Introduction
Cartilage is an important connective tissue that
exists in the muscle-skeleton system, particularly in joints, rib,
ear, nose, bronchial tubes and intervertebral discs (1). It's generally believed that cartilage
contains only one cell type called chondrocyte that produces all
cartilage extracellular matrix consisting of Type II collagen in
articular cartilage or mixture of type I and type II collagen in
fibrocartilage (2). Since cartilage
is avascularized, the metabolic activity of chondrocytes is low,
compared with other connective tissues (3). Nutrition of chondrocytes is supplied by
diffusion, by the pumping action generated by compression of the
articular cartilage during extension or flexion of joints (3). Furthermore, chondrocytes are embedded in
spaces called lacunae, which keeps them from migrating to damaged
areas. Therefore, the self-repair ability of damaged cartilage is
limited (4). Over the last decades,
stem cell based technology has been proposed for cartilage repair
joint injury (reviewed in 5). Especially when bone marrow
mesenchymal stem cells (BMSCs) were demonstrated to be able to
differentiate into chondrocytes, these were immediately considered
as the ideal source for engineering cartilage tissue (5).
However, novel findings uncovered other properties
of BMSCs rather than differentiation into specific cell types. One
of the most important is the trophic role of BMSCs in tissue repair
(4,5).
As first introduced, the terminology ‘trophic’ initially referred
to non-neurotransmitter bioactive molecules produced by nerve
terminals (6). Specifically,
‘trophic’ was first used to describe the process in which BMSCs
secrete factors stimulating neighboring cells to release
functionally bioactive molecules (7).
This term also relates to the effect of the factors produced by
BMSC on viability, proliferation, and matrix production of the
neighboring cells. This supportive effect of BMSCs on other cells
types significantly broadened the application of BMSCs in
regenerative medicine. While traditionally it was believed that
BMSCs mainly repair damaged tissue by differentiating into specific
cell types and replacing lost cells (8), nowadays the trophic role of the BMSCs in
tissue repair is considered more important than before (9).
The use of BMSCs to partially replace chondrocytes
may reduce the number of chondrocytes necessary for autologous
chondrocytes transplantation (ACT). In previously published papers,
pellet co-culture models of chondrocytes and bone marrow derived
BMSCs were employed to study the beneficial effects of co-culture
on cartilage matrix formation (10–12). In
these pellet co-cultures, it had been demonstrated that cartilage
matrix was mainly produced by chondrocytes but not BMSCs. These
studies revealed a new mechanism of cross-talk between cells in a
co-culture model of BMSCs and chondrocytes. Studies indicated that
the beneficial effects on cartilage matrix formation in co-culture
pellets were due to trophic effects of BMSCs which stimulated
chondrocyte proliferation and cartilage matrix deposition. Studies
also demonstrated that these trophic effects are independent of
culture conditions and BMSCs sources (11). Notably, it's been identified in all
these studies that the ratio of BMSCs decreased dramatically due to
BMSCs death and proliferation of chondrocytes in co-culture.
However, none of the published studies actually tracked the fate of
BMSCs or chondrocytes following co-implantation in osteo-chondral
defect models (6,7,9,10).
In the present study, the destiny of BMSCs and
chondrocytes in co-culture pellets and in co-implantation of an
osteo-chondral defect model was investigated. Data revealed that
BMSCs increased the viability of chondrocytes in co-implantation in
an osteo-chondral defect model.
Materials and methods
Cell culture
The use of experimental animals in the present study
was approved by the Medical Ethical Committee of the First
Affiliated Hospital of Bengbu Medical College (Bengbu, China).
Primary chondrocytes were obtained from knee joints of 12 neonatal
Sprague Dawley rats, all male, aged 8 weeks. The rats were kept in
12-h light/dark cycle at 37°C and a humidity of 40% and had free
access to water and food. The rats were anaesthetized with
isoflurane at a dosage of 1–3%, as previously describe (13). Cartilage biopsies were digested for
20–22 h in collagenase type II (0.15% Worthington, NJ, US)
dissolved in medium containing DMEM supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA.) and
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; basic
medium) as previously described (14). BMSCs were isolated from the bone
marrow of neonatal rats previously reported (13,14). BMSCs
were seeded in culture flasks with basic medium. Media were
replaced every 2 days to remove floating cells. When 90% confluent,
cells were digested with trypsin and passaged. The cells cultured
on culture plastic were cultured to passage 2 prior to use. All
reagents used for cell culture were purchased from Gibco (Thermo
Fisher Scientific, Inc.). Common chemicals were purchased from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany.
Pellet culture and chondrogenic
culture
For mono-cultures, 200,000 primary chondrocytes or
BMSCs were seeded in one well of a round bottom 96 wells plate
(non-tissue culture treated). For co-cultures, 200,000 chondrocytes
or BMSCs were seeded at 1:1 ratio. Cells were initially seeded in
basic medium and centrifuged at 37°C for 5 min at 500 × g. Medium
was changed to chondrogenic differentiation medium (DMEM
supplemented with 40 µg/ml of proline, 50 ug/ml ITS-premix, 50
ug/ml of AsAP, 100 ug/ml of Sodium Pyruvate, 10 ng/ml of TGFβ3,
10−7 M of dexamethasone, 500 ng/ml of BMP6, 100 U
penicillin/ml and 100 µg/ml streptomycin) one day following seeding
and stable pellets were formed. Cell pellets were cultured for 4
weeks prior to analysis.
