Introduction
Circadian rhythms include all light-sensitive
biological mechanisms that allow for the organismal alternations
that occur with 24 h. These include, but are not limited to, the
regulation and control of the intra-corporal nerves, body fluids
and immune systems. Regulation and control of biological circadian
rhythms aids in maintaining the process of normal physiology,
biochemistry and behaviors in organisms (1–3). In
mammals, the hypothalamic suprachiasmatic nucleus is the major
oscillator of circadian and biological rhythms. That same rhythm
exists in peripheral tissues and cells under the control of the
suprachiasmatic nucleus (1,4,5). The
intra-corporal mechanisms of biological rhythm control changes,
including sleep, wakefulness, feeding, temperature and blood
pressure, as well as physiological process, including cell cycle,
DNA damage response, aging and metabolism. Biological rhythms, when
disturbed, may lead to unbalanced biological functions and, in
turn, the occurrence of diseases, including cardiovascular disease
and tumors (2,6,7).
Existing in the central suprachiasmatic nucleus and
all peripheral tissues, circadian genes are the molecular
mechanisms maintaining temporal rhythm in organisms. The biological
circadian rhythm is regulated and maintained by the feedback loop
formed by a group of genes, often featuring positive or negative
regulation and control. Core circadian rhythm genes include
positive regulating genes that activate the expression of rhythm
genes (Bmal1 and Clock) and negative regulating genes
that reduce the expression of rhythm genes, including Period
(Per1, Per2 and Per3) and Cryptochrome (Cry1 and
Cry2) (3,7). Bmal1 and Clock proteins have the
structural domain of transcription factor PAS-HLH, and positive
regulation and control in the feedback loop. A Clock: Bmal1
heterodimer binds the CACGTG E-box enhancer regions of the
mPer1, mPer2, mPer3, mCry1 and
mCry2 genes; it drives the transcription and translation of
several genes but produces mPer and mCry, which are
known inhibitors of the transcriptional activity of the Clock:Bmal1
heterodimer, thereby mediating the negative feedback loop. The mRNA
and protein levels of the majority of clock genes generally exhibit
24-h cycle oscillation under the influence of their own feedback
loops, but Clock is exceptional for its constitutive
expression in tissue cells without any obvious fluctuations
(8).
Biological rhythm disorder may have negative impacts
on the physiological functions of mammals. A large amount of
clinical and experimental evidence has demonstrated that biological
rhythm disorder may lead to uncontrollable cell proliferation,
implicating the disorder and its associated mechanisms in the
etiology of cancer (7,9,10). Breast
cancer is the common malignant tumor among females, with 1.2
million women being diagnosed every year worldwide. Approximately
0.5 million women succumb to mortality as a result of breast
cancer, making it the most dangerous of all malignant tumors in
females. There are numerous risk factors for breast cancer,
including menstruation, childbearing, a high-fat diet and a family
history. Epidemiological reports have demonstrated that circadian
rhythm disorder may increase the chance of females developing
breast cancer (11–13). In general, cancer has been linked to
rhythm disorder, but a detailed molecular mechanism has yet to be
fully elucidated. The Clock gene is confirmed to be a core
member of the circadian system, and has also been demonstrated to
serve an important role in tumor growth. The present study aimed to
investigate the effects of Clock on the proliferation and
migration of breast cancer cells, in addition to elucidating the
molecular mechanisms regulating the biological actions of the gene
in a breast cancer cell line. It was reported that E-cadherin,
under the regulation of the scaffolding protein, IQ motif
containing GTPase activating protein 1 (IQGAP1), mediates the
structural and mobility features that allow tumor cells to
proliferate and become invasive. This pathway, regulated by the
Clock gene, provides a clearer picture between circadian
clock disorder and breast cancer, and lays the groundwork for
future study.
Materials and methods
Cell culture and transfection
4T1 cells were obtained from the American Type
Culture Collection cell bank (Manassas, VA, USA). They were
cultured in Dulbecco's modified Eagle's medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), containing 10%
inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Subsequently, cells were maintained in
cell incubators with 5% CO2 at a constant temperature of
37°C.
Lentiviral transfection
Mouse Clock small hairpin RNA(shRNA) was
constructed in the lentivirus gene transfer vector
pHBLV-U6-ZsGreen, and the titer of the virus was 2×108
TU/ml. lv-shRNA-Clock and lv-GFP-Puro NC viruses containing a green
fluorescent protein (GFP) sequence (Hanbio Biotechnology Co., Ltd.,
Zhejiang, China) were thawed and dissolved on ice. Once the medium
was replaced with fresh medium containing the transfection reagent
polybrene (Hanbio Biotechnology Co., Ltd.), the virus solutions
were added into the 24-well plate containing 4T1 cells at a volume
of 30 µl/well, according to the manufacturer's protocols. The cells
were cultured in the incubator at a constant temperature of 37°C
and a saturation humidity of 5% CO2 for 24 h. At 48 h
post-transfection, cells were detected using a fluorescence
microscope (magnification, ×100) (Nikon Corporation, Tokyo, Japan).
