Introduction
Osteosarcoma (OS) is one of the most common
malignant bone tumors, which occurs frequently in children and
adolescents (1). Furthermore, OS has
a strong tendency to metastasize (1).
Despite intensive efforts to identify novel treatment strategies,
survival rates have not improved in the past two decades (2). There has been considerable research on
the molecular mechanism underlying OS, and a growing body of
evidence has revealed that the regulation of oncogenes and tumor
suppressor genes is vital in the development and progression of OS
(3–5).
Therefore, there is important clinical value in identifying the
molecular targets and agents to improve the diagnosis and prognosis
of OS.
The tumor protein D52 (TACC3) gene was first
identified ~20 years ago (6). TACC3,
located at the chromosome 4p16.3, and stabilizes and organizes the
mitotic spindle to allow for proper chromosomal segregation
(7). There are three TACC proteins
that are identified in humans: TACC1, TACC2 and TACC3. TACC3,
originally isolated from the 4p16.3 region, is an Aurora and
integrin-linked kinase target, strongly concentrated at centrosomes
throughout the cell cycle and identified as a member of the
centrosomal protein family that can regulate the formation of
microtubules (8). Multiple studies
have revealed that TACC3 is a multifunctional protein involved in
various biological functions, including cell survival,
proliferation, migration, invasion, DNA repair, exocytosis and
vesicle trafficking (9,10). Previous studies have reported that
TACC3 is overexpressed in several types of cancer, including,
esophageal squamous cell carcinoma, hepatocellular carcinoma, lung,
pancreatic, cervical and gastric cancer (11,12).
However, to the best of our knowledge, this is the first study to
assess the expression of TACC3 in osteosarcoma and to investigate
the molecular mechanism underlying the TACC3-mediated regulation of
tumor progression.
The nuclear factor-κB (NF-κB) signaling pathway is
involved in immune and inflammatory responses, including,
tumorigenic processes. The deregulated activation of NF-κB is
associated with cancer (13,14). Previous research studies have reported
that NF-κB affects a number of tumor malignant behaviors, including
proliferation, invasion and metastasis by regulating the expression
of several genes relevant to tumorigenesis. For example, NF-κB
upregulates the expression of genes (cyclin D1 and c-Myc), which
are involved in anti-apoptotic processes and the regulation of cell
cycle (15,16). In osteosarcoma, previous research has
confirmed that the excessive activation of the NF-κB signaling
pathway promotes the proliferation and metastasis of tumor cells
and increases resistance to chemotherapy (17). Tang et al (18) also reported an original mechanism for
the involvement of the NF-κB signaling pathway in glycogen synthase
kinase-3β-mediated regulation of cell survival in osteosarcoma.
Therefore, it was proposed that the NF-κB pathway may be involved
in the proliferation and metastasis process of cancer.
The purpose of the present study was to investigate
the regulatory role of TACC3 in the proliferation, migration and
invasion of osteosarcoma cells, including, the potential molecular
mechanism by which TACC3 exerts its effects.
Materials and methods
Cell culture
The human osteosarcoma cell lines U2-OS, MG63 and
normal human osteoblasts (NHOst) were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). The 143B and
SAOS cells were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). All cells were
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) medium supplemented with 10% fetal bovine serum
(FBS; Gibco, Thermo Fisher Scientific, Inc.) in a humidified
incubator with 5% CO2 at 37°C.
Patient information and tissue
specimens
The present study was approved by the Institutional
Ethics Committee and Review Board of the Daqing Longnan Hospital
(Daqing, China). All study participants or their legal guardian,
provided written informed consent prior to enrollment. A total of
36 osteosarcoma specimens and matched adjacent noncancerous
osteosarcoma tissues were obtained who had undergone resection for
osteosarcoma between 2013 and 2015. Their median age was 18 years
(range, 13–38 years) and the male:female ratio was 23:13. Following
resection, matched fresh tissues were immersed immediately in
RNAlater® (Ambion, Thermo Fisher Scientific, Inc.), kept
overnight at 4°C and then stored at −80°C until RNA isolation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cells and tissues were extracted
using the TRIzol solution (Invitrogen; Thermo Fisher Scientific,
Inc.) according to manufacturer's instructions. RNA concentration
and purity were determined by absorbance at 260 nm using a NanoDrop
ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). RT was performed on 2 µg
total RNA sample using M-MLV reverse transcriptase kit (Promega
Corporation, Madison, WI, USA) at 37°C for 60 min according to the
manufacturer's protocol. Newly synthesized cDNA was amplified by
RT-qPCR to enable the expression levels of TACC3 to be detected.
