Introduction
The increase in the hepatobiliary and pancreatic
cancer surgery, the anatomic variations of the celiac axis (also
called the celiac trunk or celiac artery (CAT) in the literature)
and the hepatic arteries is of paramount importance in
hepato-pancreatico-billary (HPB) surgery. In the literature
vascular anomalies in the peripancreatic region are divided into
variations of the superior mesenteric area, the celiac trunk and
hepatic artery. The information about the abdomen arterial anatomy
is derived from the radiology and anatomy literature. Typically,
coeliac trunk arises anteriorly from abdominal aorta at the level
of twelfth thoracic vertebrae, just the aorta enters the abdomen.
Then courses anteriorly or slightly anterolateral in the lesser sac
and at the upper border of the pancreas divides into three
branches: Left gastric artery (LGA), splenic artery (SA) and common
hepatic artery (CHA) (1). A normal
CAT anatomy was found in 89.1% of patients (1). The length of coeliac axis from its
origin to the place where it gave of main branches is 1.5–2 cm. The
diameters of SA, CHA and LGA are 5, 6 and 4 mm, respectively
(2).
The patterns of hepatic arterial anatomy are not
constant. The normal anatomy of the hepatic artery is a CHA arising
from the coeliac axis and coursing to the point where the
gastroduodenal artery (GDA) arises, beyond which it becomes the
proper hepatic artery (PHA). CHA typically passes forward for a
short distance in the retroperitoneum and then emerges at the
superior border of the pancreas and left side of common hepatic
duct. After arising from CAT, CHA turns upwards and runs lateral
and adjacent to the common bile duct. GDA is the first branch of
CHA which supplies the proximal duodenum and pancreas. The right
gastric artery takes off shortly thereafter and continues within
the lesser omentum along the lesser curve of the stomach. At this
point, CHA is referred to as PHA, which courses towards the hilum
and soon divides into LHA and RHA. In 80% cases, RHA courses
posterior to the common hepatic duct before entering the hepatic
parenchyma; in 20% cases RHA may lie anterior to the common hepatic
duct (3). This normal anatomy of CHA
accounting for 25 to 75% of the cases (1,4,5). Anomalies during embryogenesis however
might result in a variety of anatomic variants and most common
variants are: From aorta: 0.5–2%; from SMA: 1.5–3.5% (1,4,5). On the basis of the literature two main
paths of CHA can be distinguished. These two variation can have a
significant impact on surgical cut: i) The extra-parynchemal path
(outside the pancreas head)-CHA is coming out SMA and is passing to
the liver on the posterior surface of the pancreas head; in this
case the dissection of this artery from the pancreas without
pancreas head injury is not difficult; ii) the intra-parynchemal
path (in the pancreas head)-CHA is coming out superior mesenteric
artery and is going to the liver hilum through head parenchyma; in
this case it might be difficult to save intra-parenchymal part of
the CHA (when saving of CHA is impossible it is necessary to
reconstruct it due to performing end to end anastomosis with
gastro-duodenal artery).
We report two cases of associated CAT and CHA
anomaly with their clinical importance. This paper is a
retrospectively prepared analysis and review of the literature
based on two independent rare cases connected with
pancreatico-billary surgery. The presented subject is highly in the
area of interests of the Department of Surgical Oncology, Medical
University of Lublin which mainly concentrate on surgery of
gastrointestinal tract cancers; moreover the Department is an
academic centre and is focused on training in surgical oncology. We
describe hepatic arterial anatomic variants: Where a hepatic
arterial system (HAS) arising directly from SMA and traveling
posterior to the pancreatic head and vena porta. We discuss the
importance of these arterial variants and implications of surgery
management.