Expression efficiency of the vectors was assessed using GFP.
Subsequently, the cells were transferred to cell culture bottles
where they were screened for successful transfection using
puromycin (J&K Scientific Ltd., Beijing, China) at a
concentration of 2 µg/ml, according to the manufacturer's
protocols. The cycle for transfection screening was one week.
Cell proliferation assay
With a total volume of 100 µl nutrient solution, the
cells screened out for successful transfection were inoculated in
96-well plates at 1,000 cells/well. Next, the starting position of
cell adherence was marked at 0 days. Cells were incubated with 10
µl Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) and placed in an incubator with a saturation
humidity of 5% CO2 at a constant temperature of 37°C for
1 h. A microplate reader (Thermo Fisher Scientific, Inc.) was used
to detect light absorption at 450 nm after 1, 2, 3, 4 and 5 days
incubation.
Cells screened for successful transfection were
inoculated in 6-well plates at 500 cells/well with a total volume
of 2 ml nutrient solution. Then cells were placed in the incubator
with a saturation humidity of 5% CO2 and a constant
temperature of 37°C for 2 weeks. After 2 weeks, plates were washed
with phosphate-buffered saline (PBS) 2–3 times and 1 ml methyl
alcohol was added for fixation for ~30 min at room temperature.
Following drying, 1 ml crystal violet was added to each well for ~2
min at room temperature.
Detection of cell cycle distribution
with flow cytometry
A single-cell suspension was made by 1,000 × g
centrifugation at 4°C for 5 min of cells successfully transfected
by the virus. Cells were centrifuged at 1,000 × g at 4°C for 5 min
and were washed three times with precooled PBS. A second
centrifugation was performed at 1,000 × g at 4°C for 5 min. The
cell sediment was resuspended with precooled 75% ethyl alcohol for
cell fixation at 4°C for 12 h, followed by staining with 50 µl
propidium iodide (PI; 0.4 mg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 50 µl RNase (Sigma-Aldrich; Merck KGaA).
Incubation was performed for 30 min at 37°C in the dark.
Subsequently, the percentage of cells in the G0/G1 phase and S
phase, respectively, was calculated using a FACSCanto II flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and ModFit LT
software (Verity Software House, Inc., Topsham, ME, USA). Each
experiment was repeated three times.
Cell migration assay
Serum-free Dulbecco's modified Eagle's medium (200
µl; Hyclone; GE Healthcare Life Sciences) was added to ~50,000
cells in the upper chamber of the Transwell filters (8.0-µm pores).
Subsequently, 900 µl Dulbecco's modified Eagle's medium, containing
20% fetal bovine serum, was loaded into the lower chamber.
Following inoculation, cells were cultured for 24 h at 37°C, were
washed three times with PBS and were fixed with 100% methyl alcohol
for 10–15 min at room temperature. Following drying, invaded cells
on the bottom surface of the Transwell were stained with crystal
violet (Sigma-Aldrich; Merck KGaA) for 30 sec at room temperature,
washed with PBS until the membrane was clear of dye. Next, the
membrane was cut and observed under a light microscope
(magnification, ×100; Nikon, Japan). Data are presented as the mean
± standard deviation of the number of the cells/field in 3
individual experiments.
Western blot analysis
Cells were collected during the logarithmic phase
and were lysed with radioimmunoprecipitation assay buffer,
containing 1 mM PMSF, Halt protease inhibitor cocktail and Halt
phosphatase inhibitor (Beyotime Institute of Biotechnology, Haimen,
China) for 30 min on ice. The resolved proteins underwent
centrifugation at 14,000 × g for 20 min at 4°C. Protein
concentrations were determined using a bicinchoninic acid protein
assay (Beyotime Institute of Biotechnology). Equal amounts of
protein (0.5 mg/ml concentration; control and experimental) were
loaded in each experiment. Protein was separated by 10–12%
SDS-PAGE, followed by transfer to polyvinylidene difluoride
membranes (Millipore, USA). The membrane was blocked with 5% skim
milk for 2 h at room temperature, incubated with the following
primary antibodies: Monoclonal anti-β-actin (1:5,000 dilution; cat
no. 60008-1-Ig; ProteinTech Group, Inc., Chicago, IL, USA),
anti-Clock (1:10,000 dilution; cat no. ab3517; Abcam, Cambridge,
MA, USA), anti-cluster of differentiation (CD)44 (1:1,000 dilution;
cat no. CY5138; Shanghai Abways Biotechnology Co., Ltd., Shanghai,
China), anti-tumor protein p53 (1:1,000 dilution; cat no. AB3125;
p53; Shanghai Abways Biotechnology Co., Ltd.), anti-E-cadherin
(1:2,000 dilution; cat no. CY1155; Shanghai Abways Biotechnology
Co., Ltd.), anti-cyclin D1 (1:1,000 dilution; cat no. CY5404;
Shanghai Abways Biotechnology Co., Ltd.), anti-IQGAP1 (1:2,000
dilution; cat no. 22167-1-AP; ProteinTech Group, Inc.), anti-heat
shock protein 27 (1:1,000 dilution; cat no. CY5934; Hsp27; Shanghai
Abways Biotechnology Co., Ltd.) and anti-PCNA (1:2,000 dilution;
cat no. BM3888; Boster Biological Technology, Pleasanton, CA, USA).