The primers used were as follows: TACC3 forward,
5′-CCTCTTCAAGCGTTTTGAGAAAC-3′ and reverse,
5′-GCCCTCCTGGGTGATCCTT-3′; GAPDH, forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′, and reverse,
5′-CCCAATACGACCAAATCCGTT-3′. qPCR amplification was performed in an
ABI 7900HT Real-time PCR system (Thermo Fisher Scientific, Inc.).
The PCR was conducted in a final volume of 15 µl, consisting of 7.5
µl of 2X SYBR Green Master Mix (Invitrogen; Thermo Fisher
Scientific, Inc.), 2 µl of each primer (1.5 pmol/µl), 0.5 µl sample
cDNA and 5 µl water. The thermocycling conditions were as follows:
One cycle of 95°C for 10 min, followed by 95°C for 30 sec and 60°C
for 60 sec for 45 cycles. The relative expression levels of TACC3
were normalized to that of the internal control gene, GAPDH. The
data were analyzed using the comparative threshold cycle
(2−ΔΔCq) method (19).
Western blot analysis
Osteosarcoma cell lines were lysed in RIPA lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China). The
lysates were harvested by centrifugation (12,000 × g for 20 min at
4°C. The protein samples (20 µg) were resolved in 12% sodium
dodecyl sulfate polyacrylamide gel by electrophoresis and
transferred to a polyvinylidene difluoride (PVDF) membrane. After
blocking non-specific binding sites for 60 min with 8% non-fat milk
in TBST, the membranes were incubated with primary antibodies,
rabbit polyclonal anti-TACC3 (1:1,000; Abcam, Cambridge, UK; cat.
no. ab134154), anti-p65 (1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA; cat. no. 8242) anti-matrix metalloproteinase-9
(MMP9; 1:1,000; Cell Signaling Technology, Inc.; cat. no. 13667),
anti-cellular FLICE-like inhibitory protein (c-FLIP; 1:500; Cell
Signaling Technology, Inc.; cat. no. 56343) or GAPDH (1:10,000;
ProteinTech Group, Inc., Chicago, IL, USA; cat. no. 10494-1-AP)
overnight at 4°C. The membranes were washed four times with
TRIS-buffered saline with Tween-20 for 10 min. After washing, the
membranes were probed with horseradish peroxidase-conjugated goat
anti-rabbit antibody (1:5,000; EMD/Merck KGaA, Darmstadt, Germany;
cat. no. AP307P) at room temperature for 1 h, and an enhanced
chemiluminescence detection kit (Cell Signaling Technology, Inc.),
was used to visualize the proteins. Band intensity was analyzed
using the Quantity One software 4.6.2 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Transfection of osteosarcoma
cells
For TACC3 functional analysis, MG63 and U2-OS cells
were transfected with TACC3 siRNA or pcDNA3.1 TACC3 plasmid using
Lipofectamine® iMAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
MG63 and U2-OS (2×105) cells were seeded into 6-well
plates, incubated for 24 h and then transfected with 12.5 nM RNA
duplex and 5 µl Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
cells were harvested for further experiments after 48 or 72 h.
siRNA oligo-ribonucleotides were purchased from Guangzhou RiboBio
Co., Ltd., (Guangzhou, China). The effective siRNA sequences were
as follows: TACC3-siRNA-(5′-GCATGCACGGTGCAAATGA-3′). In addition,
the cDNA of the human TACC3 gene, a fragment encoding the
TACC3-sequence plus 1,439 bp at both 5′- and 3′-flanking regions
was amplified with the primers:
5′-GAGGATCCCCGGGTACCGGTCGCCACCATGAGTCTGCAGGTCTTAAACGAC-3′ (forward)
and 5′-TCCTTGTAGTCCATACCGATCTTCTCCATCTTGGAGATGAG-3′ (reverse) by
PCR from human genomic DNA and then cloned into the
AgeI/NheI sites of GV358.
Cell proliferation assay
MTS assay was used to analyze the proliferation of
TACC3 siRNA-transfected and TACC3 lentivirus-transfected MG63 and
U2-OS cells. The cells were cultured in 96-well plates at 2,500
cells/well. At each time-point (0, 24, 48, 72, 96 and 120 h), the
cells incubated with 20 µl MTS (5 mg/ml; Sigma-Aldrich, Merck KGaA)
for 4 h in 5% CO2 at 37°C. Finally, the A490 value of
each sample was determined using a microplate reader. Statistical
analyses were carried out using a two-tailed unpaired Student's
t-test.
Cell migration and invasion
assays
Cell migration and invasion assays were performed
using polycarbonate filters (pore size, 8-mm) in 24-well Transwell
chambers (Corning Inc., Corning, NY, USA). The cells were seeded at
5×104 in 200 µl serum-free medium in the upper chamber,
and 500 µl RPMI 1640 medium containing 5% FBS was placed in the
lower chamber. Following incubation for 24 h at 37°C, the cells
remaining in the upper chamber were removed with cotton swabs. The
cells that migrated to the lower surface of the membrane were fixed
with methanol and stained with crystal violet at room temperature
for 15 min. The cells in ≥5 random fields of view at ×100
magnification were counted (CKX41; Olympus Corporation, Tokyo,
Japan). Each experiment was performed in triplicate. Statistical
analyses were performed using the two-tailed unpaired Student's
t-test.