Case reports
Case 1
(CHA originated from SMA, hepatomesenteric trunk). A
44-year-old man presented with tumour of the pancreatic head
[diameter 1.4×1.1 cm on computed tomography (CT) scan]. Endoscopic
retrograde cholangiopancreatography (ERCP) revealed a common bile
duct structure and atypical cells, and a stent was placed. CT scan
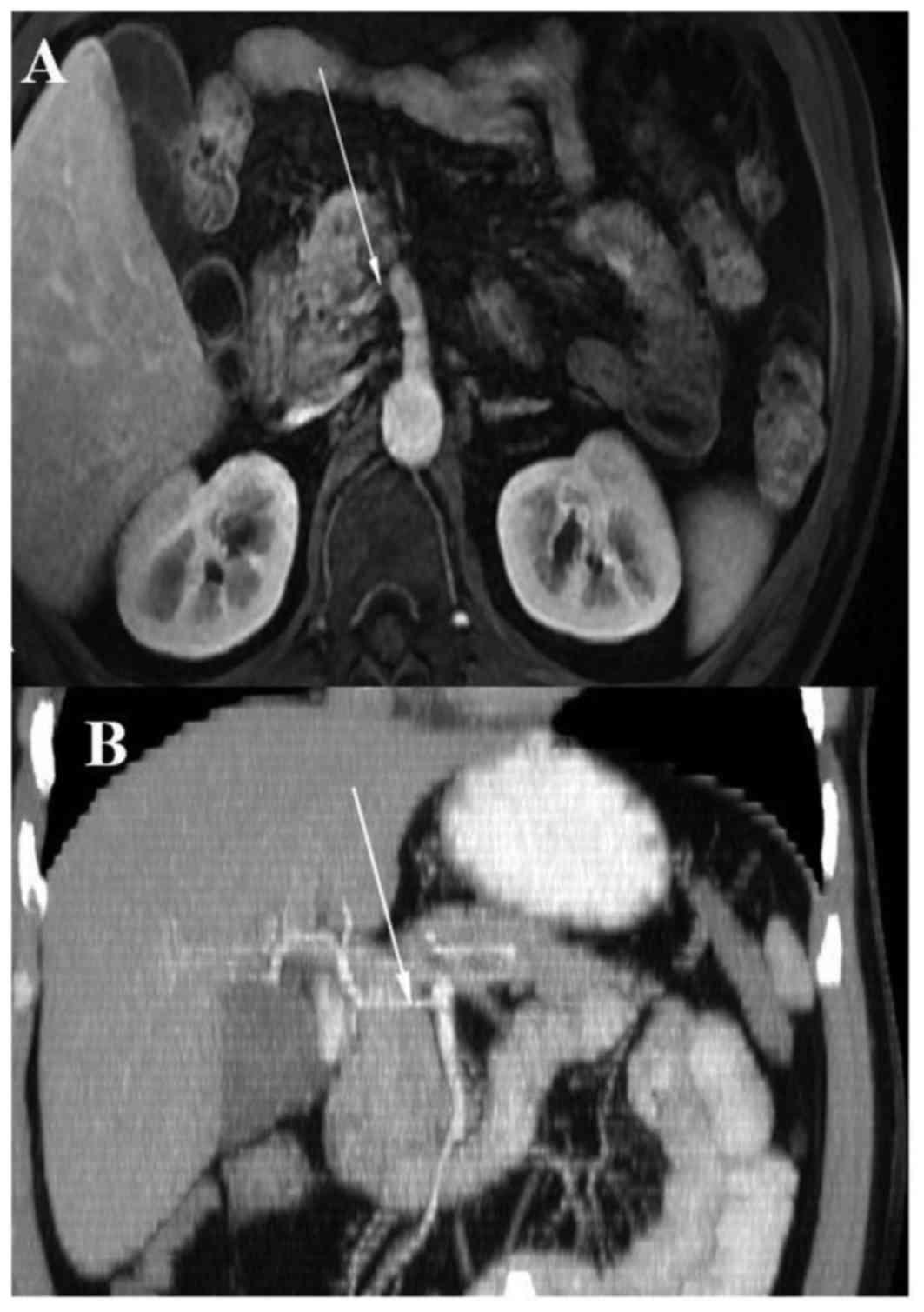
demonstrated a CHA arising directly from the SMA (Fig. 1). Intraoperatively, a palpable mass
3×4 cm in the pancreatic head was found and suspected anomalies of
the hepatic artery were confirmed after extended Kocher's manoeuvre
and careful dissection of peripancreatic and retroperitoneal space
with so-called ‘artery first’ approach. ‘Radical’ pylorus
preserving pancreatoduodenectomy with curative intent was
performed. No postoperative complications were observed and patient
was discharged from the hospital on 10th postoperative day.
Pathologic evaluation revealed a T4N0M0 pancreatic tubular
adenocarcinoma resected with positive retroperitoneal margin (R1).
