Introduction
Lung cancer is one of the most common malignancies
worldwide and non-small-cell lung cancer (NSCLC) accounts for ~80%
of all lung cancers (1). The majority
of patients with NSCLC are at an advanced stage of disease when
initially diagnosed. The standard treatment for advanced disease
includes chemotherapy and, for patients with sensitive gene
mutations, targeted therapy (2).
Multiple studies have demonstrated that patients with sensitive
epidermal growth factor receptor (EGFR) mutations respond to
tyrosine kinase inhibitors (TKIs) well, with an overall response
rate of 55–80% (3,4). However, patients with EGFR mutant NSCLC
develop disease progression 8–16 months after TKI administration,
which has been defined as ‘acquired resistance’ (5). Mechanisms underlying the phenomenon of
acquired resistance include a secondary mutation T790M, c-Met
amplification, PIK3CA mutation and epithelial-to-mesenchymal
transition (EMT) (6). However, these
account for only 50–70% of the mechanisms responsible (7), and the remaining mechanisms still
require elucidation. A previous study demonstrated that an
erlotinib resistant NSCLC cell line expressed markers of cancer
stem cell (CSC) CD44high/CD24low, and could form spheres more
efficiently (8). Consequently, during
a long exposure to TKIs, the appearance and enrichment of CSCs may
be one of the causes of acquired resistance (9).
CSCs are a small reservoir of tumor cells with
ability of self-renewal, unlimited proliferation and differential
potential, and are the principal causes of tumor growth, relapse,
metastasis and drug-resistance (10).
Dependent on the clonogenic ability of CSCs, sphere cells rich in
CSCs could be acquired under a suspension culture deprived of
serum. Based on this hypothesis, the aim of the present study was
to culture lung sphere cells, examine the features of CSCs and
identify an association, if present, between stem cell-like cells
and TKI resistance.
MAP17, also termed PDZKIIP1, is a
membrane-associated non-glycosylated protein of 17 kDa located on
the cell membrane and the Golgi apparatus. MAP17 protein includes a
PDZ combining domain and two transmembrane regions. MAP17 is
abnormally overexpressed in several malignant tumors including
carcinoma of the breast, thyroid, cervix and lung (11). Tumors overexpressing MAP17 exhibited
increased malignant phenotypes, including ability of proliferation,
invasion, migration and tumorigenesis (12–14).
However, the function of MAP17 in lung CSCs remains unclear. The
present study identified that MAP17 expression was upregulated in
lung CSC-like cells. MAP17 expression was positively associated
with self-renewal and TKI resistance. Collectively, MAP17 may
promote TKI resistance through the regulation of CSCs.
Materials and methods
Cell culture
The PC9 cell line were provided by Tianjin Lung
Cancer Institute (Tianjin, China) and cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(FBS) (HyClone; GE Healthcare Bio-Sciences, Logan, UT, USA), 100
U/ml penicillin, and 100 mg/ml streptomycin at 37°C in 5%
CO2 atmosphere. Cells were stained with 0.1% crystal
violet at room temperature for 15 min and observed with an Inverted
Microscope TS100 (Nikon Corporation, Tokyo, Japan) at ×400
magnification.
Sphere formation assay
PC9 Cells were digested with 0.25% trypsin for 3 min
and single cells were cultured in DMEM/F12 supplemented with 50×
B-27 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 20
mg/ml epidermal growth factor (EGF) and 10 ng/ml basic fibroblast
growth factor (bFGF) (PeproTech, Inc., Rocky Hill, NJ, USA) without
serum on ultralow attachment plates (Corning Incorporated, Corning,
NY, USA) at a density of 5,000 cells/ml. After 3–7 days, spheres
began to form. Cells that exceeded 4 passages were used for
subsequent experimentation.
Limited dilution
Sphere and parent cells were diluted via a 10×
gradient with the corresponding medium (sphere cells, DMEM/F12
supplemented with 50× B-27, 20 mg/ml EGF and 10 ng/ml bFGF; parent
cells, DMEM with 10% FBS) to 5 cells/ml, then 100 µl of cell
suspension was added into 96-well plate and incubated at 37°C for
24 h. The wells containing a single cell were marked. Following a
7-day incubation, cells were observed with an inverted microscope
at ×400 magnification, and a clone with >10 cells was considered
to be a sphere. Sphere formation rate was calculated as=wells
containing a sphere/wells containing a single cell ×100 (%).
Fluorescence-activated cell sorting
(FACS)
Sphere and parent cells were digested with 0.25%
trypsin into single cell suspension and incubated with 10 µl
phycoerythrin-conjugated CD133 (1:10; cat. no. 130-109-166) or CD44
(1:20; cat. no. 130-110-121) antibody (Miltenyi Biotech GmbH,,
Bergisch Gladbach, Germany) for 30 min at 4°C. Mouse
IgG2-phycoerythrin (1:10; cat. no. 130-092-215; Miltenyi Biotech
GmbH) was used as an isotype control. The labeled cells were
analyzed by BD FASCCanto II flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer's protocol
and data was analyzed with FlowJo v10 software (Tree Star, Inc.,
Ashland, OR, USA). Gating was set on the basis of negative-control
staining profiles.
MTT assay
Sphere and parent cells were seeded in 96-well
plates at a density of 3×103 cells/well in triplicate.
Cells were incubated for either 24, 72, 120 or 168 h at 37°C. Cells
were then incubated with MTT (5 mg/ml) for 4 h at 37°C. The
formazan crystals were solubilized with 150 µl DMSO for 10 min. The
level of MTT/formazan was determined by measuring absorbance at a
wavelength of 570 nm using a microplate reader (SPECTRA; Tecan
Group, Ltd., Mannedorf, Switzerland).
Drug treatmenst
Sphere and parent cells were treated with different
concentrations of cisplatin (1, 5, 10, 20, 40, and 100 µg/l) and
erlotinib (0.1, 1, 10, 40, and 100 µmol/l) in 200 µl medium for 48
h at 37°C. Cells were then treated with MTT as previously
described. The 50% inhibitive concentration (IC50) of
cisplatin for sphere cells and parent cells were 17.81±1.13 and
8.73±0.56 µg/l respectively. A concentration of 8 µg/l cisplatin
was selected for subsequent experimentation. The IC50 of
erlotinib for sphere cells and parent cells were 53.61±1.10 and
3.71±0.42 µmol/l. A concentration of of 3 µmol/l erlotinib was
selected for the subsequent experimentation. As for SGLT1 inhibitor
phloridzin, cells were treated with 50 µmol/l phloridzin for 48 h
for the following experiments.
