Introduction
Osteosarcoma is a very common osteoblast malignant
tumor, which is prone to metastasis, especially lung tissue
metastasis. It is highly malignant and the prognosis is not
satisfactory (1,2). Two to three out of one million
individuals suffer from osteosarcoma each year, mainly male
minors.
The treatment methods are becoming increasingly
advanced and the prognosis of the patient has improved obviously
since the 1870s (3). The currently
used comprehensive treatment mode is preoperative
chemotherapy-surgery-postoperative adjuvant chemotherapy and the
highest 5-year survival rate of the patients has reached 80%.
Nevertheless, there are patients who die after treatment failure
(4). More research is needed to
further understand the occurrence and the development of
osteosarcoma, the pathogenesis of osteosarcoma, the development of
the disease and the transfer mechanism to identify an ideal
therapeutic target. The human cell division cycle gene 2
(CDC2), and its encoded cyclin CDC2 protein participates in
regulating the transition of phase G2 into phase M in the
interphase of mitosis (5,6).
The pathogenesis and progression of cancer are
related to the abnormal regulation of the cell cycle, and there is
a high expression of CDC2 in many malignant tumors (7–18).
However, there is less research on the expression level and
clinical significance of CDC2 in osteosarcoma (19). In the present study, quantitative
polymerase chain reaction (RT-PCR) was used to detect the
expression of CDC2 in order to explore its clinical
significance.
Materials and methods
Clinical data
Specimens of cancer, paracancerous tissues and serum
from 47 patients hospitalized at the Department of Orthopedics at
The Third Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China) from January, 2010 to January, 2015 and serum of 35 normal
subjects were collected. The expression of CDC2 was detected
via PCR and the relationship between CDC2 expression and clinical
features of patients with osteosarcoma was analyzed. The
instruments and reagents used in this study are shown in Table I. The study was approved by the Ethics
Committee of The Third Affiliated Hospital of Sun Yat-sen
University, and the patient or their families signed informed
consent.
 | Table I.Instruments and reagents. |
Table I.
Instruments and reagents.
| Instruments and
reagents | Manufacturer |
|---|
| PCR instrument | Applied Biosystems;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA) |
| Spectrophotometer
SMA5000 | Merinton Instrument,
Inc. (Ann Arbor, MI, USA) |
| Reverse transcription
kit | Fermentas; Thermo
Fisher Scientific, Inc. |
| U6 internal
reference | Guangzhou Shangeng
Biological Technology Co., Ltd. (Guangzhou, China) |
| 100 bp DNA
Marker | Tiangen Biotech Co.,
Ltd. (Beijing, China) |
| 2X Taq PCR
MasterMix | Tiangen Biotech Co.,
Ltd. |
| TRIzol | Tiangen Biotech Co.,
Ltd. |
| DEPC | Sigma Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) |
Detection of the CDC2 expression via
RT-PCR
CDC2 was extracted from tissues and serum in
strict accordance with the instructions provided by Sigma-Aldrich
(St. Louis, MO, USA) and Merck KGaA (Darmstadt, Germany),
respectively, and the purity of RNA was expressed by the ratio of
the absorbance value from 260 to 280 nm. Purity was satisfactory if
the result was between 1.9 and 2.1; otherwise, the purification was
repeated until it was up to the standard.
