Introduction
Despite decreasing rates of morbidity, gastric
cancer (GC) remains a high-incidence neoplastic disease that is
particularly prevalent in Eastern Asia (1); it is the third-leading cause of
cancer-associated mortality in the world (2). GC rates in men are approximately twice
as high as in women (1). To date,
although patients with early GC can receive effective treatment and
achieve a clinical cure, GC patients with moderate or advanced GC
lack an effective form of therapy.
MicroRNAs (miRNAs/miRs) are small, noncoding RNAs
~22 nucleotides in length that have notable functions in
development, cell differentiation and regulation of cell cycle and
apoptosis (3). miRNAs regulate gene
expression generally by inhibiting translation or degrading mRNA
transcripts (4). miRNA expression is
deregulated in cancer via a variety of mechanisms such as
amplification, deletion, mutation, and epigenetic silencing
(3).
miRNA-124 is abundantly and specifically expressed
in the brain, but is expressed at a low level in other tissues
(5); it has a critical role in
central nervous system development and function, as well as the
progression of various diseases in brain (5). Recently, miR-124 has been found
down-regulated in multiple tumor types, including cancer (6), prostate (7), lung (8)
and colorectal cancer (9). It also
been found that miR-124 could inhibit the proliferation of GC cells
proliferation by targeting Rho-associated protein kinase 1,
enhancer of zeste homolog 2 (EZH2) and sphingosine kinase 1 (SPHK1)
(10–12). A previous study demonstrated that
overexpression of miR-124 by transfection with miR-124 mimics could
inhibit GC cell growth, migration and invasion, and induce cell
cycle arrest by directly targeting the Notch ligand Jagged1 (JAG1)
in vitro (13). Furthermore,
Notch activation by the ectopic expression of intracellular domain
of Notch1 (NICD) negatively regulates miR-124 expression and
promotes cell growth, migration and invasion in GC cells (13). A recent study revealed that miR-124
was downregulated in GC tissues and was associated with lymph node
metastasis and tumor stages (14).
The results of the present study demonstrated that
miR-124 genes were methylated and the expression of miR-124 was
downregulated in GC specimens. Lentivirus-mediated overexpression
of miR-124 inhibited GC cell growth, migration and invasion, and
suppressed of GC cell xenografts growth in a nude mouse model. In
addition, overexpression of miR-124 suppressed JAG1 and EZH2
protein expression in GC cells. Silencing of JAG1 or EZH2 by RNA
interference inhibited GC cell growth, migration and invasion. The
expression of fibronectin and vimentin proteins was downregulated
by miR-124 overexpression or silencing of JAG1 or EZH2 in GC cells.
These data provided additional evidence of a pivotal role for
miR-124 in GC, and indicated that overexpression of miR-124 using
lentiviruses may be a feasible approach to treatment of GC.
Materials and methods
Clinical specimens and cell
culture
Gastric specimens were collected from 51 GC patients
that had received surgery in the First Affiliated Hospital of
Wenzhou Medical University (Wenzhou, Zhejiang, China) between
January 2014 and December 2015. These GC cases included 40 male and
11 female patients with a mean age of 65 years (range 37–86 years).
All patients had not received radiotherapy or chemotherapy prior to
surgery. Tissue samples were immediately snap-frozen in liquid
nitrogen after resection and stored at −80°C until subsequent
analyses. The tissues were verified by a trained pathologist.
Normal gastric samples were taken at a distance of at least 5 cm
from the tumor. The collection and analysis of clinical specimens
were approved by the Institutional Review Board of the First
Affiliated Hospital of Wenzhou Medical University. Written informed
consent was obtained from the patients prior to participation.
GC SGC-7901 and AGS cell lines, and 293T cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). SGC-7901 and AGS cells were cultured
in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 293T was cultured in Dulbecco's Modified Eagle's
Medium (DMEM) (Invitrogen; Thermo Fisher Scientific, Inc.), and
each medium were supplemented with 10% fetal bovine serum (FBS)
(Invitrogen; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). All cells were maintained in a humidified incubator at 37°C
with 5% CO2.
Methylation analysis
Genomic DNA was extracted from tissue samples using
a DNeasy Blood & Tissue kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. An EpiTect bisulfite
reagent kit (Qiagen, Inc.) was used to transform 120–200 ng of DNA
before it was subjected to polymerase chain reaction (PCR)
amplification of the specific promoter region using a primer set
(Table I) designed to amplify
methylated and unmethylated sequences of the miR-124 genes. This
reaction was performed by 2×Taq PCR Master Mix (Tiangen Biotech
Co., Ltd., Beijing, China). The thermocycling conditions were as
follows: 94°C for 5 min, 30 cycles of 94°C for 30 sec, 60°C for 30
sec, and 72°C for 30 sec, followed by a final extension at 72°C for
10 min. The results were analyzed by 1.5% agarose gel
electrophoresis and ethidium bromide was used to visualize DNA.
 | Table I.Primers for methylation-specific
polymerase chain reaction. |
Table I.