Transwell invasion assay
Sphere and parent cells (2×104
cells/well) were cultured in triplicate in the upper chamber of
Transwell plates that were loaded with 100 µl of diluted matrigel
(BD Biosciences, Franklin Lakes, NJ, USA). DMEM with 10% FBS was
added in the lower chamber. Following a 48 h incubation at 37°C,
the migrated cells at the bottom of the membrane were fixed with
95% ethanol and visualized using 10% crystal violet at room
temperature for 15 min. Cells were counted using an inverted
microscope TS100 (Nikon Corporation, Tokyo, Japan) at a ×400
magnification. A total of 5 random fields were selected in each
chamber to quantify the invading cells.
In vivo xenograft assay
Tumorigenicity was assayed by the subcutaneous
injection of 5×105 sphere or parent cells into the back
legs of 4 week-old female non-obese diabetic-severe combined
immunodeficiency (NOD/SCID) mice purchased from Laboratory Animal
Center of Beijing Tumor Research Institute (Beijing, China). A
total of 24 mice were randomly divided into two groups. There were
12 mice in each group and mice were kept in 23±2°C
temperature-controlled environment with a 12 h dark/12 h light
cycle and free access to food and water. The overall health, body
weights of the animals and the tumor volumes were examined twice
weekly, and every other day following the establishment of tumors.
Tumor formation rate was defined as the ratio of the number of mice
with tumor formation compared with the total number of mice. The
tumor size was calculated using the formula: V (volume) = 1/2 ×
length × width2. The permitted maximum tumor size was 1
cm3. All mice were anesthetized and sacrificed with an
overdose of anesthetic on day 56 after injection or when the tumor
reached the maximum projective size. All animal experiments were
performed according to Animal Research: reporting of in vivo
experiments (ARRIVE) guidelines, the Animal Welfare Act 2006, and
the experimental protocol were reviewed and approved by the
Experimental Animal Ethical Committee of Tianjin Medical University
(Tianjin, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of sphere and parent cells was
extracted using TRIzol Reagent (Thermo Fisher Scientific, Inc.).
The first strand cDNA was synthesized using a PrimeScript RT
reagent kit (Takara Bio, Inc., Otsu, Japan) at 37°C for 15 min,
then 85°C for 5 sec, according to the manufacturer's protocol. The
following primers were synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China) to amplify specific cDNA regions: Oct-4, forward:
5′-GGTGGAAGCTGACAACA-3′ and reverse: 5′-ATCTGCTGCAGTGTGGGTTT-3′;
ABCG2, forward: 5′-CACCTTATTGGCCTCAGGAA-3′ and reverse:
5′-CCTGCTTGGAAGGCTCTATG-3′; CD133, forward:
5′-CAGATGCTCCTAAGGCTTG-3′ and reverse: 5′-GCAAAGCATTTCCTCAGG-3′;
MAP17, forward: 5′-CAGCCATGTCGGCCCTCA-3′ and reverse:
5′-TTATTTCACAGAAATTAGGGCC-3′; β-actin, forward:
5′-AGGCCAACCGCGAGAAGATGAC-3′ and reverse:
5′-GAAGTCCAGGGCGACGTAGCA-3′. qPCR was performed in the ABI PRISM
7500 Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using SYBR® Fast qPCR Mix (Takara Bio,
Inc.). Relative expression level was determined using the
2−ΔΔCq method (15). The
thermocycling conditions were as follows: 95°C for 15 sec, 60°C for
60 sec, 72°C for 30 sec, for a total of 40 cycles. Agarose gel
electrophoresis was performed following the reaction, and the
products were observed using an ultraviolet imaging system.
Stable transfection with MAP17
PC9 cells were seeded on 6-well plates at a density
of 1×105 cells/well. When cell distribution reached
60–70%, cells were transfected with the pcDNA3.1-MAP17 and pcDNA3.1
plasmids using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc). Following 48 h, the transfected cells were
cultured with a medium containing G418 (800 µg/ml; Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany) to eliminate nontransfected cells.
G418-resistant colonies were isolated and expanded. Following this,
positively-transfected cells were identified by determining whether
MAP17 was expressed stably by qPCR and western blot analysis. The
subsequent experiments were performed 72 h after transfection.
Western blot analysis
The cells were lysed in RIPA buffer (Roche, Basel.
Switzerland) and centrifuged at 14,000 × g for 15 min at 4°C. The
protein was quantified with the BCA Protein Assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). The individual cell
lysates (20 µg/lane) were separated by 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% fat-free dried milk in TBST at room temperature for 1 h and then
incubated with relevant primary antibodies (Oct-4, dilution 1:500,
cat. no. sc-101534; cABCG2, 1:500, cat. no. sc-69989; β-actin,
dilution 1:1,000, cat. no. sc-130065; Santa Cruz Biotechnology,
Inc., Dalas, TX, USA; MAP17, 1:400, cat. no. ab31405; SGLT1,
dilution 1:400, cat. no. ab14686; Abcam, Cambridge, UK) at 4°C
overnight. After washing with PBS with 0.1% Tween-20, the membranes
were incubated with the horseradish peroxidase-conjugated goat
anti-mouse secondary antibodies (1:2,000; cat. no. GTX213111-01;
GeneTex, Irvine, CA, USA) at 37°C for 1 h. The bands were detected
by enhanced chemiluminescence detection reagents (Applygen
Technologies, Inc., Beijing, China).
Cell cycle analysis
The cell cycle was examined by a Cell Cycle
Detection kit (BD Biosciences), according to the manufacturer's
protocol. A total of 1×106 sphere or parent cells were
centrifuged at 1,000 × g for 5 min and washed twice with PBS. The
cells were then suspended in 500 µl ice-cold 70% ethanol and
incubated at 4°C overnight. The fixed cells were centrifuged at
1,000 × g for 5 min and then washed with PBS. Following incubation
with 200 µl RNase A (1 mg/ml) at 37°C for 30 min in the dark, the
cells were resuspended in 800 µl propidium iodide (50 µg/ml) and
placed in the dark at 4°C for 30 min. The stained cells were
analyzed using BD FASCCanto II flow cytometer (BD Biosciences).
RNA knockdown
Cells were transfected with the small interfering
RNAs (siRNAs) in 6-well plates. For each transfection, 250 µl of
100 pmol siRNA was mixed with 250 µl of HiPerFect Transfection
reagent (Qiagen GmbH, Hilden, Germany) and 2 ml of Optimem medium
(Invitrogen, Thermo Fisher Scientific, Inc). After 10 min of
incubation at room temperature, the 2.5 ml transfection mix was
distributed to each well and incubated at 37°C for 72 h. The
following specific siRNAs were used: siRNA PDZK1 (cat. no.