Statistical analyses
Statistical analyses were conducted using SPSS
(version 18.0; SPSS, Inc., Chicago, IL, USA). The comparisons
between groups were analyzed using two-tailed unpaired Student
t-test, unless otherwise specified. The data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
TACC3 is upregulated in osteosarcoma
tissues
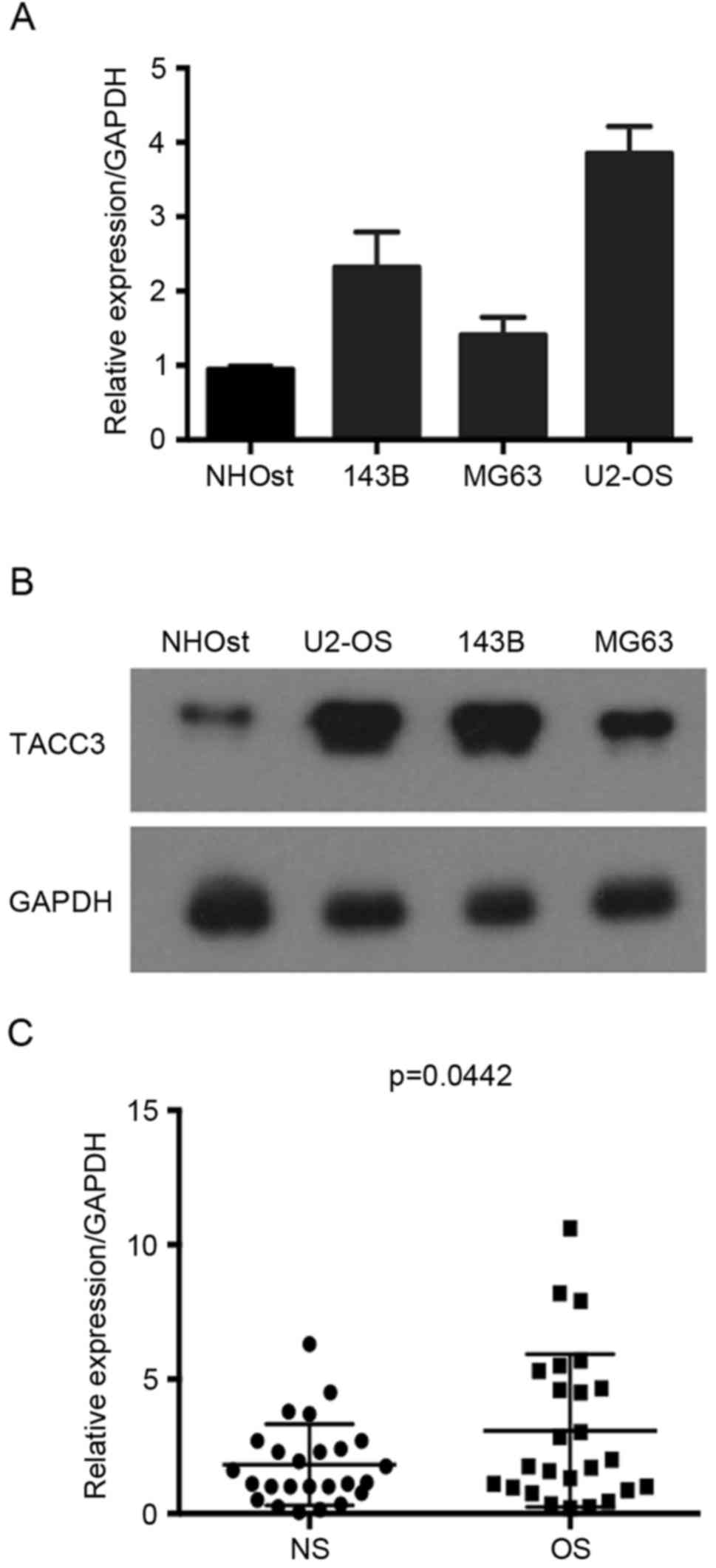
In the present study, the levels of TACC3 in
osteosarcoma tissues and osteosarcoma cell lines U2-OS, MG63, 143B
and SAOS, were examined. RT-qPCR and western blot analysis
indicated that TACC3 mRNA and protein expression levels were
markedly higher in the osteosarcoma cell lines compared with normal
NHOst cell line, particularly in U2-OS cells (Fig. 1A and B). In addition, the mRNA
expression level of TACC3 was upregulated in osteosarcoma tissues
compared with the normal adjacent tissues (Fig. 1C). The present data suggested that
increased TACC3 expression is associated with osteosarcoma.
