Introduction
Hepatitis B virus (HBV) is a DNA virus belonging to
the Hepadnaviridae family with an enveloped nucleocapsid containing
a partially double-stranded relaxed circular DNA of ~3.2 kb in
length with four partially overlapping open-reading frames (S/PreS,
C/PreC, P and X) that encode for the viral proteins (1). HBV infection can cause acute and chronic
infection, which may ultimately lead to cirrhosis and
hepatocellular carcinoma (HCC) (2–4).
Worldwide, more than two billion people are infected with HBV, of
which 350 million are chronic HBV carriers. Chronic HBV infection
is a major risk factor for liver disease, including liver cancer.
The total number of people dying from liver fibrosis and HCC caused
by HBV each year has reached one million (5,6).
The mechanisms involved in the progression and
development of HBV-related HCC are not fully understood. Currently,
the pathogenicity of HBV is not considered to be attributable to
the direct killing of liver cells; instead, it is attributed to the
immune dysfunction that occurs subsequent to HBV infection
(7). The complement system consists
of a group of globulins with enzymatic activity and no heat
tolerance; the most important components of the complement system
are C3 and C4. As part of the body's innate immune system, these
components are involved in regulating the body's immunity against
invasion from foreign pathogens (8).
However, viruses have developed a number of strategies to evade
attack by the complement components (9); for example, certain types of viruses can
incorporate the regulatory proteins for host complement into their
viral envelope and regulate the expression of these proteins in
infected cells (10–12).
In the present study, the serological levels of C3
and C4 were compared between healthy controls and patients with
chronic hepatitis B (CHB), and the molecular mechanisms at the
cellular level were explored, which is expected to lay the
foundation for understanding the pathogenesis of HBV.
Materials and methods
Subjects
A total of 226 patients with a clinical diagnosis of
HBV hepatitis from Renmin Hospital of Wuhan University (Wuhan,
China) from March 2010 to January 2016 were included in the present
study, including 136 male and 90 female patients, with a mean age
of 53.7±14.2 years; this included 153 cases of CHB and 73 cases of
HCC. A total of 116 healthy individuals were selected to form the
control group, including 73 male and 43 female patients, with a
mean age of 49.8±13.6 years. The included patients did not exhibit
disease of the heart, brain, kidneys and other vital organs, or
infection with any other hepatotropic virus. The present study was
approved by the Ethical Committee of Renmin Hospital of Wuhan
University and written informed consent was obtained from all
patients.
Cell culture and transfection
Huh7 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured with RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 mg/l streptomycin in a 37°C incubator with
5% CO2. Prior to transfection, the Huh7 cells were
seeded in 6-well plates. For transfection, 2.4 µg pHBV1.3 or
pBlue-ks plasmid (Agilent Technologies, Inc., Santa Clara, USA) DNA
and 5 µl Lipofectamine 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) were diluted in 100 µl serum- and
antibiotic-free RPMI-1640 medium. Subsequent to incubation at room
temperature for 20 min, the obtained transfection solution was
added to the cell culture medium, and the cells were incubated for
48 h.
Reverse transcription-semiquantitative
polymerase chain reaction (RT-sqPCR)
The transfected Huh7 cells were collected for total
RNA extraction, which was performed using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol; the total RNA was subsequently
subjected to sqPCR with primers for C3 and C4. The sequences of the
primers were as follows: C3 forwards, 5′-ACGGCATCCTCTGTCATCT-3′,
reverse 5′-ACGGCATCCTCTGTCATCT-3′; C4 forwards
5′-CGAGGACAGGTAGTGAAAGG-3′, reverse 5′-GGCCAGGGTTGTAAATGG-3′;
β-actin forwards 5′-ATGATATCGCCGCGCTCG-3′, reverse
5′-CGCTCGGTGAGGATCTTCA-3′. β-actin served as an internal reference
for densitometry ImageQuant™ TL 7.0 software (GE
Healthcare, Chicago, Il, USA). The PCR products were detected by 1%
agarose gel electrophoresis.
Western blotting
Huh7 cells were harvested and sonicated in
radioimmunoprecipitation assay lysis buffer (BioTeke Corporation,
Beijing, China) and the protein concentration was determined using
the Coomassie brilliant blue G250 method. SDS-PAGE (12%) was
performed using 30 mg protein mixed with equal volume of sample
loading buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1 mM
EDTA, 10% glycerol, and protease inhibitor cocktail, pH 7.4).
