Introduction
Colon cancer is a disease with a high incidence
among malignant tumors globally, and its incidence rate ranks third
among malignant tumors in developed countries in Europe and America
(1). At present, with the improvement
of China's economic level and changes in living habits, dietary
structure and lifestyle, the number of individuals suffering from
colon cancer in China shows an increasing trend.
Colon cancer is a malignant disease, which is a
malignant lesion not induced by a single factor but by a variety of
genes and with complex mechanisms of action. It seriously threatens
human health and the quality of life. However, colon cancer is
characterized by slow progression and a high cure rate in the early
stage. If effective interventions are conducted in the early stage,
the 5-year survival rate may reach 90% (2). Therefore, if the pathogenesis of colon
cancer can be effectively studied, and early screening and
diagnosis carried out, the timely treatment of colon cancer,
improvement of prognosis and the quality of life of patients would
be significantly influenced. The study of Olaku and White (3) showed that traditional Chinese medicines
and their active ingredients with obvious pharmacological activity
can significantly inhibit the proliferation of cancer cells or
induce cancer cell apoptosis with high efficiency and low toxicity
reducing the adverse effects of chemotherapy drugs. Therefore, the
active ingredients extracted from Chinese herbal medicines with
antitumor activity are very important screening methods for
antitumor drugs (4–6).
Aesculetin belongs to the natural coumarin
compounds, and studies have shown that aesculetin can significantly
inhibit the proliferation of melanoma cells, lung cancer cells,
gastric cancer cells and other tumor cells (7–10).
This study aimed to investigate the relevant
possible mechanisms in the process of growth and inhibition of
human colonic cancer cell line SW480, which provides a basis for
the clinical treatment of colon cancer using aesculetin.
Materials and methods
Materials and reagents
Aesculetin, bromodeoxyuridine (BrdU) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma-Aldrich, St. Louis, MO, USA); human colon cancer cell line
SW480 (Cell Bank, Chinese Academy of Sciences, Shanghai, China);
BrdU kit, rabbit anti-human glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; cat. no. 10494-1-AP), β-catenin (cat. no.
17565-1-AP), c-Myc (cat. no. 10828-1-AP) and cyclin D1 (cat. no.
60186-1-Ig) primary polyclonal antibodies and goat anti-rabbit
horseradish peroxidase (HRP)-labeled secondary polyclonal antibody
(cat. no. SA00001-2) (ProteinTech Group Inc., Wuhan, China);
Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA,
USA); ribonucleic acid (RNA) extraction kits, reverse transcription
kits, reverse transcription-polymerase chain reaction (RT-PCR) kits
(Invitrogen, Carlsbad, CA, USA); primer synthesis (Takara, Dalian,
China); and human immunization (Biotechnology Research Institute,
Nantong, China), bicinchoninic acid (BCA) protein quantification
kits and cell lysate (Beyotime Institute of Biotechnology, Nantong,
China) were used in the present study.
Cell culture
Colon cancer SW480 cells were cultured in DMEM
containing 10% fetal bovine serum, amino acid and double-antibodies
with 100 kU/l penicillin and 0.1% streptomycin in an incubator at
37°C, 5% CO2 and saturated humidity. The cells were
continuously subcultured, and the culture medium was regularly
replaced. When the cells grew to 80% confluence, trypsin was used
for digestion, and follow-up experiments were conducted.
Detection of the inhibition rate of
cell proliferation by cell counting kit-8 (CCK-8) assay
Colon cancer SW480 cells in the logarithmic growth
phase were used. After digestion, the cells were counted using a
cell counter, and 100 µl single cell suspension containing
1×105 cells was added to 96-well plates. After cells
adhered to the wall, the original culture medium was removed, and
100 µl drugs at the corresponding concentration were added
according to the experimental grouping. CCK-8 solution (10 µl) was
added to the 96-well plates at 10, 48 and 72 h. After 4 h, the
optical density (OD) of each well at the wavelength of 450 nm was
measured using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The inhibition rate was calculated as: (1-OD
value of the experimental group/OD value of the blank control
group) ×100%.