Regional lymphadenectomy enabled pathological examintion of 27
lymph nodes without metastases. On 19th postoperative day the
patient was readmitted to the hospital due to pancreatic fistula
type B. The complication was effectively managed conservatively for
16 days. Thirty five days after operation patient was scheduled for
systemic adjuvant chemotherapy.
 | Figure 1.Anomalous origin of common hepatic
artery from SMA. (A) Axial scan, abdominal computed tomography,
shows proximal common hepatic artery running posterior to portal
vein. (B) Volume rendering, abdominal computer tomography. (C)
Intraoperative view: 1, portal vein; 2, SMA; 3, common hepatic
artery; 4, splenic artey; 5, superior mesenteric vein; 6, splenic
vein; 7, liver; 8, proper hepatic artery; 9, bile ducts; 10, tail
of pancreas; 11, gastroduodenal artery stump. SMA, superior
mesenteric artery. |
Case 2
(CHA originated from SMA, hepatomesenteric trunk). A
67-year-old man presented with a pancreatic head cancer. CT scan
demonstrated focal tumour in the head of the pancreas. CT
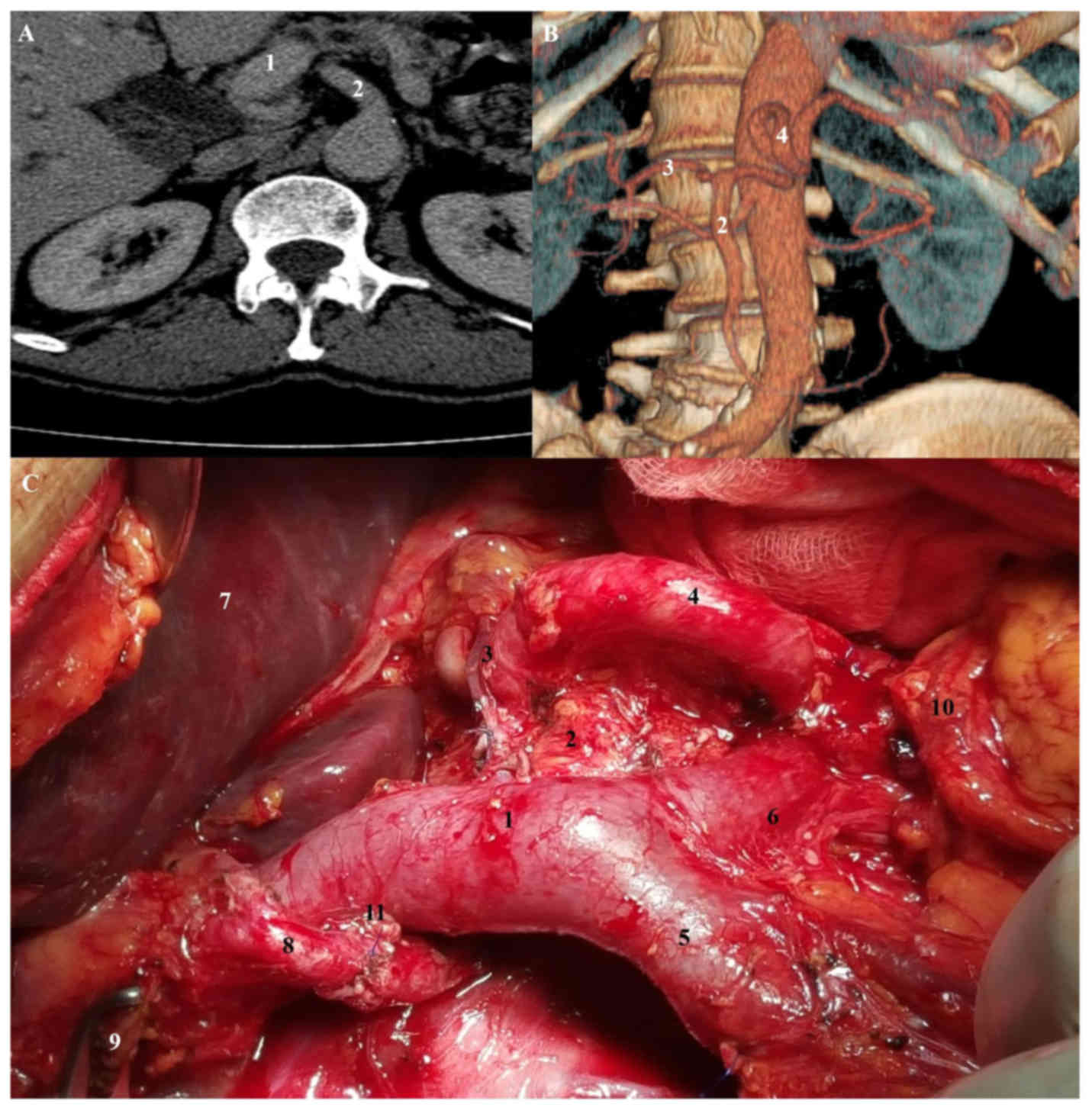
arteriography showed a CHA originating from the SMA and traveling
posterior to the head of the pancreas as well as posterior to Vena
Porta (Fig. 2). After division of the
pancreatic neck anterior to the superior mesenteric vein,
mobilization of the pancreas head and uncinated process exposed the
CHA and GDA. We performed pylorus preserving pancreatoduodenectomy
sp. Traverso-Longmire and cholecystectomy. Patient's postoperative
hospitalization lasted 10 days. He did well postoperatively and
pathologic evaluation confirmed adenocarcinoma tubulare et
papillare partim gelatinosum (G1), pT2N1M0 with negative margins.
