|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang J, Li H and Ren G:
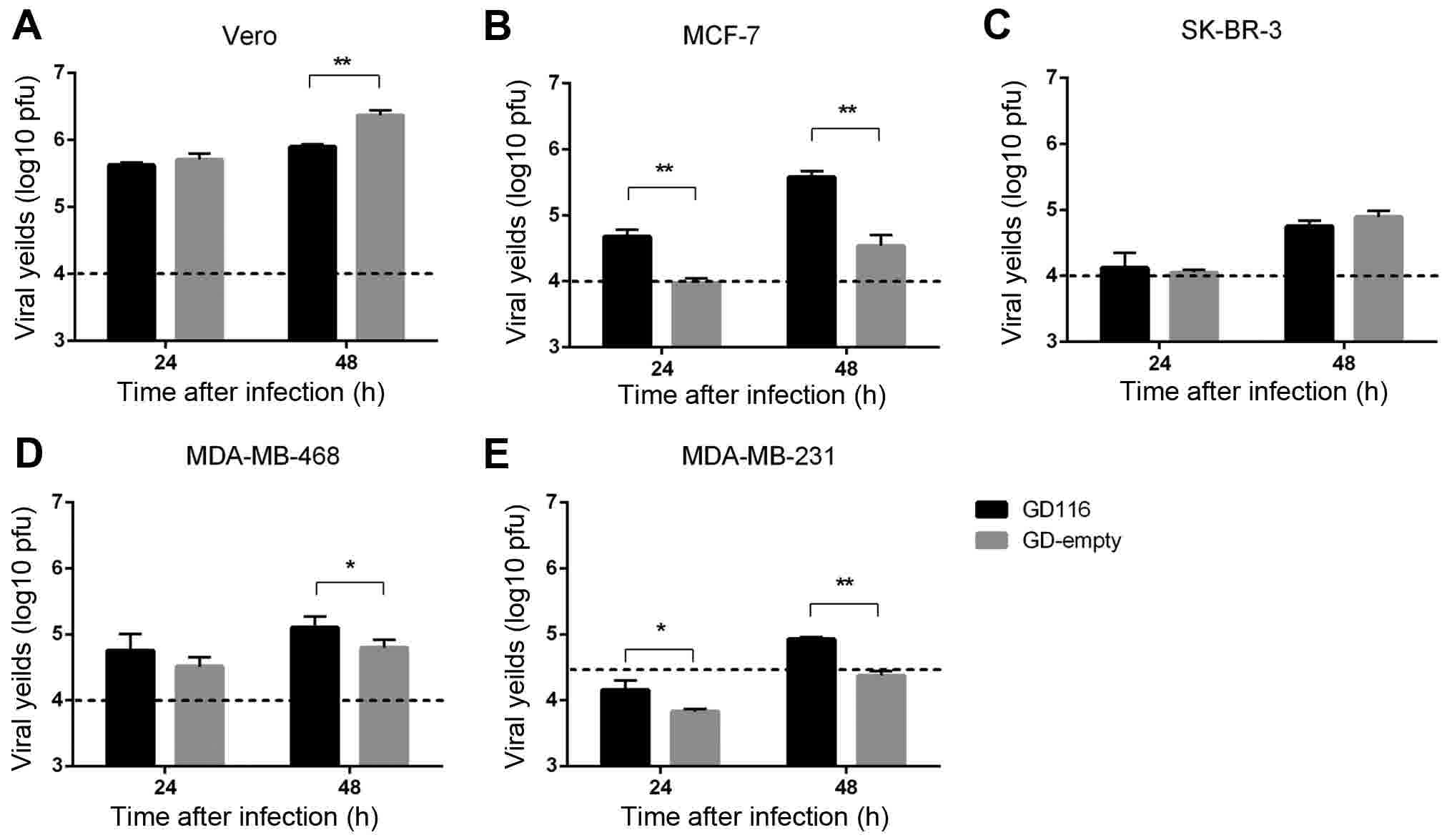
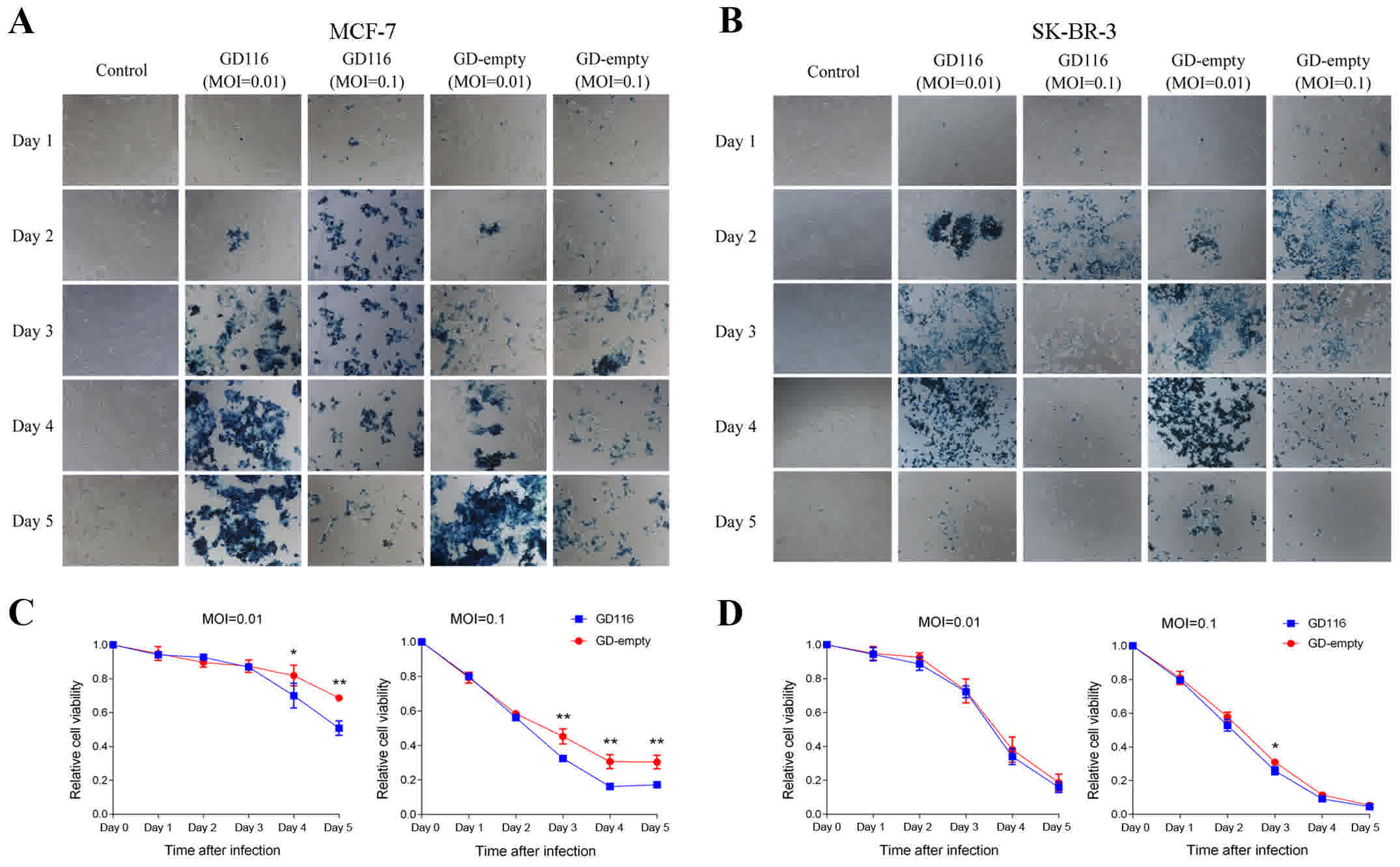
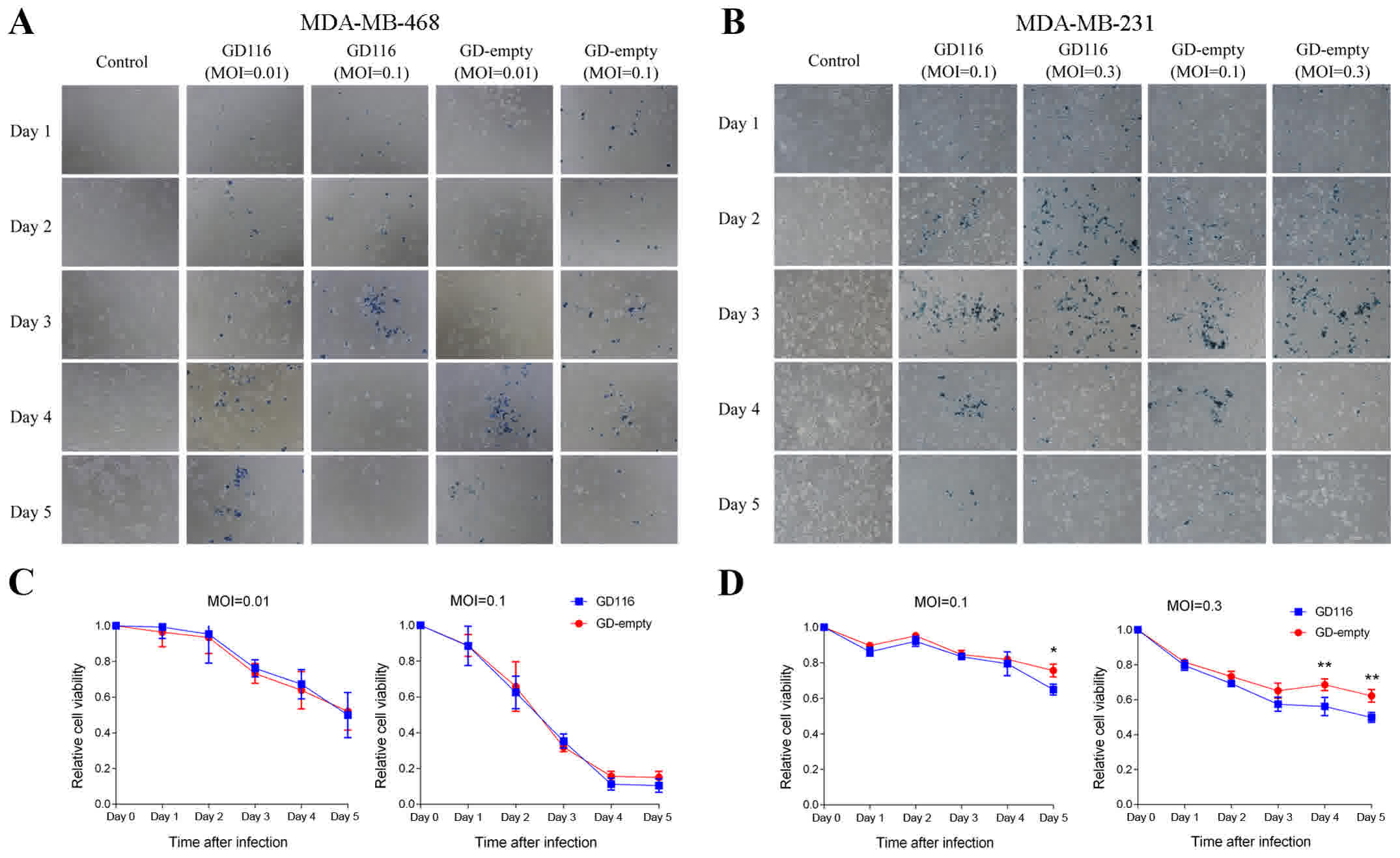
Epithelial-mesenchymal transition and drug resistance in breast
cancer (Review). Int J Oncol. 47:840–848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T9, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleibl Z and Kristensen VN: Women at high
risk of breast cancer: Molecular characteristics, clinical
presentation and management. Breast. 28:136–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartkopf AD, Fehm T, Wallwiener D and
Lauer UM: Oncolytic virotherapy of breast cancer. Gynecol Oncol.
123:164–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuruppu D and Tanabe KK: HSV-1 as a novel
therapy for breast cancer meningeal metastases. Cancer Gene Ther.
22:506–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andtbacka RH, Kaufman HL, Collichio F,
Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I,
Agarwala SS, et al: Talimogene laherparepvec improves durable
response rate in patients with advanced melanoma. J Clin Oncol.
33:2780–2788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campadelli-Fiume G, Petrovic B, Leoni V,
Gianni T, Avitabile E, Casiraghi C and Gatta V: retargeting
strategies for oncolytic herpes simplex viruses. Viruses. 8:632016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Fulci G, Wakimoto H, Cheema TA,
Buhrman JS, Jeyaretna DS, Rachamimov Stemmer AO, Rabkin SD and
Martuza RL: Combination of oncolytic herpes simplex viruses armed
with angiostatin and IL-12 enhances antitumor efficacy in human
glioblastoma models. Neoplasia. 15:591–599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
U.S. Food and Drug Administration: FDA
approves first-of-its-kind product for the treatment of melanoma.