SI04314723), and AllStars negative control siRNA (cat. no.
SI03650318, both purchased from Qiagen GmbH).
Statistical analysis
All the experiments were performed in triplicate and
the data are presented as mean ± standard deviation. A two-tailed
paired Student's t-test was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses and graphics were produced using
the GraphPadPrism software version 5 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Tumor spheres exhibit stemness
CSCs can be enriched with suspension cultivation in
anchorage independent conditions based on their clonogenic ability
(16). Consequently, the present
study examined whether spheres could be formed in a serum-deprived
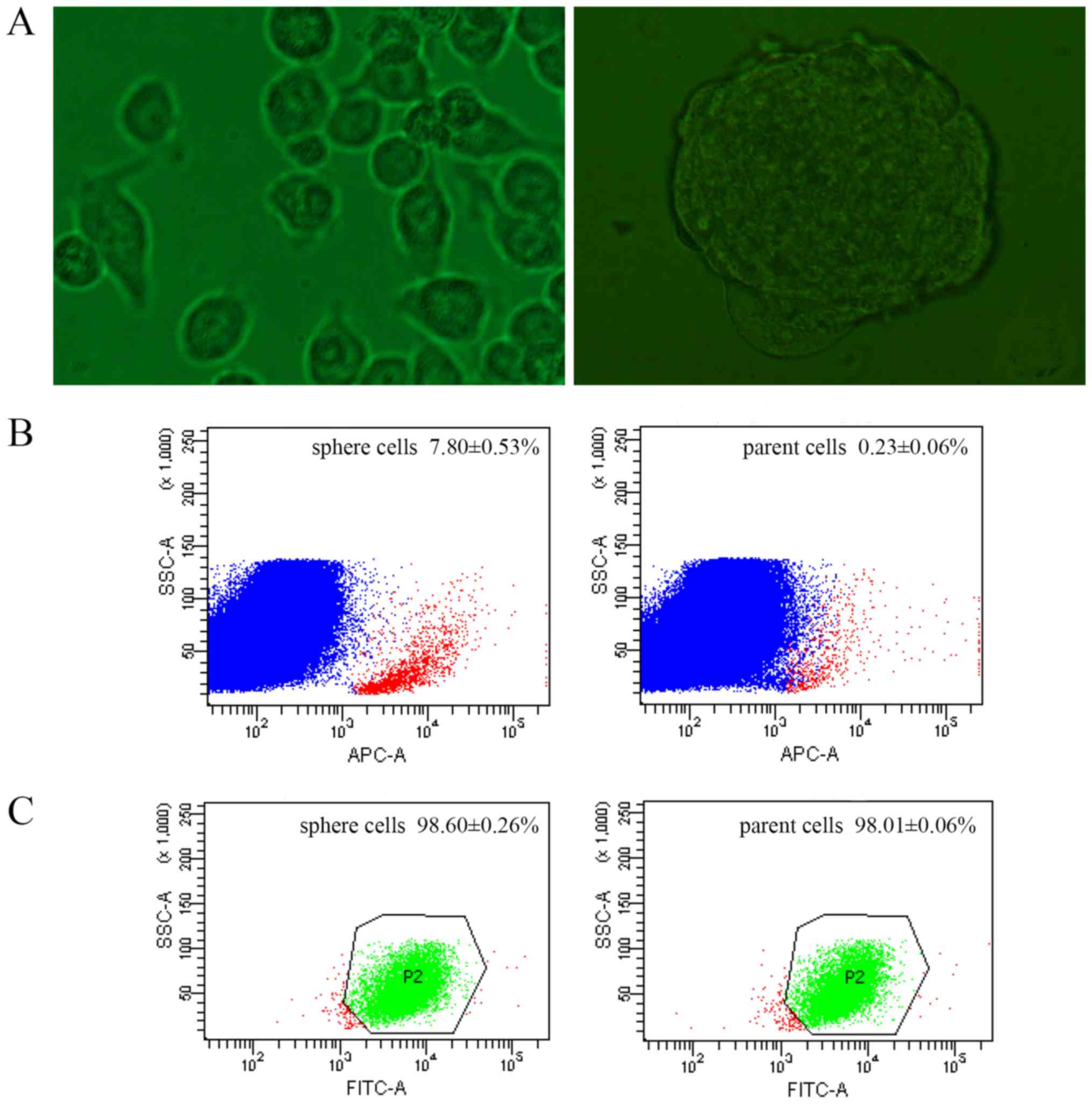
suspension culture. Following 3 days of culture, the spheres formed
and could generate >8 generations stably (Fig. 1A). When seeded in clonogenic density,
single cell could still form second-generation spheres, which
excluded the possibility of cell adhesion and proved single
clonality.
CSCs were previously identified using stem cell
surface markers, among which CD133 and CD44 are the most frequently
used (17,18). The expression of CD133 and CD44 were
compared between sphere and parent cells by FACS. The CD133
positivity of sphere cells was 7.80±0.53%, while 0.23±0.06% of
parent cells (Fig. 1B). The rate of
sphere cells expressing CD44 was 98.60±0.26%, and 98.01±0.06% in
parent cells (Fig. 1C).