GAG staining
Cell pellets for co-cultures were fixed with 10%
formalin for 28 h, decalcified, dehydrated and embedded in paraffin
using routine procedures (14). A
microtome (Thermo Fischer Scientific, Inc.) was used to cut 5 µm
thick sections. Slides were then deparaffinized and stained for
sulfated glycosaminoglycans (GAG) with Toluidine blue for 2 h at
37°C.
Quantitative GAG and DNA content
assays
Cell pellets were washed with PBS and stored at
−80°C for 16–20 h. Subsequently, they were digested with proteinase
K solution [1 mg/ml proteinase K in Tris/EDTA buffer (pH 7.6)] for
>16 h at 56°C. GAG content was spectrophotometrically determined
with 1,9-dimethylmethylene blue chloride (DMMB) staining for 2 h at
37°C in PBE buffer (14.2 g/l Na2HPO4 and 3.72
g/l Na2EDTA, pH 6.5) using a microplate reader (TECAN
group, Ltd., Mannedorf, Switzerland) at an absorbance of 520 nm
using standard curves generated with chondroitin sulfate. Total DNA
content was determined using a CyQuant DNA Kit (Molecular Probes;
Thermo Fisher Scientific, Inc.).
DNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
DNA samples from cell pellets were isolated with
DNeasy Mini Kit (Qiagen GmbH, Hilden, Germany). Total genomic RNA
was used for RT-qPCR using the iQ SYBR Green Supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). PCR Reactions were carried
out on MyiQ2 Two-Color Real-Time PCR Detection System (Bio-Rad,
Laboratories, Inc.) under the following conditions: cDNA was
denatured for 5 min at 95°C, followed by 45 cycles, consisting of
15 sec at 95°C, 15 sec 60°C and 30 sec at 72°C. For each reaction,
a melting curve was generated to test primer dimer formation and
non-specific amplification. Primer sequences were as follows: Green
fluorescence protein (GFP) Forward, 5′-ACGACGGCAACTACAAGACC-3′ and
Reverse, 5′-TTGTACTCCAGCTTGTGCCC-3′; red fluorescence protein (RFP)
Forward, 5′-AAGCTGAAGGTGACCAAGGG-3′ and Reverse,
5′-CAAGTAGTCGGGGATGTCGG-3′; GAPDH Forward,
5′-GATGGTGAAGGTCGGTGTGA-3′ and Reverse, 5′-TTCTCAGCCTTGACTGTGCC-3′.
Relative gene copies were calculated using the 2−ΔΔCq
method (15). GAPDH was used for
normalization.
Rat osteochondral defect model
Twelve athymic nude rats (all male) of 8-weeks old,
weighing 25–30 g, were kept in 12-h light/dark cycle at a
temperature of 37°C and a humidity of 40% and had free access to
water and food. They were anaesthetized with isoflurane with dosage
of 1–3% and a medial parapatellar incision was made so that the
knee joint was exposed. The patella was dislocated laterally and
the anterior articular surface of the distal femur was exposed. A
1-mm-diameter full-thickness cylindrical osteochondral defect was
made using an electrical trephine in the articular surface of the
femoral patellar groove. Then 200,000 cell pellets were put into
the defects. Suturing the knee joint capsule and the skin
layer-by-layer closed the wound. Rats were allowed to move freely
following surgery. Each rat carried one pellet. Pellets made of
BMSCs or chondrocytes, or co-cultures were implanted into the three
experimental groups, containing 4 rats per group. All samples were
used for histological staining.
Cell tracking with green and red
fluorescence proteins
To track cells in co-cultures and co-implantation,
BMSCs were labeled with red fluorescence protein by lenti-viral
transduction, while chondrocytes were labeled with green
fluorescence protein. Lenti-GFP and Lenti-RFP virus were purchased
from lenti-virus were carried out by Hanbio biotechnology, Co, Ltd.
(Shanghai, China). Both lenti-viral vectors contained resistant
genes against puromycin. Infection of cells with Lenti-GFP or
Lenti-RFP (4%) was performed in 1.0 ml of serum-free basic medium
for 4 h at 37°C. Following infection, the remaining supernatant was
removed and replaced with basic medium supplemented with 10% fetal
bovine serum. Stably transduced cells were selected by adding
puromycin (1 µg/ml) in culture medium on day 2 following infection.
Antibiotic selection lasted for 1 week. Selection efficiency was
verified by visual examination under a fluorescence microscope. To
examine GFP and RFP signals in co-culture pellets, cryosections
were made with a cryotome (Leica CM1520, Leica Microsystems GmbH,
Wetzlar, Germany).