Detection of cell proliferation by
BrdU test
Cells (2×105) were inoculated in 24-well
plates with the volume of 200 µl, and three control wells were set.
The cells were incubated at 37°C with 5% CO2 for 24 h.
After cells adhered to the wall, and the solution was replaced with
serum-free medium, BrdU at the final concentration of 30 µg/l was
added before the termination of culture, and 40 min later, the
cells were fixed and added with mouse-derived BrdU monoclonal
antibodies overnight after blocking by goat serum. Secondary
antibodies were added, the anti-fluorescent quencher was added
dropwise after the washing with phosphate-buffered saline (PBS),
and the number of positive cells was observed under an inverted
phase microscope.
Detection of the expression of
β-catenin c-Myc and cyclin D1 messenger ribonucleic acid (mRNA) by
RT-PCR
The cultured SW480 cells were inoculated in 6-well
plates with 1×104/well. After 24 h, the supernatant was
aspirated, and cells were cultured for 48 h in the culture medium
containing 0.56, 1.12 and 2.24 mmol/l aesculetin, respectively.
Cells were collected from each group, and the total RNA was
extracted from tissues according to the protocol of the RNA
extraction kit. The concentration and purity of the total RNA were
detected by an ultraviolet-visible spectrophotometry (Hitachi,
Tokyo, Japan) (A260/A280 >1.8 results qualified). Complementary
deoxyribonucleic acid (cDNA) was obtained from the reverse
transcription according to the instructions of the reverse
transcription kit. Then the expression of β-catenin, c-Myc and
cyclin D1 mRNA was detected by RT-qPCR with cDNA as the template.
Primer sequences are shown in Table
I. Reaction conditions: at 95°C for 10 min, at 95°C for 15 sec,
at 60°C for 1 min, and the amplification was for 40 cycles. The Ct
value was calculated from the instrument software, and the relative
expression was calculated using the 2−ΔCq method
according to the following formula: ΔCq (target gene) = Cq (target
gene)-Cq (control gene).
 | Table I.Primer sequences of RT-qPCR. |
Table I.
Primer sequences of RT-qPCR.
| Gene | Primer sequences |
|---|
| β-catenin | F:
5′-GCTTGGAATGAGACTGCTGA-3′ |
|
| R:
5′-CTGGCCATATCCACCAGAGT-3′ |
| c-Myc | F:
5′-AGCGACTCTGAGGAGGAACA-3′ |
|
| R:
5′-TCCAGCAGAAGGTGATCCA-3′ |
| Cyclin D1 | F:
5′-TGCCACAGATGTGAAGTTCATT-3′ |
|
| R:
5′-CAGTCCGGGTCACACTTGAT-3′ |
| GAPDH | F:
5′-CAAGGTCATCCATGACAACTTTG-3′ |
|
| R:
5′-GTCCACCACCCTGTTGCTGTAG-3′ |
Detection of the expression of
β-catenin, c-Myc and cyclin D1 proteins by western blotting
The cultured cells were inoculated in 6-well plates
with 1×104/well. After 24 h, the supernatant was
aspirated, and the cells were cultured for 48 h in the culture
medium containing 0.56, 1.12 and 2.24 mmol/l aesculetin,
respectively. Cells were collected from each group and lysed using
cell lysates. The supernatant was collected after the
centrifugation by a high-speed centrifugal machine at 8,000 × g, at
a low temperature for 15 min. The concentration of extracted
proteins was determined by the BCA kit.
Sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) was used to separate 50 µg protein, and
the isolated protein was transferred to polyvinylidene difluoride
(PVDF) membranes. Proteins were sealed using blocking solution at
room temperature for 1 h, and incubated by primary antibodies
(1:1,000) at 4°C overnight. After that, the secondary antibodies
(1:2,000) were added for incubation at room temperature for 1 h,
and enhanced chemiluminescence (ECL) was used for development in
the dark. The gel imager (Bio-Rad Laboratories, Hercules, CA, USA)
was applied to scan records, and GADPH was taken as the
internal reference for gray-scale analysis and comparison.