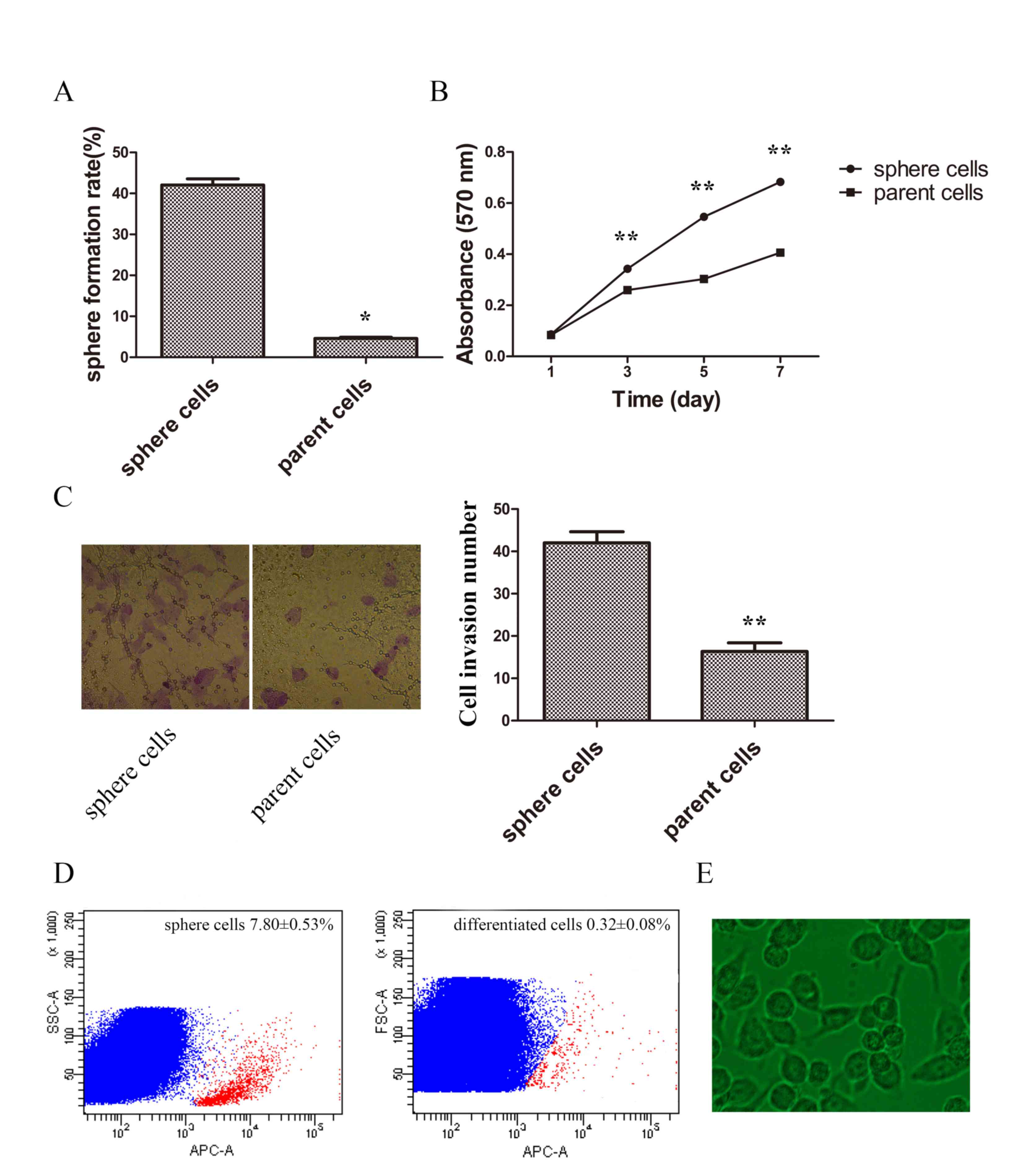
Stem-like cells have been demonstrated to have the
ability of self-renewal, invasion, proliferation and
differentiation (19). To determine
whether sphere cells harbor these stem-like properties, the
potential of self-renewal was compared by limited dilution. The
results demonstrated that sphere cells had improved sphere
formation capabilities (Fig. 2A). The
proliferation rate was compared by MTT assay, and the growth curve
demonstrated a higher proliferation ability in sphere cells
(Fig. 2B). Cell invasion ability was
also examined. The invasive ability of spheres was significantly
higher when compared with parent cells (Fig. 2C). Sphere cells were then planted in
normal cultural condition in DMEM medium containing 10% FBS for 48
h and the appearance of differentiated cells were similar to parent
tumor cells (Fig. 2D). Following a
5-day culture the percentage of CD133-positive cells of sphere
cells was 7.80±0.53%, and that of differentiated cells was
0.32±0.08%, which demonstrated sphere cells could differentiate
into common tumor cells (Fig. 2D and
E).
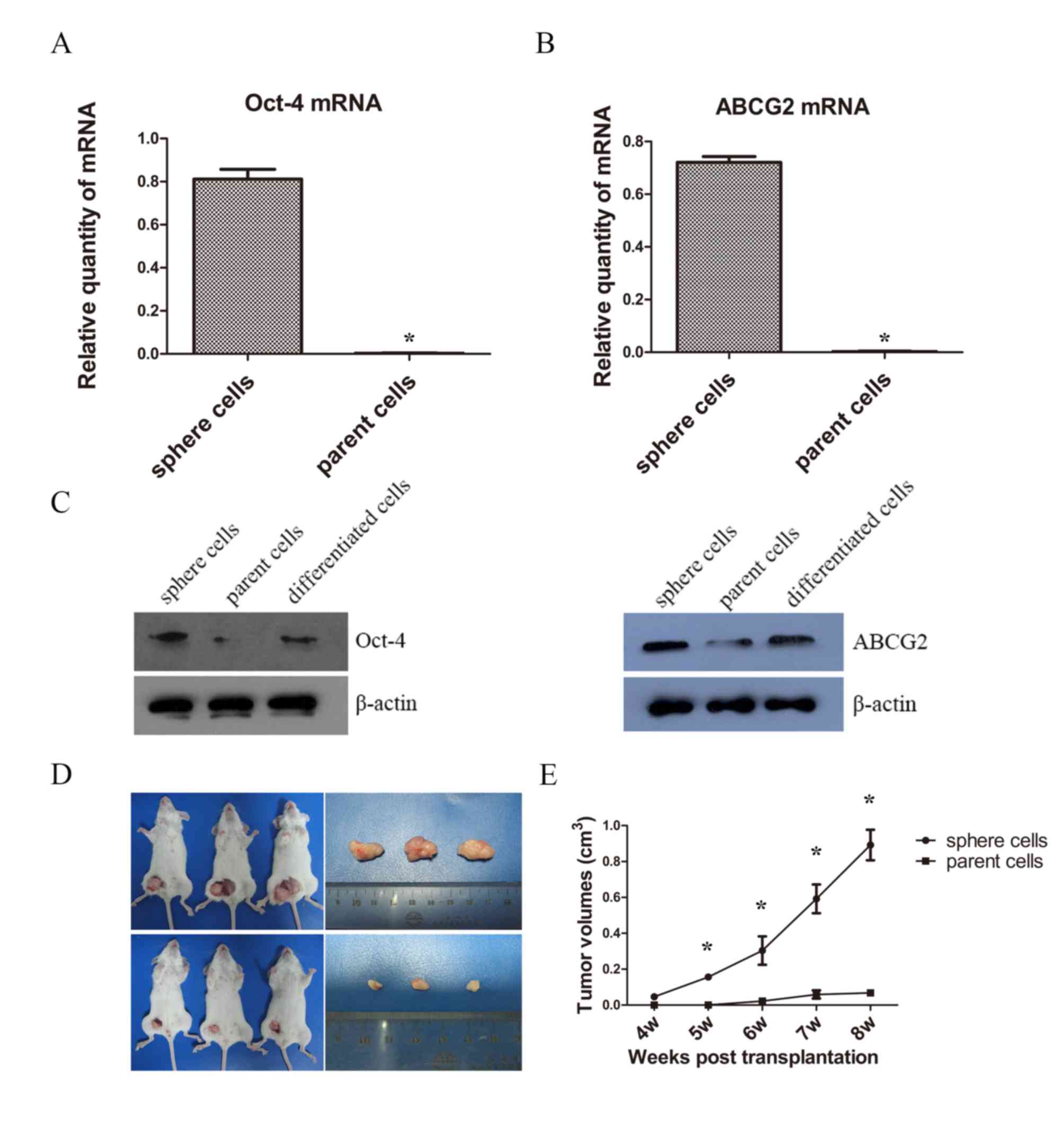
A number CSCs overexpress Oct-4 and ABCG2, which are
respectively associated with maintenance of self-renewal and drug
resistance (20,21). Therefore, the present study examined
the mRNA levels of Oct-4 and ABCG2 of spheres by RT-qPCR. The mRNA
expressions of Oct-4 and ABCG2 of sphere cells were statistically
higher than that of parent cells (Fig. 3A
and B). Then western blotting was used for the measurement of
protein expression and a notable difference of Oct-4 and ABCG2
levels were detected between sphere, parent and differentiated
cells. The expression level of Oct-4 in spheres was markedly higher
when compared with those of parent and differentiated cells;
however, no evident difference was observed between the parent and
differentiated cells. Similarly, the expression of ABCG2 in spheres
was higher than that of the parent and differentiated cells, and
the difference in expression of ABCG2 in parent cells was not
evident compared with differentiated cells (Fig. 3C). Although insignificant, the
expressions of Oct-4 and ABCG2 were higher in differentiated cells
than parent cells, potentially 48 h is not sufficient time for
CSC-like cells to fully differentiate.
