Introduction
The metastatic spread of tumor cells to vital organs
is the principle cause of mortality in patients with colon cancer.
The tumor microenvironment is important in regulating the
metastatic capacity of numerous types of cancer (1). Interactions of cancer cells with the
tumor microenvironment throughout the epithelial-mesenchymal
transition (EMT) are important determinants of cancer progression
toward metastasis (2). The EMT
program has emerged as a crucial early event for promoting the
invasive and metastatic behavior of various types of carcinoma
(3). EMT is the process by which the
epithelial phenotype of a cell is converted to a mesenchymal
phenotype. Specifically, adherens junction proteins, including
E-cadherin and cytokeratin, are downregulated, and the expression
of mesenchymal markers, including vimentin and N-cadherin, are
upregulated (4). Furthermore,
morphologically, the cell-cell junctions are weakened and cell
polarity is altered to reduce cell-cell interactions. The
morphology of cells becomes more fibroblast-like and cells become
more invasive.
The exact mechanisms underlying EMT have yet to be
elucidated; however, several cytokines, including transforming
growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α),
interleukin-6 and macrophage migration inhibitory factor, have
previously been demonstrated to be associated with EMT in
experimental models and human cancer. These factors may either
promote or inhibit tumor development by modulating EMT (5). TGF-β1, a member of the TGF-β
super-family, is involved in embryonic development and also
contributes to tumor progression by inducing EMT (6). Several studies have demonstrated that
TNF-α, which is produced by macrophages, upregulates various
adhesion molecules on endothelial cells that contribute to tumor
cell adhesion and migration (7,8).
Furthermore, in primary bronchial epithelial cells, TNF-α can
accentuate TGF-β1-induced EMT, resulting in the dysregulated wound
repair of injured lung epithelium (9). TNF-α has also been demonstrated to
increase the metastatic potential in human colonic epithelial
organoid models of colon cancer by promoting EMT, indicating a
potential link between EMT and inflammation (10). TGF-β1-induced EMT is promoted by TNF-α
via signaling between the Smad and nuclear factor-κB (NF-κB)
pathways in the A549 lung adenocarcinoma epithelial cell line
(11). However, the signaling
pathways involved in the synergistic effect of TGF-β1 and TNF-α in
colon adenocarcinoma epithelial cells remain to be elucidated, and
further investigation is required to identify potential therapeutic
targets to disrupt or even reverse EMT. Thus, the present study was
designed to determine whether TNF-α and TGF-β1, two stroma-derived
factors, facilitated EMT in colon cancer cells, and to understand
the underlying mechanisms.
Materials and methods
Cell culture
SW480 colon cancer cells were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in an incubator with 5% CO2.
Antibodies and reagents
Recombinant human TNF-α and human TGF-β1 were
purchased from PeproTech, Inc. (Rocky Hill, NJ, USA) and
Prospec-Tany TechnoGene, Ltd. (East Brunswick, NJ, USA),
respectively. Rabbit polyclonal anti-N-cadherin (cat. no. ab12221),
mouse monoclonal anti-E-cadherin (cat. no. ab1416) and
anti-vimentin (cat. no. ab8069) antibodies were all purchased from
Abcam (Cambridge, UK). FITC AffiniPure Goat Anti-Mouse IgG (H+L)
(cat. no. E031210-01) and Cy3 AffiniPure Goat Anti-Mouse IgG (cat.
no. E031610-01) were obtained from EarthOx Life Sciences (Millbrae,
CA, USA). The rabbit monoclonal anti-extracellular signal-regulated
kinase (ERK) antibody (cat. no. 4695), phospho-ERK (cat. no. 4370)
and phospho-p38 mitogen-activated protein kinase (MAPK) antibodies
(cat. no. 2387) were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and the antibody against GAPDH was from
Sigma-Aldrich (Merck KGaA). IKKβ4i (cat. no. CAS
507475-17-4), a small molecule inhibitor against inhibitor of NF-κB
kinase subunit β (IKKβ), an ERK1/2 inhibitor (PD98059; cat. no.
sc-3532) and a p38 MAPK inhibitor (SB203580; cat. no. sc-3533) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Inhibitors were dissolved in dimethyl sulfoxide and aliquots were
stored at −80°C prior to use.
Cytokine and inhibitor assays
A total of 5×104 SW480 cells were seeded
in 24-well plates with either TNF-α (10 ng/ml) or TGF-β1 (20
ng/ml), or a combination of the two. The morphology of the colon
cancer cells was assessed by light microscopy (Olympus Corporation,
Tokyo, Japan). For inhibition assays, cells were pretreated for 20
min with PD98059 ERK inhibitor (20 µM) or for 1 h with SB203580 p38
MAPK inhibitor (40 µM), and then seeded in culture plates in the
presence of cytokines. In certain experiments, cells were
pretreated with the IKKβ inhibitor, IKKβ4i (10 µM), for 2 h
prior to the addition of cytokines and subsequent culture in
24-well plates.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde at room
temperature for 10 min and incubated with anti-E cadherin (1:100)
and anti-vimentin (1:100) at 4°C overnight followed by incubation
with the secondary antibodies of FITC AffiniPureGoat Anti-Mouse IgG
(1:100) or Cy3AffiniPure Goat Anti-Mouse IgG (1:200) at room
temperature for 1 h. DAPI was used as a nuclear counterstain.
Images were acquired using a Leica TCS-SP-2UV laser scanning
confocal microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Migration assay
The migratory capacity of SW480 cells was assessed
using a Transwell chamber assay (8 µm pore size, polycarbonate
filters, 6.5 mm diameter; Costar Corporation, Cambridge, MA, USA)
in 24-well plates. Briefly, 4×104 cells were treated
with TGF-β1 (10 ng/ml), TNF-α (20 ng/ml) or a combination of the
two for 72 h, incubated overnight in 250 µl of serum-free DMEM,
then added to the upper chamber; and DMEM medium with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) was used as a
chemoattractant in the lower chamber. Following incubation at 37°C
for 24 h, the nonmigrating cells were removed from the upper
surface of each Transwell using a cotton swab. Transwell membranes
were then stained with crystal violet at room temperature for 5–10
min. The cells that migrated through the membrane to the lower
surface were counted by light microscopy.