Primers for methylation-specific
polymerase chain reaction.
| Gene name | Methylation
status | Direction | Sequence, 5′-3′ |
|---|
| miR-124-1 | M | Forward |
AAAGAGTTTTTGGAAGACGTC |
|
|
| Reverse |
AATAAAAAACGACGCGTATA |
|
| N | Forward |
AATAAAGAGTTTTTGGAAGATGTT |
|
|
| Reverse |
AAAAAAATAAAAAACAACACATATAC |
| miR-124-2 | M | Forward |
GGGTAATTAATTTGGATTTACGTC |
|
|
| Reverse |
ACCGCTATTAATTAATCTATTCCG |
|
| N | Forward |
GGGGTAATTAATTTGGATTTATGTT |
|
|
| Reverse |
AAAACCACTATTAATTAATCTATTCCA |
| miR-124-3 | M | Forward |
GCGAGGATTTTACGTAAGTTC |
|
|
| Reverse |
CCGCGTACCTTAATTATATAA |
|
| N | Forward |
GGGTGAGGATTTTATGTAAGTTT |
|
|
| Reverse |
TTCACCACATACCTTAATTATATAAAC |
Lentiviral vector construction,
production and transduction
To construct lentiviral vectors expressing miR-124,
miR-124 precursors and their native context sequences (upstream and
downstream flanking genomic sequences) were amplified by PCR from
293T cell genomic DNA. The PCR products were digested with
SalI/EcoRI restriction enzymes and inserted into
corresponding sites of human U6 promoter-containing pBluescript SK
(+) plasmid (Stratagene; Aglient Technologies, Inc., Santa Clara,
CA, USA) (15). Next the obtained
constructs were digested with CalI/EcoRI and cloned
the U6-miRNA cassettes into a previously described lentiviral
vector (provided by Dr Chen Yangchao, Chinese University of Hong
Kong) (15). Lentivirus carrying
green fluorescent protein (GFP) (15)
were used as the control. Lentivirus carrying short hairpin RNA
(shRNA) targeting firefly luciferase (shLuc,
5′-TGCGCTGCTGGTGCCAACCCTATTCT-3′) or EZH2 (shEZH2,
5′-GACTCTGAATGCAGTTGCTTCAGTACCC-3′) was constructed as previously
described (16). Cells were
transduced with the lentiviruses using polybrene (8 µg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
siRNA transfection
siRNAs for JAG1 (target sequence,
5′-CCUGUAACAUAGCCCGAAA-3′) (17) and
negative control (NC) siRNA (target sequence,
5′-UUCUCCGAACGUGUCACGU-3′) were purchased from GenePharma
(GenePharma Co., Ltd., Shanghai, China). Cells were transfected
with 50 nM siRNA using Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manuscript introduction.
Subsequent experimentations were performed 48 h after
transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissue samples or cells
using TRIzol (Thermo Fisher Scientific Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The miR-124 RT
reaction was performed with specific RT primer using Revert Aid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific Inc.).
The expression levels of miR-124, JAG1 and EZH2 were evaluated by
qPCR using SYBRGreen PCR Master Mix (Takara Biotechnology Co.,
Ltd., Dalian, China) and an ABI7500 Real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: Initial denaturation 95°C for 30 sec,
followed by 40 cycles: 95°C for 5 sec and 60°C for 34 sec. The U6
small nuclear RNA was used for normalization of the mRNA expression
level of miR-124. GAPDH, an endogenous housekeeping gene, was used
for normalization of the mRNA expression levels of JAG1 and EZH2.
The specific RT primers and PCR primers are shown in Table II. The relative difference in
expression of studied genes between normal and GC samples was
represented by -ΔCq. To determine over-expression of miR-124 in GC
cells transduced with Lenti-miR-124 relative to control, the
2−ΔΔCq method was used to calculate fold changes values
(18).
 | Table II.Primers for RT-quantitative
polymerase chain reaction. |
Table II.
Primers for RT-quantitative
polymerase chain reaction.
| Gene name | Direction | Sequence,
5′-3′ |
|---|
| miR-124 | RT |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCATTCA |
|
| Forward |
ATGGTTGGTTGGTAAGGCACGCGG |
|
| Reverse |
GCAGGGTCCGAGGTATTC |
| U6 | RT |
AACGCTTCACGAATTTGCGT |
|
| Forward |
CTCGCTTCGGCAGCACA |
|
| Reverse |
AACGCTTCACGAATTTGCGT |
| JAG1 | Forward |
GGGGCAACACCTTCAACCTC |
|
| Reverse |
CCACGCCTCCACAAGCAAC |
| EZH2 | Forward |
CAAAGAGAAAAACCGACTGCG |
|
| Reverse |
GGCGACGAAGGCTTTGC |
| GAPDH | Forward |
TCCCATCACCATCTTCCAGG |
|
| Reverse |
GATGACCCTTTTGGCTCCC |
Cell proliferation assay
Cell proliferation was determined using the Cell
Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Briefly, 3,000 cells (100 µl/well) were seeded
into 96-well plates and cultured at 37°C. At 1, 2, 3 and 4 days
following cell seeding, 10 µl CCK-8 solution was added to each
well, cells were incubated for another 4 h at 37°C. After
incubation, a microplate reader was used to measure the
corresponding absorbance at 450 nm. Each condition was determined
in quintuplicate, and all experiments were repeated three
times.
Western blot analysis
Tissue samples or cells were lysed by
Radioimmunoprecipitation Assay (RIPA) buffer (Thermo Fisher
Scientific, Inc.) supplemented with Protease Inhibitor (Thermo
Fisher Scientific, Inc.). The protein concentration was quantified
using BCA protein assay kit (Shanghai Qcbio Technologies Co., Ltd.,
Shanghai, China) according to the manufacturer's protocol. The
proteins (40 µg per lane) were separated by 10% SDS-PAGE.
Electrophoresed proteins were transferred to a polyvinylidene
fluoride membrane and blocked for 2 h with 5% skimmed milk at room
temperature, and then incubated with rabbit anti-GAPDH (cat. no.