Statistical analysis
Data were expressed as mean ± standard deviation and
processed by Statistical Product and Service Solutions (SPSS) 17.0
(International Business Machines Corp., Armonk, NY, USA). One-way
analysis of variance (ANOVA) was used to statistically analyze
data, and the post-hoc test used was SNK test. P<0.05 was set as
the statistically significant difference.
Results
Detection of the effects of aesculetin
on the inhibition rate of SW480 cells by CCK-8
The proliferation of SW480 cells was significantly
inhibited in each group after they were cultured in the culture
medium containing 0.56, 1.12 and 2.24 mmol/l aesculetin for 24, 48
and 72 h, respectively. By contrast, the proliferation inhibition
rate was significantly increased with the increase of concentration
and time in a significant dose-dependent manner (P<0.01)
(Table II). In the follow-up
experiments 0.56, 1.12 or 2.24 mmol/l aesculetin were selected as
the concentrations of administration, and the action time was 48
h.
 | Table II.Inhibitory effects of aesculetin at
different concentrations on the proliferation of SW480 cells (mean
± SD, n=30). |
Table II.
Inhibitory effects of aesculetin at
different concentrations on the proliferation of SW480 cells (mean
± SD, n=30).
|
| Proliferation
inhibition rate (%) |
|---|
|
|
|
|---|
| Concentration
(mmol/l) | 24 h | 48 h | 72 h |
|---|
| Control group
(0) | 0 | 0 | 0 |
| 0.56 |
17.29±1.25a |
35.61±2.25a |
39.53±3.12a |
| 1.12 |
26.64±1.56a |
50.12±3.32a |
60.25±2.56a |
| 2.24 |
36.72±1.96a |
68.22±3.83a |
72.65±3.08a |
Detection of the effects of aesculetin
on the proliferation of SW480 cells by BrdU tests
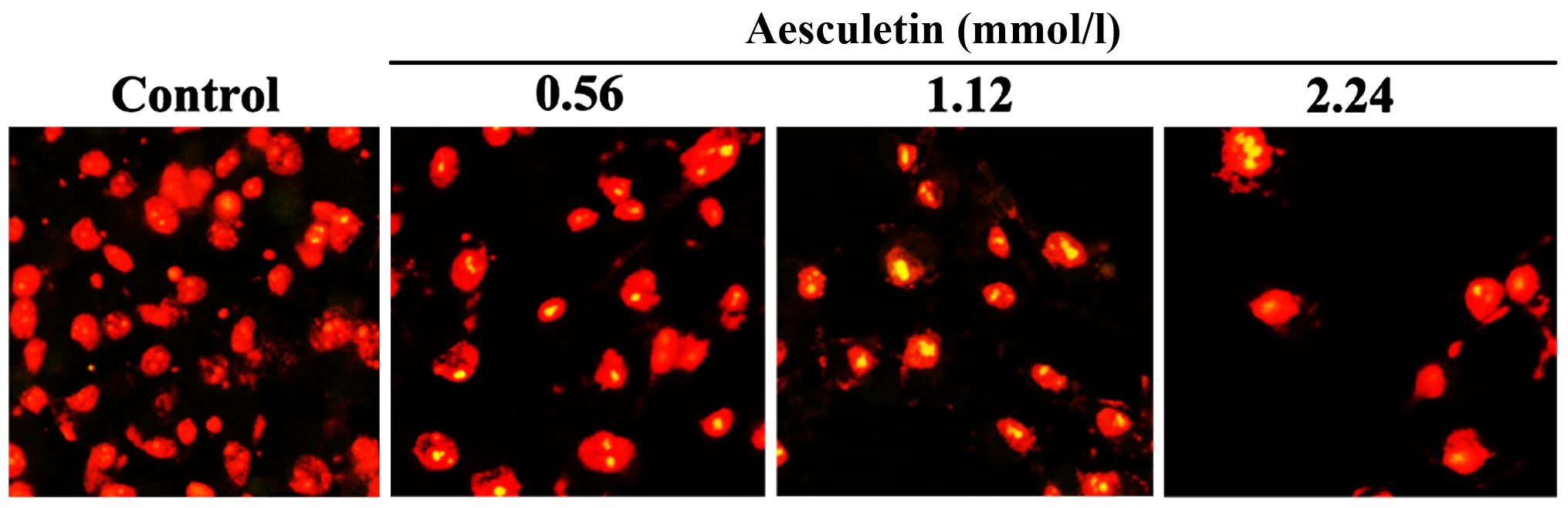
As shown in Fig. 1,
BrdU test results showed that compared with the control group, the
number of BrdU-positive cells in each group was significantly
different after the treatment with BrdU for the same period. The
number of the aesculetin group was significantly less than that in
the control group (P<0.01).