Western blotting
Proteins were extracted from cells in a Triton-X
lysis buffer (1% Triton-X, 50 mM Tris and 150 mM NaCl) containing
protease inhibitors (pepstatin, phenylmethane sulfonyl fluoride,
aprotinin and leupeptin) for 1 h in 4°C. The protein concentration
in the samples was determined using a Bradford protein assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts of
cell lysates (30 µg/lane) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were probed with antibodies
against E-cadherin (1:1,000), vimentin (1:500), N-cadherin (1:500),
ERK (1:1,000), phospho-ERK (1:500) and phospho-p38 MAPK (1:11,000)
overnight at 4°C. Membranes were incubated with horseradish
peroxidase-conjugated anti-mouse antibody (cat. no. NXA931;
1:5,000) and anti-rabbit antibody (cat. no. NA934; 1:5,000) (GE
Healthcare Life Sciences, Little Chalfont, UK) for 1 h at room
temperature. Immune complexes were detected by enhanced
chemiluminescence (Cell Signaling Technology, Inc.). GAPDH
(1:1,000) was detected as a reference protein for equal sample
loading, and Image Lab 5.0 software (Bio-Rad Laboratories, Inc.)
was used for quantitative analysis.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean. The significance of differences was assessed by a one-way
analysis of variance followed by Tukey's post hoc test, using the
computer software package GraphPad Prism (version 5.0; GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of TNF-α and TGF-β1 on EMT in
SW480 cells
For further confirmation that the SW480 cells had
undergone EMT, the effect of TGF-β1 and TNF-α on the invasive
behavior of cells was investigated individually and simultaneously.
In contrast to previous findings in colon cancer cells (12), TGF-β1 or TNF-α alone did not alter
SW480 morphology within 72 h compared with the untreated cells
(Fig. 1A); the cancer cells
maintained uniform cobblestone morphology with tight cell-cell
contact (Fig. 1A-a-i). However, the
combined addition of TGF-β1 and TNF-α to SW480 cells for 72 h
resulted in a complete mesenchymal transition, with cells
exhibiting fibroblastic morphology, with a bipolar, spindle-like
shape (Fig. 1A-j-l). Consistent with
this change, the results of immunofluorescent staining demonstrated
that E-cadherin immunoreactivity was predominantly on the cell
membrane, with some immunoreactivity in the plasma. The expression
of E-cadherin was markedly decreased (Fig. 1B) and the induction of vimentin was
increased with a weak trend in the cancer cells treated with both
cytokines (Fig. 1C). By contrast, no
significant effect on cell phenotype was observed when SW480 cells
were treated with TNF-α and TGF-β1 individually compared with the
untreated control cells.
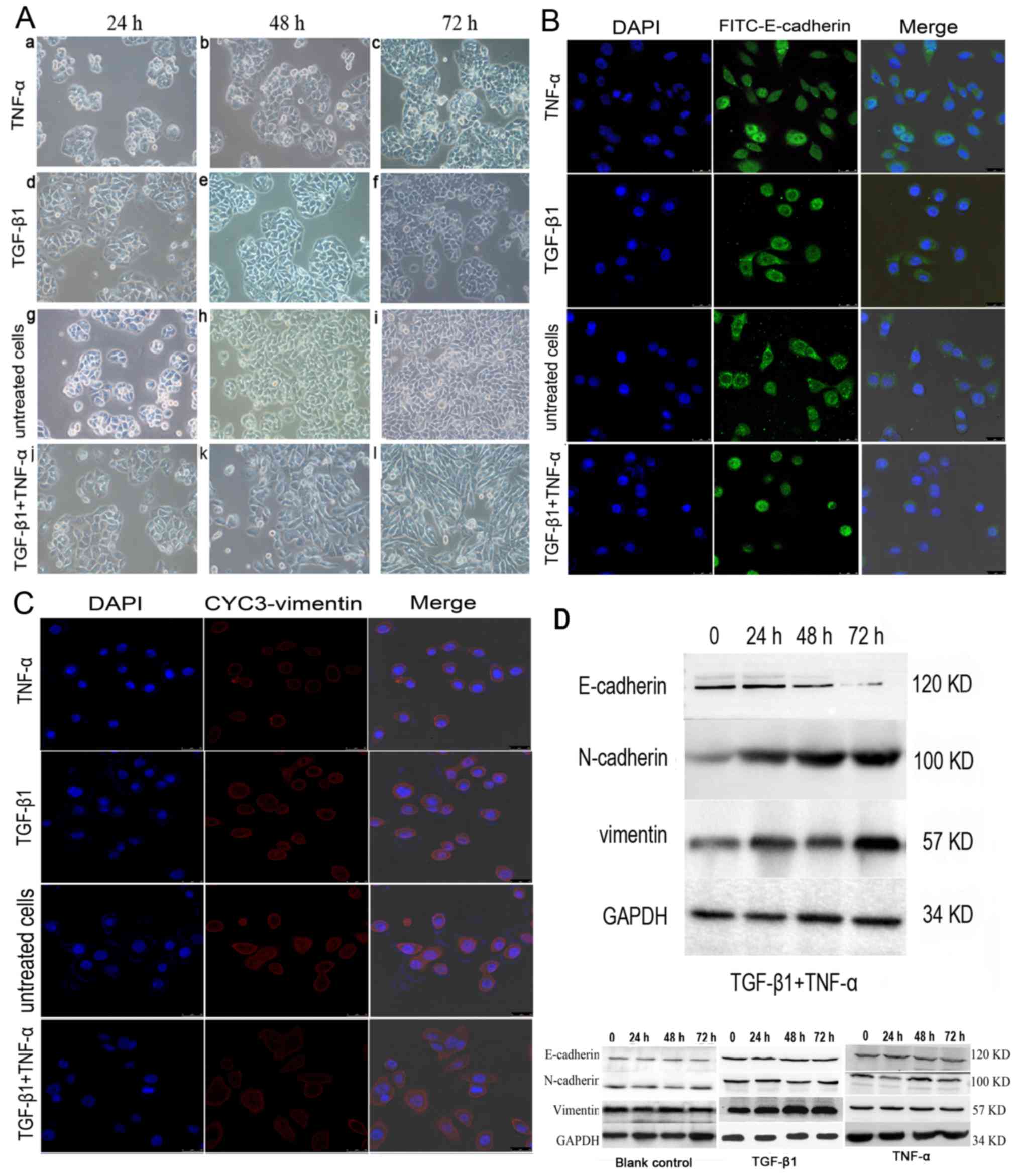 | Figure 1.Effects of TGF-β1 and TNF-α on SW480