2118; dilution, 1:1,000; Cell Signaling Technology, Europe, B.V.,
Leiden, The Netherlands), rabbit anti-JAG1 (cat. no. SC8303;
dilution, 1:300; Santa Cruz Biotechnology, Dallas, TX, USA,),
rabbit anti-EZH2 (dilution, 1:1,000; Abcam, Cambridge, MA, USA),
mouse anti-vimentin (cat. no. 550513; dilution, 1:1,000; BD
Biosciences, San Jose, CA, USA), and mouse anti-fibronectin (cat.
no. SC18825; dilution, 1:1,000; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) primary antibodies at 4°C overnight. The next day,
the membranes were washed using Tris-buffered saline with 1%
Tween-20 (TBST), and incubated with horseradish peroxidase-labeled
secondary anti-mouse (cat. no. 31430; dilution, 1:2,000; Thermo
Fisher Scientific, Inc. Waltham, MA, USA) or anti-rabbit IgG
antibody (cat. no. 31460; dilution, 1:2,000; Thermo Fisher
Scientific, Inc.) at room temperature for 1 h. After washing with
TBST, all blots were visualized using enhanced chemiluminescence
substrate kit (Amersham, GE Healthcare, Chicago, IL, USA). GAPDH
was used as a loading control. The western blot figures were
acquired using ChemiDoc™ XRS+ Imaging Systems (BioRad
Laboratories, Inc., Hercules, CA, USA) and densitometry was
analyzed using Image Lab 4.1 software (BioRad Laboratories,
Inc.).
Cell migration and invasion
assays
Cell migration and invasion assays were performed
using a Transwell chamber (Corning Incorporated, Corning, NY, USA).
Cells (5×105/200 µl) incubated in serum-free RPMI-1640
medium were seeded in the upper chamber; the lower chamber
contained complete RPMI-1640 medium supplemented with 10% FBS. For
invasion assays, the upper chambers were pre-coated with Matrigel.
After incubation at 37°C for 16 h (migration) or 48 h (invasion),
migratory and invasive cells on the bottom surface were fixed with
4% paraformaldehyde in room temperature for 30 min and stained with
0.1% crystal violet solution, while the cells on the upper membrane
were removed. Five fields from each membrane were counted using a
×20 objective of light microscope (CKX41; Inverted Microscope,
Olympus Corporation, Tokyo, Japan).
Tumor xenografts growth in nude
mice
All animal studies complied with current ethical
considerations and were approved by the Laboratory Animal Ethics
Committee of Wenzhou Medical University. A total of 12 BALB/c nude
mice (male, 4–6 weeks old, 18–20 g) were obtained from the Shanghai
LAC Laboratory Animal Co. Ltd. (Shanghai, China) and housed in a
specific-pathogen-free grade animal center in a 12 h light/dark
cycle under controlled temperature (22–25°C) and given free access
to food and water. Mice were subcutaneously injected with