TACC3 promotes the proliferation of
osteosarcoma cells in vitro
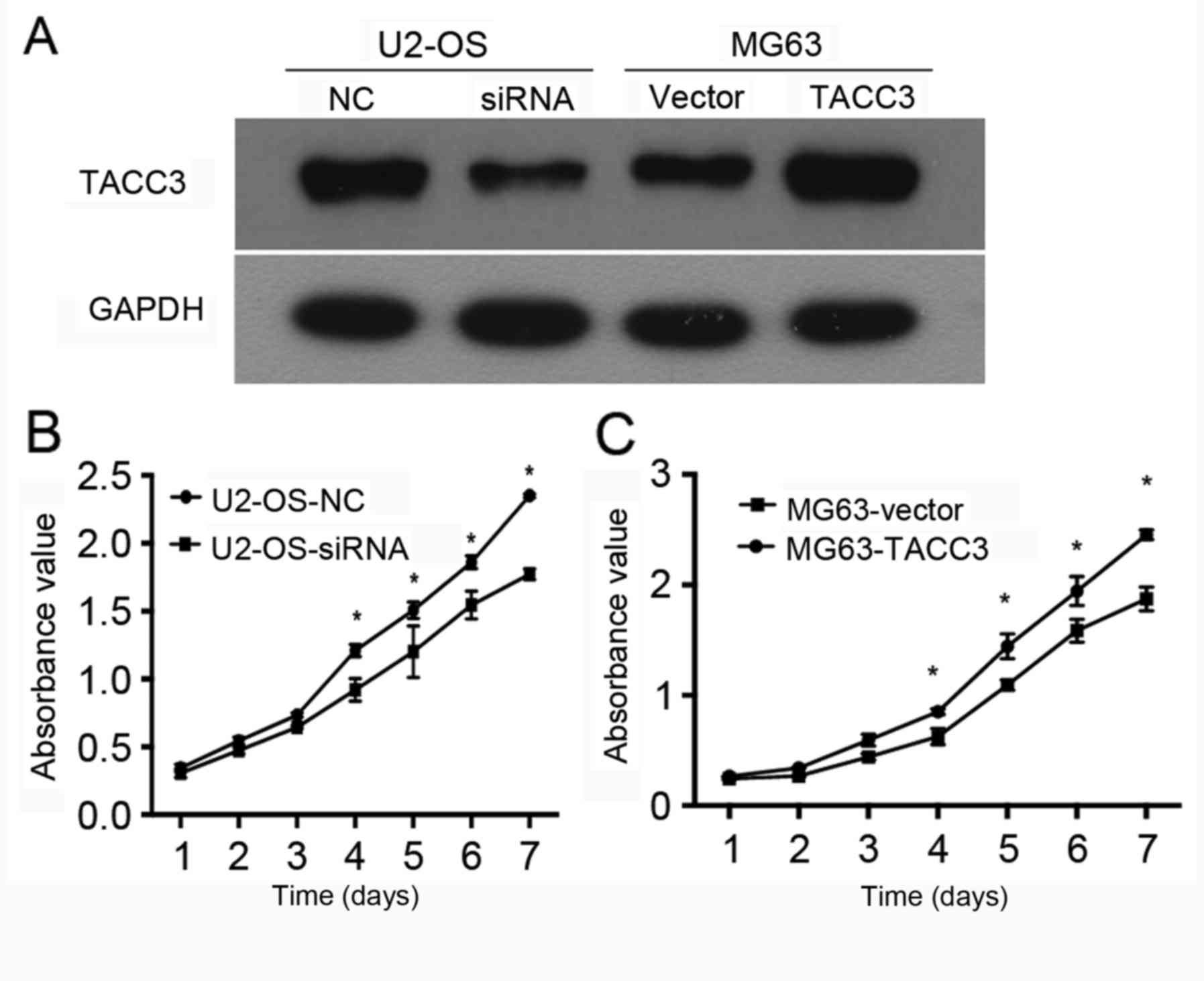
In order to examine the function of TACC3 in the
progression of osteosarcoma in vitro, U2-OS cells were
transfected with TACC3 siRNA, and MG63 cells were infected with
TACC3 lentivirus to silence and overexpress TACC3, respectively.