Next pathologist examined 44 lymph nodes and in 3 found component
of cancer cells. Patient was qualified for systemic
chemotherapy.
Discussion
Nowadays, there are many improvements and
developments in abdomen surgical techniques: upper abdominal
videolaparoscopic surgeries, liver transplantation and radiological
procedures (6,7). All of invasive procedures in the abdomen
need for professional and wide knowledge of the anatomy of the CAT,
HAS and their main variations. The frequency of inadvertent or
iatrogenic hepatic vascular injury increases in the event of
aberrant anatomy and variations. The knowledge of anatomical liver
vascular variants is crucial for decreasing operative and
postoperative morbidity and mortality during the performance of
hepatic and pancreatic surgeries (7–9).
Fortunately, along with the development of surgical techniques come
the improvement in radiological visualization. Pre-operative
imaging can detect even up to 60–80% of all artery anomalies
(8). The gold standard for arterial
supply visualization remains angiography, nevertheless the huge
impact of multidetector computer tomography (CT) angiography and
modern reconstruction programs should be noted. Usage of maximum
intensity projection and three-dimensional volume rendering
techniques allows the non-invasive visualization of small arteries
in multidetector CT angiography (10).
Arterial vascularisation of the gastrointestinal
tract is provided by anterior branches at three different levels of
the abdominal aorta (coeliac trunk, superior and inferior
mesenteric arteries). Haller (1756) was described CAT as the
trifurcation which originates on the LGA, SA and CHA (6,11). Since
that observation many variations and anomalous were described
(7). The normal anatomy of CAT and it
branches is observed in 60–89,1% of cases, while normal hepatic
arterial supply can be seen in 52–80.3% of cases (10). Most of anatomical variations come from