Immunofluorescence staining
For immunocytochemistry, sections of co-culture
pellets of BMSCs and chondrocytes were deparaffinized, incubated
with 3% hydrogen peroxide and blocked in 1% bovine serum albumin
and 1.5% normal goat serum at 37°C. Slides were subsequently
incubated overnight at 4°C with mouse monoclonal antibodies against
GFP (GF28R; Novagen; Merck KGaA) or RFP (cat no. 69831-3; Novagen;
Merck KGaA). Sequentially, primary antibodies were visualized by
incubating with fluorochrome-labeled secondary antibodies at 37°C
(L21998; Invitrogen; Thermo Fisher Scientific, Inc).
Counterstaining was performed with DAPI under a light microscope
(Olympus IX51; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Statistical significance between different groups
was examined with one-way analysis of variance followed by Tukey
Honestly Significant Difference Test. All data were presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Isolation and labeling of rat BMSCs
and chondrocytes
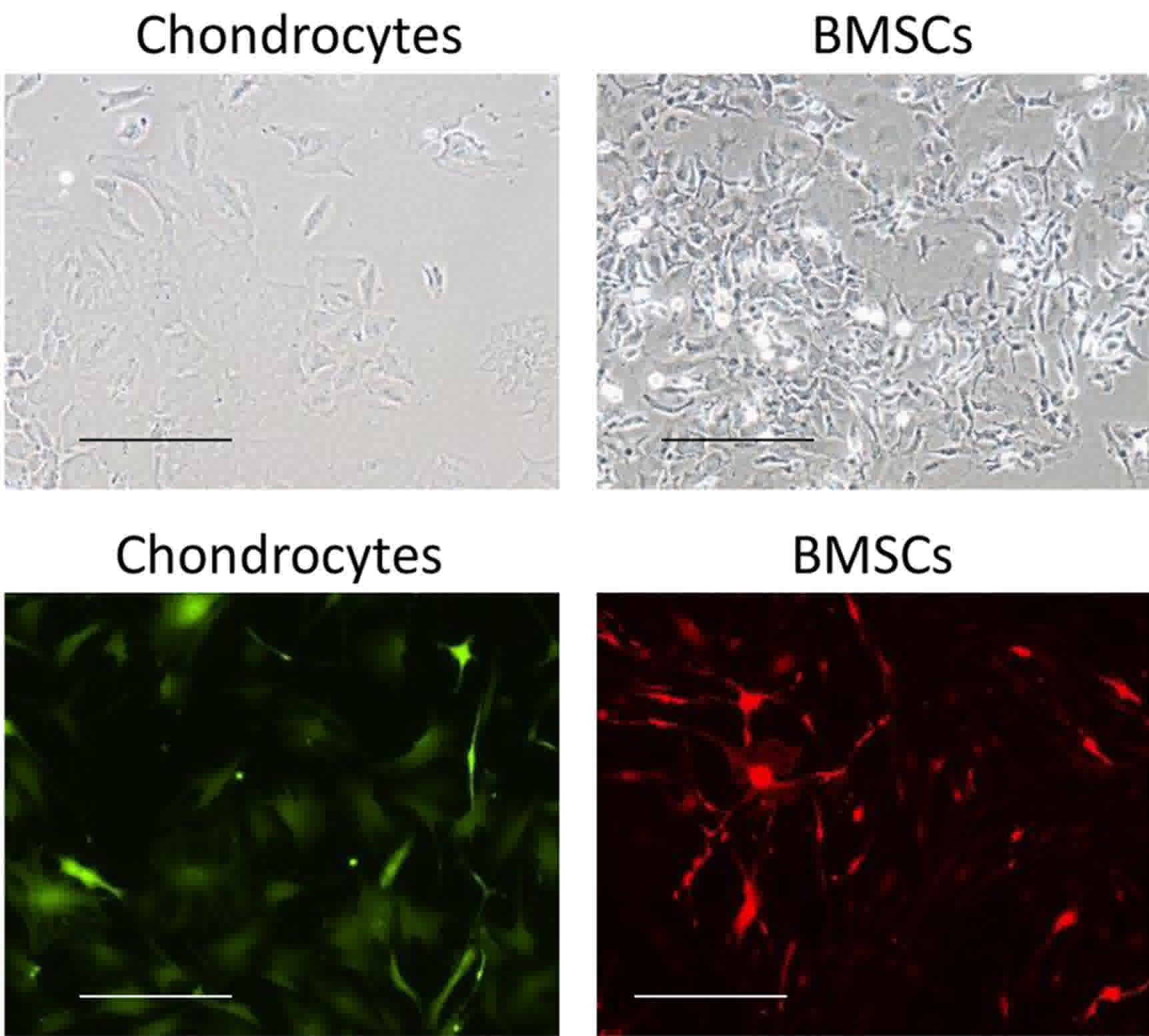
Articular chondrocytes were isolated from knee
joints of neonatal rats. Following in vitro expansion for
two passages, cells displayed a typical morphology of chondrocytes
(Fig. 1). Chondrocytes first spread
in a bipolar fashion for a few days, then most cells displayed
polygonal morphology with few filopodia. BMSCs presented a
spindle-like shape. With time in culture, cells gradually adopted a
more fibroblastic morphology (Fig.