Western blot analysis was used to verify knockdown and
overexpression efficiency in U2-OS cells and MG63 cells. The
results indicated that the expression levels of TACC3 were
upregulated in MG63 cells that were transfected with TACC3
lentivirus, and reduced in U2-OS cells that were transfected with
TACC3 siRNA compared with the control cells (Fig. 2A). To examine the effect of TACC3
expression on tumorigenicity, the growth of MG63 cells that were
infected with TACC3 lentivirus or U2-OS cells transfected with
TACC3 siRNA was assessed. The results demonstrated a significant
decrease in the growth rate of TACC3 siRNA-transfected cells
compared with the control cells (P<0.05; Fig. 2B). The overexpression of TACC3 in MG63
cell line significantly promoted the cell proliferation rate
compared with the control (P<0.05; Fig. 2C). Therefore, these findings indicated
that TACC3 may serve a carcinogenic role in progression of
osteosarcoma.
TACC3 promotes the migration and
invasion of osteosarcoma cells in vitro
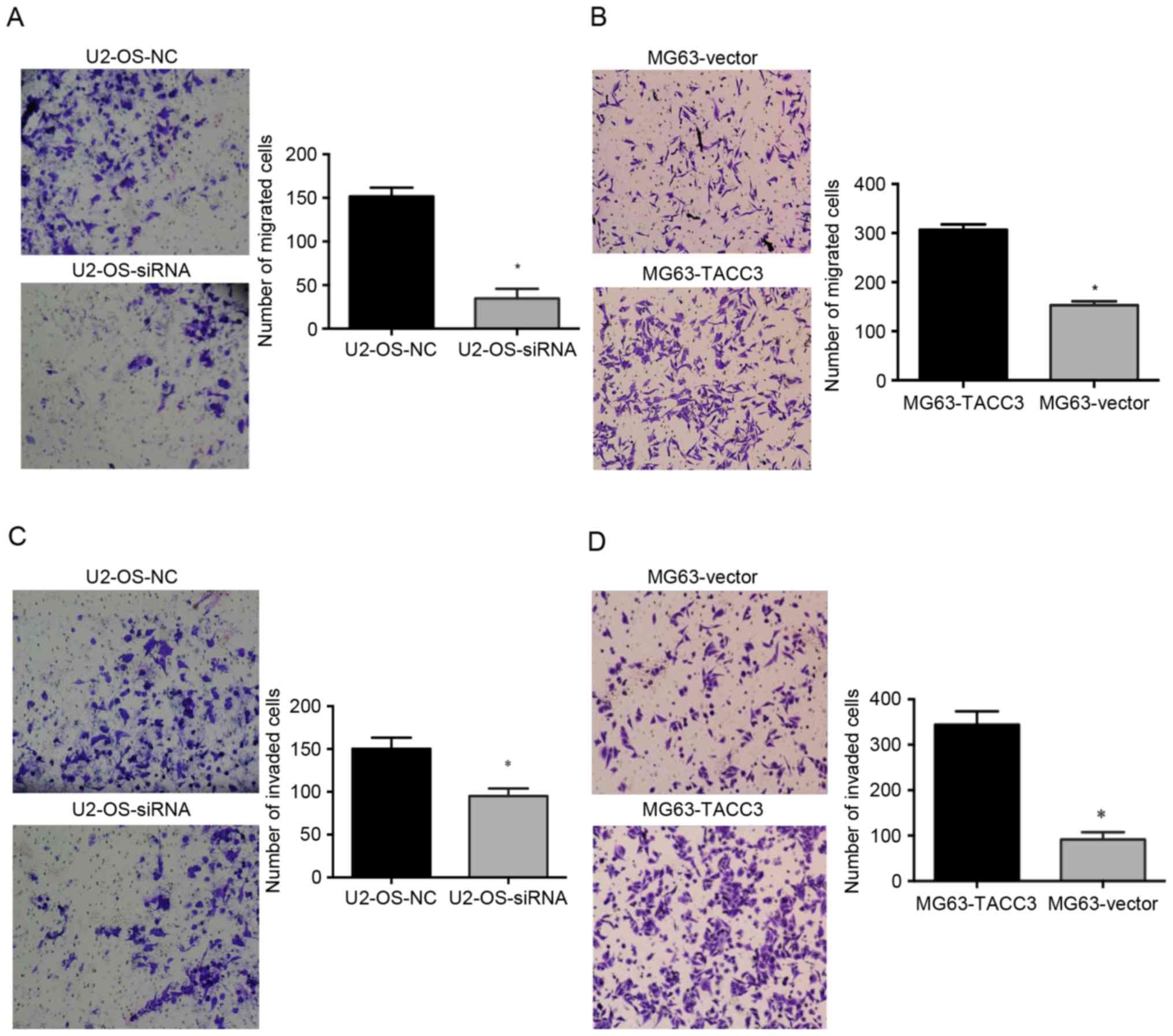
To determine the role of TACC3 in cell migration and
invasion, Transwell assays of MG63 and U2-OS cells were performed
in vitro. The silencing of TACC3 expression significantly
reduced the migration of U2-OS cells compared with the control
cells (P<0.05; Fig. 3A). Compared
with the control cells, the overexpression of TACC3 induced an
increase in the migration of MG63 osteosarcoma cell line compared
with control cells (P<0.05; Fig.