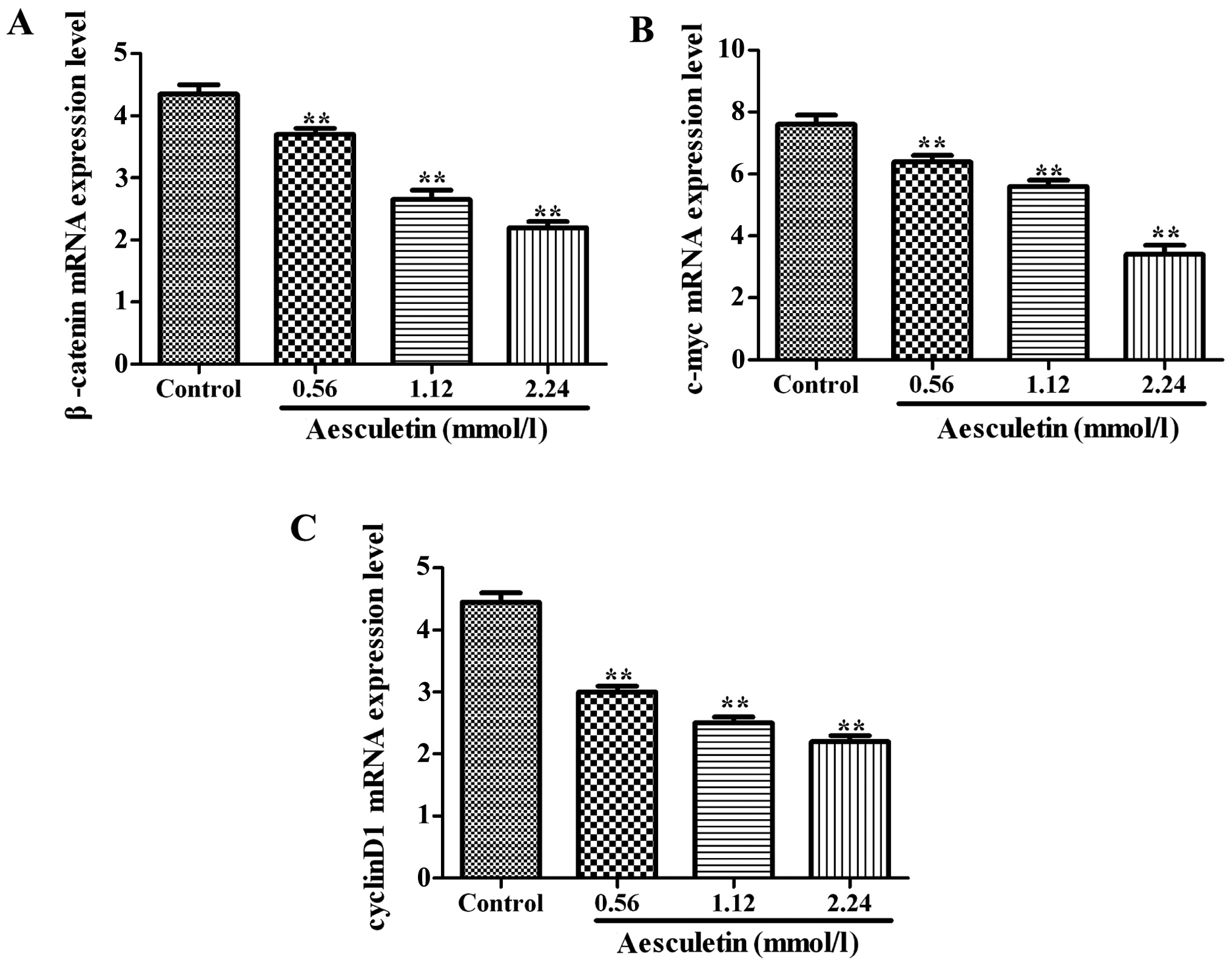
Effects of aesculetin on the levels of
β-catenin, c-Myc and cyclin D1 mRNA
As shown in Fig. 2,
the levels of β-catenin, c-Myc and cyclin D1 mRNA in each group
were significantly inhibited compared with those in the control
group after the cells were cultured in the culture medium
containing 0.56, 1.12 and 2.24 mmol/l aesculetin for 24, 48 and 72
h, respectively (P<0.01).