cells. (A) Morphological characteristics of EMT in SW480 cells
treated with (a-c) TGF-β1 or (d-f) TNF-α alone, or in (g-i)
combination. (a-f) The majority of TGF-β1 or TNF-α-treated, or
(g-i) untreated SW480 cells demonstrated a pebble-like shape and
tight cell-cell adhesion at 72 h. (j-l) Cotreatment with TGF-β1 and
TNF-α decreased cell-cell contacts and resulted in a more elongated
morphological shape compared with individual treatments. (l) TGF-β1
and TNF-α treatment resulted in EMT of SW480 cells, with cells
exhibiting a bipolar, spindle-like shape, characteristic of
fibroblasts, by 72 h. Magnification, ×200. (B) Immunofluorescent
images of SW480 cells treated with TGF-β1, TNF-α or a combination
of both for 48 h. Cells were stained with an antibody against
E-cadherin (green), and the nucleus was counterstained with DAPI
(blue). Subpanels are representative images of the nuclear
staining, E-cadherin staining, and a merge of the two,
respectively, in SW480 cells. There was a decrease in the an
immunostaining of E-cadherin in TGF-β1-and TNF-α-treated SW480
cells compared with cells treated with TNF-α, TGF-β1 alone or
untreated cells group. Magnification, ×200. (C) Cells were stained
with antibody against vimentin (red), and the nucleus was
counterstained with DAPI. Subpanels are representative images of
nuclear staining, vimentin staining and a merge of the two,
respectively, in SW480 cells. There was an increase in
immunostaining of vimentin in TGF-β1- and TNF-α-treated SW480 cells
compared with cells treated with TNF-α or TGF-β1 alone, or
untreated. Magnification, ×200. (D) Western blotting of the
treatment of SW480 cells with TGF-β1 and TNF-α. SW480 cells were
incubated with TGF-β1 and TNF-α for 0–72 h and E-cadherin
expression was visibly depleted at 72 h. N-cadherin and vimentin
expression levels were increased from 24 h. TGF-β1, transforming
growth factor-β1; TNF-α, tumor necrosis factor-α; EMT,
epithelial-mesenchymal transition. |
E-cadherin expression was almost undetectable at 72
h in the presence of the two cytokines based on a western blotting
analysis, which indicated that simultaneous stimulation with TNF-α
and TGF-β1 promoted EMT (Fig. 1D). By
contrast, E-cadherin expression remained discernible following a
72-h treatment with TGF-β1 or TNF-α alone (Fig. 1D). Additionally, E-cadherin protein
levels were downregulated and the N-cadherin expression was
upregulated following incubation with TNF-α or TGF-β1 alone
compared with the control cells (n=3; P<0.05) (data not shown).
Vimentin expression levels were marginally increased compared with
the control; however, the difference was not significant (n=3;
P>0.05).
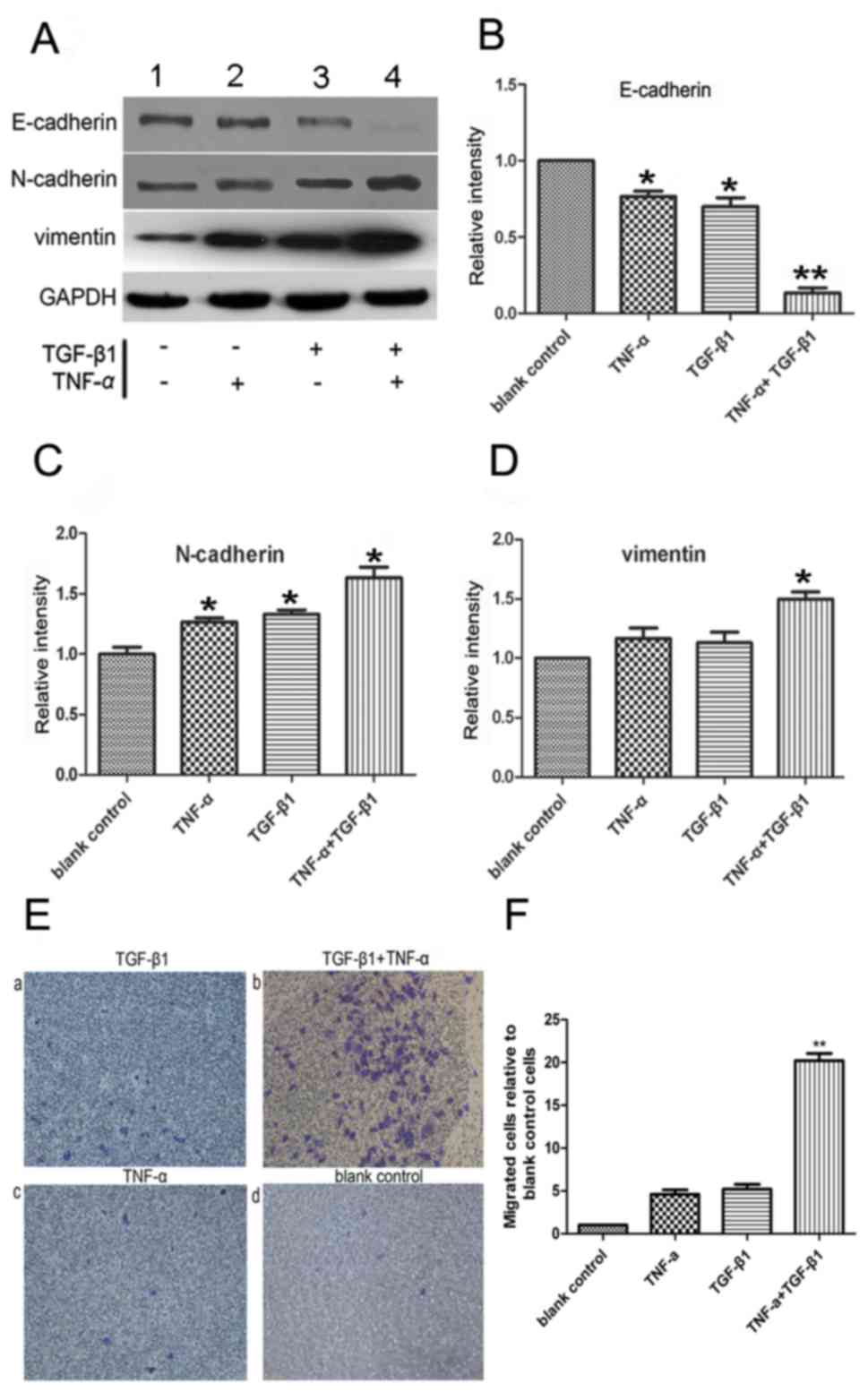
The expression levels of the mesenchymal markers
vimentin and N-cadherin were upregulated following combined
treatment with TNF-α and TGF-β1 (Fig.