Tumorigenesis is the definitive indicator of CSCs
(17). Therefore the present study
examined the ability of tumorigenesis of sphere cells and parent
PC9 cells in vivo. The results demonstrated that tumors
began to form in the experimental group in the fourth week, while
in the control group, tumors did not begin to form until the sixth
week. The rate of tumor formation in sphere groups was 100%, and
66.7% in parent cells group. The tumor growth curve showed the
sphere cells gave rise to significantly higher tumor volumes than
the parent cells (Fig. 3D and E).
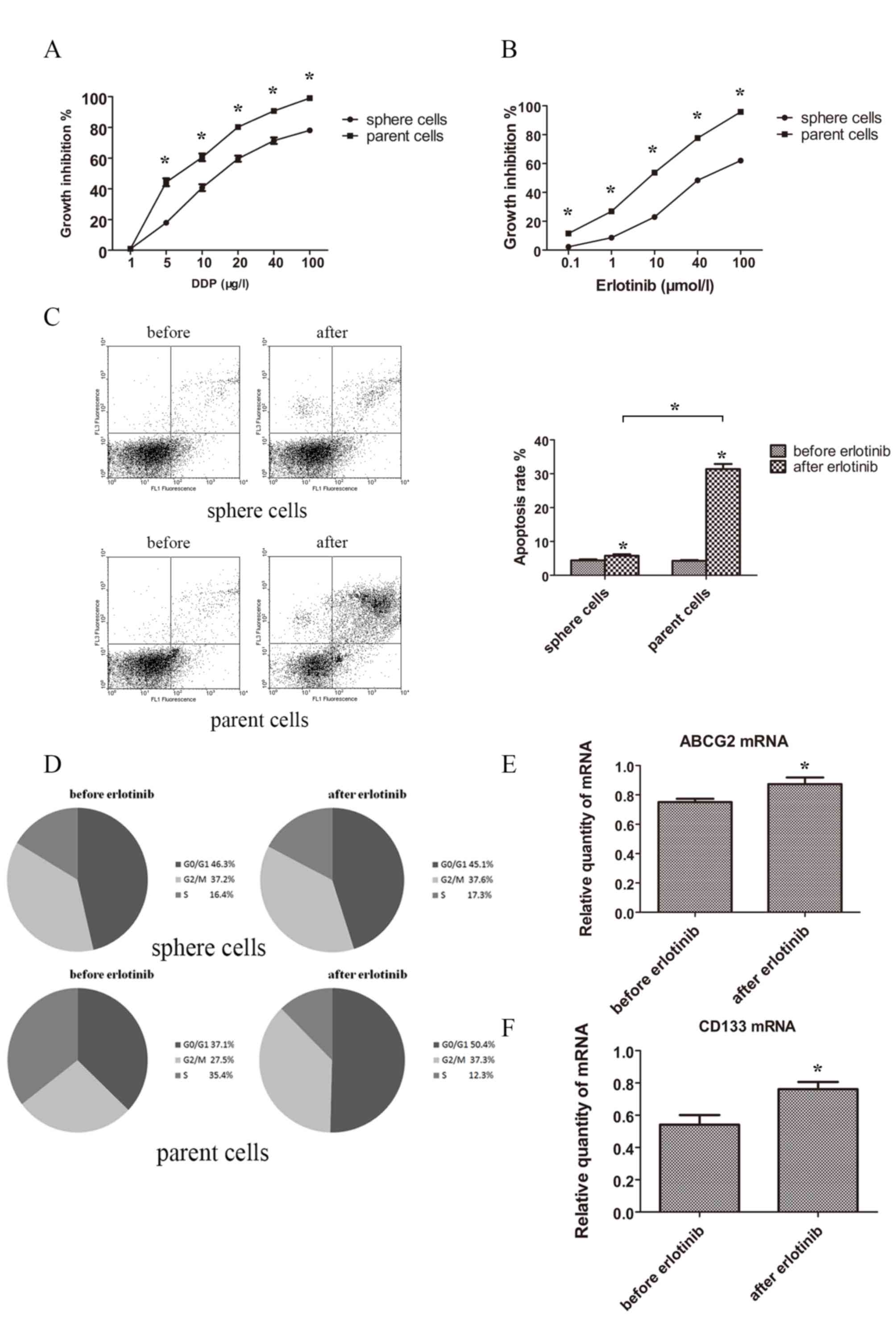
Drug resistance and influences of TKI
on sphere and parent tumor cells
To elucidate whether stem cells had an increased
resistance to drugs, sphere and parent cells were treated with the
chemotherapeutic drug cisplatin or the targeted drug erlotinib. The
results demonstrated that sphere cells possessed a stronger
capacity for drug resistance in cisplatin and erlotinib when
compared with parent cells. Spheres and parent cells treated with
different concentrations of cisplatin and erlotinib for 48 h showed
statistically different growth inhibition rate curve (Fig. 4A and B).