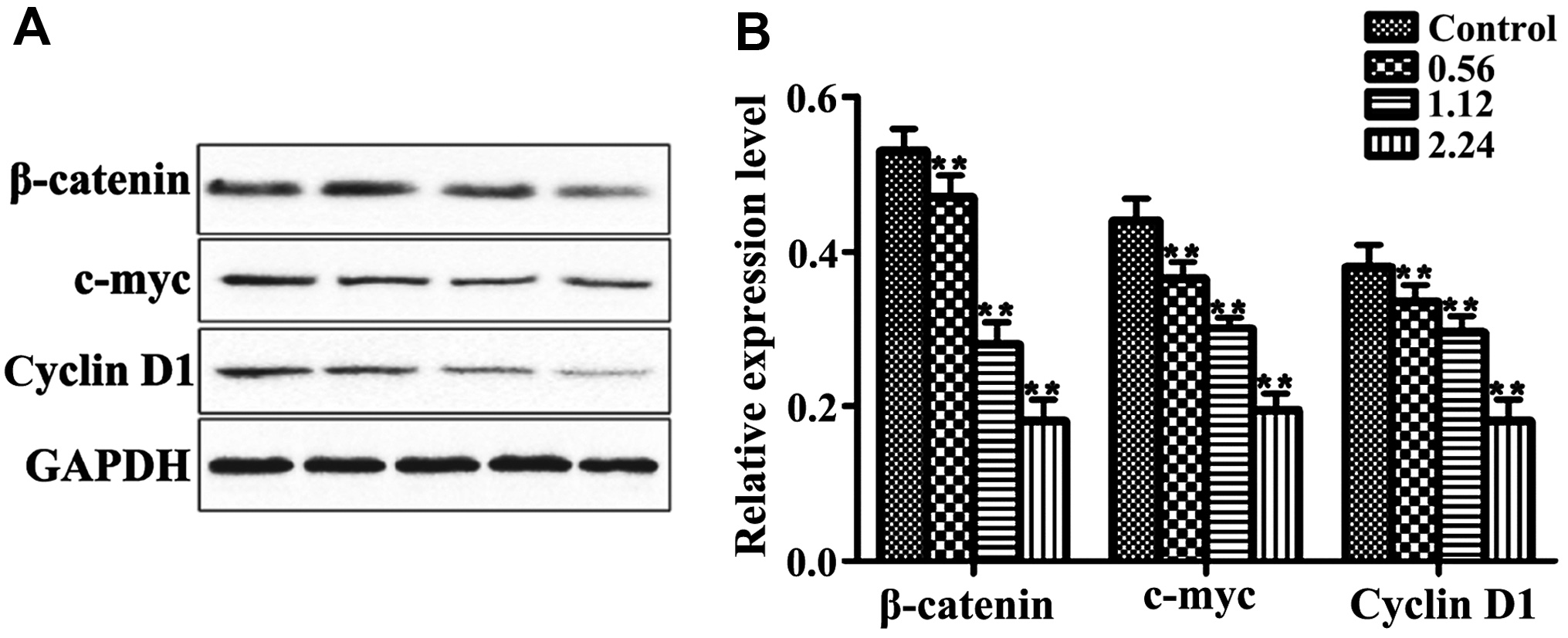
Effects of aesculetin on the
expression levels of β-catenin, c-Myc and cyclin D1 proteins
As shown in Fig. 3,
the levels of β-catenin, c-Myc and cyclin D1 proteins in each group
were significantly inhibited compared with those in the control
group after the cells were cultured in the culture medium
containing 0.56, 1.12 and 2.24 mmol/l aesculetin for 24, 48 and 72
h, respectively in a dose-dependent manner to a certain degree
(P<0.01).