Subsequent to electrophoresis, the separated proteins were
transferred onto a nitrocellulose (NC) membrane, which was blocked
with 5% skimmed milk for 2 h, followed by incubation with C3 (cat.
no. C6025) and C4 monoclonal antibodies (cat. no. SAB1403623) for 2
h at room temperature (dilution, 1:2,000; Sigma Aldrich; Merck
KGaA, Darmstadt, Germany). The NC membrane was washed three times
in PBS with Tween (PBST), followed by incubation with a horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (cat. no.
7074; dilution, 1:5,000; Cell Signaling Technology, Inc., Danvers,
MA, USA) for 1 h at room temperature; then the membrane was washed
four times with PBST. Development was performed using an
electrogenerated chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.).
Measurement of complement C3 and
C4
Assays for Complement C3 and C4 in serum of patients
with HBV infection and healthy controls were conducted using a
Human Complement C3 and Complement C4 Multiplex EFSIA kit (cat. no
ABIN1774745; Beijing 4A Biotech Co., Ltd, Beijing, China) according
to the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS Inc., Chicago, IL, USA). The measurement data were presented
as the mean ± standard deviation. A one-way analysis of variance
was used for comparisons of C3 and C4 levels between the 3 disease
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
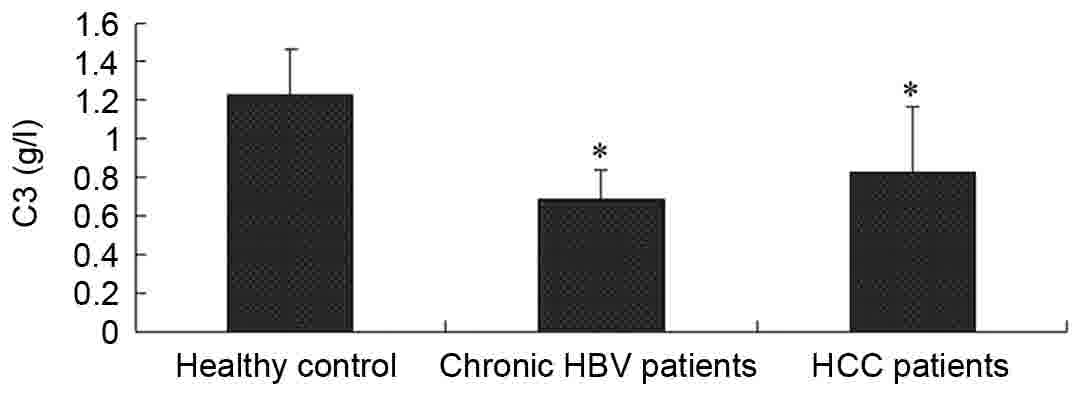
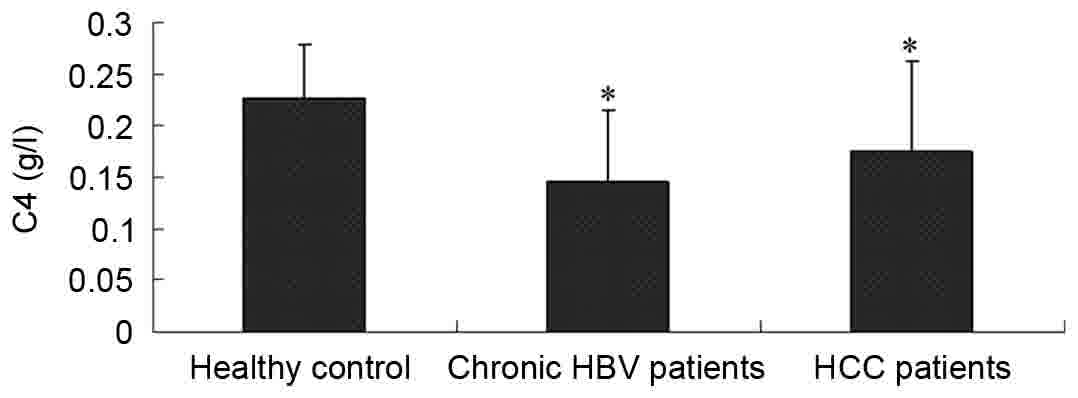
The serum levels of complement C3 and
C4 are decreased in patients with HBV
The clinical parameters of the controls and case
subjects enrolled in the study are included in Table I. The serum levels of complement C3
and C4 for the healthy subjects and the patients with HBV were
detected by an immunoturbidimetric assay. As included in Table I, the serum levels of C3 and C4 were
1.223±0.237 and 0.226±0.052 g/l in the healthy controls,
0.687±0.150 and 0.145±0.070 g/l in the patients with CHB, and
0.829±0.332 and 0.174±0.088 g/l in the patients with HCC,
respectively. Compared with the healthy control group, the levels
of complement C3 and C4 in the patients with CHB and HCC were
significantly lower (P<0.05), as demonstrated in Figs. 1 and 2.