Then the apoptosis rate was tested. Following
treatment with erlotinib 3 µmol/l for 48 h, the apoptosis rates of
sphere and parent cells increased. Apoptosis rate of spheres prior
to and following treatment with erlotinib were 4.42±0.28 and
5.79±0.43%, while apoptosis rate of normal tumor cells prior to and
following treatment with erlotinib were 4.27±0.23 and 31.37±1.50%.
Normal tumor cells showed a much higher proportion of apoptosis
following treatment with erlotinib (Fig.
4C).
As for cell cycle distribution, following treatment
with erlotinib (3 µmol/l) for 48 h, the proportions of G0/G1, S and
G2/M of sphere cells were not statistically different. However, the
proportions of G0/G1 and G2/M were notably increased in parent
cells and the propotion of S of parent cells was decreased
(Fig. 4D).
The change of levels of ABCG2 and CD133 following
erlotinib treatment were investigated in sphere cells, and the
expression of ABCG2 and CD133 were significantly elevated following
erlotinib treatment (Fig. 4E and
F).
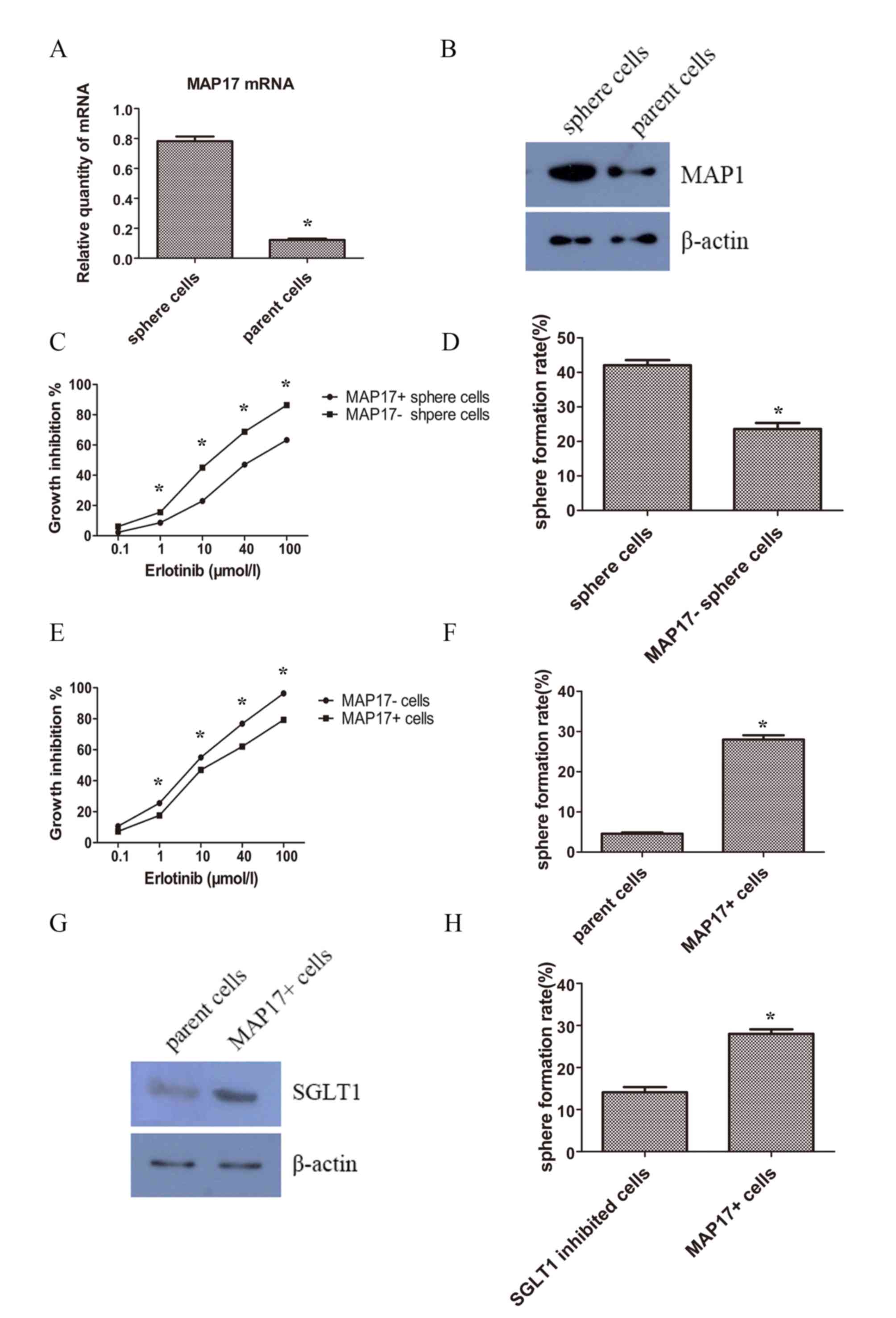
MAP17 expression was upregulated and
associated with TKI resistance in CSCs
In order to investigate whether MAP17 participates
in the regulation of lung CSCs, the expression pattern of MAP17 on
CSC-like and parent cancer cells were examined using RT-qPCR and
western blot analysis. The results revealed that the expression
level of MAP17 was increased in sphere cells, in mRNA (Fig. 5A) and protein levels (Fig. 5B). To determine whether MAP17 had a
role in CSC phenotype of self-renewal, MAP17 was knocked-down in
sphere cells using siRNA. MTT assays were performed 48 h after
erlotinib treatment to examine cell viability. The results showed
that following MAP17 knockdown, sphere cells became less resistant
to erlotinib (Fig. 5C). Additionally,
the results demonstrated that knockdown of MAP17 decreased the
sphere formation efficiency of CSC-like cells (Fig. 5D).
To further confirm the function of MAP17, MAP17 was
overexpressed in parent cells. After a 48 h treatment with
erlotinib, cells overexpressing MAP17 were increasingly resistant
to erlotinib compared with their control counterparts (Fig. 5E). Self-renewal was also examined and
cells overexpressing MAP17 exhibited a higher sphere formation
efficiency than the control group (Fig.
5F).
Na-dependent glucose transporter 1
(SGLT1) overexpression is associated with MAP17 and CSC
phenotypes
It was reported that MAP17 harbored the ability of
tumorigenesis by increasing endogenous reactive oxygen species
(ROS) (22). The increased ROS
activates AKT and significantly decreases c-myc-induced apoptosis
through SGLT1 activation (23), and
the suppression of SGLT1 inhibits MAP17-induced ROS increase and
tumor cell proliferation (24).
Therefore, examined SGLT1 expression levels of the tumor cells. It
was revealed that SGLT1 expression was increased in MAP17
overexpressed tumor cells (Fig. 5G).