RT-PCR
The experiment was conducted in strict accordance
with the instructions of reverse transcription kits (Fermentas;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The PCR reaction
system was measured as: 25 µl, CDC2 annealing at 53°C, 25
cycles. Primer sequences are shown in Table II. For the statistical analysis,
three parallel wells were set for all samples, and the average was
taken. With U6 as the internal reference, the relative expression
level of CDC2 was expressed as 2−ΔΔCq.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Primer | U6 internal
reference | CDC2 |
|---|
| F |
5′-CTCGCTTCGGCAGCACA-3 |
5′-TACCTATGGAGTTGTGTATAA-3′ |
| R |
5′-AACGCTTCACGAATTTGCGT-3′ |
5’′-ATTCCACTTCTGGCCACACTT-3′ |
Statistical analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used for
data analysis. The post hoc test was SNK test. Measurement data are
presented as mean ± SD, and the analysis of variance was used for
the comparison among groups. The t-test was used for the
comparisons of CDC2 expression levels in specimens of cancer,
paracancerous tissues and serum and the serum of 35 normal
subjects, and the Chi-square test was used for the comparison of
parametres including sex and age. The receiver operating
characteristic (ROC) curve was drawn to assess the diagnosis value
of serum CDC2 in patients with osteosarcoma and the relationship
between CDC2 and osteosarcoma was analyzed via univariate and
multivariate Cox regression analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of CDC2 in tissues,
cells and blood
CDC2 was highly expressed in cancer tissues, which
was higher than that in paracancerous tissues (P<0.05). The
expression level in the blood of patients was higher than that in
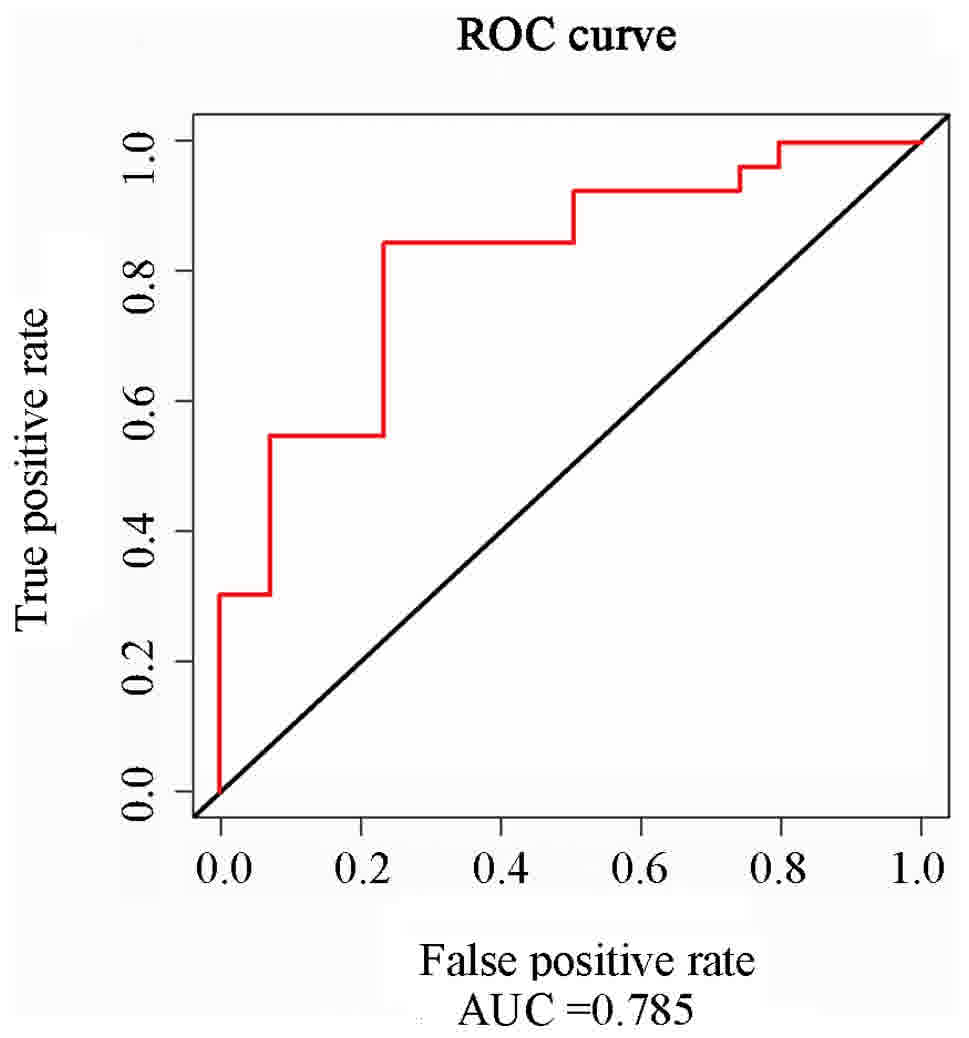
normal subjects (P>0.05). The ROC curve analysis revealed that
CDC2 had a high value in the diagnosis of osteosarcoma (AUC =
0.785, 95% CI = 0.729–0.834) (Fig. 1
and Table IIIA and B).
 | Table III.Expression levels of CDC2 in tissues
and blood. |
Table III.