1). With lenti-virus infection, chondrocytes were labeled with
GFP while BMSCs were labeled with RFP. Both cells were selected by
puromycin for 1 week prior to reaching a labeling efficiency
closing to 100%, as examined by fluorescent microscope (Fig. 1).
Co-culture pellets deposited more GAGs
and collagen than mono-cultures pellets
Labeled chondrocytes and BMSCs were used to make
cartilage tissue in pellet cultures. Four weeks following seeding,
cell pellets of both mono- and co-culture were harvested for
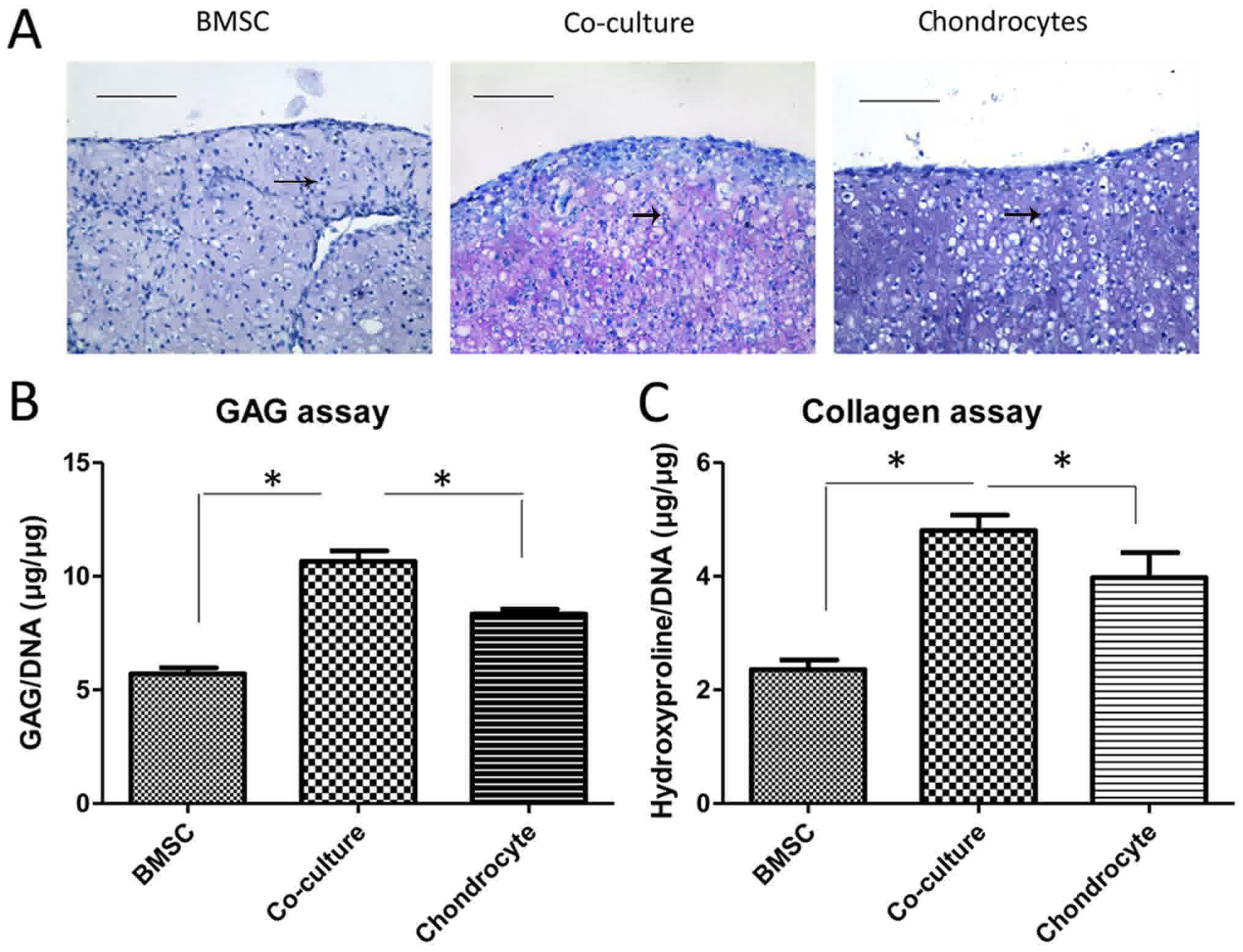
histology, GAG assay. As demonstrated in Fig. 2A, BMSCs pellets were able to deposit
some GAG into extracellular matrix. Chondrocytes pellets, however,
deposited much more GAG than BMSCs. When co-cultured, chondrocytes
and BMSCs together produced extracellular matrix containing
abundant GAG in the pellets which was significantly more than that
in mono-culture pellets, as measured by toluidine blue staining
(Fig. 2B). The same was observed in
the collagen assay by quantifying hydroxyproline (Fig. 2C).