2A-D), indicating the occurrence of EMT. To measure cell
migration, the ability of SW480 cells to penetrate Transwell
membranes was examined (Fig. 2E). Few
non-treated cells spontaneously penetrated the filters, and the
addition of either TNF-α or TGF-β1 alone for 72 h did not
observably initiate migration (Fig. 2E-a
and -c) compared with the untreated control (Fig. 2E-d). The addition of the two cytokines
together increased the rate of migration compared with the control
(P<0.05; n=3; Fig. 2E-b and -F),
which corroborated the morphological observations of EMT, as
demonstrated in Fig. 2E-b.
Collectively, the data of the present study suggested that TNF-α
and TGF-β1 cooperated to reduce the level of E-cadherin, induce
mesenchymal phenotypes and, thus, promote EMT.
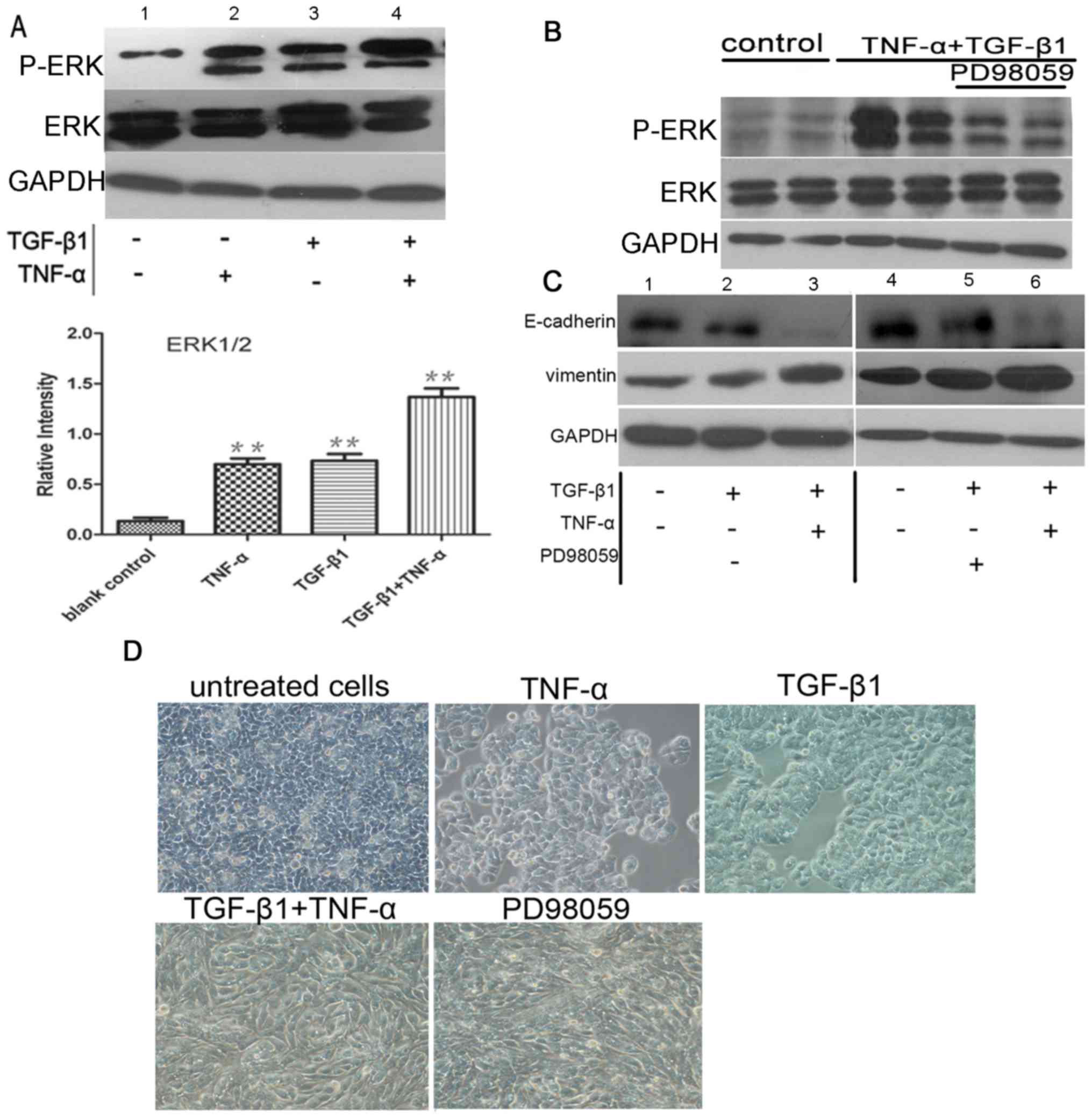
TNF-α promotes ERK activation, with no
effect on EMT
To elucidate the cytokine-stimulated signaling
pathways that induce EMT, the present study investigated the ERK1/2
signaling pathway. This signaling cascade can be stimulated by
TNF-α (13) and has previously been
demonstrated to regulate epithelial cell plasticity and EMT induced
by TGF-β1 (14,15). Western blotting for phospho-ERK1/2
demonstrated that ERK1/2 was activated in SW480 cells exposed to
TNF-α or TGF-β1. Furthermore, TGF-β1 and TNF-α treatment
demonstrated an additive effect on phospho-ERK1/2 activation
(P<0.05; n=3). A negligible effect of ERK isoform was detected
in control cells (Fig. 3A). To
identify the relative contribution of ERK1/2 signaling to EMT,
selective inhibitors were used. Pretreatment of SW480 cells with
PD98059 eliminated ERK1/2 phosphorylation induced by the
co-treatment with TGF-β1 and TNF-α (Fig.