3B). Consistent with the results of the migration assay, the
invasion assay demonstrated that the knockdown of TACC3,
significantly inhibited cell invasion (P<0.05; Fig. 3C). The cell invasion assay also
indicated that cell invasion was significantly increased when TACC3
was overexpressed (P<0.05; Fig.
3D).
TACC3 regulates the NF-κB signaling
pathway in osteosarcoma cells
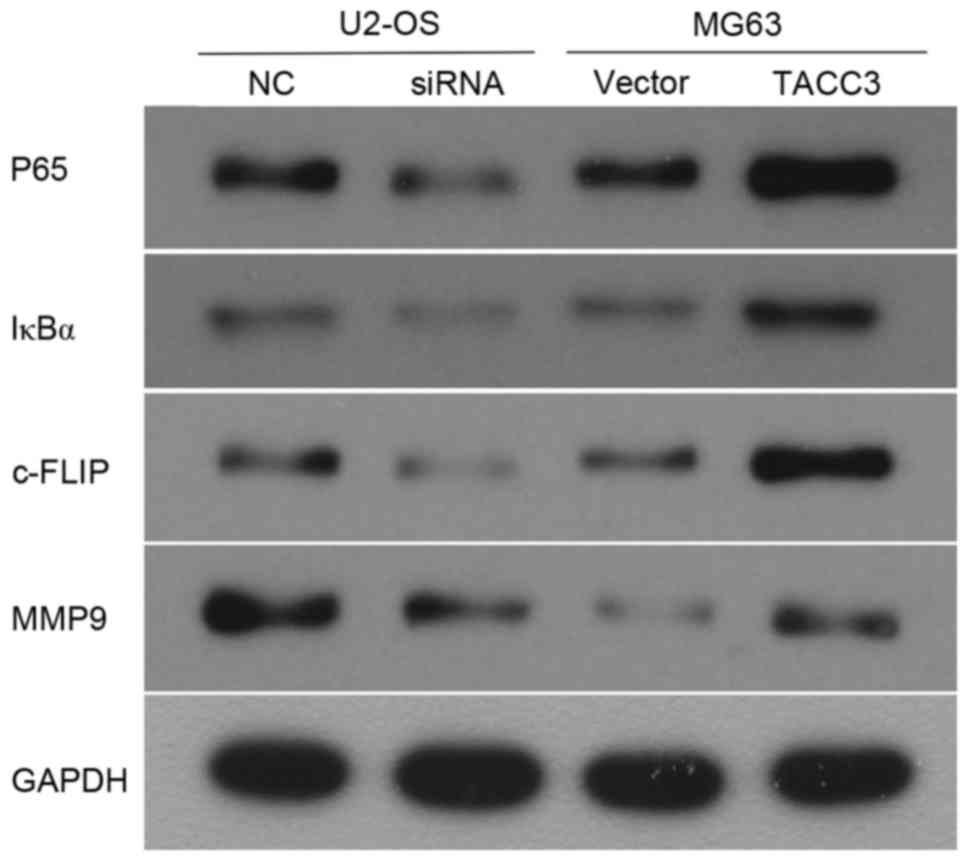
It has been reported that the activation of NF-κB
promotes the development of osteosarcoma (18), which suggests that the effect of TACC3
on osteosarcoma may be involved with the NF-κB signaling pathway.
Therefore, a further aim of the present study was to investigate
whether the upregulation of TACC3 promotes metastasis in
osteosarcoma through activation of the NF-κB pathway. The western
blot analysis revealed that the overexpression of TACC3 in MG63
cells increased the protein expression levels of IκBα and p65
(Fig. 4). Consequently, the target
genes of NF-κB, including, MMP9 and c-FLIP were increased following
TACC3 overexpression. By contrast, the opposing expression patterns
of these aforementioned genes were observed in TACC3-knocked down
cells (Fig. 4).
Discussion
Previous studies have demonstrated that the
transforming acidic coiled-coil protein (TACC) family members,
notably TACC3, serve a critical function in tumor development and
progression (20). However, the
number of studies on the role of TACC3 in osteosarcoma is
limited.
Therefore, there is an urgent requirement to
identify novel molecular targets with therapeutic potential for the
treatment of osteosarcoma. In the present study, it was confirmed
that TACC3 is upregulated in osteosarcoma tissues and cell lines
compared with normal NHOst cell line and adjacent noncancerous
osteosarcoma tissues. The results from the present in vitro
studies revealed that TACC3 promoted the proliferation, migration
and invasion of osteosarcoma cells by activating the NF-κB
signaling pathway.