BMSCs and chondrocytes in
co-cultures
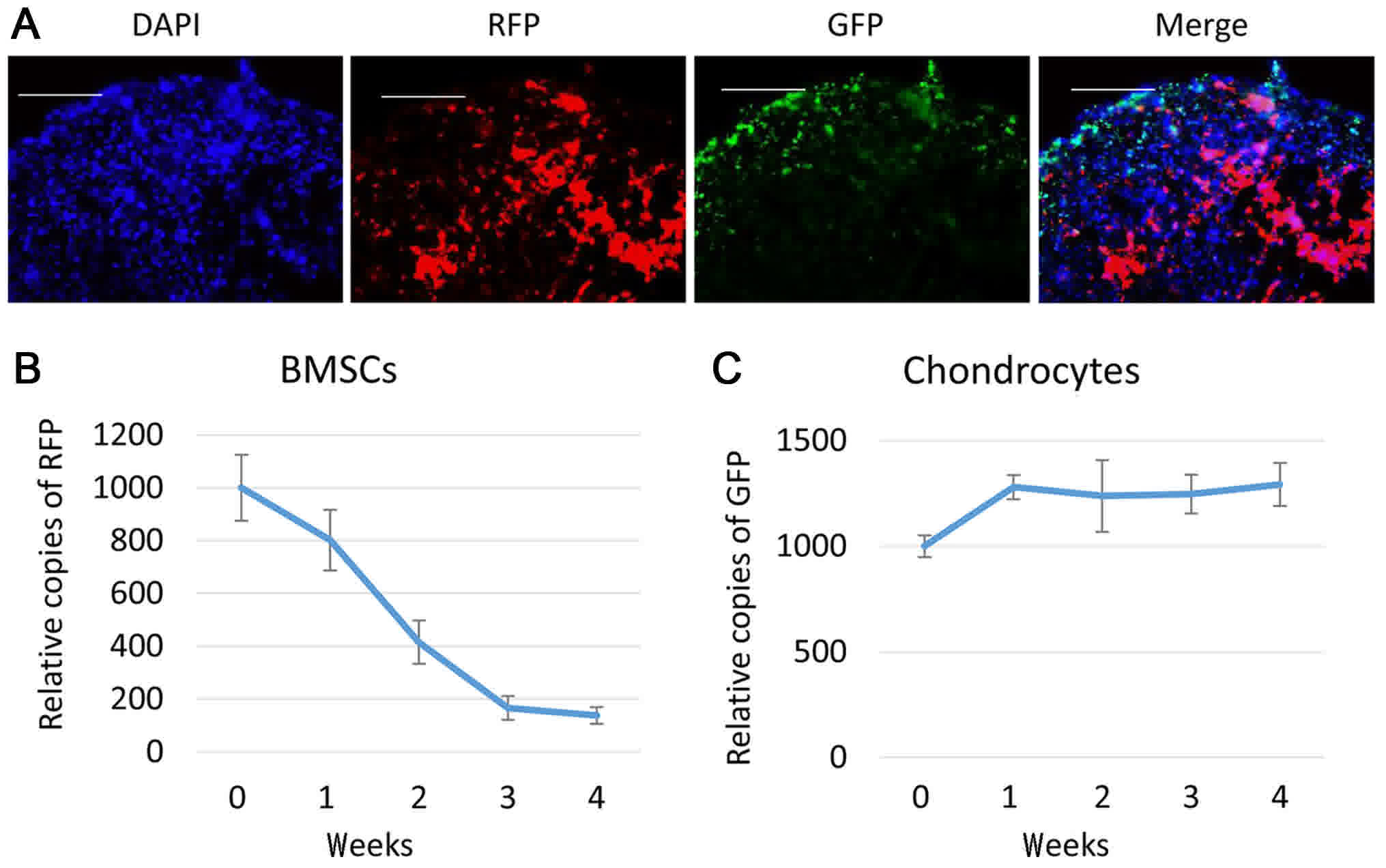
To track BMSCs and chondrocytes following
co-culture, cryosections were made to examine GFP and RFP signals
in the pellets, 1 week after the pellets were made. As depicted in
Fig. 3A, both RFP and GFP positive
cells were present in co-culture pellets. RFP labeled BMSCs tended
to present in the center of the pellets, while GFP labeled
chondrocytes were distributed more on the periphery of the pellet.
Subsequently, RT-qPCR was performed to track RFP and GFP DNA
contents in them. Compared with the cells initially seeded (week
0), the RFP positive cells dropped following 1 week of co-culture
to roughly 80%, continued to decrease until week 3 and kept stable
at week 4 at about 15% (Fig. 3B). On
the other hand, GFP positive cells increased at week 1 to about
120% of week 0, and remained stable thereafter (Fig. 3C).