foetal developmental changes in the ventral segmental arteries.
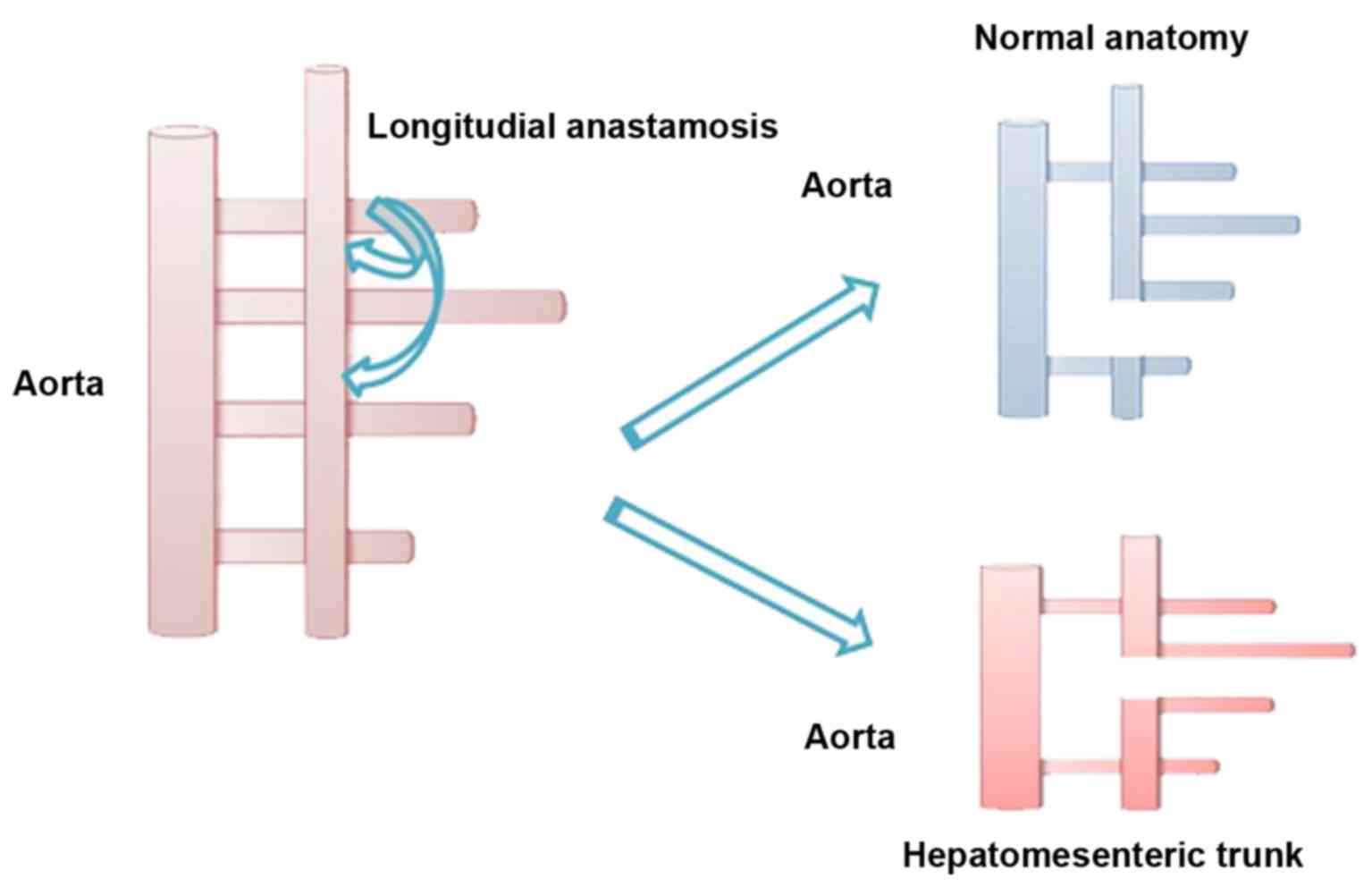
Tandler (1904) gave an embryological explanation for anatomical
variations of CAT (11) where
explained that the ventral branches develop initially from the
abdominal aorta as paired vessels, which form four roots connected
by ventral longitudinal anastomosis. LGA is usually formed by the
first root, the second root gives the beginning for the SA and the
third root creates CHA; SMA develops from the last root, which
migrates caudally with the gut (6,11). Quoting
Morita, the disappearance of primitive ventral splanchnic arteries
and longitudinal anastomosis is the reason for numerous anomalous
of the CAT, as it was schematically presented in Fig. 3 (11).
In the paper we aimed to illustrate several types of
anatomical variations of celiac trunk, hepatic artery and its main
branches, based on description of rare clinical cases. We described
two independent cases of such rare anomalies. Moreover, we also
focused on surgical implications and an establishment of practical
tips for surgeons during abdominal surgery especially HPB
surgery.
In the literature classical course of CAT is
reported with a frequency of 72–90% (6,12).
According to the Uflacker's classification (6,13) the most
commonly observed CAT variations are: Hepatosplenic trunk (3% of
cases), splenogastric trunk (4%), hepatogastric trunk (1%),
hepatomesenteric trunk (<1%), the absence of CAT is the most
rare (0,1-4,0%) (6,10). Among vessel anomalities ‘accessory’
and ‘replaced’ vassels can also be qualified, which examples are
replaced right hepatic artery (11–21% cases) and replaced left
hepatic artery (3,8–10%) (8). In the
literature coeliac trunk bifurcation is reported at a rate of about
12% (12).
The mostly chosen classifications for description of
anatomical findings and possible surgical implications of CAT
variation are presented in Table I.