Different oncogene expression profiles in
osteosarcoma have been identified previously. TACC3 have involved
in the tumorigenesis of different cancer types (21,22). The
overexpression of TACC3 has been verified to be associated with a
poor overall survival (OS) and poor recurrence-free survival of
patients with non-small cell lung cancer (23). Nahm et al (24) demonstrated that high protein levels of
TACC3 were associated with histological differentiation, tumor
size, microvascular invasion and pathological tumor-node metastasis
stage in 188 hepatocellular carcinoma (HCC) tissue specimens. In
addition, the authors demonstrated that the silencing of TACC3 by
siRNA decreased the invasive ability of HCC cells, indicating that
TACC3 may be a major contributory factor in tumor development.
TACC3 is involved with several types of human
cancer, including osteosarcoma. However, the exact role of TACC3 in
the regulation of proliferation and migration of osteosarcoma cells
has not been clarified. In the present study, it was revealed that
the knockdown of TACC3 significantly suppressed the proliferation
and migration of osteosarcoma cells, and the overexpression of
TACC3 notably enhanced the proliferation and migration of
osteosarcoma cells. Consequently, the results from the present
study suggest that TACC3 may serve an oncogenic role in the
regulation of osteosarcoma cells.
It has previously been reported that TACC3 is able
to promote cell proliferation by regulating several signaling
pathways. For example, Zhou et al (12) reported that TACC3 expression is
frequently increased in HCC tumor tissues compared with matched
non-cancerous samples. Furthermore, the knockdown of TACC3
suppressed tumor stem cell-like characteristics through the
Wnt/β-catenin and phosphatidylinositide 3-kinase/protein kinase B
(PI3K/AKT) signaling pathways. Huang et al (25) reported that TACC3 is upregulated in
human esophageal squamous cell carcinoma (ESCC) and promotes the
proliferation, colony formation and migration of esophageal
squamous cell carcinoma cells.
TACC3 may also act as a potential oncogene that
promotes cell proliferation by epidermal growth factor
(EGF)-mediated epithelial-mesenchymal transition (EMT) in cervical
cancer cells (26). In accordance
with these previous results, it was verified in the present study
that TACC3 regulates the proliferation, migration and invasion of
osteosarcoma cells. Therefore, targeting TACC3 may be an attractive
strategy for the treatment of osteosarcoma. However, the specific
regulatory mechanism of how TACC3 exerts its function remains
unclear.
There were several limitations to the present study.
Firstly, the scope of the experiments was limited to proliferation,
migration and invasion. Therefore, future aims are to investigate
the role of TACC3 with respect to the cell cycle, apoptosis and
colony formation of osteosarcoma cell lines. Secondly, while it was
demonstrated that TACC3 is able to modulate the proliferation and
migration of osteosarcoma cells via the NF-κB signaling pathway,
the effect of a NF-κB pathway inhibitor on TACC3 was not assessed.
Finally, the present study focused solely on the association
between TACC3 and prognosis, and potential application in clinical
practice were not investigated.
In conclusion, this present study demonstrates that
TACC3 may act as a tumor oncogene in osteosarcoma. TACC3 is able to
promote the proliferation, migration and invasion of osteosarcoma
cells via the NF-κB signaling pathway. Therefore, TACC3 may be a
potential therapeutic target for the treatment of osteosarcoma in
the future.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TACC
|
transforming acidic coiled-coil
protein
|
|
OS
|
osteosarcoma
|
|
OS
|
overall survival
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al: Neoadjuvant chemotherapy with high-dose Ifosfamide,
high-dose methotrexate, cisplatin, and doxorubicin for patients
with localized osteosarcoma of the extremity: A joint study by the
Italian and Scandinavian Sarcoma Groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu J, Song G, Tang Q, Zou C, Han F, Zhao
Z, Yong B, Yin J, Xu H, Xie X, et al: IRX1 hypomethylation promotes
osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J
Clin Invest. 125:1839–1856. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou CH, Lin FL, Hou SM and Liu JF: Cyr61
promotes epithelial-mesenchymal transition and tumor metastasis of
osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol
Cancer. 13:2362014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ
and Tang CH: CTGF increases matrix metalloproteinases expression
and subsequently promotes tumor metastasis in human osteosarcoma
through down-regulating miR-519d. Oncotarget. 5:3800–3812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Still IH, Vince P and Cowell JK: The third
member of the transforming acidic coiled coil-containing gene
family, TACC3, maps in 4p16, close to translocation breakpoints in
multiple myeloma, and is upregulated in various cancer cell lines.
Genomics. 58:165–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiemeney LA, Sulem P, Besenbacher S,
Vermeulen SH, Sigurdsson A, Thorleifsson G, Gudbjartsson DF, Stacey
SN, Gudmundsson J, Zanon C, et al: A sequence variant at 4p16.3
confers susceptibility to urinary bladder cancer. Nat Genet.