3B). However, pre-incubation with the ERK1/2 inhibitor exerted
no significant effect on E-cadherin and vimentin expression
examined by western blotting (Fig.
3C) or morphological phenotype analysis (Fig. 3D) of the cells in which EMT was
induced by TGF-β1 alone or TGF-β1 plus TNF-α (P>0.05; n=3).
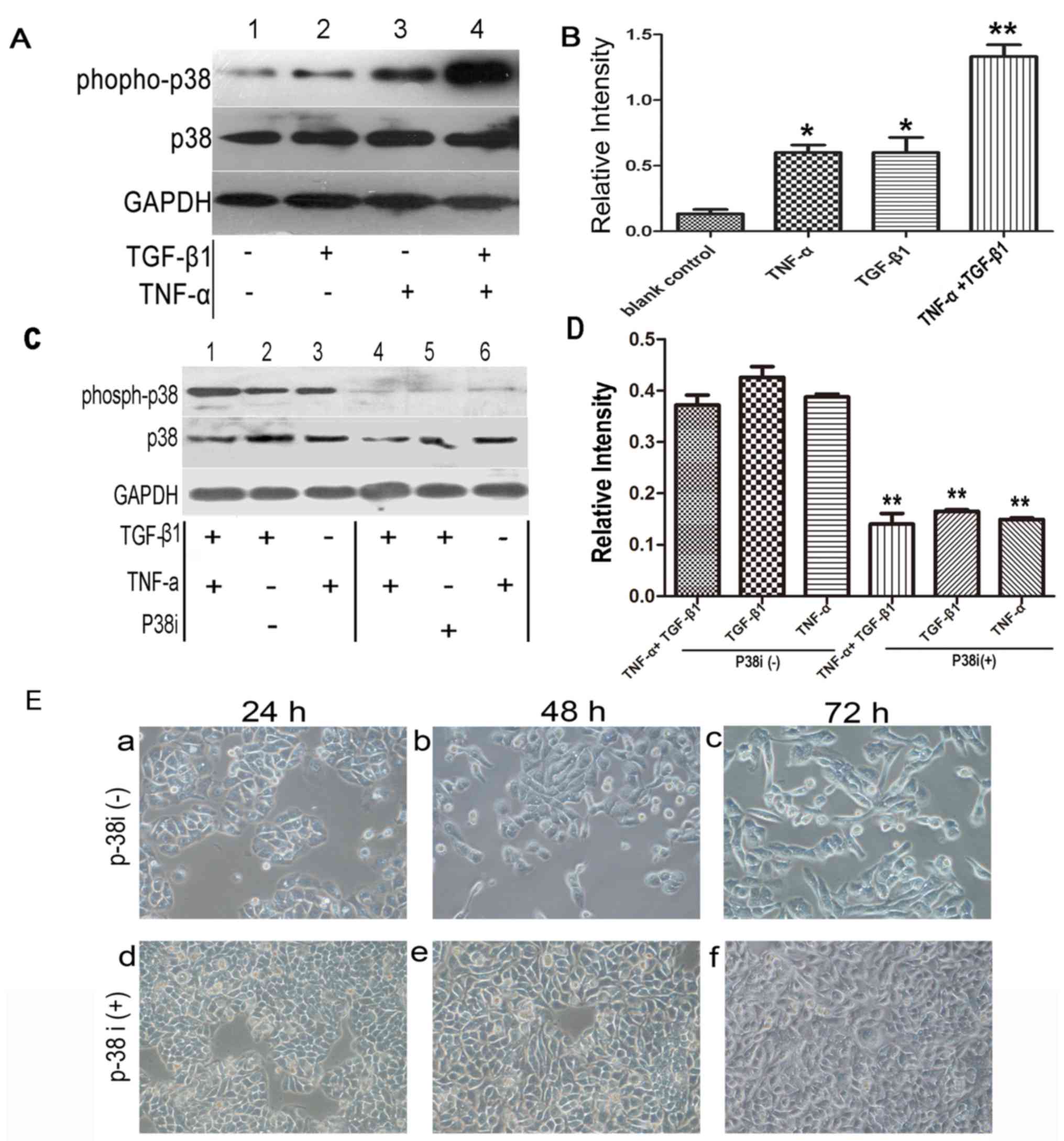
TNF-α activates p38 MAPK signaling but
does not promote EMT
Another pathway activated by TNF-α which has
previously been proposed to be important in EMT is the p38 MAPK
signaling pathway, which is known to be a convergence point for
TNF-α and TGF-β signaling (16).