Effect of BMSCs on the survival rate
of chondrocytes following co-implantation in rat osteo-chondral
defect model
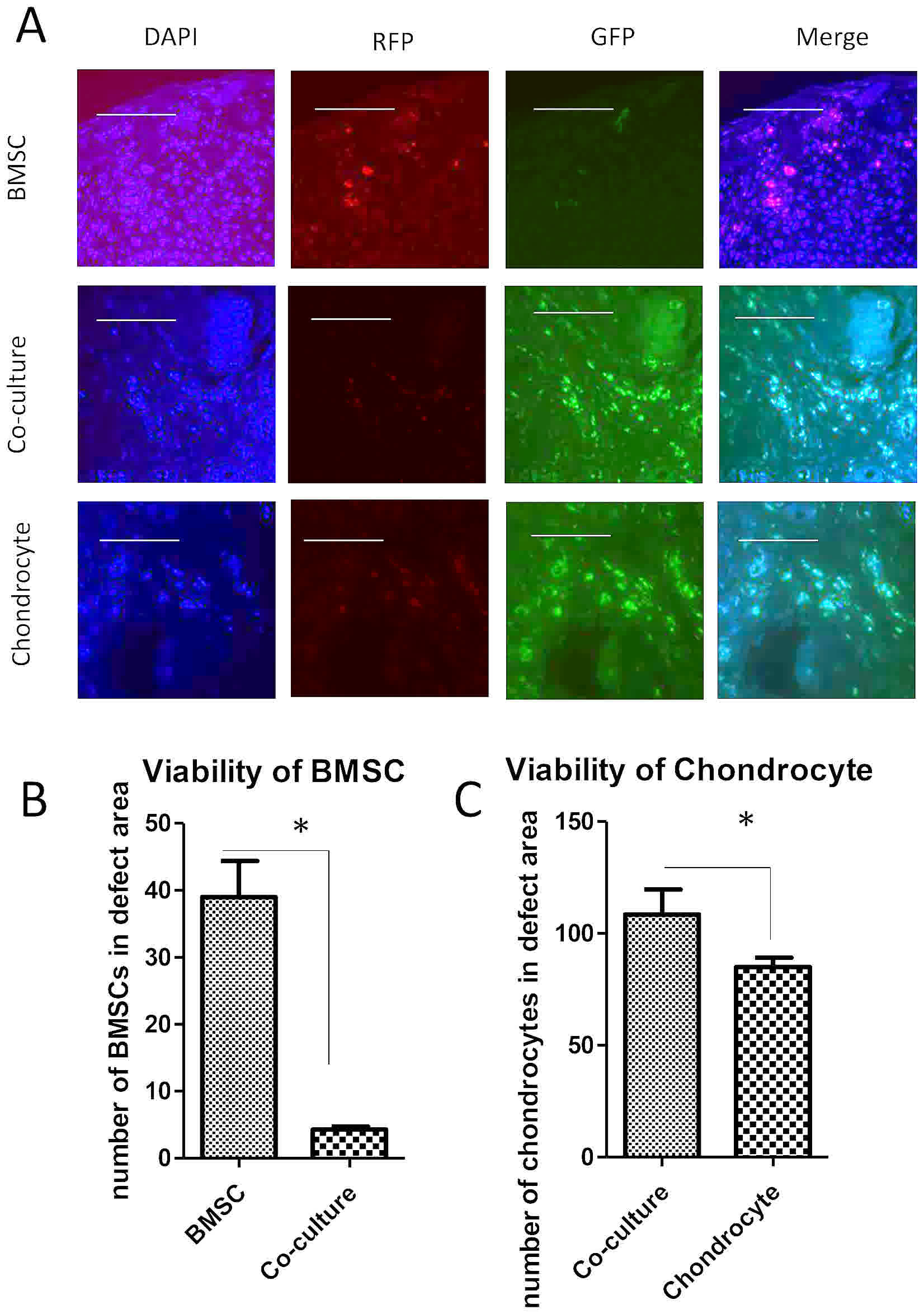
To test the viability of cells following
implantation into osteo-chondral defects, pellets made of BMSCs,
chondrocytes or co-cultures were implanted into a nude rat knee
injury model. Cell tracking by DNA content using GFP and RFP
indicated that a few BMSCs survived in defects when implanted alone
(Fig. 4A). In one section, less than
40 cells were found to be red in the defect area (Fig. 4B). Chondrocytes however, survived
compared with BMSCs when implanted alone into the defects, with
about 80 cells labeled with green in one section. Notably, the
viability of BMSCs in co-culture pellets was extremely low, with
roughly 4 cells found in defect area (Fig. 4C).
Discussion
In recent years, trophic effects of BMSCs have
attracted much attention in cartilage engineering (reviewed in 16).
However, very few studies successfully revealed the viability of
BMSCs or chondrocyte after co-implantation into osteo-chondral
defects (13,15). In the present study, BMSCs and
chondrocytes were labeled with fluorescence proteins and were
tracked in pellet co-cultures and in a osteo-chondral defect
model.