Furthermore, treatment with the SGLT inhibitor phloridzin could
decrease the sphere formation slightly but significantly (Fig. 5H).
Discussion
At present, there is growing concern about the
resistance to EGFR-TKIs. In the present study, CSC-like cells were
enriched with a serum-deprived suspension culture, and found the
sphere cells bore stem cell-like properties and were resistant to
erlotinib. Sphere cells expressed higher levels of MAP17, and MAP17
was associated with self-renewal and TKI resistance.
CSCs are defined as tumor cells capable of
self-renewal and able to generate heterogenetic tumor cells
(10). Cancer is regarded as a
stem-cell disease, and CSCs serve an important role in
tumorigenesis, proliferation, metastases and drug resistance
(25). CSCs are usually identified
based on their biological functions and specific cell surface
markers, and stem cell surface markers have been used to purify
CSCs. Among which, CD133, CD44, Oct-4, sox-2 are the most commonly
used (17,18,20,21).
Additionally side population (SP) cells manifest significant
abilities of invasiveness and tumorigenesis, and express
drug-resistance related proteins such as ABCG2, therefore SP cells
are considered to exhibit stemness. SP cells are capable of
‘pumping out’ dyes including Hoechst 33324, therefore the unstained
cells are identifiable by fluorescence activated cell sorting
(FACS) (26). Furthermore, CSCs hold
the ability of colony forming, so enriching spheroid cells in an
undifferentiated suspension condition is an effective way of
purifying and identifying CSC-like cells. Under these conditions,
cells form spheres, express stem cell markers like Oct-4 and Bmi1,
and the ability of self-renewal could also be tested (16). In the present study, sphere cells
manifested an enhanced stem cell like ability for self-renewal,
invasion, proliferation, tumorigenesis, drug resistance and
increased expression of stem cell markers, including CD133, CD44,
Oct-4 and ABCG2. Notably, although the expression levels of CD44 in
sphere and parent cells were statistically different, the levels of
both were increased when compared with the control group. Previous
studies have reported that the significance of CD44 was also
ambivalent (27,28). Therefore the optimal combination of
stem cells markers for specific tumors still require further
examination.
CSCs may serve a role in TKI resistance. A previous
study reported gefitinib resistant cells exhibited stem cell-like
properties, including increase of SP and self-renewal capabilities
(9). Another study by Kobayashi et
al (29) reported that Oct-4, the
putative stem cell marker, could induce gefitinib resistance by
regulating the CSC properties in aNSCLC cell line with EGFR
mutation, and the samples from patients with acquired resistance to
TKIs manifested a high level of Oct-4 expression. Similarly, cells
resistant to the second generation TKI afatinib manifested CSC
phenotypes, including an enhanced ability of colony formation and
proliferation, and increased level of ALDH1 and CD44 (30). So it was implied that resistance to
TKI could partly arise from CSCs. This was corroborated by the
present study, where sphere cells were demonstrated to develop
resistance to chemotherapeutic drugs and TKI. The possible
mechanisms are as follows: Firstly, in EGFR mutant NSCLC, EGFR
downstream signals including phosphatidylinositol 3-kinase
(PI3K)/Akt are activated (31). Upon
the treatment of TKIs, these signals are inhibited. Meanwhile,
PI3K/Akt may serve a critical role in CSC phenotypes (32). Therefore, the abnormal activation of
PI3K/Akt in CSCs may contribute to TKI resistance. Secondly, genes
including Oct-4 and nanog have been demonstrated to promote EMT and
therefore induce stem cell properties (33). EMT can also trigger aquired resistance
of TKIs (6). CSCs are resistant to
chemotherapeutic drugs, partially owing to elevated expression of
drug transporters of the ATP-binding cassette (ABC) family
(34). However the role of the ABC
family in TKI resistance requires further study. Lastly, under the
selective pressure of TKIs, parent tumor cells underwent apoptosis
and multi-drug resistant CSCs were retained and enriched. In this
work, after treatment of erlotinib, the elevated levels of CD133
and ABCG2 may reflect the elevated proportion of TKI-resistant
CSCs.
MAP17 is an established oncogene working through ROS
induction (12–14). The N-terminus of MAP17 is composed of
13 amino acids that encode a PDZ-binding domain (35). MAP17 can bind to a number of PDZ
domain-containing proteins, including PDZK1 (NHRF3) and other NHRF
proteins, and MAP17 is abnormally overexpressed in several
malignancies, including laryngoesophageal, breast, lung and thyroid
tumors (12,36,37).
Tumors overexpressing MAP17 showed increased ability for
proliferation, invasion, migration and tumorigenesis, suggesting
that there may be an association of MAP17 and CSC phenotypes.
However, the function of MAP17 in CSCs has, to the best of our
knowledge, yet to be reported. In the present study, the MAP17
level was demonstrated to be increased in sphere cells, MAP17
knockdown resulted in decreased ability of drug resistance and
sphere formation, while MAP17 overexpression induced an increase in
malignant phenotypes, therefore demonstrating the role of MAP17 in
CSCs.
The function of MAP17 is associated with production
of ROS, and dependent on the PDZ-binding domain. MAP17 protects
cells from Myc-induced apoptosis through ROS-dependent activation
of the PI3K/AKT pathway (38).
Furthermore, MAP17 can cause the activation of AKT, independent of
PI3K activity (12). Previous studies
have demonstrated that MAP17 induced ROS though SGLT1 (23), and the inhibition of SGLT1 inhibits
MAP17-induced ROS increase and cell proliferation (12). Furthermore, MAP17 and SGLT1 were
prognostic markers for treatment with cisplatin and radiation
(24). In the present study, the
SGLT1 level was elevated in cells overexpressing MAP17, inhibition
of SGLT1 resulted in decreased ability of sphere formation,
indicating MAP17 induced CSC phenotypes, partially through ROS and
dependent on SGLT1.
In conclusion, the present study suggested that
CSC-like cells could be enriched under an undifferentiated
suspension culture. CSC-like cells exhibit increased resistance to
TKIs, and the enrichment of CSCs may be a reason for TKI
resistance. MAP17 was aberrantly elevated in CSC-like cells and
associated with TKI resistance, and the regulatory function of
MAP17 is partially dependent on SGLT1.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS was involved in study conception, study design,
experimentation, data analysis and manuscript writing. HL conducted
literature research, experiments and statistical analysis, and was
involved in manuscript preparation. DZ was involved in study
conception and data analysis, and reviewed the manuscript. QZ was
involved in study conception and data analysis, and revised the
manuscript.