 | Table I.Clinical parameters of the subjects
enrolled in the study. |
Table I.
Clinical parameters of the subjects
enrolled in the study.
| Clinical
parameters | Healthy controls
(n=116) | Chronic HBV patients
(n=153) | HCC patients
(n=73) |
|---|
| Age (years) | 49.8±13.6 | 46.2±14.7 | 58.3±14.2 |
| Gender
(male/female) | 73/43 | 97/56 | 49/24 |
| BMI | 25.2±1.6 | 24.9±1.4 | 24.4 ± 1.5 |
| ALT (IU/l) | <30 | 168.3±114.6 | 63.3±44.7 |
| AST (IU/l) | <30 | 216.2±116.7 | 72.5±58.1 |
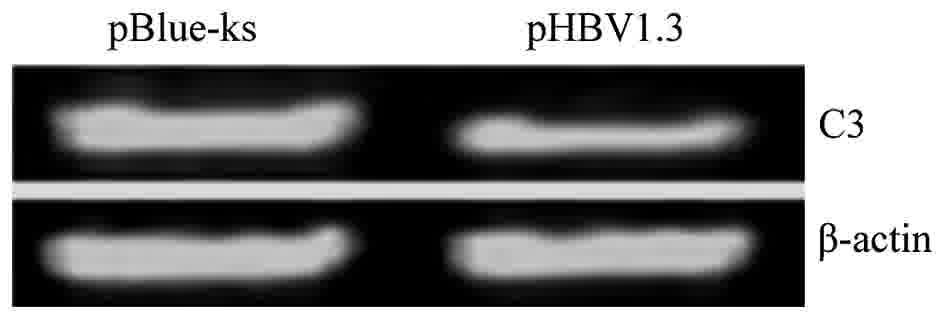
HBV inhibits the mRNA and protein
expression of complement C3 and C4
To investigate the molecular mechanism of HBV in
regulating the expression of complement C3 and C4, the HBV
infectious clone pHBV1.3 was transfected into the Huh7 cells. The
empty vector pBlue-ks was used as a negative control. Huh7 cells
transfected with pHBV1.3, an HBV infectious clone, synthesize and
secrete HBV viral particles, as previously described (13).
The expression levels of C3 and C4 mRNA were
detected using RT-sqPCR. The results showed that the expression
levels of C3 and C4 mRNA were reduced in Huh7 cells transfected
with pHBV1.3 (C3/β-actin ratio of 0.96 and C4/β-actin ratio of
0.90; data not shown) compared to Huh7 cells transfected with
pBlue-ks (C3/β-actin ratio of 0.33 and C4/β-actin ratio of 0.29;
data not shown, representative images of the PCR products are
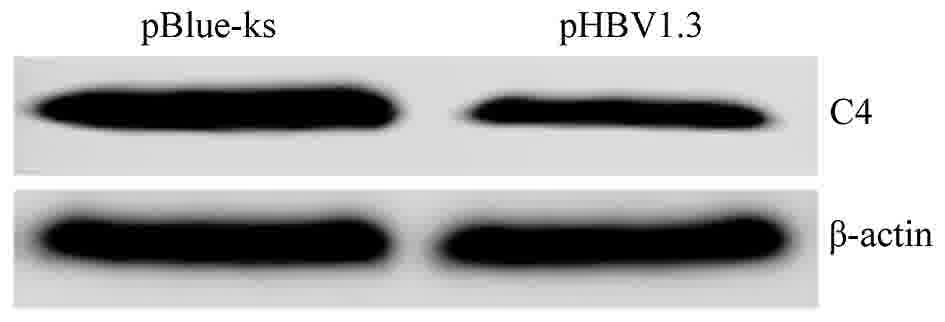
included in Figs. 3 and 4). In order to test the effect of HBV on the
protein expression of C3 and C4, the protein expression levels of
C3 and C4 were detected by western blotting. The results
demonstrated that the expression levels of C3 and C4 proteins were
reduced in Huh7 cells transfected with pHBV1.3 (C3/β-actin ratio of
0.78 and C4/β-actin ratio of 1.09; data not shown) compared to Huh7
cells transfected with pBlue-ks (C3/β-actin ratio of 0.36 and
C4/β-actin ratio of 0.31; data not shown, representative images of
the western blots are included in Figs.