2.5×106 SGC7901 cells in the right side of the back;
tumor sizes were then recorded at the indicated times. The humane
endpoints were defined as a tumor size exceeding 2,000
mm3 or >10% body weight loss. Euthanasia was
performed at the endpoint and tumor nodules were dissected.
Statistical analysis
All experiments were performed in triplicate, and
data are expressed as the mean ± standard deviation. Statistical
analysis was performed using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA) or GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA). Statistically significant differences were calculated using
independent samples t-test, or one-way ANOVA followed by post-hoc
Tukey's analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression and methylation analyses of
miR-124 in human GC tissues
The expression level of miR-124 in 28 paired GC
tissues and adjacent normal tissue were evaluated by RT-qPCR. As
Fig. 1 depicts, the miR-124 levels in
GC tissues were significantly lower when compared with the normal
gastric tissues. miR-124 is present in three genomic loci
(miR-124-1 (8p23.1), miR-124-2 (8q12.3) and miR-124-3 (20q13.33))
(19), and miR-124 has been reported
to be inactivated by methylation in several tumor types, including
hepatocellular carcinoma (20).
Therefore, the DNA methylation status of the three CPG sites was
detected in 20 paired GC tissues and adjacent normal tissue using
methylation-specific PCR. The methylation levels of miR-124-1
(Fig. 1B), miR-124-2 (Fig. 1C) and miR-124-3 (Fig. 1D) were much higher in GC tissues than
in normal gastric tissues. These results indicated that miR-124 was
hypermethylated and therefore its expression was downregulated in
GC.
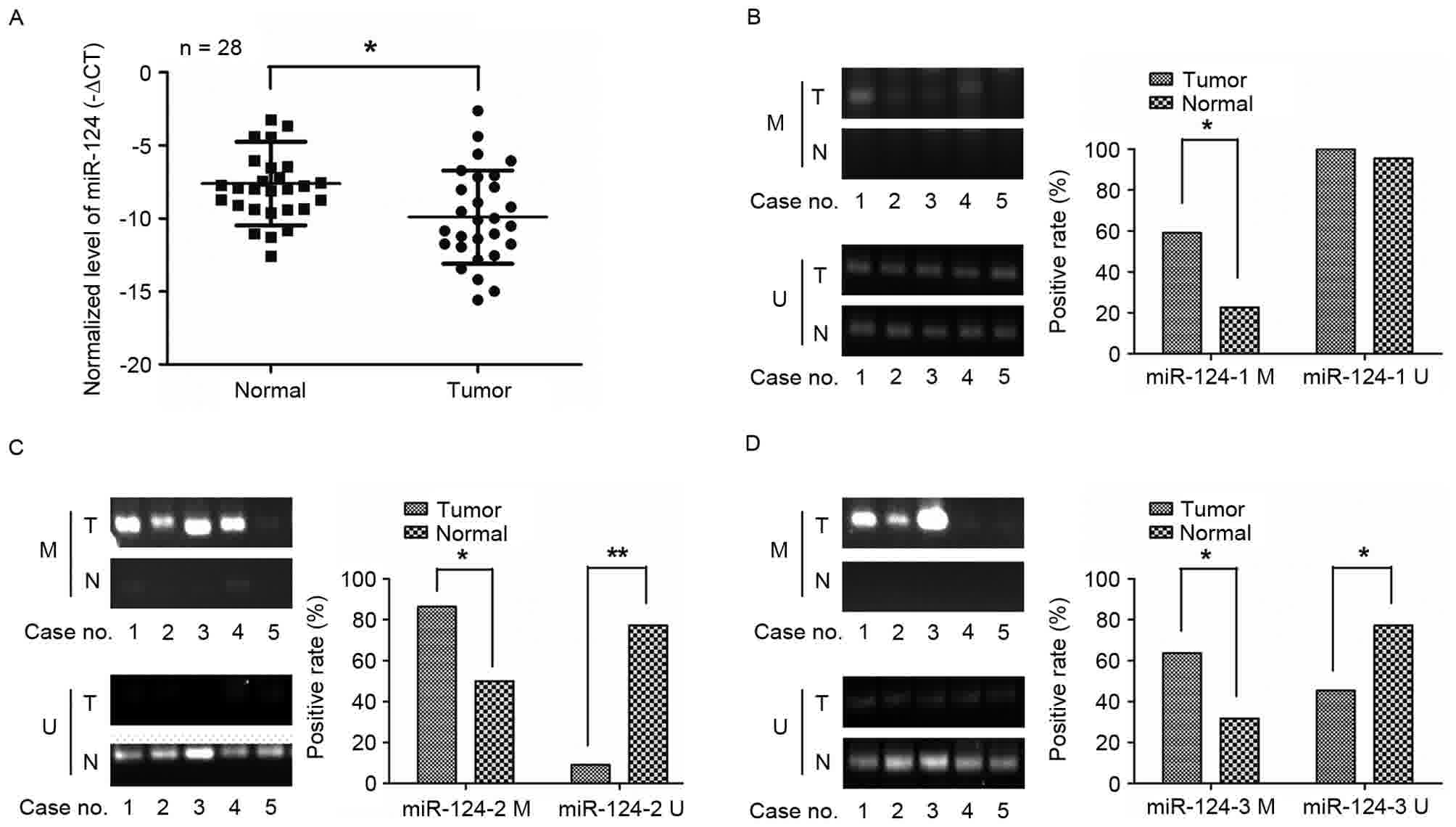 | Figure 1.miR-124 genes were frequently
methylated and the expression of miR-124 was downregulated in GC
tissues. (A) The relative expression levels of miR-124 were
detected by reverse transcription-quantitative PCR in 28 cases of
GC tissues and matched normal gastric tissues; (B-D) Comparison of
miR-124 genes promoter methylation states between GC and matched
normal gastric tissues. Methylation of the three miR-124 family
genes (miR-124-1, miR-124-2, and miR-124-3) was detected by
methylation-specific PCR. miR-124, microRNA-124; M, methylated; U,
unmethylated; GC, gastric cancer; miR-124, microRNA-124; T, tumor;
N, node; PCR, polymerase chain reaction. *P<0.05,
**P<0.01. |
Lentivirus-mediated overexpression of
miR-124 inhibited GC cell growth, migration and invasion
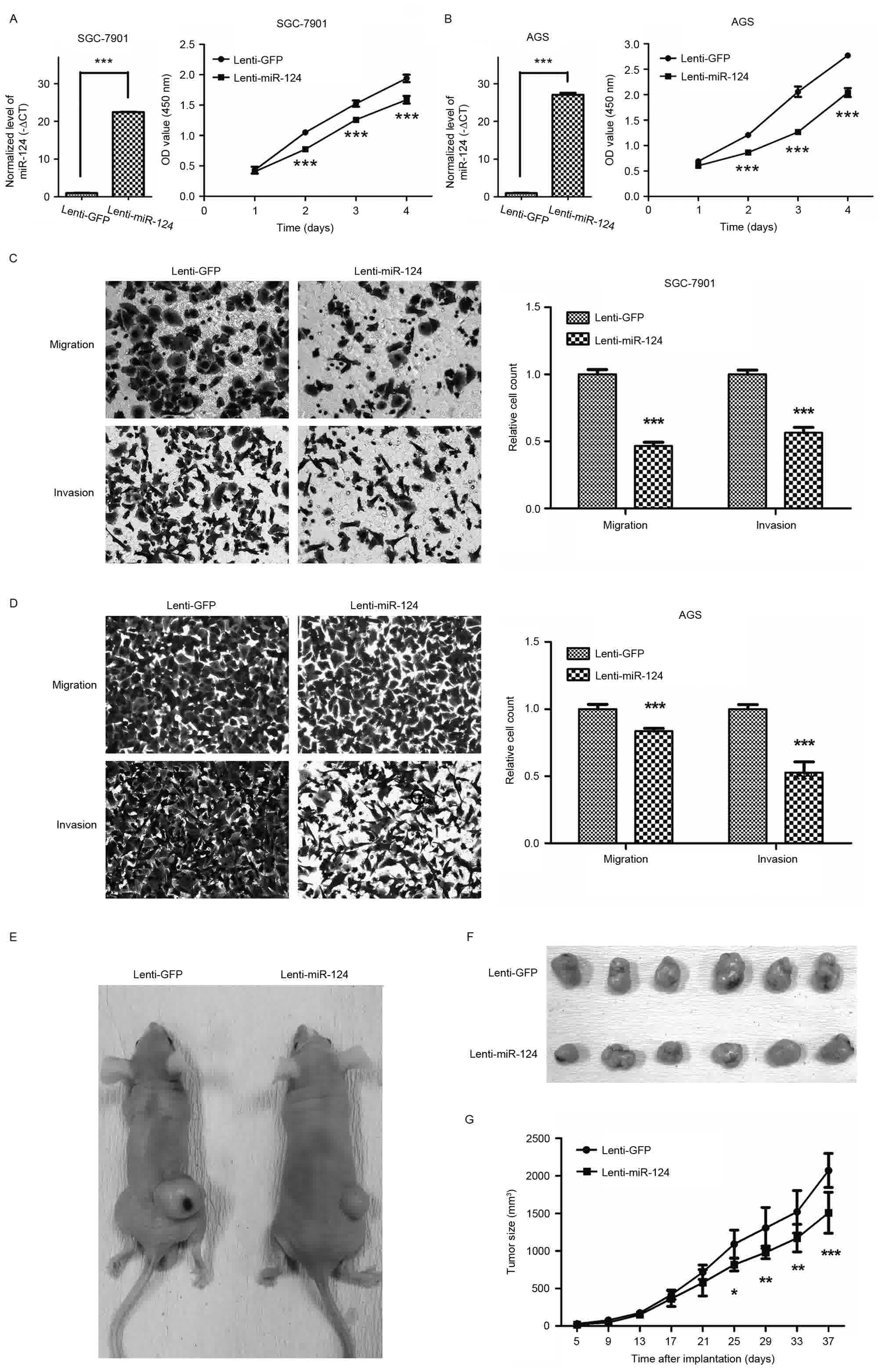
GC cell lines SGC-7901 and AGS cells were
transfected with the lentiviral vector expressing miR-124.