Expression levels of CDC2 in tissues
and blood.
| A, Tissue |
|
|
|---|
| Cancer tissue | Paracancerous
tissue | P-value |
| 2.31±0.306 | 0.91±0.251 | 0.013 |
|
| B, Blood |
|
|
|
| Patient | Normal subject | P-value |
| 1.58±0.149 | 0.67±0.136 | 0.024 |
Clinical features of 47 patients with
osteosarcoma
Of the 47 patients, 23 cases were osteoblastic; 13
were osteogenic; and 11, were fibroblastic osteosarcoma,
respectively. In osteosarcoma cells, the expression level of CDC2
had no difference in terms of sex, age and occurrence site
(P>0.05). Osteosarcoma was divided into 3 levels: Parosteal (I),
periosteal (II) and conventional osteosarcoma (III) according to
the fourth edition of World Health Organization (WHO) bone tumor
classification. The expression level of CDC2 was increased with the
increase of level (P<0.05). KPS was scored according to the
evaluation standards of physical condition (20). The results showed that the expression
level of CDC2 was increasingly higher with the decrease of
KPS score. The CDC2 expression level was closely associated with
tumor diameter (P<0.05). Finally, the expression level of CDC2
was increased with the increase of tumor lymph nodes metastasis
(TNM) staging (P<0.05) (Table
IV).
 | Table IV.Clinical characteristics of 447
patients with osteosarcoma. |
Table IV.
Clinical characteristics of 447
patients with osteosarcoma.
| Item | No. | CDC2 expression
level | P-value |
|---|
| Sex |
|
|
|
| Male | 31 | 2.38±0.317 | 0.685 |
|
Female | 16 | 2.24±0.305 |
|
| Age (years) |
|
|
|
|
<12 | 25 | 2.31±0.313 | 0.314 |
| ≥12 | 22 | 1.91±0.206 |
|
| Histological
subtypes |
|
|
|
|
Osteoblastic osteosarcoma | 23 | 2.11±0.331 | 0.412 |
|
Osteogenic osteosarcoma | 13 | 1.98±0.285 |
|
|
Fibroblastic osteosarcoma | 11 | 2.06±0.446 |
|
| KPS score |
|
|
|
|
<70 | 30 | 3.01±0.363 | 0.032 |
| ≥70 | 17 | 1.81±0.106 |
|
| Tumor location |
|
|
|
| Upper
limb bone | 9 | 2.43±0.278 | 0.647 |
| Lower
limb bone | 38 | 2.31±0.306 |
|
| Tumor size (cm) |
|
|
|
|
<10 | 36 | 2.84±0.267 | 0.042 |
| ≥10 | 11 | 1.94±0.348 |
|
| WHO
classification |
|
|
|
|
I | 15 | 1.57±0.124 |
|
|
II | 24 | 2.79±0.217 | 0.039 |
|
III | 8 | 1.97±0.135 |
|
| TNM staging |
|
|
|
|
I/II | 35 | 1.65±0.152 | 0.035 |
|
III/IV | 12 | 2.87±0.225 |
|
| Pathological
fracture |
|
|
|
|
Yes | 11 | 2.48±0.165 | 0.752 |
| No | 36 | 2.74±0.274 |
|
Association of TNM staging and CDC2
level with survival rate of patients and its effect on
prognosis
The median expression level of CDC2 in 47 patients
with osteosarcoma was 2.49. Thus, the patients were divided into
the high-expression CDC2 (>2.49) and low-expression CDC2
(<2.49) groups. The univariate and multivariate Cox regression
analysis revealed that the increase of the TNM staging of
osteosarcoma and the high expression of CDC2 were both risk factors
affecting the prognosis of osteosarcoma patients (P<0.05)
(Table V).
 | Table V.Univariate and multivariate
analysis. |
Table V.
Univariate and multivariate
analysis.
| Variables | Univariate HR
(95%CI) | Multivariate
P-value | HR (95%CI) | P-value |
|---|
| CDC2 (low vs.
high) | 1.647
(1.122–2.896) | 0.009 | 1.969
(0.9505–4.0765) | 0.012 |
| Age (<12 vs. ≥12
years) | 1.014
(0.999–1.029) | 0.062 |
|
|
| Sex (male vs.