The membrane was incubated overnight at 4°C, followed by incubation
with a horseradish peroxidase-conjugated anti-rabbit IgG or
anti-mouse IgG secondary antibody (1:5,000 dilution; cat no.
SA00001-2&SA00001-1; ProteinTech Group, Inc.) at 37°C for 2 h.
Membranes were developed using an enhanced chemiluminescence
reagent from the EasySee Western Blot kit (Beijing Transgen Biotech
Co., Ltd., Beijing, China) and imaged using a gel imager (GE
Healthcare Life Sciences, Little Chalfont, UK). The images were
further analyzed with ImageJ software (National Institutes of
Health, Bethesda, MD, USA) to assess the differences in protein
expression.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and were analyzed by one-way analysis of variance followed
by the Student-Newman-Keuls post hoc test. All data were averaged
from three independent assays and were analyzed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA and SPSS 19.0
statistics software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
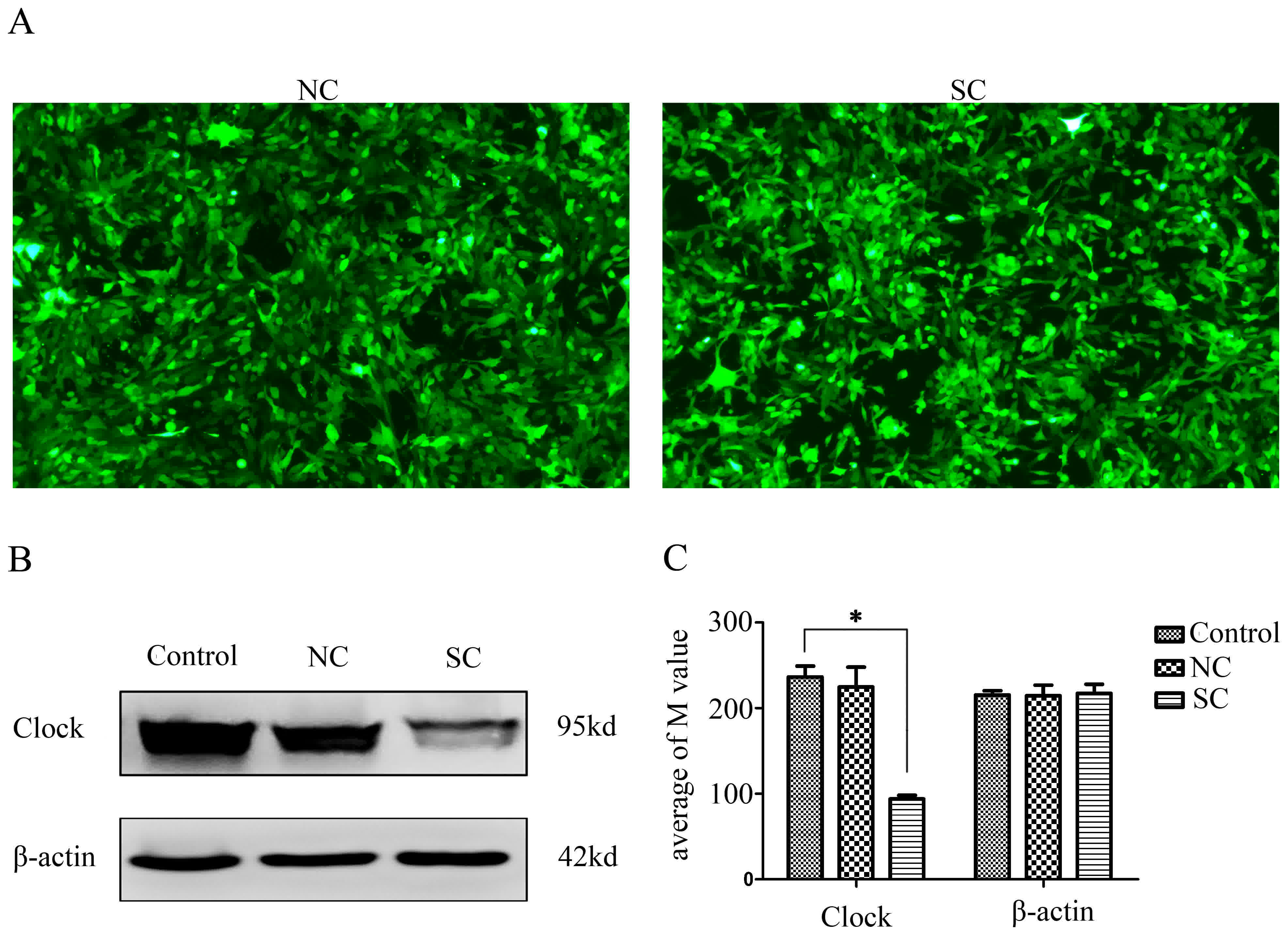
Clock expression was efficiently
decreased in 4T1 cells
Stably-transfected 4T1 cells were named as follows:
Control group (untransfected 4T1 cells), NC group (4T1 cells
transfected with lv-GFP-Puro NC virus) and SC group (4T1 cells
transfected with lv-shRNA-Clock virus). Observation of green
fluorescence indicated that the number of cells successfully
transfected was >90% (Fig. 1A).