Therefore, it was hypothesized that elevated p38 MAPK activity,
induced by TNF-α, may provide a mechanism by which the cytokine
promoted EMT. Western blotting with phospho-specific antibody
demonstrated that p38 MAPK activity was increased following
treatment with TGF-β1 or TNF-α alone compared with the control.
Notably, cotreatment of the cells with TGF-β1 and TNF-α exerted an
additive effect (Fig. 4A and B)
compared with TGF-β1 or TNF-α treatment alone.
To elucidate the effect of p38 MAPK activation on
EMT, a specific p38 MAPK inhibitor, SB203580, was used. As
demonstrated in Fig. 4C and D, the
expression of phospho-p38 MAPK was inhibited by SB203580 following
treatment with TNF-α/TGF-β1 for 1 h, and a significant difference
was observed between groups with or without treatment of p38 MAPK
inhibitor (P<0.01). Subsequently, the effects of SB203580 on
morphology were examined at 48 h following addition of the
cytokines (the initial discernible effects of accelerated EMT could
be observed at this time-point). At 48 h, the morphology of the
SW480 cells changed from epithelial to fibroblast-like following
treatment with TNF-α plus TGF-β1. However, treatment with SB203580
appeared to reduce the early transitional effects, completely
inhibiting cellular elongation and spreading. Over a longer time
period (72 h), the SB203580-treated cells underwent EMT, similar to
those examined at 48 h after the addition of the cytokines
(Fig. 4E).
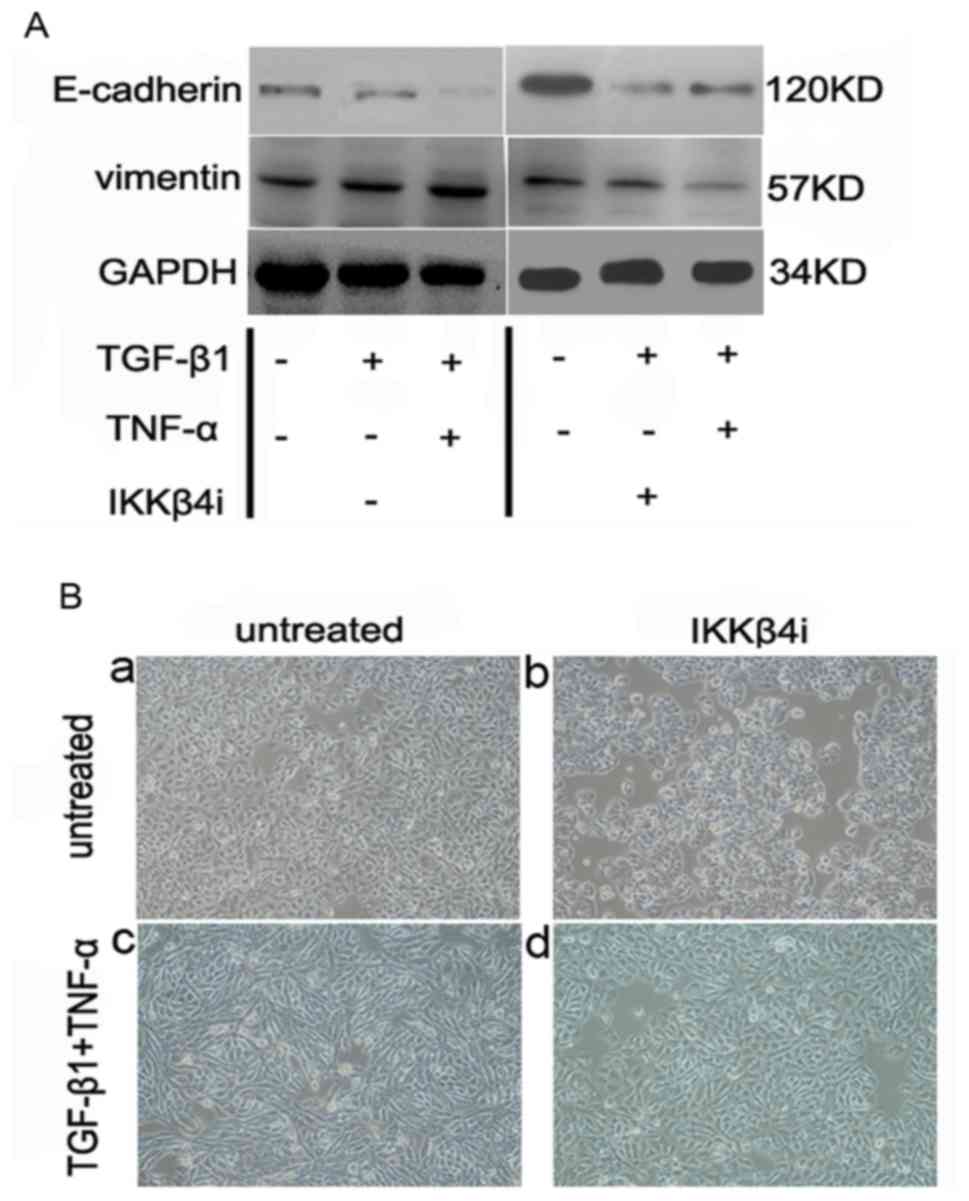
TNF-α activates NF-κB signaling to
promote EMT
As inhibition of the p38 MAPK pathway demonstrated
no effect on EMT, alternative pathways initiated by TNF-α were
investigated. An apparent candidate was the NF-κB pathway, which is
important for TNF-α-induced tumor initiation and promotion
(8).
SW480 cells were incubated with IKKβ4i and
the effect on EMT was examined. Pretreatment with IKKβ4i
significantly inhibited EMT marker expression in cells incubated
with TGF-β1 plus TNF-α compared with the control (E-cadherin,
62±15%; vimentin, 75±15%; P<0.05; n=3; Fig. 5A). Additionally, IKKβ4i
inhibited the change in cell phenotype induced by TGF-β1 and TNF-α.