Discussion
Colon cancer is a common tumor with a high incidence
rate clinically. In recent years, the number of deaths and the
mortality rate of colon cancer have shown a clear upward trend both
in China and other countries and regions. Colon cancer is an
important factor affecting human health and life. Tumor cell
metastasis or the loss of sensitivity to chemotherapeutic drugs is
the leading cause of death caused by colon cancer, and the
mechanisms of cell proliferation and invasion and the metastasis of
colon cancer are complex and involve changes in different genes
(11,12).
β-catenin is an intercellular adhesion molecule. It
participates in the cell adhesion on the cell membrane, but once
the nucleus is translocated or degraded, its adhesion activity
disappears (13). A study showed that
β-catenin, not only mediates cell adhesion, but also transducts
signals. The abnormal activation of Wnt/β-catenin signaling pathway
is one of the important mechanisms of human tumorigenesis. The
overexpression of β-catenin is also the main manifestation of the
activation of the signaling pathway (14). The proto-oncogenes, cyclin D1
and c-Myc, play important roles in the process of cell
proliferation, differentiation and apoptosis, and are related to
the development of various tumors. In recent years, a study showed
that cyclin D1 and c-Myc are very important target
genes in the Wnt signaling pathway (15). Immunohistochemistry confirmed that the
abnormal expression of cyclin D1, c-Myc and β-catenin are
correlated with the activation of the Wnt signaling pathway
(16). When the Wnt signaling pathway
is activated, the number of β-catenin entering the nucleus is
increased, further activating the expression of cyclin D1,
c-Myc and other genes and promoting cell proliferation
(17). In the nucleus, if β-catenin
is abnormally accumulated and activates genes, β-catenin becomes an
oncogene. A study indicated that β-catenin is related to the
occurrence of a variety of digestive system, hematological and
reproductive system tumors (18).
In this study, colon cancer cell line SW480 was
added with aesculetin at different final concentrations, and 24, 48
and 72 h later, the indexes were detected. The results of CCK-8
showed that compared with the control group, aesculetin effectively
inhibited the proliferation of SW480 cells. The BrdU test results
indicated that the number of BrdU-positive cells in all the groups
treated with drugs was significantly decreased. The detection
results of RT-PCR suggested that aesculetin reduced the expression
level of β-catenin mRNA and inhibited the expression of mRNA in Wnt
signaling pathway target genes, c-Myc and cyclin D1;
western blotting detection results revealed that aesculetin
downregulated the expression level of β-catenin, c-Myc and cyclin
D1 proteins. Utsuki et al (19) analyzed the expression levels of cyclin
D1 and β-catenin in tumor tissues and found that with tumor
deterioration, the expression levels of cyclin D1 and β-catenin
were gradually increased. In addition, Ehrlich et al
(20) showed that the positive
expression rates of β-catenin, cyclin D1 and c-Myc in
nephroblastoma cells were significantly increased compared with
those in normal renal tissues, indicating that the Wnt/β-catenin
signaling pathway plays an important role in the formation of
nephroblastoma. Studies of Hu et al (21) and Liu et al (22) showed that aesculetin can reduce the
level of β-catenin abnormally aggregated in tumor cells, thus
inhibiting the expression of cyclin D1 and c-Myc to inhibit the
growth of colon cancer cells. The results provide a theoretical
basis for the therapeutic effect of aesculetin on colon cancer.