Western blot analysis further demonstrated that the expression
level of Clock protein in the SC group was significantly lower than
that in the control and NC groups (P<0.05; Fig. 1B and C).
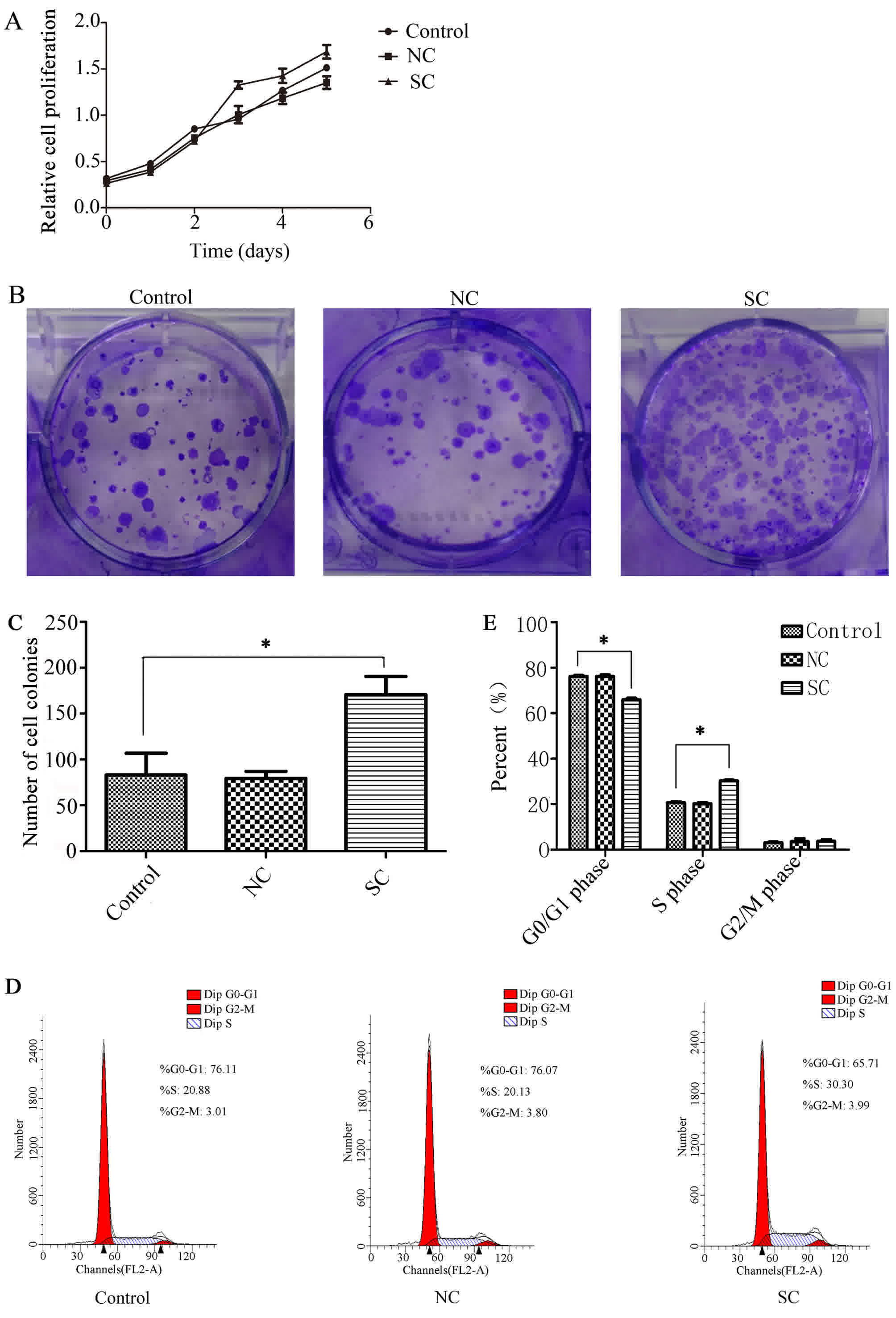
Effects of Clock on cell
proliferation
4T1 cell lines knocked down by Clock-shRNA
and control vectors were analyzed using a CCK-8 cell proliferation
assay for 6 consecutive days. Data were used to form a longitudinal
proliferation curve. Results demonstrated that the proliferation
ability of cells knocked down by Clock-shRNA was enhanced
compared with that of cells in the control group (P<0.05;
Fig. 2A). The monoclonal cell mass
that had formed in the 6-well plates was dyed with crystal violet,
which revealed that the monoclonal cell number in the SC group was
significantly increased compared with cells in the control groups
(Fig. 2B and C). Following further
analysis of the cells by flow cytometry, cycle phases of the cells
were determined using Modfit software (Verity Software House,
Inc.). The cycle phase of the cells in the SC group was different
to that of the control cells. When cells in the SC group were
compared with those in NC group, the proportion of cells in the
G0/G1 phase was lower, while the number of cells in the S phase
increased (P<0.05; Fig. 2D and E).