http://www.webcitation.org/6drvCltG7October
27–2015
|
|
11
|
Todo T: Active immunotherapy: Oncolytic
virus therapy using HSV-1. Adv Exp Med Biol. 746:178–186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nigim F, Esaki SI, Hood M, Lelic N, James
MF, Ramesh V, Stemmer-Rachamimov A, Cahill DP, Brastianos PK,
Rabkin SD, et al: A new patient-derived orthotopic malignant
meningioma model treated with oncolytic herpes simplex virus. Neuro
Oncoly. 18:1278–1287. 2016. View Article : Google Scholar
|
|
13
|
Fan J, Jiang H, Cheng L and Liu R: The
oncolytic herpes simplex virus vector, G47Δ, effectively targets
tamoxifen-resistant breast cancer cells. Oncol Rep. 35:1741–1749.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng WG, Li JJ, Hu P, Lei L, Wang JN and
Liu RB: An oncolytic herpes simplex virus vector, G47Δ, synergizes
with paclitaxel in the treatment of breast cancer. Oncol Rep.
29:2355–2361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu R, Varghese S and Rabkin SD: Oncolytic
herpes simplex virus vector therapy of breast cancer in C3(1)/SV40
T-antigen transgenic mice. Cancer Res. 65:1532–1540. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Hu P, Zeng M, Rabkin SD and Liu R:
Oncolytic herpes simplex virus treatment of metastatic breast
cancer. Int J Oncol. 40:757–763. 2012.PubMed/NCBI
|
|
17
|
Alexander DE, Ward SL, Mizushima N, Levine
B and Leib DA: Analysis of the role of autophagy in replication of
herpes simplex virus in cell culture. J Virol. 81:12128–12134.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanai R, Zaupa C, Sgubin D, Antoszczyk SJ,
Martuza RL, Wakimoto H and Rabkin SD: Effect of γ34.5 deletions on
oncolytic herpes simplex virus activity in brain tumors. J Virol.
86:4420–4431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
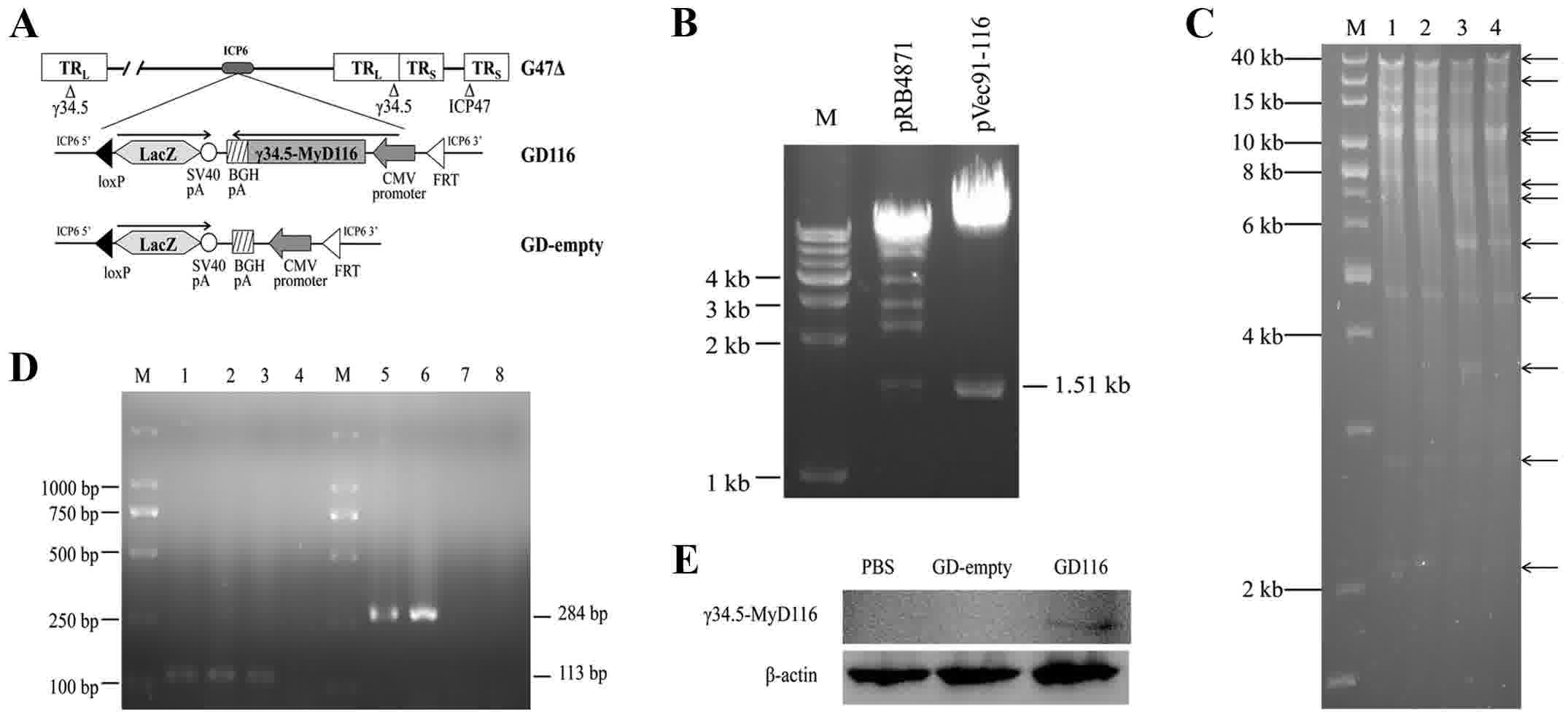
He B, Chou J, Liebermann DA, Hoffman B and
Roizman B: The carboxyl terminus of the murine MyD116 gene
substitutes for the corresponding domain of the gamma(1)34.5 gene
of herpes simplex virus to preclude the premature shutoff of total
protein synthesis in infected human cells. J Virol. 70:84–90.