It also reduced the extent of EMT stimulated by TGF-β1 and TNF-α
(Fig. 5B). This suggested that NF-κB
signaling is a critical pathway involved in TGF-β1 and
TNF-α-induced EMT.
Discussion
Despite advances in clinical treatment, recurrence
occurs in ~50% of all patients with colorectal cancer, including
~25% of patients who are theoretically curable during the early
stages of disease development (17).
A potential mechanism to explain metastatic progression is EMT, a
complex cellular reprogramming process in which epithelial cells
acquire a mesenchymal phenotype; EMT is crucial for tumor invasive
and metastatic behaviour (18), and
facilitates metastasis to distant organs (2). EMT is promoted by stimulatory signals
(19). The results of the present
study demonstrated that TNF-α or TGF-β1 alone exerted almost no
effect on SW480 cell morphology. By contrast, the addition of both
cytokines resulted in a complete mesenchymal transition, upon which
cells changed to exhibit a spindle-shaped, fibroblast-like
morphology. Previous research has indicated that TGF-β1 stimulation
alters endothelial morphology; however, this is a rare occurrence
(20). Furthermore, downregulation of
E-cadherin expression is a crucial event in EMT (21). The findings of the present study
demonstrated that combined treatment with TGF-β1 and TNF-α resulted
in the reduced expression of E-cadherin and the increased
expression of vimentin and N-cadherin.
In the present study, the migration of SW480 cells
treated with both the cytokines was increased, which is
characteristic of an invasive phenotype. By contrast, the addition
of either TNF-α or TGF-β1 alone did not significantly induce
migration. It was also demonstrated that TNF-α promoted phenotypic
transition and increased the expression of invasive markers of EMT
compared with TGF-β1 alone, which suggested that a pro-inflammatory
tumor microenvironment enriched in TNF-α may accentuate
TGF-β1-induced EMT. Notably, it is well established that levels of
pro-inflammatory cytokines are strongly associated with tumor
progression in patients with colorectal cancer and positive lymph
node metastasis (22). Furthermore,
the levels of TGF-β1 and TNF-α have previously been demonstrated to
be increased in the serum of patients with colorectal cancer, which
also indicates an association with tumor progression of colon
adenocarcinomas (6,23). Potentially, an increased level of
TNF-α, coupled with the increased levels of TGF-β1, may initiate
EMT and contribute to disease pathogenesis. The results of the
present study indicated that TNF-α and TGF-β1 are crucial for the
initiation of EMT, and suggested that an inflammatory
microenvironment rich in TNF-α is important for the regulation of
EMT in colon adenocarcinoma.
Signaling cascades involved in the effect of TNF-α-
and TGF-β1-induced EMT were investigated in the present study.
Several studies have previously demonstrated that the ERK1/2
pathway activity is required for TGF-β1-induced EMT in
non-transformed cells (24–26). To further elucidate the signaling
pathways involved in EMT, the present study examined the effect of
TGF-β1 and TNF-α on ERK signaling. However, inhibition of ERK
exerted no effect on TGF-β1- and TNF-α-induced EMT. An alternative
candidate pathway is the p38 MAPK signaling pathway, which is a
point of convergence in TNF-α and TGF-β1 signaling and is
responsive to both cytokines (27).
The results of the present study indicated that p38 MAPK activation
decreased E-cadherin levels, but did not promote mesenchymal
transition.
NF-κB is a heterodimeric transcription factor
composed of members of the Rel family of transcription factors,
including RelA (p65) (28), and is
involved in numerous biological processes. In resting cells, NF-κB
protein is retained in an inactive state in the cytoplasm,
complexed with inhibitor of κB proteins (IκBs). When cells are
exposed to cytokines, including TNF-α, interleukin-1 or epidermal
growth factor, NF-κB dissociates from IκBs, and is subsequently
translocated to the nucleus to activate the transcription of target
genes. Activated NF-κB promotes the expression of various proteins,
including proteins that facilitate metastasis in the tumor
microenvironment (29,30). In the present study, pretreatment of
SW480 cells with IKKβ4i significantly reduced EMT stimulated
by the combination of TGF-β1 and TNF-α. This suggested that
inflammation-induced EMT is closely associated with NF-κB
activation. Notably, NF-κB activation has been previously indicated
to be involved in inflammation-induced cancer or metastatic
progression (31). NF-κB has been
previously identified as an important regulator of EMT during
breast cancer progression (32),
which highlights NF-κB as a potential therapeutic target for
suppressing EMT in cancer. In addition, it is notable that the
results of the present study show IKK inhibitor enhanced E-cadherin
expression without TGF-β1 and TNF-α treatment, though the
expression of vimentin was not altered. To a certain degree, NF-κB
constitutive activation in the cell line used in the present study
may account for this phenomenon. The alternative pathway may be
involved, including WNT/β-catenin-mediated target gene expression,
which participates in NF-κB activation pathway and eventually
regulates EMT (3).
In conclusion, the present study investigated the
ability of TNF-α to promote TGF-β1-induced EMT and the potential
pathways involved in this crucial transition in SW480 colon cancer
cells. The results indicated that the effects of TNF-α and TGF-β1
on EMT are not associated with p38 activation and ERK1/2 signaling;
however, the effects may be mediated by activation of the NF-κB
pathway. These findings provide potential therapeutic targets for
the treatment of metastatic colon cancer. However, further
investigation is required.
Acknowledgements
The present study was supported by the Shan'xi
Provincial Health Department of Science and Technology Research
Projects (grant no. 201301062). The authors thank Professor Shao
Lee (Chinese University of Hong Kong, China) for critically
reviewing the manuscript in advance of submission.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
ERK
|
extracellular regulated protein
kinases
|
|
FBS
|
fetal bovine serum
|
|
IKKβ
|
inhibitor of nuclear factor-κB
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear-factor κB
|
|
SEM
|
standard error of the mean
|
|
TNF
|
tumor necrosis factor
|
|
TGF
|
transforming growth factor
|
References
|
1
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33 Suppl 1:S79–S84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao D, Vahdat LT, Wong S, Chang JC and
Mittal V: Microenvironmental regulation of epithelial-mesenchymal
transitions in cancer. Cancer Res. 72:4883–4889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwitalla S, Fingerle AA, Cammareri P,
Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ,
Moreaux G, et al: Intestinal tumorigenesis initiated by
dedifferentiation and acquisition of stem-cell-like properties.