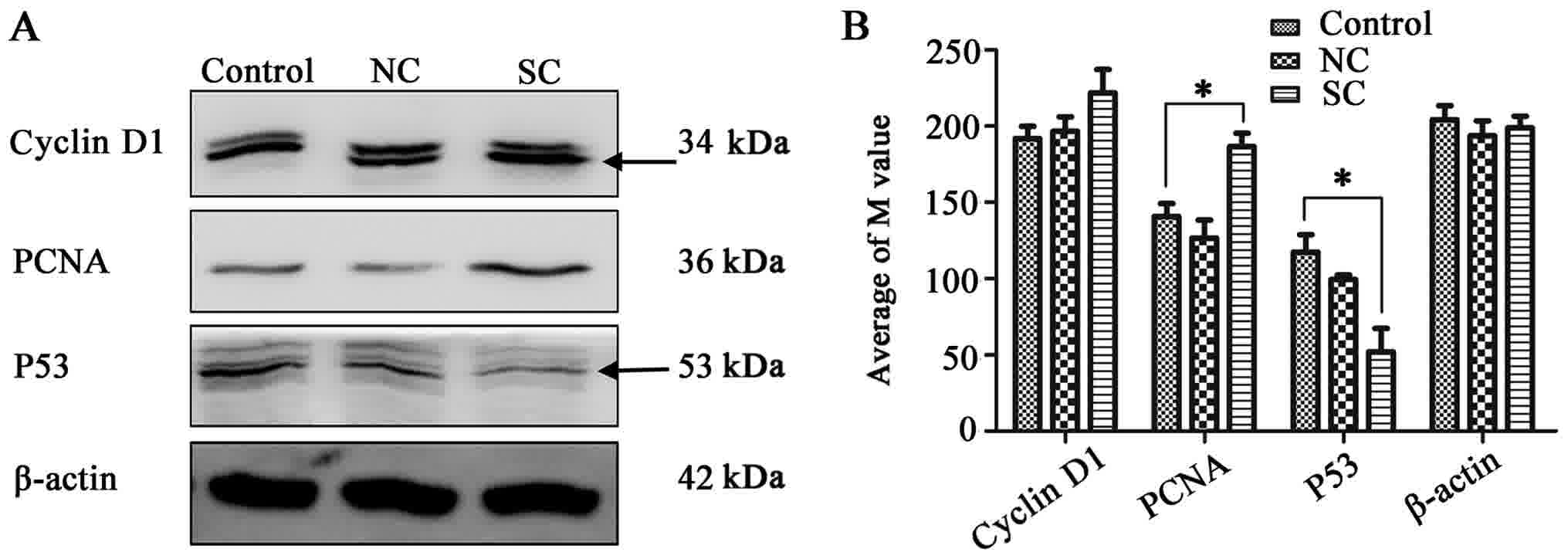
Western blot analysis revealed that the expression of cyclin D1,
PCNA and p53 proteins was increased in the SC group
(Fig. 3A and B), which further
illustrated the enhanced proliferation ability of cells following
Clock interference.