Overexpression of miR-124 in GC cells was confirmed by RT-qPCR
(Fig. 2A and B). To investigate the
role of miR-124 in GC cell growth, CCK8 assays were performed. As
depicted in Fig. 2A and B,
overexpression of miR-124 inhibited SGC-7901 and AGS cell
growth.
The effects of miR-124 on migration and invasion of
GC cells were evaluated using Transwell migration and invasion
assays. As Fig. 2C and D depicts,
compared with the empty-vector groups, those transfected with
miR-124 significantly inhibited the migratory and invasive
abilities of SGC-7901 and AGS cells (P<0.001). Ectopic stable
expression of miR-124 in SGC-7901 and AGS cells reduced the number
of migrated cells by 53.4 and 16.4% compared with GC cells
expressing GFP, respectively, and in the invasion assay by 43.6 and
47.3%, respectively.
To confirm the effect of miR-124 on GC cell growth
in vivo, xenograft models were generated by implanting
SGC-7901 cells transfected with lentivirus stably overexpressing
miR-124 or a control lentiviral vector into nude mice. The results
revealed that overexpression of miR-124 led a marked decrease in
the rate of subcutaneous xenograft tumor growth (Fig. 2E-G). Together, these findings
confirmed that miR-124 inhibits miR-124 GC cell growth, migration
and invasion.
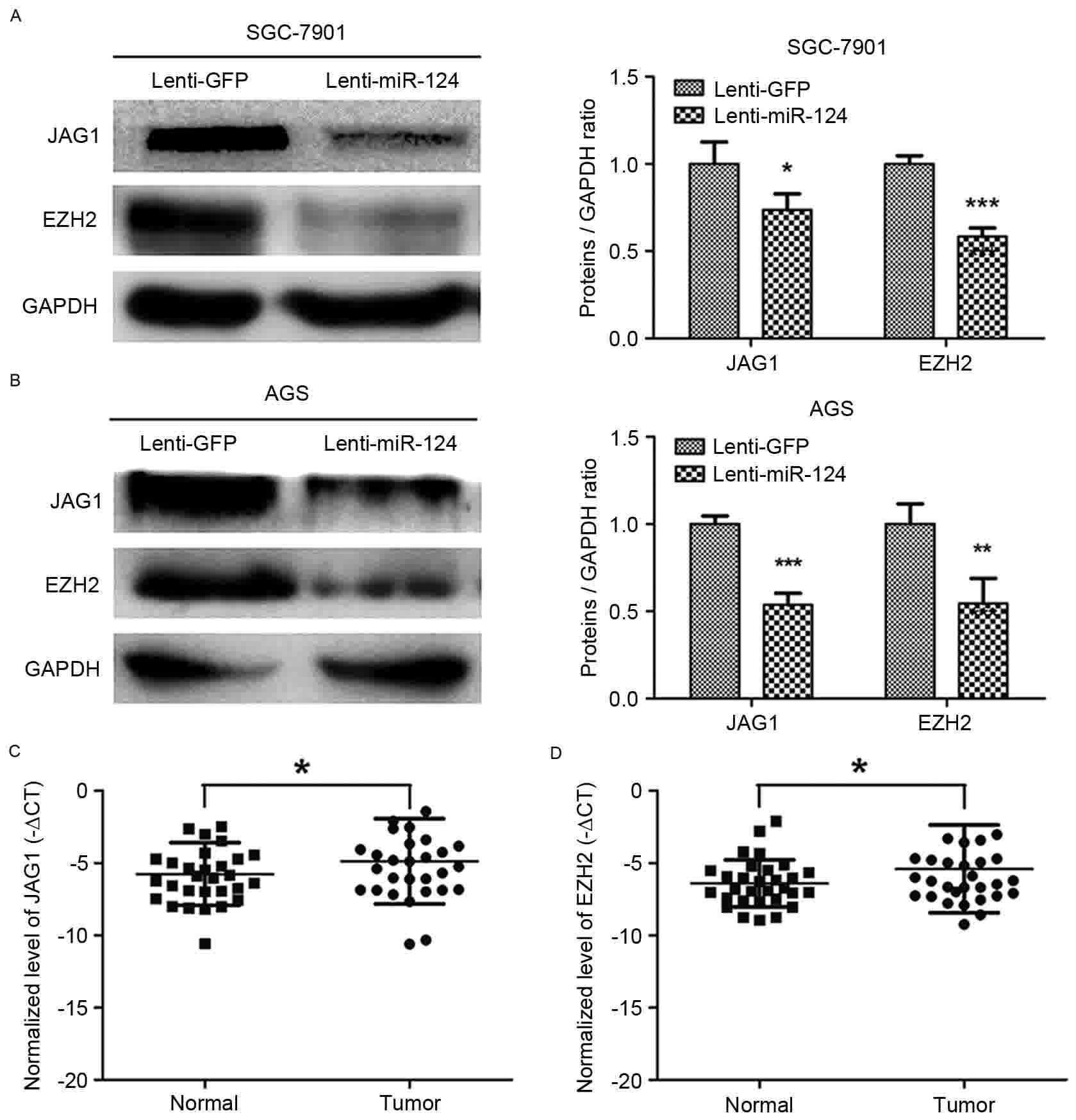
Overexpression of miR-124 repressed
the expression of the JAG1 and EZH2 in GC cell lines
JAG1 and EZH2 have been reported to be targets of
miR-124 (7,10,21). JAG1
and EZH2 protein expression levels were downregulated by
overexpression of miR-124 in SGC-7901 and AGS GC cell lines
(Fig. 3A and B). RT-qPCR was used to
quantify the levels of JAG1 and EZH2 in 30 GC and paired normal
gastric mucosa tissues, which confirmed the significant
upregulation of JAG1 and EZH2 in GC tissues (Fig. 3C and D). These results indicated that
the overexpression of miR-124 could repress JAG1 and EZH2
expression in GC cells.
