, Forbes S, Bindal N, Beare D, Smith JA, Thompson IR, et
al: Genomics of drug sensitivity in cancer (GDSC): A resource for
therapeutic biomarker discovery in cancer cells. Nucleic Acids Res.
41:D955–D961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tu LC, Chou CK, Chen CY, Chang YT, Shen YC
and Yeh SF: Characterization of the cytotoxic mechanism of
Mana-Hox, an analog of manzamine alkaloids. Biochim Biophys Acta.
1672:148–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin YH, Kim H, Oh M, Ki H and Kim K:
Regulation of Notch1/NICD and Hes1 expressions by GSK-3alpha/beta.
Mol Cells. 27:15–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freire AG, Waghray A, Soares-da-Silva F,
Resende TP, Lee DF, Pereira CF, Nascimento DS, Lemischka IR and
Pinto-do-Ó P: Transient HES5 activity instructs mesodermal cells
toward a cardiac fate. Stem Cell Reports. 9:136–148. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kitagawa M, Hojo M, Imayoshi I, Goto M,
Ando M, Ohtsuka T, Kageyama R and Miyamoto S: Hes1 and Hes5
regulate vascular remodeling and arterial specification of
endothelial cells in brain vascular development. Mech Dev.
130:458–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Somi MH, Ghojazadeh M, Bagheri M and
Tahamtani T: Clinicopathological factors and gastric cancer
prognosis in the Iranian population: A meta-analysis. Asian Pac J
Cancer Prev. 16:853–857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung DH, Bae YS, Yoon SO, Lee YC and Kim
H, Noh SH, Park H, Choi SH, Kim JH and Kim H: Poorly differentiated
carcinoma component in submucosal layer should be considered as an
additional criterion for curative endoscopic resection of early
gastric cancer. Ann Surg Oncol. 22 Suppl 3:S772–S777. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Turkoz FP, Solak M, Kilickap S, Ulas A,
Esbah O, Oksuzoglu B and Yalcin S: Bone metastasis from gastric
cancer: The incidence, clinicopathological features and influence
on survival. J Gastric Cancer. 14:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu C, Wang ZN, Sun Z and Xu HM:
Clinicopathologic features and prognosis of gastric cancer in young
adults. Zhonghua Wai Ke Za Zhi. 46:1468–1471. 2008.(In Chinese).
PubMed/NCBI
|
|
38
|
Zu H, Wang H, Li C and Xue Y:
Clinicopathologic characteristics and prognostic value of various
histological types in advanced gastric cancer. Int J Clin Exp
Pathol. 7:5692–5700. 2014.PubMed/NCBI
|
|
39
|
Yoshinori N and Hideyuki O: New insight
into cancer therapeutics: Induction of differentiation by
regulating the Musashi/Numb/Notch pathway. Cell Res. 20:1083–1085.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng P, Kumar V, Liu H, Youn JI, Fishman
M, Sherman S and Gabrilovich D: Effects of notch signaling on
regulation of myeloid cell differentiation in cancer. Cancer Res.
74:141–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sikandar SS, Pate KT, Anderson S, Dizon D,
Edwards RA, Waterman ML and Lipkin SM: NOTCH signaling is required
for formation and self-renewal of tumor-initiating cells and for
repression of secretory cell differentiation in colon cancer.
Cancer Res. 70:1469–1478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Danza G, Di Serio C, Rosati F, Lonetto G,
Sturli N, Kacer D, Pennella A, Ventimiglia G, Barucci R, Piscazzi
A, et al: Notch signaling modulates hypoxia-induced neuroendocrine
differentiation of human prostate cancer cells. Mol Cancer Res.
10:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sjölund J, Manetopoulos C, Stockhausen MT
and Axelson H: The Notch pathway in cancer: Differentiation gone
awry. Eur J Cancer. 41:2620–2629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li LC, Peng Y, Liu YM, Wang LL and Wu XL:
Gastric cancer cell growth and epithelial-mesenchymal transition
are inhibited by γ-secretase inhibitor DAPT. Oncol Lett.
7:2160–2164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Ye J, Chen Z, Wen J, Li F, Su P, Lin
Y, Hu B, Wu D, Ning L, et al: Annonaceous acetogenins mediated
up-regulation of Notch2 exerts growth inhibition in human gastric
cancer cells in vitro. Oncotarget. 8:21140–21152. 2017.PubMed/NCBI
|
|
46
|
Davide T and Livio T: Oncogene addiction
as a foundational rationale for targeted anti-cancer therapy:
Promises and perils. EMBO Mol Med. 3:623–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wong H and Yau T: Targeted therapy in the
management of advanced gastric cancer: Are we making progress in
the era of personalized medicine? Oncologist. 17:346–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang J, Zhang L, Su X, Li M, Xie L,
Malchers F, Fan S, Yin X, Xu Y, Liu K, et al: Translating the
therapeutic potential of AZD4547 in FGFR1-amplified non-small cell
lung cancer through the use of patient-derived tumor xenograft
models. Clin Cancer Res. 18:6658–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guo LY, Li YM, Qiao L, Liu T, Du YY, Zhang
JQ, He WT, Zhao YX and He DQ: Notch2 regulates matrix
metallopeptidase 9 via PI3K/AKT signaling in human gastric
carcinoma cell MKN-45. World J Gastroenterol. 18:7262–7270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X,
Lu XH, Shen L, Liu BN, Liu J, Luo HS, et al: The
AKT1/NF-kappaB/Notch1/PTEN axis has an important role in
chemoresistance of gastric cancer cells. Cell Death Dis.
4:e8472013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang Y, Lim SK, Choong LY, Lee H, Chen Y,
Chong PK, Ashktorab H, Wang TT, Salto-Tellez M, Yeoh KG and Lim YP:
Cathepsin S mediates gastric cancer cell migration and invasion via
a putative network of metastasis-associated proteins. J Proteome
Res. 9:4767–4778. 2010. View Article : Google Scholar : PubMed/NCBI
|