42:415–419. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hood FE and Royle SJ: Pulling it together:
The mitotic function of TACC3. Bioarchitecture. 1:105–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sadek CM, Pelto-Huikko M, Tujague M,
Steffensen KR, Wennerholm M and Gustafsson JA: TACC3 expression is
tightly regulated during early differentiation. Gene Expr Patterns.
3:203–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao R, Natsume Y and Noda T: TACC3 is
required for the proper mitosis of sclerotome mesenchymal cells
during formation of the axial skeleton. Cancer Sci. 98:555–562.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capelletti M, Dodge ME, Ercan D, Hammerman
PS, Park SI, Kim J, Sasaki H, Jablons DM, Lipson D, Young L, et al:
Identification of recurrent FGFR3-TACC3 fusion oncogenes from lung
adenocarcinoma. Clin Cancer Res. 20:6551–6558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou DS, Wang HB, Zhou ZG, Zhang YJ, Zhong
Q, Xu L, Huang YH, Yeung SC, Chen MS and Zeng MS: TACC3 promotes
stemness and is a potential therapeutic target in hepatocellular
carcinoma. Oncotarget. 6:24163–24177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D,
Wu Y, Yin J, Xie X, Shen J, et al: Downregulation of MCT1 inhibits
tumor growth, metastasis and enhances chemotherapeutic efficacy in
osteosarcoma through regulation of the NF-κB pathway. Cancer Lett.
342:150–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schon S, Flierman I, Ofner A, Stahringer
A, Holdt LM, Kolligs FT and Herbst A: β-catenin regulates NF-κB
activity via TNFRSF19 in colorectal cancer cells. Int J Cancer.
135:1800–1811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bera A, Ghosh-Choudhury N, Dey N, Das F,
Kasinath BS, Abboud HE and Choudhury GG: NFκB-mediated cyclin D1
expression by microRNA-21 influences renal cancer cell
proliferation. Cell Signal. 25:2575–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao N, Lu Z, Zhang Y, Wang M, Li W, Hu Z,
Wang S and Lin Y: Interleukin-8 upregulates integrin 3 expression
and promotes estrogen receptor-negative breast cancer cell invasion
by activating the PI3K/Akt/NF-κB pathway. Cancer Lett. 364:165–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Korber MI, Staribacher A, Ratzenbock I,
Steger G and Mader RM: NFκB-Associated pathways in progression of
chemoresistance to 5-fluorouracil in an in vitro model of colonic
carcinoma. Anticancer Res. 36:1631–1639. 2016.PubMed/NCBI
|
|
18
|
Tang QL, Xie XB, Wang J, Chen Q, Han AJ,
Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al: Glycogen synthase
kinase-3, NF-κB signaling, and tumorigenesis of human osteosarcoma.
J Natl Cancer Inst. 104:749–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ha GH, Park JS and Breuer EK: TACC3
promotes epithelial-mesenchymal transition (EMT) through the
activation of PI3K/Akt and ERK signaling pathways. Cancer Lett.
332:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lauffart B, Vaughan MM, Eddy R, Chervinsky
D, DiCioccio RA, Black JD and Still IH: Aberrations of TACC1 and
TACC3 are associated with ovarian cancer. BMC Women's Health.
5:82005. View Article : Google Scholar
|
|
22
|
Yun M, Rong J, Lin ZR, He YL, Zhang JX,
Peng ZW, Tang LQ, Zeng MS, Zhong Q and Ye S: High expression of
transforming acidic coiled coil-containing protein 3 strongly
correlates with aggressive characteristics and poor prognosis of
gastric cancer. Oncol Rep. 34:1397–1405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang F, Kuang B, Que Y, Lin Z, Yuan L,
Xiao W, Peng R and Zhang X and Zhang X: The clinical significance
of transforming acidic coiled-coil protein 3 expression in
non-small cell lung cancer. Oncol Rep. 35:436–446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nahm JH, Kim H, Lee H, Cho JY, Choi YR,
Yoon YS, Han HS and Park YN: Transforming acidic
coiled-coil-containing protein 3 (TACC3) overexpression in
hepatocellular carcinomas is associated with ‘stemness’ and
epithelial-mesenchymal transition-related marker expression and a
poor prognosis. Tumour Biol. 37:393–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang ZL, Lin ZR, Xiao YR, Cao X, Zhu LC,
Zeng MS, Zhong Q and Wen ZS: High expression of TACC3 in esophageal
squamous cell carcinoma correlates with poor prognosis. Oncotarget.
6:6850–6861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ha GH, Kim JL and Breuer EK: TACC3 is
essential for EGF-mediated EMT in cervical cancer. PLoS One.
8:e703532013. View Article : Google Scholar : PubMed/NCBI
|