Multiple studies have investigated the effects of
BMSCs on chondrocytes (13–15), but few have provided data to indicate
the viability of BMSCs and chondrocytes in the content of direct
cell-cell contact neither in vitro nor in vivo
(13–16). The present study demonstrated that
co-culture of BMSCs and chondrocytes led to a decrease in BMSCs
cell numbers which can be explained by reduced cell proliferation
or cell death, but this needs to be confirmed. Since previous study
demonstrated massive cell death of BMSCs by apoptosis (10). The decrease of BMSCs numbers following
1 week of co-culture may suggest that chondrocytes were able to
change their behavior. Similar results have been demonstrated in
studies using co-culture of BMSCs with chondrocyte pellets from
different sources, during 3 to 4 weeks of culture, in which BMSCs
numbers decreased progressively (17,18).
Besides the dramatic decrease of BMSCs following co-culture, the
proliferation of chondrocytes demonstrated a trend of slowdown of
the increasing rate following one week of co-culture. In fact, in a
previous study employing a pellet co-culture system of BMSCs and
chondrocytes, an increase in chondrogenic markers was observed at
later stage of culture, following day 7 (19). This is in line with the present
finding that proliferation of chondrocytes occurred in the first
week of co-culture (20).
A previous report demonstrated that significant
numbers of TUNEL positive BMSCs were detected in co-culture pellets
(21). This result suggests that the
BMSCs may have died by apoptosis upon contacting with chondrocytes.
This may have occurred due to cell compaction, and nutrition or
space limitation in pellets. The cell labeling experiments
demonstrated that the majority of BMSCs reside in the center while
most chondrocytes presented on the edge of the pellets. BMSCs may
undergo apoptosis simply due to lack of nutrients or oxygen, which
was more likely to happen in the center of the pellet (22). However, this is not sufficient to
explain why the number of BMSCs deceased so much since some of
BMSCs did survive in the middle of a pellet made by BMSCs alone.
This suggested that besides to location of BMSCs in pellets, the
presence of chondrocytes may have also contributed to the apoptosis
of BMSCs. It's known that chondrocytes may secrete some apoptotic
cytokines (23), which may have
induced the death of BMSCs, but his needs to be further confirmed.
Furthermore, it is likely that direct cell-cell contact between
chondrocytes and BMSCs may have contributed to increased cell death
(24).
Viability of cells in tissue engineering is an
important issue for regenerative medicine (25). The present data indicated that
viability of chondrocytes increased a lot with co-implantation
compared with chondrocyte implanted alone, despite the death of
BMSCs. A lot of factors are involved in the death of chondrocytes
during autologous chondrocyte transplantation into joint
environment (26). Mechanical stress,
low oxygen and lack of nutrients may cause apoptosis or necrosis of
chondrocytes. Small chemicals and bio-compatible scaffolds are
designed to increase the viability of chondrocytes (27,28).
Findings of the present study may provide an alternative solution
to reduce the cell death of chondrocytes after implantation, which
is mixing chondrocytes with BMSCs. This may benefit the matrix
deposition, but also increase chondrocytes viability, but this
needs to be studied further.
To conclude, the present data indicated that BMSCs
were overtaken by chondrocytes in the pellet co-culture. Results
from the in vivo study demonstrated that BMSCs increased the
viability of chondrocytes following implantation in osteo-chondral
defects. Co-implantation of BMSCs and chondrocytes may be a
promising procedure in repairing osteo-chondral defects in clinical
settings.
Acknowledgements
Not applicable.
Funding
The present study was funded by a seed grant for
scientific innovation from Bengbu Medical College (Bengbu, Anhui,
China).
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available due to statutory
provisions regarding data and privacy protection, the dataset
supporting the conclusions of this article is available upon
reasonable request directed to the corresponding author.
Authors' contributions
ZZ, XZ and JG were involved in the conception and
design of the study, in the collection, assembly, analysis and
interpretation of the data, and in drafting of the article; they
also provided statistical expertise. JZ, MW and ZZ contributed to
final approval of the article, provision of study materials, and
administrative, technical and logistical support, as well as
critical revision of the article for important intellectual
content.
Ethics approval and consent to
participate
The use of experimental animals in the present study
was approved by the Medical Ethical Committee of the First
Affiliated Hospital of Bengbu Medical College (Bengbu, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ulrich-Vinther M, Maloney MD, Schwarz EM,
Rosier R and O'Keefe RJ: Articular cartilage biology. J Am Acad
Orthop Surg. 11:421–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mollenhauer JA: Perspectives on articular
cartilage biology and osteoarthritis. Injury. 39 Suppl 1:S5–S12.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee DA, Salih V, Stockton EF, Stanton JS
and Bentley G: Effect of normal synovial fluid on the metabolism of
articular chondrocytes in vitro. Clin Orthop Relat Res. 228–238.
1997.PubMed/NCBI
|
|
4
|
Umlauf D, Frank S, Pap T and Bertrand J:
Cartilage biology, pathology, and repair. Cell Mol Life Sci.
67:4197–4211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schindler OS: Current concepts of
articular cartilage repair. Acta Orthop Belg. 77:709–726.