Cell. 152:25–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung HY, Fattet L and Yang J: Molecular
pathways: Linking tumor microenvironment to epithelial-mesenchymal
transition in metastasis. Clin Cancer Res. 21:962–968. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hawinkels LJ, Verspaget HW, van der
Reijden JJ, van der Zon JM, Verheijen JH, Hommes DW, Lamers CB and
Sier CF: Active TGF-beta1 correlates with myofibroblasts and
malignancy in the colorectal adenoma-carcinoma sequence. Cancer
Sci. 100:663–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borthwick LA, McIlroy EI, Gorowiec MR,
Brodlie M, Johnson GE, Ward C, Lordan JL, Corris PA, Kirby JA and
Fisher AJ: Inflammation and epithelial to mesenchymal transition in
lung transplant recipients: Role in dysregulated epithelial wound
repair. Am J Transplant. 10:498–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bates RC and Mercurio AM: Tumor necrosis
factor-alpha stimulates the epithelial-to-mesenchymal transition of
human colonic organoids. Mol Biol Cell. 14:1790–1800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borthwick LA, Gardner A, De Soyza A, Mann
DA and Fisher AJ: Transforming Growth Factor-β1 (TGF-β1) driven
epithelial to mesenchymal transition (EMT) is accentuated by tumour
necrosis factor α (TNFα) via crosstalk between the SMAD and NF-κB
pathways. Cancer Microenviron. 5:45–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pino MS, Kikuchi H, Zeng M, Herraiz MT,
Sperduti I, Berger D, Park DY, Iafrate AJ, Zukerberg LR and Chung
DC: Epithelial to mesenchymal transition is impaired in colon
cancer cells with microsatellite instability. Gastroenterology.
138:1406–1417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kyriakis JM and Avruch J: Protein kinase
cascades activated by stress and inflammatory cytokines. Bioessays.
18:567–577. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ellenrieder V, Hendler SF, Boeck W,
Seufferlein T, Menke A, Ruhland C, Adler G and Gress TM:
Transforming growth factor beta1 treatment leads to an
epithelial-mesenchymal transdifferentiation of pancreatic cancer
cells requiring extracellular signal-regulated kinase 2 activation.
Cancer Res. 61:4222–4228. 2001.PubMed/NCBI
|
|
15
|
Zavadil J, Bitzer M, Liang D, Yang YC,
Massimi A, Kneitz S, Piek E and Bottinger EP: Genetic programs of
epithelial cell plasticity directed by transforming growth
factor-beta. Proc Natl Acad Sci USA. 98:6686–6691. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Obata T, Brown GE and Yaffe MB: MAP kinase
pathways activated by stress: The p38 MAPK pathway. Crit Care Med.
28 4 Suppl:N67–N77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Young PE, Womeldorph CM, Johnson EK,
Maykel JA, Brucher B, Stojadinovic A, Avital I, Nissan A and Steele
SR: Early detection of colorectal cancer recurrence in patients
undergoing surgery with curative intent: Current status and
challenges. J Cancer. 5:262–271. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuxe J and Karlsson MC: TGF-β-induced
epithelial-mesenchymal transition: A link between cancer and
inflammation. Semin Cancer Biol. 22:455–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown KA, Aakre ME, Gorska AE, Price JO,
Eltom SE, Pietenpol JA and Moses HL: Induction by transforming
growth factor-beta1 of epithelial to mesenchymal transition is a
rare event in vitro. Breast Cancer Res. 6:R215–R231. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamadien MA, Khan Z, Vaali-Mohammed MA,
Zubaidi A, Al-Khayal K, McKerrow J and Al-Obeed O: Polymorphisms of
tumor necrosis factor alpha in Middle Eastern population with
colorectal cancer. Tumour Biol. 37:5529–5537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al Obeed OA, Alkhayal KA, Al Sheikh A,
Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH and Abdulla
MH: Increased expression of tumor necrosis factor-α is associated
with advanced colorectal cancer stages. World J Gastroenterol.
20:18390–18396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buonato JM and Lazzara MJ: ERK1/2 blockade
prevents epithelial-mesenchymal transition in lung cancer cells and
promotes their sensitivity to EGFR inhibition. Cancer Res.
74:309–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Xiao W, Wang W, Luo L, Ye S and
Liu Y: The complex interplay between ERK1/2, TGFβ/Smad, and
Jagged/Notch signaling pathways in the regulation of
epithelial-mesenchymal transition in retinal pigment epithelium
cells. PLoS One. 9:e963652014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-beta1-induced EMT in vitro. Neoplasia. 6:603–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Itoigawa Y, Harada N, Harada S, Katsura Y,
Makino F, Ito J, Nurwidya F, Kato M, Takahashi F, Atsuta R and
Takahashi K: TWEAK enhances TGF-β-induced epithelial-mesenchymal
transition in human bronchial epithelial cells. Respir Res.
16:482015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong H, May MJ, Jimi E and Ghosh S: The
phosphorylation status of nuclear NF-kappa B determines its
association with CBP/p300 or HDAC-1. Mol Cell. 9:625–636. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bradford JW and Baldwin AS: IKK/nuclear
factor-kappaB and oncogenesis: Roles in tumor-initiating cells and
in the tumor microenvironment. Adv Cancer Res. 121:125–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Epanchintsev A, Shyamsunder P, Verma RS
and Lyakhovich A: IL-6, IL-8, MMP-2, MMP-9 are overexpressed in
Fanconi anemia cells through a NF-κB/TNF-α dependent mechanism. Mol
Carcinog. 54:1686–1699. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar : PubMed/NCBI
|