Silencing JAG1 or EZH2 prevented GC
cells growth, migration and invasion, and downregulated the
expression levels of fibronectin and vimentin
To investigate the function of JAG1 in GC, specific
siRNAs were used to silence the expression of JAG1 in SGC-7901
cells (Fig. 4A). The CCK8-based
viability assay revealed a significant decrease in the
proliferation of SGC-7901 cells following knockdown of JAG1,
compared with the NC group (Fig. 4B).
Transwell migration and invasion assays revealed a significant
decrease in the migratory and invasive ability of GC cells knocked
down for JAG1 expression (Fig. 4C).
EZH2 was also silenced in SGC-7901 using a lentiviral
vector-mediated shRNA against EZH2 (Fig.
4D). As Fig. 4E depicts,
suppression of EZH2 led a marked decrease in the GC cell growth.
Moreover, knockdown of EZH2 markedly decreased the migration and
invasion ability of GC cells (Fig.
4F). Combined with the aforementioned experiments, which
demonstrated the antitumor function of miR-124 in GC, these results
indicated that miR-124 serves a tumor-suppressive role, at least
partly through directly repressing JAG1 and EZH2 in GC.
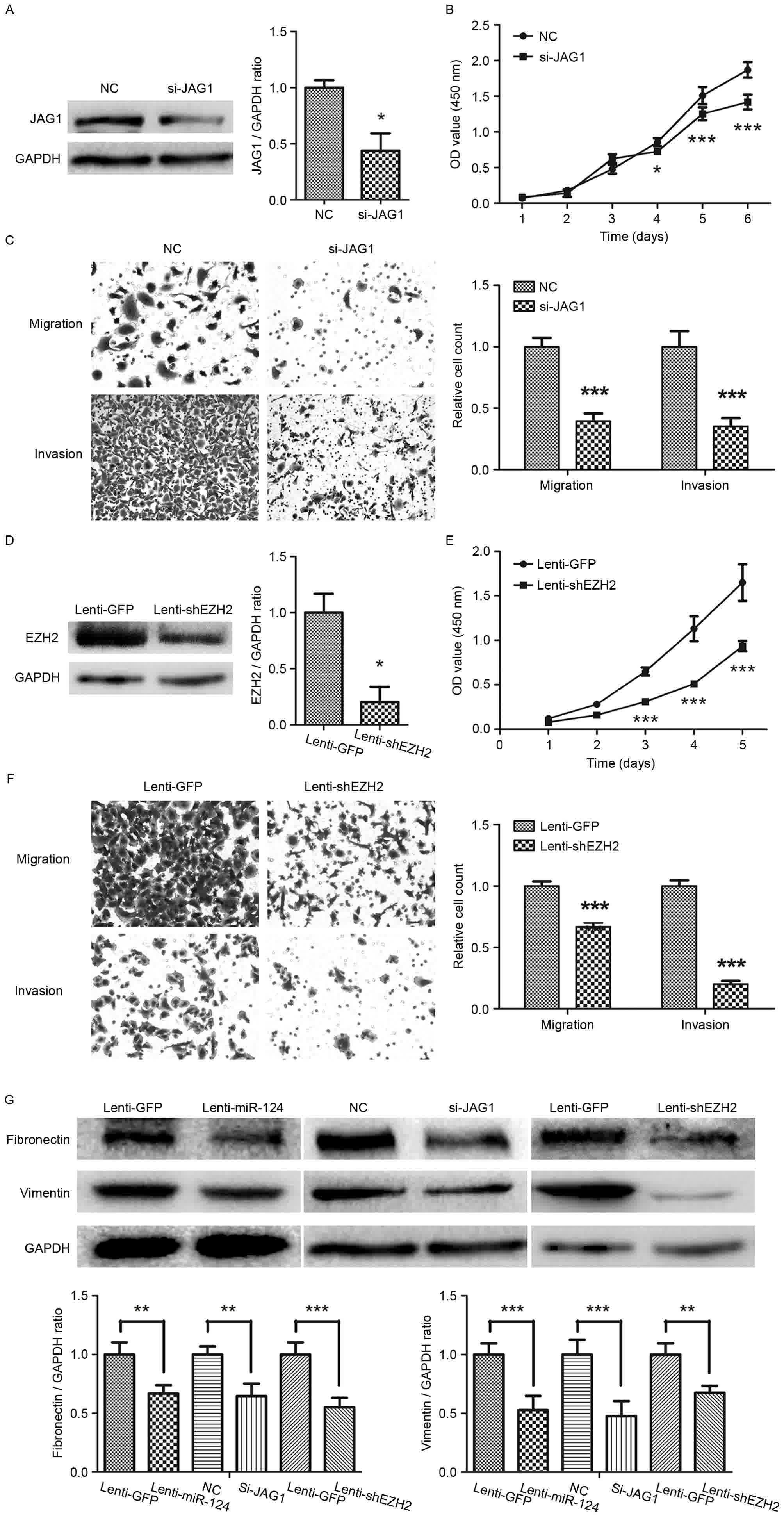 | Figure 4.Silencing JAG1 or EZH2 prevents
gastric cancer cell growth, migration and invasion. (A) Western
blotting revealing the result of JAG1 knockdown with JAG1 siRNA in
gastric cancer SGC-7901 cells; (B) CCK8 assays showed that
knockdown of JAG1 inhibited growth of SGC-7901 cells; (C) Knockdown
of JAG1 inhibited the migration and invasion abilities of SGC-7901
cell (magnification, ×200); (D) Western blotting depicting the
knockdown of EZH2 by lentiviral vector-mediated shRNA in SGC-7901.