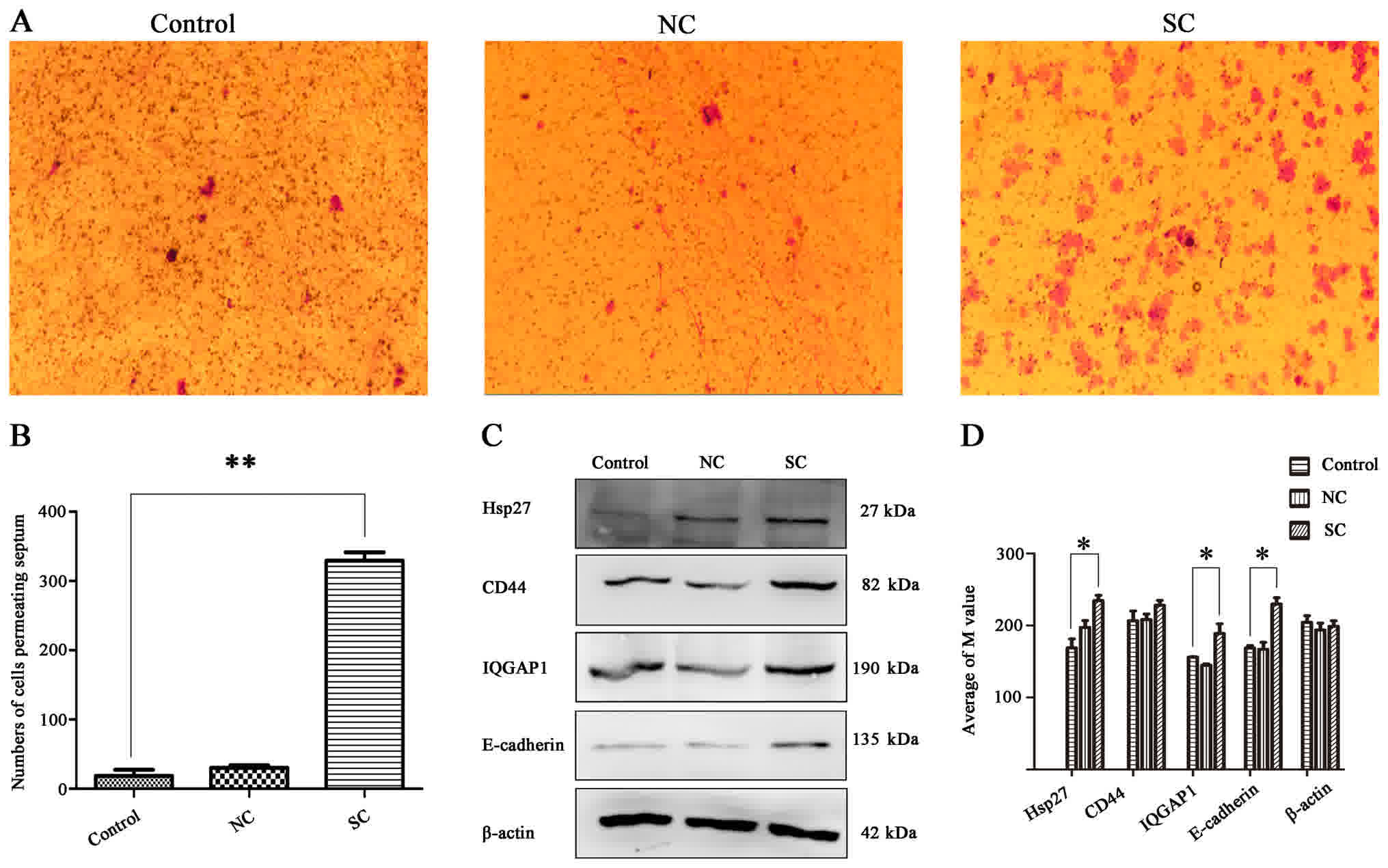
Effects of CLOCK on migration
A thin layer of membrane from the cells of each
group were cut and fixed on a glass slide following fixation and
crystal violet staining. Results demonstrated that the rate of
migration of the cells in the SC group was significantly higher
than that of the control cells (Fig. 4A
and B). Furthermore, western blot analysis demonstrated that
the expression level of E-cadherin in the SC group was
similarly increased. E-cadherin is an important adherence
factor on the surface of the cytomembrane, and has important
functions in proliferation and migration of cells. The expression
of IQGAP1 protein, which directly interacts with E-cadherin,
was also markedly increased in the SC group compared with the
control group. Finally, an increased expression of
cell-migration-associated protein Hsp27 and membrane surface
adhesion-associated protein CD44 was detected following
Clock knockdown, compared with the control cells (Fig. 4C and D).
Discussion
In the present study, inhibition of clock gene
expression was achieved in 4T1 cells by lentiviral transfection
with Clock-shRNA. Cell proliferation and migration, and the
expression of Clock-related proteins were assessed in a breast
cancer cell line in order to more clearly understand the
association between circadian rhythm regulatory genes and the
development of cancer. Following Clock downregulation, it
was revealed that the proliferation of breast cancer cells
improved, as determined by CCK-8 and clone formation assays. Cell
cycle distribution analysis using flow cytometry revealed that the
number of cells in the S phase was increased in the knockdown
group, providing further evidence that the interference of
Clock facilitates the proliferation of cells to a certain
degree. This, coupled with the observed increase in migration of
the knocked down cells, illustrates an endogenous inhibitory role
of Clock on cellular proliferation and migration.
Cyclin D1, an important positive regulatory
factor of the cell cycle, serves an important role in the
occurrence and development of tumors (14,15).
Binding and activating the unique cyclin-dependent kinase, CDK4,
during the G1 phase, cyclin D1 is responsible for the
phosphorylation of retinoblastoma protein (Rb) during the G1 phase
(16). Rb protein is subsequently
dissociated from its bound E2F transcription factors, which
initiates gene transcription and activation of cell cycle genes,
driving the cycle from the G1 phase to the S phase (16). The p53 gene is a tumor
suppressor gene that has been revealed to be correlated with human
tumors. It is a cyclin-dependent gene and has been implicated in
cell proliferation (15). A previous
study demonstrated that cells expressing wild-type p53,
following irradiation, become locked in the G1 phase, likely due to
the abnormal expression of cyclin D (15). Proliferating cell nuclear antigen
(PCNA), an intra-nuclear polypeptide synthesized or
expressed only in proliferating cells, serves a role in the
regulation of the cell cycle. PCNA is primarily expressed during
the S, G1 and G2 phases, the primary phases of proliferating cells.