Regarding liver arterial supply, it is described as ‘normal
anatomy’ when the CHA originates the PHA after the emergence of the
GDA; next, the PHA separates into right and left hepatic arteries
within the hepatoduodenal ligament. The knowledge of hepatic artery
supply is essential to avoid iatrogenic complications during HPB
surgery; in the presence of anatomical variations accidental
ligation, provoking hepatic necrosis, ischemic biliary injury and
anastomotic fistula can complicate the peri- and postoperative
course (14). With the grooving
number of liver transplantation, the importance of the hepatic
artery anatomy become crucial and many authors proposed
classifications describing liver vascular variations based on their
studies (7). The most often described
hepatic artery anatomical variations are: i) An anomalous RHA from
the SMA (10–21%); ii) displaced LHA from the LGA (4–10%); iii)
displaced RHA and LHA; iv) an accessory RHA and/or LHA (1–8%); v)
displaced CHA from SMA or aorta (0,4-4,5%); or vi) quadrifurcation
of hepatic artery (14). In our study
we described two independent cases of hepatic arterial anatomic
variants where a HAS arised directly from SMA and traveling
posterior to the pancreatic head and vena porta. It is named as
hepatomesenteric trunk and is the second most common variation of
HA (2–3%) (14). Described cases
belong to type V in Hiatt's classification (Table II).
 | Table I.Uflacker's classification of CAT and
its possible surgical implications. |
Table I.
Uflacker's classification of CAT and
its possible surgical implications.
|
| Description |
|---|
|
|
|
|---|
| Classifications of
CAT variations | Type | Variation | Possible surgical
implications |
|---|
| Uflacker's
classification (5,11,12) | I | Classic coeliac
trunk | Frequency of
72.0–89,0% (5,11). |
|
| II | Hepatosplenic
trunk | Most common CAT
variation (3–4,4%) (5). |
|
| III | Hepatogastric
trunk |
|
|
| IV |
Hepatosplenomesenteric trunk | Occurrence rate of
below 1%. Crucial when performing pancreatic surgery (blood supply
to the duodenum come only from SMA): Accidental ligation of the SMA
or branches of the common trunk can lead to the ischemia/necrosis
of liver or duodenum (5). |
|
| V | Gastrosplenic
trunk |
|
|
| VI | Coeliac-mesenteric
trunk | During
pancreatoduodenectomy (pancreatic/peripancreatic cancers
treatment): Increases a perioperative mordibity by 20–30% (5). |
|
| VII | Coeliac-colic
trunk | It is formed when the
middle colic artery originates from coeliac trunk instead of the
SMA. Difficulties and complications during transverse colon
surgery: Unexpected bleeding during surgery (coeliac-colic trunk
gets blood from SMA and inferior mesenteric artery) (5). |
|
| VIII | No coeliac trunk | No coeliac trunk can
be recognised when left gastric, common hepatic and splenic
arteries arise from the abdominal aorta(5). |
 | Table II.Anatomical variations of the hepatic
artery: Hiatt's classification and its possible surgical
implications. |
Table II.
Anatomical variations of the hepatic
artery: Hiatt's classification and its possible surgical
implications.
|
| Description |
|---|
|
|
|
|---|
| Classsification of
hepatic artery variations | Type | Variation | Possible surgical
implications |
|---|
| Hiatt's
classification (9,11) | I | Normal anatomy | Most frequent type:
59–79,1% (9), 51–80% (11). |
|
| II | Left hepatic artery
or accessory left hepatic artery relocation | Gastrectomy should be
cautiously performed: Left hepatic artery emerges from the left
hepatic artery (ischemia of the left hepatic lobe after section of
the left gastric artery) (9). |
|
| III | Right hepatic artery
or accessory right hepatic artery relocation | The most frequent
described variation (7–18%). Procedures involving liver surgery.