(E) CCK8 assays showed that knockdown of EZH2 inhibited cell growth
of SGC-7901 cells. (F) The migratory and invasive abilities of
gastric cancer cell line SGC-7901 are reduced by transfection with
shRNA for EZH2 (magnification, ×200). (G) The levels of fibronectin
and vimentin protein expression, as determined by western blot
analysis. JAG1, jagged1; EZH2, enhancer of zeste homolog 2; siRNA,
small interfering RNA; CCK8, Cell Counting kit-8; shRNA, short
hairpin RNA. *P<0.05, **P<0.01, ***P<0.001. |
Fibronectin and vimentin, the mesenchymal markers,
were then detected by western blotting. As shown in Fig. 4G, there was significant decrease in
the expression levels of fibronectin and vimentin in GC cells
overexpressing miR-124 (Fig. 4G).
Knockdown of JAG1 or EZH2 also downregulated fibronectin and
vimentin expression in GC cells (Fig.
4G).
Discussion
miRNAs are small, noncoding RNAs ~22 nucleotides in
length that have notable functions in development, cell
differentiation and regulation of the cell cycle and apoptosis
(3). miRNAs are dysregulated in
various types of cancer, and detection of miRNA levels promises to
be a novel diagnostic indicator (22). miRNAs can function as oncogenes or
tumor suppressor genes in cancer, aiming at restoration of tumor
suppressor genes and silence of oncogenes would be a novel
anticancer therapy (23). The
inhibitory effect of miR-124 in tumor growth and migration, as well
as dysregulation of miR-124 has been reported in various tumor
types (6–9). In a previous (13) and the present study, miR-124 was
downregulated in GC tissues and cell lines. DNA hypermethylation at
CpG sites is believed to lead to the inactivation of tumor
suppressor genes or miRNAs in human cancer (24). To determine whether the methylation of
miR-124 occurs predominantly in GC, the methylation status of three
CpG sites of miR-124 gene was evaluated using methylation-specific
PCR in 20 GC tissues. The degree of methylation of three CpG sites
of the miR-124 gene in cancer tissues was higher than those in
normal gastric mucosa. These data indicated that the deregulation
of miR-124 in GC maybe due to the hypermethylation status of
miR-124.
Previous studies have revealed that downregulation
of miR-124 promotes cancer progression, and forced expression of
miR-124 suppresses cancer cell proliferation and inhibits
metastasis in specific cancer types including lung, breast and
prostate cancer (7,25–28). In
the present study, miR-124 was overexpressed using a lentiviral
vector system in GC cells; it was found that miR-124 inhibited GC
cell growth in vitro and in vivo, and prevented GC
cells migration and invasion. These findings indicated that miR-124
may be a potential therapeutic target of GC.
A prior study reported that miR-124 directly targets
JAG1(13). It has also been reported that miR-124 directly targets
EZH2 by binding the 3′-untranslated region of EZH2 (21). JAG1, as a Notch ligand, was found to
be upregulated in GC tissues compared with matched normal tissues
in the present study. Notch signaling is a highly conserved
cell-signaling pathway that is involved in cell proliferation,
differentiation and survival (29).
Dysregulation of the Notch signaling pathway often promotes tumor
formation and progression. Silencing of JAG1 by siRNA inhibited GC
cell growth, migration and invasion. Similar results were found in
GC cells transduced with shRNAs targeting EZH2. The present study
also determined that EZH2 was upregulated in GC tissues compared
with corresponding normal gastric tissues. Silencing EZH2 has also
been found to inhibit the proliferation of GC cells and the
invasive ability of hepatocellular carcinoma (HCC) cells (10,21).
Epithelial-mesenchymal transition is known to be a key event in the
invasion and metastasis of cancer cells (10,30). Owing
to the effects of miR-124 on GC cell migration and invasion,
fibronectin and vimentin, as the mesenchymal markers, were detected
in GC cells transduced with Lenti-miR-124. The expression of
fibronectin and vimentin were downregulated by miR-124 in GC cells.
Similarly, knockdown of JAG1 and EZH2 suppressed fibronectin and
vimentin expression. These results provide evidence that the
antitumor ability of miR-124 could be partly due to targeting of
JAG1 and EZH2 expression. However, further study is required to
confirm this conclusion.
In summary, the results of the present study
revealed that miR-124 genes were frequently methylated and the
expression of miR-124 was downregulated in GC tissues. The
lentivirus-mediated overexpression of miR-124 suppressed GC cell
growth, migration and invasion, and downregulated the expressions
of fibronectin and vimentin through repression of JAG1 and EZH2.
These findings indicate that the lentivirus-mediated overexpression
of miR-124 may be a potential therapeutic stategy against GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81672385), the
Science Research Foundation of National Health and Family Planning
commission of China (no. WKJ-ZJ-1416) and Zhejiang Provincial
Natural Science Foundation of China (no. LY14H16004).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YP, AW, FX, and CC conducted the study, performed
the statistical analysis. YP, LJ and RJ performed the study design
and drafted the manuscript. All the authors participated in the
discussion have read and approved the final manuscript.