Ethical approval and consent to
participate
All procedures performed in studies involving
animals were in accordance with the ethical standards of the
institution or practice at which the studies were conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small-cell lung cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
TKI
|
tyrosine kinase inhibitor
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
CSC
|
cancer stem cell
|
|
ROS
|
reactive oxygen species
|
|
SGLT1
|
Na-dependent glucose transporter 1
|
|
SP
|
side population
|
|
FACS
|
fluorescence activated cell
sorting
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
ABC
|
ATP-binding cassette
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jackman D, Pao W, Riely GJ, Engelman JA,
Kris MG, Jänne PA, Lynch T, Johnson BE and Miller VA: Clinical
definition of acquired resistance to epidermal growth factor
receptor tyrosine kinase inhibitors in non-small-cell lung cancer.
J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh G, Lian X, Kron SJ and Palecek SP:
Properties of resistant cells generated from lung cancer cell lines
treated with EGFR inhibitors. BMC Cancer. 12:952012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shien K, Toyooka S, Yamamoto H, Soh J,
Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, et al:
Acquired resistance to EGFR inhibitors is associated with a
manifestation of stem cell-like properties in cancer cells. Cancer
Res. 73:3051–3061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kocher O, Cheresh P and Lee SW:
Identification and partial characterization of a novel
membrane-associated protein (MAP17) up-regulated in human
carcinomas and modulating cell replication and tumor growth. Am J
Pathol. 149:493–500. 1996.PubMed/NCBI
|
|
12
|
Guijarro MV, Vergel M, Marin JJ,
Muñoz-Galván S, Ferrer I, Ramon y Cajal S, Roncador G,
Blanco-Aparicio C and Carnero A: p38α limits the contribution of
MAP17 to cancer progression in breast tumors. Oncogene.
31:4447–4459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Maro G, Orlandella FM, Bencivenga TC,
Salerno P, Ugolini C, Basolo F, Maestro R and Salvatore G:
Identification of targets of Twist1 transcription factor in thyroid
cancer cells. J Clin Endocrinol Metab. 99:E1617–1626. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inoue J, Otsuki T, Hirasawa A, Imoto I,
Matsuo Y, Shimizu S, Taniwaki M and Inazawa J: Overexpression of
PDZK1 within the 1q12-q22 amplicon is likely to be associated with
drug-resistance phenotype in multiple myeloma. Am J Pathol.
165:71–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
18
|
Al-Hajj M: Cancer stem cells and oncology
therapeutics. Curr Opin Oncol. 19:61–64. 2007.PubMed/NCBI
|
|
19
|
Liu S, Dontu G and Wicha MS: Mammary stem
cells, self-renewal pathways, and carcinogenesis. Breast Cancer
Res. 7:86–95. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boiani M and Schöler HR: Regulatory
networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell
Biol. 6:872–884. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carnero A: MAP17 and the double-edged
sword of ROS. Biochim Biophys Acta. 1826:44–52. 2012.PubMed/NCBI
|
|
23
|
Perez M, Praena-Fernandez JM, Felipe-Abrio
B, Lopez-Garcia MA, Lucena-Cacace A, Garcia A, Lleonart M, Roncador
G, Marin JJ and Carnero A: MAP17 and SGLT1 protein expression
levels as prognostic markers for cervical tumor patient survival.
PLoS One. 8:e561692013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guijarro MV, Leal JF, Blanco-Aparicio C,
Alonso S, Fominaya J, Lleonart M, Castellvi J, Ramon Y Cajal S and
Carnero A: MAP17 enhances the malignant behavior of tumor cells
through ROS increase. Carcinogenesis. 28:2096–2104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2-cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takanami I, Takeuchi K and Naruke M:
Expression and prognostic value of the standard CD44 protein in
pulmonary adenocarcinoma. Oncol Rep. 7:1065–1067. 2000.PubMed/NCBI
|
|
29
|
Kobayashi I, Takahashi F, Nurwidya F, Nara
T, Hashimoto M, Murakami A, Yagishita S, Tajima K, Hidayat M,
Shimada N, et al: Oct4 plays a crucial role in the maintenance of
gefitinib-resistant lung cancer stem cells. Biochem Biophys Res
Commun. 473:125–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hashida S, Yamamoto H, Shien K, Miyoshi Y,
Ohtsuka T, Suzawa K, Watanabe M, Maki Y, Soh J, Asano H, et al:
Acquisition of cancer stem cell-like properties in non-small cell
lung cancer with acquired resistance to afatinib. Cancer Sci.
106:1377–1384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho R, Minturn JE, Hishiki T, Zhao H, Wang
Q, Cnaan A, Maris J, Evans AE and Brodeur GM: Proliferation of
human neuroblastomas mediated by the epidermal growth factor
receptor. Cancer Res. 65:9868–9875. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang G, Yan H, Ye S, Tong C and Ying QL:
STAT3 phosphorylation at tyrosine 705 and serine 727 differentially
regulates mouse ESC fates. Stem Cells. 32:1149–1160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei
X, Gao J, Zhao Z and Liu C: Oct-4 and Nanog promote the
epithelial-mesenchymal transition of breast cancer stem cells and
are associated with poor prognosis in breast cancer patients.
Oncotarget. 5:10803–10815. 2014.PubMed/NCBI
|
|
34
|
Lage H: An overview of cancer multidrug
resistance: A still unsolved problem. Cell Mol Life Sci.
65:3145–3167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lanaspa MA, Giral H, Breusegem SY,
Halaihel N, Baile G, Catalán J, Carrodeguas JA, Barry NP, Levi M
and Sorribas V: Interaction of MAP17 with NHERF3/4 induces
translocation of the renal Na/Pi IIa transporter to the
trans-Golgi. Am J Physiol Renal Physiol. 292:F230–F242. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Miguel-Luken MJ, Chaves-Conde M,
Quintana B, Menoyo A, Tirado I, de Miguel-Luken V, Pachón J,
Chinchón D, Suarez V and Carnero A: Phosphorylation of gH2AX as a
novel prognostic biomarker for laryngoesophageal dysfunction-free
survival. Oncotarget. 7:31723–31737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang N, Zhou F, Xiong H, Du S, Ma J, Okai
I, Wang J, Suo J, Hao L, Song Y, et al: Screening and
identification of distant metastasis-related differentially
expressed genes in human squamous cell lung carcinoma. Anat Rec
(Hoboken). 295:748–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Miguel-Luken MJ, Chaves-Conde M, de
Miguel-Luken V, Muñoz-Galván S, López-Guerra JL, Mateos JC, Pachón
J, Chinchón D, Suarez V and Carnero A: MAP17 (PDZKIP1) as a novel
prognostic biomarker for laryngeal cancer. Oncotarget.
6:12625–12636. 2015. View Article : Google Scholar : PubMed/NCBI
|