2011.PubMed/NCBI
|
|
6
|
Singer M: Trophic functions of the neuron.
VI. Other trophic systems. Neurotrophic control of limb
regeneration in the newt. Ann N Y Acad Sc. 228:308–322. 1974.
View Article : Google Scholar
|
|
7
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruder SP, Fink DJ and Caplan AI:
Mesenchymal stem cells in bone development, bone repair, and
skeletal regeneration therapy. J Cell Biochem. 56:283–294. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kassis I, Vaknin-Dembinsky A and Karussis
D: Bone marrow mesenchymal stem cells: Agents of immunomodulation
and neuroprotection. Curr Stem Cell Res Ther. 6:63–68. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L, Leijten JC, Georgi N, Post JN, van
Blitterswijk CA and Karperien M: Trophic effects of mesenchymal
stem cells increase chondrocyte proliferation and matrix formation.
Tissue Eng Part A. 17:1425–1436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu L, Prins HJ, Helder MN, van
Blitterswijk CA and Karperien M: Trophic effects of mesenchymal
stem cells in chondrocyte co-cultures are independent of culture
conditions and cell sources. Tissue Eng Part A. 18:1542–1551. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acharya C, Adesida A, Zajac P, Mumme M,
Riesle J, Martin I and Barbero A: Enhanced chondrocyte
proliferation and mesenchymal stromal cells chondrogenesis in
coculture pellets mediate improved cartilage formation. J Cell
Physiol. 227:88–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jurgens WJ, Oedayrajsingh-Varma MJ, Helder
MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ and van
Milligen FJ: Effect of tissue-harvesting site on yield of stem
cells derived from adipose tissue: Implications for cell-based
therapies. Cell Tissue Res. 332:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SY, Nakagawa T and Reddi AH:
Mesenchymal progenitor cells derived from synovium and
infrapatellar fat pad as a source for superficial zone cartilage
tissue engineering: Analysis of superficial zone protein/lubricin
expression. Tissue Eng Part A. 16:317–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoddart MJ, Bara J and Alini M: Cells and
secretome-towards endogenous cell re-activation for cartilage
repair. Adv Drug Deliv Rev. 84:135–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song X, Xie Y, Liu Y, Shao M and Wang W:
Beneficial effects of coculturing synovial derived mesenchymal stem
cells with meniscus fibrochondrocytes are mediated by fibroblast
growth factor 1: Increased proliferation and collagen synthesis.
Stem Cells Int. 2015:9263252015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai JH, Rogan H, Kajiyama G, Goodman SB,
Smith RL, Maloney W and Yang F: Interaction between osteoarthritic
chondrocytes and adipose-derived stem cells is dependent on cell
distribution in three-dimension and transforming growth factor-β3
induction. Tissue Eng Part A. 21:992–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aung A, Gupta G, Majid G and Varghese S:
Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic
differentiation of human mesenchymal stem cells. Arthritis Rheum.
63:148–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mardones R, Jofré CM and Minguell JJ: Cell
therapy and tissue engineering approaches for cartilage repair
and/or regeneration. Int J Stem Cells. 8:48–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meretoja VV, Dahlin RL, Wright S, Kasper
FK and Mikos AG: Articular chondrocyte redifferentiation in 3D
co-cultures with mesenchymal stem cells. Tissue Eng Part C Methods.
20:514–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao L, Pike SE, Pittaluga S, Cherney B,
Gupta G, Jaffe ES and Tosato G: Anti-tumor activities of the
angiogenesis inhibitors interferon-inducible protein-10 and the
calreticulin fragment vasostatin. Cancer Immunol Immunother.
51:358–366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Secchiero P, Melloni E, Corallini F,
Beltrami AP, Alviano F, Milani D, D'Aurizio F, di Iasio MG,
Cesselli D, Bagnara GP and Zauli G: Tumor necrosis factor-related
apoptosis-inducing ligand promotes migration of human bone marrow
multipotent stromal cells. Stem Cells. 26:2955–2963. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song IH, Caplan AI and Dennis JE:
Dexamethasone inhibition of confluence-induced apoptosis in human
mesenchymal stem cells. J Orthop Res. 27:216–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guthrie K, Bruce A, Sangha N, Rivera E and
Basu J: Potency evaluation of tissue engineered and regenerative
medicine products. Trends Biotechnol. 31:505–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hindle P, Hall AC and Biant LC: Viability
of chondrocytes seeded onto a collagen I/III membrane for
matrix-induced autologous chondrocyte implantation. J Orthop Res.
32:1495–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q, Lu Z, Wu H and Zheng L:
Chondroprotective effects of taurine in primary cultures of human
articular chondrocytes. Tohoku J Exp Med. 235:201–213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shui W, Yin L, Luo J, Li R, Zhang W, Zhang
J, Huang W, Hu N, Liang X, Deng ZL, et al: Characterization of
chondrocyte scaffold carriers for cell-based gene therapy in
articular cartilage repair. J Biomed Mater Res A. 101:3542–3550.
2013. View Article : Google Scholar : PubMed/NCBI
|