5 and 6), indicating that HBV
could inhibit the mRNA and protein expression of C3 and C4.
Discussion
The complement system is the first line of immune
defense against foreign pathogens in the host, including the
defense against viruses (14). The
complement constituents adhere to the surface of the pathogen,
promoting the phagocytosis of the host cell, the formation of the
membrane attack complex, the dissolution of pathogens and the
release of anaphylatoxin to cause inflammation and promote the
elimination of pathogens (15,16).
However, in the process of evolving with the host, certain viruses
have established strategies to escape the complement system,
including encoding the membrane complement regulatory proteins from
the host, and entering the host cells using the membrane complement
receptor of the host (17–20).
In the present study, serum was collected from
clinically diagnosed HBV-infected patients and healthy controls,
and the levels of C3 and C4 were determined using
immunoturbidimetric assays. Statistical analysis indicated that the
expression levels of C3 and C4 in patients with HBV were
significantly lower than in the healthy controls, and the serum
levels of C3 and C4 in the patients with HCC were higher than in
the patients with CHB. To investigate the molecular mechanism of
HBV in regulating the expression of C3 and C4, pHBV1.3 was
transfected into the Huh7 cells. As an HBV infectious clone, cells
transfected with pHBV1.3 can synthesize and secrete HBV viral
particles, and HBV DNA can be detected in the cell culture
supernatant (13). RT-PCR and western
blot analyses were applied to detect the changes in the mRNA and
protein expression levels of C3 and C4; the results demonstrated
that HBV suppressed the expression of C3 and C4 in vivo.
The serum levels of complement components C3 and C4
in the patients with CHB were decreased. We hypothesize that there
are two possible explanations: Firstly, the liver synthesizes the
majority of blood proteins with the exception of γ-globin, and
damage to the liver would reduce the synthesis of C3 and C4
(21); secondly, the infection with
HBV may induce the formation of various antigen-antibody complexes
(22), leading to the activation of
the complement system and resulting in the excessive consumption of
the complement components, including C3 and C4.
The complement system is widely involved in the
body's defense against the invasion of foreign pathogens, the
maintenance of the internal environment and the regulation of
immunity (23); however, HBV can
incorporate the complement regulatory protein of the host into its
outer membrane to evade the host's complement attack (11,12). HBV
may also inhibit the expression of complement C3 and C4 through the
aforementioned hypotheses. Additionally, the decline in the
synthesis by liver cells and excessive complement component
consumption would lead to reduced levels of complement C3 and C4 in
the serum of the patients with CHB. However, the serum levels of C3
and C4 in the patients with HCC were higher than in the patients
with CHB, which may be associated with the alteration in the
expression level following the malignant transformation of the
liver cells; the mechanism for this change requires further
investigation.
In summary, the present study demonstrated that HBV
can downregulate the synthesis and secretion of C3 and C4 both
in vitro and in vivo. The detection of C3 and C4 in
the serum of patients with HPV infection may provide a basis for
the diagnosis of HBV-associated diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Pudong New
Area Science and Technology Development Fund (grant no.
PKJ2016-Y56), he Discipline Group Construction Project of Pudong
Health Bureau of Shanghai (grant no. PWZxq2017-15(grant no.
PWZxq2017-15), the Key Specialty Construction Project of Shanghai
Municipal Health Bureau (grant no. ZK2015B16), the National Science
Foundation of China (grant nos. 81672079, 30973073 and 81172042)
and the Open Research Program of the State Key Laboratory of
Virology of China (grant nos. 2015KF002, 2015KF007, 2015KF005 and
2016KF003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
CZ participated in the cell culture, transfection
and reverse transcription-semiquantitave polymerase chain reaction.
HS and FX participated in the sample collection and measurement of
complement C3 and C4, WY and FL performed the western blotting and
statistical analysis. XL participated in the design. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This work was approved by the Ethical Committee of
Renmin Hospital of Wuhan University (Wuhan, China), and all
patients provided written informed consent.