Its expression intensity is often used as an indicator of
proliferation activity and as an assessment tool in the malignancy
of tumor cells. The present study revealed that, following
Clock knockdown, the expression level of p53 was
decreased, while the expression of cyclin D1 and PCNA
was increased, indicating that the proliferation ability of cells
was enhanced by the inhibition of Clock.
Downregulation of Clock in breast cancer
cells may promote extracorporeal cell migration and the present
study investigated the molecular drivers of this phenomenon. During
the course of the study, it was revealed that the expression of
IQGAP1 was increased following Clock knockdown.
IQGAP1 is an actin scaffold protein that has been implicated
in the migration of cancer cells, and it harbors a direct
E-cadherin binding site. Consequently, epithelial-mesenchymal
transition (EMT)-associated protein assessment revealed that cell
adhesion-associated proteins, CD44 and E-cadherin, were increased
by knockdown of Clock in 4T1 cells, indicating that
Clock, through IQGAP1, may regulate cell-adhesion
proteins to influence cell motility.
Previous reports have supported a role for IQGAP1 in
the regulation of the cytoskeleton (though actin and tubulin),
impacting migration, invasion and fission of colorectal cells. It
has also demonstrated regulatory action on vascular endothelial
growth factor, a regulator of endothelial cell migration (17,18).
IQGAP1 has also been documented to bind to and regulate the
localization of Dia 1, another factor of cell migration. Finally,
IQGAP1 has been reported to promote the migration and invasiveness
of E-cadherin-mediated homologous cells, regulating cell adhesion
and plasma-membrane-mediated transposition (19–21).
The present study revealed that the expression of
CD44 and E-cadherin is regulated by Clock. E-cadherin, a
well-known EMT marking molecule that serves a role in maintaining
cell morphology, movement and adhesion, reduced expression of which
may arrest cells in their rounded state, weakening the ability of
the cells to migrate and become invasive (22,23). CD44,
like E-cadherin, is a membrane-associated protein that regulates
connectivity among heterogeneous cells, connecting tightly to the
cytoskeleton and participating in the formation of cell pseudopods
which are associated with cell movement and migration (24). Positive expression of CD44 has been
reported to be associated with vascular infiltration and distant
metastasis (25,26). The present study revealed that,
following knockdown of Clock, the ability of the cells to
migrate was enhanced. Furthermore, Hsp27, reportedly associated
with lymph node metastasis, organ metastasis, tumor size and
staging (27), was similarly revealed
to be upregulated by the inhibition of Clock.
In conclusion, the results of the present study
suggested that the downregulation of Clock may promote the
proliferation and migration of mouse breast cancer cells. Clock may
affect the expression of the adhesion protein, E-cadherin, on cell
membranes, likely through regulation of the expression of the
skeletal-associated protein, IQGAP1. Collectively, the pathway
affects the proliferation and migration ability of breast cancer
cells. Clarifying and understanding the function and mechanisms of
Clock is an important step toward elucidating the
therapeutic potential of Clock and other circadian rhythm factors
in cancer and other biological rhythm disorders. The present study
sheds light on an important but elusive epidemiological association
between circadian rhythm dysregulation and cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31371180) and the
Scientific and Technological Project of Sichuan Province (grant no.
2014SZ0193).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
All authors contributed to this study, LXX, WZR, and
JZ designed experiment; LXX, WSY, YSH conducted the experiments;
LXX, WSY, YJJ, YH analyzed experimental results. YCL, LYY, WYH,
CST, XJ and GHL participated in solving the problems in the
experiment. LXX, WZR and JZ wrote the paper. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Clock
|
circadian locomotor output cycles
kaput
|
|
IQGAP1
|
IQ motif containing GTPase activating
protein 1
|
References
|
1
|
Fujioka A, Takashima N and Shigeyoshi Y:
Circadian rhythm generation in a glioma cell line. Biochem Biophys
Res Commun. 346:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng D and Lazar M: Clocks, metabolism,
and the epigenome. Molecular Cell. 47:158–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reppert SM and Weaver DR: Coordination of
circadian timing in mammals. Nature. 418:935–941. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doi M, Ishida A, Miyake A, Sato M, Komatsu
R, Yamazaki F, Kimura I, Tsuchiya S, Kori H, Seo K, et al:
Circadian regulation of intracellular G-protein signalling mediates
intercellular synchrony and rhythmicity in the suprachiasmatic
nucleus. Nat Commun. 2:3272011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamura H: Suprachiasmatic nucleus clock
time in the mammalian circadian system. Cold Spring Harb Symp Quant
Biol. 72:551–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu L, Pelicano H, Liu J, Huang P and Lee
C: The circadian gene Period2 plays an important role in tumor
suppression and DNA damage response in vivo. Cell. 111:41–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schibler U and Sassone-Corsi P: A web of
circadian pacemakers. Cell. 111:919–922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reppert SM and Weaver DR: Molecular
analysis of mammalian circadian rhythms. Annu Rev Physiol.