1996.PubMed/NCBI
|
|
20
|
Fukuhara H, Ino Y, Kuroda T, Martuza RL
and Todo T: Triple gene-deleted oncolytic herpes simplex virus
vector double-armed with interleukin 18 and soluble B7-1
constructed by bacterial artificial chromosome-mediated system.
Cancer Res. 65:10663–10668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuji T, Nakamori M, Iwahashi M, Nakamura
M, Ojima T, Iida T, Katsuda M, Hayata K, Ino Y, Todo T and Yamaue
H: An armed oncolytic herpes simplex virus expressing
thrombospondin-1 has an enhanced in vivo antitumor effect against
human gastric cancer. Int J Cancer. 132:485–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuroda T, Martuza RL, Todo T and Rabkin
SD: Flip-Flop HSV-BAC: Bacterial artificial chromosome based system
for rapid generation of recombinant herpes simplex virus vectors
using two independent site-specific recombinases. BMC Biotechnol.
6:402006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korom M, Davis KL and Morrison LA: Up to
four distinct polypeptides are produced from the γ34.5 open reading
frame of herpes simplex virus 2. J Virol. 88:11284–11296. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown SM, Harland J, MacLean AR, Podlech J
and Clements JB: Cell type and cell state determine differential in
vitro growth of non-neurovirulent ICP34.5-negative herpes simplex
virus types 1 and 2. J Gen Virol. 75:2367–2377. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zemp FJ, Corredor JC, Lun X, Muruve DA and
Forsyth PA: Oncolytic viruses as experimental treatments for
malignant gliomas: Using a scourge to treat a devil. Cytokine
Growth Factor Rev. 21:103–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He B, Gross M and Roizman B: The
gamma(1)34.5 protein of herpes simplex virus 1 complexes with
protein phosphatase 1alpha to dephosphorylate the alpha subunit of
the eukaryotic translation initiation factor 2 and preclude the
shutoff of protein synthesis by double-stranded RNA-activated
protein kinase. Proc Natl Acad Sci USA. 94:843–848. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhang C, Chen X, Yu J, Wang Y, Yang
Y, Du M, Jin H, Ma Y, He B and Cao Y: ICP34.5 protein of herpes
simplex virus facilitates the initiation of protein translation by
bridging eukaryotic initiation factor 2alpha (eIF2alpha) and
protein phosphatase 1. J Biol Chem. 286:24785–24792. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis KL, Korom M and Morrison LA: Herpes
simplex virus 2 ICP34.5 confers neurovirulence by regulating the
type I interferon response. Virology. 468–470:330–339. 2014.
View Article : Google Scholar
|
|
29
|
Tang S, Guo N, Patel A and Krause PR:
Herpes simplex virus 2 expresses a novel form of ICP34.5, a major
viral neurovirulence factor, through regulated alternative
splicing. J Virol. 87:5820–5830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Orvedahl A, Alexander D, Tallóczy Z, Sun
Q, Wei Y, Zhang W, Burns D, Leib DA and Levine B: HSV-1 ICP34.5
confers neurovirulence by targeting the Beclin 1 autophagy protein.
Cell Host Microbe. 1:23–35. 2007. View Article : Google Scholar : PubMed/NCBI
|