Consent for publication
Written informed consent for publication was
obtained from these patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang AY, Lai CL, Poon RT, Huang FY, Seto
WK, Fung J, Wong DK and Yuen MF: Hepatitis B virus full-length
genomic mutations and quasispecies in hepatocellular carcinoma. J
Gastroenterol Hepatol. 31:1638–1645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seeger C and Mason WS: Molecular biology
of hepatitis B virus infection. Virology. 479–480:672–686. 2015.
View Article : Google Scholar
|
|
3
|
Chang ML and Liaw YF: Hepatitis B flares
in chronic hepatitis B: Pathogenesis, natural course, and
management. J Hepatol. 61:1407–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang X and Hollinger FB: Occult hepatitis
B virus infection and hepatocellular carcinoma: A systematic
review. J Viral Hepat. 21:153–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Custer B, Sullivan SD, Hazlet TK, Iloeje
U, Veenstra DL and Kowdley KV: Global epidemiology of hepatitis B
virus. J Clin Gastroenterol. 38 10 Suppl 3:S158–S168. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang YQ and Guo JS: Antiviral therapies
for hepatitis B virus-related hepatocellular carcinoma. World J
Gastroenterol. 21:3860–3866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ali YM, Lynch NJ, Haleem KS, Fujita T,
Endo Y, Hansen S, Holmskov U, Takahashi K, Stahl GL, Dudler T, et
al: The lectin pathway of complement activation is a critical
component of the innate immune response to pneumococcal infection.
PLoS Pathog. 8:e10027932012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andrade F: Non-cytotoxic antiviral
activities of granzymes in the context of the immune antiviral
state. Immunol Rev. 235:128–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biswas M, Johnson JB, Kumar SR, Parks GD
and Elankumarana S: Incorporation of host complement regulatory
proteins into Newcastle disease virus enhances complement evasion.
J Virol. 86:12708–12716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shan C, Zhang S, Cui W, You X, Kong G, Du
Y, Qiu L, Ye L and Zhang X: Hepatitis B virus X protein activates
CD59 involving DNA binding and let-7i in protection of hepatoma and
hepatic cells from complement attack. Carcinogenesis. 32:1190–1197.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu Z, Liang X, Liu Y, Du J, Liu S and Sun
W: Hepatitis B virus sensitizes hepatocytes to complement-dependent
cytotoxicity through downregulating CD59. Mol Immunol. 47:283–289.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu CL, Cheng DZ, Liu F, Yan XH, Wu KL,
Wang FB and Liu XH: Hepatitis B virus upregulates the expression of
kinesin family member 4A. Mol Med Rep. 12:3503–3507. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tripathi S, White MR and Hartshorn KL: The
amazing innate immune response to influenza A virus infection.
Innate Immun. 21:73–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Persson BD, Schmitz NB, Santiago C, Zocher
G, Larvie M, Scheu U, Casasnovas JM and Stehle T: Structure of the
extracellular portion of CD46 provides insights into its
interactions with complement proteins and pathogens. PLoS Pathog.
6:e10011222010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rus H, Cudrici C and Niculescu F: The role
of the complement system in innate immunity. Immunol Res.
33:103–112. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Girgis NM, Dehaven BC, Xiao Y, Alexander
E, Viner KM and Isaacs SN: The Vaccinia virus complement control
protein modulates adaptive immune responses during infection. J
Virol. 85:2547–2556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mastellos D, Morikis D, Isaacs SN, Holland
MC, Strey CW and Lambris JD: Complement: Structure, functions,
evolution, and viral molecular mimicry. Immunol Res. 27:367–386.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernet J, Mullick J, Singh AK and Sahu A:
Viral mimicry of the complement system. J Biosci. 28:249–264. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urquiza M, Lopez R, Patiño H, Rosas JE and
Patarroyo ME: Identification of three gp350/220 regions involved in
Epstein-Barr virus invasion of host cells. J Biol Chem.
280:35598–35605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao B, Jeong WI and Tian Z: Liver: An
organ with predominant innate immunity. Hepatology. 47:729–736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu KQ: Occult hepatitis B virus infection
and its clinical implications. J Viral Hepat. 9:243–257. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carroll MC and Isenman DE: Regulation of
humoral immunity by complement. Immunity. 37:199–207. 2012.
View Article : Google Scholar : PubMed/NCBI
|