63:647–676. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Choo K, Hou M, Yeh K, Kuo S and
Chang J: Deregulated expression of the PER1, PER2 and PER3 genes in
breast cancers. Carcinogenesis. 26:1241–1246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roenneberg T and Lucas R: Light, endocrine
systems, and cancer-a view from circadian biologists. Neuro
Endocrinol Lett. 23 Suppl 2:S82–S83. 2002.
|
|
11
|
Stevens R: Circadian disruption and breast
cancer: From melatonin to clock genes. Epidemiology. 16:254–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moser M, Schaumberger K, Schernhammer E
and Stevens RG: Cancer and rhythm. Cancer Causes Control.
17:483–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hansen J: Risk of breast cancer after
night- and shift work: Current evidence and ongoing studies in
Denmark. Cancer Causes Control. 17:531–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou JX, Niehans GA, Shar A, Rubins JB,
Frizelle SP and Kratzke RA: Mechanisms of G1 checkpoint
loss in resected early stage non-small cell lung cancer. Lung
Cancer. 32:27–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rose SL and Buller RE: The role of p53
mutation in BRCA1-associated ovarian cancer. Minerva Ginecol.
54:201–209. 2002.PubMed/NCBI
|
|
16
|
Motokura T, Bloom T, Kim HG, Jüppner H,
Ruderman JV, Kronenberg HM and Arnold A: A novel cyclin encoded by
a bcl1-linked candidate oncogene. Nature. 350:512–515. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarparanta J, Jonson PH, Golzio C, Sandell
S, Luque H, Screen M, McDonald K, Stajich JM, Mahjneh I, Vihola A,
et al: Mutations affecting the cytoplasmic functions of the
co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat
Genet. 44(450–455): S451–452. 2012.
|
|
18
|
Hunter PJ, Swanson BJ, Haendel MA, Lyons
GE and Cross JC: Mrj encodes a DnaJ-related co-chaperone that is
essential for murine placental development. Development.
126:1247–1258. 1999.PubMed/NCBI
|
|
19
|
Hayashi H, Nabeshima K, Aoki M, Hamasaki
M, Enatsu S, Yamauchi Y, Yamashita Y and Iwasaki H: Overexpression
of IQGAP1 in advanced colorectal cancer correlates with poor
prognosis-critical role in tumor invasion. Int J Cancer.
126:2563–2574. 2010.PubMed/NCBI
|
|
20
|
Lin DC, Zhang Y, Pan QJ, Yang H, Shi ZZ,
Xie ZH, Wang BS, Hao JJ, Zhang TT, Xu X, et al: PLK1 Is
transcriptionally activated by NF-κB during cell detachment and
enhances anoikis resistance through inhibiting β-catenin
degradation in esophageal squamous cell carcinoma. Clin Cancer Res.
17:4285–4295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Revenu C, Ubelmann F, Hurbain I, El-Marjou
F, Dingli F, Loew D, Delacour D, Gilet J, Brot-Laroche E, Rivero F,
et al: A new role for the architecture of microvillar actin bundles
in apical retention of membrane proteins. Mol Biol Cell.
23:324–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song H, Li Y, Lee J, Schwartz AL and Bu G:
Low-density lipoprotein receptor-related protein 1 promotes cancer
cell migration and invasion by inducing the expression of matrix
metalloproteinases 2 and 9. Cancer Res. 69:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh Y and Seiki M: MT1-MMP: A potent
modifier of pericellular microenvironment. J Cell Physiol. 206:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alemayehu M, Dragan M, Pape C, Siddiqui I,
Sacks DB, Di Guglielmo GM, Babwah AV and Bhattacharya M:
β-Arrestin2 regulates lysophosphatidic acid-induced human breast
tumor cell migration and invasion via Rap1 and IQGAP1. PLoS One.
8:e561742013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiaoping L, Xiaowei Z, Leizhen Z and
Weijian G: Expression and significance of CD44 and p-AKT in
pancreatic head cancer. World J Surg Oncol. 13:3342015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McFarlane S, McFarlane C, Montgomery N,
Hill A and Waugh DJ: CD44-mediated activation of α5β1-integrin,
cortactin and paxillin signaling underpins adhesion of basal-like
breast cancer cells to endothelium and fibronectin-enriched
matrices. Oncotarget. 6:36762–36773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaigorodova EV, Zavyalova MV, Bychkov VA,
Perelmuter VM and Choynzonov EL: Functional state of the Hsp27
chaperone as a molecular marker of an unfavorable course of larynx
cancer. Cancer Biomark. 17:145–153. 2016. View Article : Google Scholar : PubMed/NCBI
|