Confusing course of the RHA: After originating from SMA, the right
hepatic artery runs posteriorly to the portal vein (9). |
|
| IV | Left hepatic
artery/accessory left hepatic artery relocation and right hepatic
artery/accessory right hepatic artery relocation |
|
|
| V | Common hepatic artery
originating from superior mesenteric artery | Known as a
hepatomesenteric trunk (2–3%). Altering the surgical approach
(interference with resection or lymphadenectomy); unexpected
bleeding; ischemia; biliary leak; liver dysfunction (7). |
|
| VI | Common hepatic artery
originating from the aorta |
|
The paper illustrates several types of anatomical
variations of celiac trunk, hepatic artery and its main branches,
based on description of two of such findings in our own clinical
practice. However, in past decade there are several valuable case
reports and papers (especially from liver transplant centers) which
share a proper approach to the subject of the hepatic arterial
variants. The first publication which mentioned CHA passing through
pancreatic parenchyma comes from Michels (1951), but it still
remains unclear why CHA penetrates the pancreas (it might be that
CHA is developed before the fusion of dorsal and ventral pancreas)
(15). Rammohan et al
(8), emphasize that hepatomesenteric
trunk that courses through the pancreatic parenchyma can be spared
by dividing the pancreas, but there is always a risk of not
achieving tumor-free margins, what is essential in oncological
surgery. If the hepatomesenteric trunk courses ventral to the
pancreas, it can be displaced and dissected from the surface of the
pancreas and a standard pancreaticoduodenectomy is performed. When
a hepatomesenteric trunk has anastomotic connection to the LGA or
another accessory artery, ligation will result in no compromise to
the blood supply. In cases where the CHA is divided either
inadvertently or for oncological purposes, it should be
reconstructed using an autologous vascular graft such as the GDA or
saphenous vein (8,16). The knowledge of anatomical anomalies
is of the great value during surgical interventions. The issue is
crucial for, liver transplantation and resection, hepatic artery
chemotherapy, gastrectomy, biliary reconstruction and especially
for pancreatoduodenectomy (17,18).
According to Pallisera et al (14) problems related to anatomical
variations of hepatic artery and coeliac axis stenosis are most
prevalent arterial complications during HPB surgery. It is
highlighted that intraoperative arterial complications generate
longer operative time, higher transfusion rate and more
postoperative complications (19).
There are some key tips which knowledge and
application in clinical practice can be useful for HPB surgery to
avoid unnecessary complications. First and foremost, multidetector
CT with multidimensional reconstruction should be make in the
preoperative management (14). Next,
complete kocherisation and opening the cavity, the porta hepatis
should be palpated to determine the localization of the arterial
pulsation (8). Afterwards, the
decision about the surgical approach and intraoperative proceeding
is dependent on discovered arterial anatomical variations. The most
important hepatic artery anatomical variations that the surgeon
must take into consideration during pancreatoduodenectomy are:
accessory RHA, accessory or displaced CHA, both arising from the
SMA. If we encounter hepatic arterial anomalies the possible
options for intraoperative management are ligation, dissection and
traction away from the site of dissection, division and anastomosis
(14). There are numerous
difficulties during surgery and postoperative complications which
can occur if arterial anomalies are identified: i) Partial liver
ischemia and necrosis-the main problem with ligature of the
displaced RHA and replaced CHA. During pancreatoduodenectomy
ligation of the GDA should be delayed until the retropancreatic
dissection and proper identification of the artery is complete;
desirable is preoperative clamping of arteries which are going to
be ligatured and post-ligature control of the blood flow (8,14); ii)
modification of the resection area and a risk of not achieving
tumor-free margins-tough oncological compromise between safety of
the procedure and radical removal of tumour (8); iii) pancreatic or biliary anastomotic
leak-postoperative hepatic enzymes elevation is possible (8); and iv) unexpected bleeding-iatrogenic
post- or intraoperative loss of blood (6).
In summary, we have described a two rare cases of a
CHA originating from the SMA in combination with the topography.
However, this anatomical variant is very rare (with frequency 1–3%)
should be known to surgical oncologist. In our opinion this paper
is of great value for surgeons during their training period as well
as the experts. Multiple arterial anomalies in a single person are
found rarely. When performing operations of the pancreatic region,
it is necessary to have a knowledge of anatomy including those
patterns that have been rarely observed. The awareness of the
possible extra- or intra-parenchymal path of CHA has a huge effect
on decisions connected with next steps of surgery, achieving
tumor-free margins, complications, patient's quality of life and
costs of hospitalization. Careful review of preoperative imaging
especially during multidisciplinary meeting may prevent injury to
these vascular structures and later complications.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computer tomography
|
|
CAT
|
celiac artery trunk
|
|
CHA
|
common hepatic artery
|
|
ERCP
|
cholangiopancreatography
|
|
GDA
|
gastroduodenal artery
|
|
HAS
|
hepatic arterial system
|
|
HPB
|
hepato-pancreatico-billary
|
|
LGA
|
left gastric artery
|
|
LHA
|
left hepatic artery
|
|
SA
|
splenic artery
|
|
SMA
|
superior mesenteric artery
|
|
PHA
|
propia hepatic artery
|
|
RHA
|
right hepatic artery
|
References
|
1
|
Song SY, Chung JW, Yin YH, Jae HJ, Kim HC,
Jeon UB, Cho BH, So YH and Park JH: Celiac axis and common hepatic
artery variations in 5002 patients: Systematic analysis with spiral
CT and DSA. Radiology. 255:278–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Standring S: Gray's Anatomy: The
Anatomical Basis of Clinical Practice. Elsevier; Churchill
Livingstone: 2005
|
|
3
|
Bhart S: Srb's Surgical Operations. Text
Atlas: Jaypee Brothers Medical Pub; 2014
|
|
4
|
Hiatt JR, Gabbay J and Busuttil RW:
Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg.