Ethics approval and consent to
participate
The collection and analysis of clinical specimens
were approved by the Institutional Review Board of the First
Affiliated Hospital of Wenzhou Medical University. Written informed
consent was obtained from the patients prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin M, Shi C, Lin X, Pan J, Shen S, Xu Z
and Chen Q: sMicroRNA-1290 inhibits cells proliferation and
migration by targeting FOXA1 in gastric cancer cells. Gene.
582:137–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in Cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayes EL and Lewis-Wambi JS: Mechanisms of
endocrine resistance in breast cancer: An overview of the proposed
roles of noncoding RNA. Breast Cancer Res. 17:402015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Luo ZM, Guo XM, Su DF and Liu X: An
updated role of microRNA-124 in central nervous system disorders: A
review. Front Cell Neurosci. 9:1932015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng T, Shao F, Wu Q, Zhang X, Xu D, Qian
K, Xie Y, Wang S, Xu N, Wang Y and Qi C: miR-124 downregulation
leads to breast cancer progression via LncRNA-MALAT1 regulation and
CDK4/E2F1 signal activation. Oncotarget. 7:16205–16216.
2016.PubMed/NCBI
|
|
7
|
Shi XB, Ma AH, Xue L, Li M, Nguyen HG,
Yang JC, Tepper CG, Gandour-Edwards R, Evans CP, Kung HJ, et al:
miR-124 and androgen receptor signaling inhibitors repress prostate
cancer growth by downregulating androgen receptor splice variants,
EZH2 and Src. Cancer Res. 75:5309–5317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zu L, Xue Y and Wang J, Fu Y, Wang X, Xiao
G, Hao M, Sun X, Wang Y, Fu G and Wang J: The feedback loop between
miR-124 and TGF-β pathway plays a significant role in non-small
cell lung cancer metastasis. Carcinogenesis. 37:333–343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Liu S, Tian L, Wu M, Ai F, Tang W,
Zhao L, Ding J, Zhang L and Tang A: miR-124 and miR-506 inhibit
colorectal cancer progression by targeting DNMT3B and DNMT1.
Oncotarget. 6:38139–38150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia J, Wu Z, Yu C, He W, Zheng H, He Y,
Jian W, Chen L, Zhang L and Li W: miR-124 inhibits cell
proliferation in gastric cancer through down-regulation of SPHK1. J
Pathol. 227:470–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu CB, Li QL, Hu JF, Zhang Q, Xie JP and
Deng L: miR-124 inhibits growth and invasion of gastric cancer by
targeting ROCK1. Asian Pac J Cancer Prev. 15:6543–6546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Lin T, Xu C, Hu S, Pan Y and Jin
R: miR-124 interacts with the Notch1 signalling pathway and has
therapeutic potential against gastric cancer. J Cell Mol Med.
20:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Ye JX, Qin YZ, Chen QH and Ge LY:
Evaluation of miR-29c, miR-124, miR-135a and miR-148a in predicting
lymph node metastasis and tumor stage of gastric cancer. Int J Clin
Exp Med. 8:22227–22236. 2015.PubMed/NCBI
|
|
15
|
Jiang L, Lai YK, Zhang J, Wang H, Lin MC,
He ML and Kung HF: Targeting S100P inhibits colon cancer growth and
metastasis by Lentivirus-mediated RNA interference and proteomic
analysis. Mol Med. 17:709–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Lin MC, Wang H, Chan CY, Jiang L,
Ngai SM, Yu J, He ML, Shaw PC, Yew DT, et al: Proteomic analysis of
EZH2 downstream target proteins in hepatocellular carcinoma.
Proteomics. 7:3097–3104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steg AD, Katre AA, Goodman B, Han HD, Nick
AM, Stone RL, Coleman RL, Alvarez RD, Lopez-Berestein G, Sood AK
and Landen CN: Targeting the notch ligand JAGGED1 in both tumor
cells and stroma in ovarian cancer. Clin Cancer Res. 17:5674–5685.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang P, Chen L, Zhang J, Chen H, Fan J,
Wang K, Luo J, Chen Z, Meng Z and Liu L: Methylation-mediated
silencing of the miR-124 genes facilitates pancreatic cancer
progression and metastasis by targeting Rac1. Oncogene. 33:514–524.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH,
Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, et al: The putative
tumour suppressor microRNA-124 modulates hepatocellular carcinoma
cell aggressiveness by repressing ROCK2 and EZH2. Gut. 61:278–289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saito Y: Alterations of epigenetics and
microRNAs in cancer and cancer stem cell. Front Genet. 5:2832014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang N, Huang Y, Wu F, Zhao Y, Li X, Shen
P, Yang L, Luo Y, Yang L and He G: Codelivery of a miR-124 mimic
and obatoclax by cholesterol-penetratin micelles simultaneously
induces apoptosis and inhibits autophagic flux in breast cancer in
vitro and in vivo. Mol Phar. 13:2466–2483. 2016. View Article : Google Scholar
|
|
26
|
Feng T, Xu D, Tu C, Li W, Ning Y, Ding J,
Wang S, Yuan L, Xu N, Qian K, et al: MiR-124 inhibits cell
proliferation in breast cancer through downregulation of CDK4.
Tumour Biol. 36:5987–5997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Mao YQ, Wang H, Yin WJ, Zhu SX
and Wang WC: MiR-124 suppresses cell motility and adhesion by
targeting talin 1 in prostate cancer cells. Cancer Cell Int.
15:492015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Ai X, Shen S and Lu S:
NF-κB-mediated miR-124 suppresses metastasis of non-small-cell lung
cancer by targeting MYO10. Oncotarget. 6:8244–8254. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S,
Wu GS and Wu K: Notch signaling: An emerging therapeutic target for
cancer treatment. Cancer Lett. 369:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|