In conclusion, this study demonstrates that
aesculetin can inhibit the proliferation of colon cancer cell line
SW480, and its mechanism may be achieved by inhibiting the Wnt
signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TL contributed significantly to writing the
manuscript and cell culture. LZ analyzed and interpreted CCK-8
assay. XH contributed significantly to manuscript preparation and
BrdU test. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olaku O and White JD: Herbal therapy use
by cancer patients: A literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang CJ, Hsieh YJ, Chu CY, Lin YL and
Tseng TH: Inhibition of cell cycle progression in human leukemia
HL-60 cells by esculetin. Cancer Lett. 183:163–168. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu CY, Tsai YY, Wang CJ, Lin WL and Tseng
TH: Induction of a poptosis by esculetin in human leukemia cells.
Eur J Pharmacol. 416:25–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuo HC, Lee HJ, Hu CC, Shun HI and Tseng
TH: Enhancement of esculetin on Taxol-induced apoptosis in human
hepatoma HepG2 cells. Toxicol Appl Pharmacol. 210:55–62. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leung KN, Leung PY, Kong LP and Leung PK:
Immunomodulatory effects of esculetin (6,7-dihydroxycoumarin) on
murine lymphocytes and peritoneal macrophages. Cell Mol Immunol.
2:181–188. 2005.PubMed/NCBI
|
|
8
|
Kaneko T, Tahara S and Takabayashi F:
Inhibitory effect of natural coumarin compounds, esculetin and
esculin, on oxidative DNA damage and formation of aberrant crypt
foci and tumors induced by 1,2-dimethylhydrazine in rat colons.
Biol Pharm Bull. 30:2052–2057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noguchi M, Earashi M, Minami M, Miyazaki
I, Tanaka M and Sasaki T: Effects of piroxicam and esculetin on the
MDA-MB-231 human breast cancer cell line. Prostaglandins Leukot
Essent Fatty Acids. 53:325–329. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Effect of esculetin on proliferation of
human heptocellular carcinoma cell line SMMC-7721 in vitro. Chin
JMAP. 26:6439–442. 2009.
|
|
11
|
Din FV, Theodoratou E, Farrington SM,
Tenesa A, Barnetson RA, Cetnarskyj R, Stark L, Porteous ME,
Campbell H and Dunlop MG: Effect of aspirin and NSAIDs on risk and
survival from colorectal cancer. Gut. 59:1670–1679. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyoshi K and Hennighausen L:
Beta-catenin: A transforming actor on many stages. Breast Cancer
Res. 5:63–68. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruyama K, Ochiai A, Akimoto S, Nakamura
S, Baba S, Moriya Y and Hirohashi S: Cytoplasmic beta-catenin
accumulation as a predictor of hematogenous metastasis in human
colorectal cancer. Oncology. 59:302–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
16
|
Koehler A, Schlupf J, Schneider M, Kraft
B, Winter C and Kashef J: Loss of Xenopus cadherin-11 leads to
increased Wnt/beta-catenin signaling and up-regulation of target
genes c-myc and cyclin D1 in neural crest. Dev Biol. 383:132–145.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim SC and Lee MS: Significance of
E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol
Rep. 9:915–928. 2002.PubMed/NCBI
|
|
18
|
Roh MS, Hong SH, Jeong JS, Kwon HC, Kim
MC, Cho SH, Yoon JH and Hwang TH: Gene expression profiling of
breast cancers with emphasis of beta-catenin regulation. J Korean
Med Sci. 19:275–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Utsuki S, Sato Y, Oka H, Tsuchiya B,
Suzuki S and Fujii K: Relationship between the expression of E-,
N-cadherins and beta-catenin and tumor grade in astrocytomas. J
Neurooncol. 57:187–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ehrlich D, Bruder E, Thome MA, Gutt CN,
von Knebel Doeberitz M, Niggli F, Perantoni AO and Koesters R:
Nuclear accumulation of beta-catenin protein indicates activation
of wnt signaling in chemically induced rat nephroblastomas. Pediatr
Dev Pathol. 13:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Wang S, Wu X, Zhang J, Chen R, Chen
M and Wang Y: Chinese herbal medicine-derived compounds for cancer
therapy: A focus on hepatocellular carcinoma. J Ethnopharmacol.
149:601–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|