220:50–52. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michels NA: Newer anatomy of the liver and
its variant blood supply and collateral circulation. Am J Surg.
112:337–347. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torres K, Staśkiewicz G, Denisow M,
Pietrzyk Ł, Torres A, Szukała M, Czekajska-Chehab E and Drop A:
Anatomical variations of the coeliac trunk in the homogeneous
Polish population. Folia Morphol (Warsz). 74:1–99. 2015.PubMed/NCBI
|
|
7
|
Maslarski I: Anatomical variant of the
liver blood supply. Clujul Med. 88:420–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rammohan A, Sathyanesan J, Palaniappan R
and Govindan M: Transpancreatic hepatomesenteric trunk complicating
pancreaticoduodenectomy. JOP. 14:649–652. 2013.PubMed/NCBI
|
|
9
|
Rela M, McCall JL, Karani J and Heaton ND:
Accessory right hepatic artery arising from the left: Implications
for split liver transplantation. Transplantation. 66:792–794. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Araujo Neto SA, de Mello Júnior CF, Franca
HA, Duarte CM, Borges RF and de Magalhães AG: Multidetector
computed tomography angiography of the celiac trunk and hepatic
arterial system: Normal anatomy and main variants. Radiol Bras.
49:49–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kardile PB, Ughade JM, Ughade MN, Dhende A
and Ali SS: Anomalous origin of the hepatic artery from the
hepatomesenteric trunk. J Clin Diagn Res. 7:386–388.
2013.PubMed/NCBI
|
|
12
|
Ugurel MS, Battal B, Bozlar U, Nural MS,
Tasar M, Ors F, Saglam M and Karademir I: Anatomical variations of
hepatic arterial system, coeliac trunk and renal arteries: An
analysis with multidetector CT angiography. Br J Radiol.
83:661–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uflacker R: Atlas of Vascular Anatomy: An
Angiographic Approach. Lippincott Williams & Wilkins;
Baltimore, MD: 1997
|
|
14
|
Pallisera A, Morales R and Ramia JM:
Tricks and tips in pancreatoduodenectomy. World J Gastrointest
Oncol. 6:344–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura Y, Miyaki T, Hayashi S, Iimura A
and Itoh M: Three cases of the gastrosplenic and the
hepatomesenteric trunks. Okajimas Folia Anat Jpn. 80:1–76. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosokawa I, Shimizu H, Nakajima M,
Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Takeuchi
D, Takayashiki T, et al: A case of pancreaticoduodenectomy for
duodenal carcinoma with a replaced common hepatic artery running
through the pancreatic parenchyma. Gan To Kagaku Ryoho.
39:1963–1965. 2012.(In Japanese). PubMed/NCBI
|
|
17
|
Skórzewska M, Romanowicz T, Mielko J,
Kurylcio A, Pertkiewicz J, Zymon R and Polkowski WP: Frey operation
for chronic pancreatitis associated with pancreas divisum: Case
report and review of the literature. Prz Gastroenterol. 9:175–178.
2014.PubMed/NCBI
|
|
18
|
Mielko J, Kurylcio A, Skórzewska M, Ciseł
B, Polkowska B, Rawicz-Pruszyński K, Sierocińska-Sawa J and
Polkowski WP: Duodenal obstruction due to annular pancreas
associated with carcinoma of the duodenum. Prz Gastroenterol.
11:139–142. 2016.PubMed/NCBI
|
|
19
|
Kim AW, McCarthy WJ III, Maxhimer JB,
Quiros RM, Hollinger EF, Doolas A, Millikan KW, Deziel DJ, Godellas
CV and Prinz RA: Vascular complications associated with
pancreaticoduodenectomy adversely affect clinical outcome. Surgery.
132:738–747. 2002. View Article : Google Scholar : PubMed/NCBI
|