female) | 0.819 |
|
|
|
| (0.277–2.424) | 0.788 |
|
|
|
| Diameter of tumor
(<10 vs. ≥10 cm) | 0.812 |
|
|
|
| (0.357–1.847) | 0.682 |
|
|
|
| Tumor site | 2.2611 |
|
|
|
| (Upper vs. lower
limb bone) |
|
|
|
|
| (0.9821–147.3) | 0.052 |
|
|
|
| TNM staging | 3.064 |
|
|
|
| (1.282–7.323) | 0.028 | 1.268
(0.918–2.471) | 0.041 |
|
Discussion
Since the 21st century, it has been found (21) that the abnormal regulation of cell
cycle is one of the most important causes of tumor. Researchers
have concluded that cancer is a progressive disease that is caused
by the destruction of the cell cycle regulation mechanism, and many
genes are found to be involved in the cell cycle regulation,
providing many important targets for the treatment of cancer
(22).
CDC (23) is
one of the genes that has been identified, the most important being
CDC2, and the CDC2 kinase (24) encoded by it controls the beginning of
the cell cycle and the transition from the G2 to the M phase. The
cell cycle checkpoint regulates various cell regulators, thus
completing the mitosis of cells. Previous findings have shown that
the disruption of the function of the cell cycle checkpoint may
lead to malignant differentiation of cells and produce tumors
(25).
In the present study, the expression of CDC2 in
cancer, paracancerous tissues and serum from patients and normal
controls were detected via RT-PCR. The results showed that, there
was a significant difference in the expression level of CDC2
between cancer and paracancerous tissues (P<0.05), as well as
the serum in patients and the normal control group (P<0.05). It
was also found that a high CDC2 expression may interfere with
normal cell growth and differentiation and cause malignant cell
proliferation, and the detection of CDC2 expression in serum may
predict the occurrence of osteosarcoma. Leijen et al
(21) found that the function of the
tumor cell checkpoint is incomplete and can trigger an automatic
interlocking feedback loop, which leads to further malignant cell
growth. The expression level of CDC2 was closely associated with
tumor diameter, WHO grading and KPS score, indicating that the
expression level of CDC2 is closely associated with the occurrence
and development of osteosarcoma. Chae et al (26) reported that the expression of CDC2 is
significantly different between benign and malignant breast
lesions, and the increase of CDC2 levels is associated with tumor
invasiveness. The results in the present study also showed that the
expression level of CDC2 was associated with the TNM staging of
osteosarcoma (P<0.05), suggesting that a high expression of CDC2
in osteosarcoma may promote the development of osteosarcoma, and
the detection of CDC2 expression in serum may predict the
development of osteosarcoma. Yang et al (27) found that CDC2 is associated with
squamous cell carcinoma of the larynx. The multivariate Cox
regression analysis of the prognosis of osteosarcoma patients
revealed that the expression level of CDC2 was a risk factor
affecting the prognosis of patients with osteosarcoma (P<0.05),
making it possible to predict the prognosis of osteosarcoma by
detecting the CDC2 expression level in serum. Jansen et al
(18) found that CDC2 plays a
crucial role in G2 cell cycle progression and cell proliferation,
and CDC2 may be considered as a prognostic marker for metastatic
breast cancer.
Since no relevant reports are currently available to
confirm the clinical significance of CDC2 expression in
osteosarcoma, and the sample size was small in this study with a
lack of representativeness, a larger number of samples are needed
to confirm the findings. In this study, whether patients received
chemotherapy and radiotherapy was not recorded; thus, further
verification is needed in future research.
Collectively, CDC2 is highly expressed in
osteosarcoma tumor cells. A high expression of CDC2 may be involved
in the process of tumor development and progression, which leads to
disordered mitosis and malignant proliferation of cells. The
detection of CDC2 expression in serum may predict the occurrence,
development and prognosis of osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GH and BC wrote the manuscript and assisted with
PCR. WX and KL designed the primer sequences and analyzed specimens
of patients. HZ and HY contributed significantly to statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China), and the patients or their families signed informed
consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu M, Zhang YY, Wang HF and Yang GS: The
expression and function of miRNA-106 in pediatric osteosarcoma. Eur
Rev Med Pharmacol Sci. 21:715–722. 2017.PubMed/NCBI
|
|
2
|
Hua Y, Jin Z, Zhou F, Zhang YQ and Zhuang
Y: The expression significance of serum MiR-21 in patients with
osteosarcoma and its relationship with chemosensitivity. Eur Rev
Med Pharmacol Sci. 21:2989–2994. 2017.PubMed/NCBI
|
|
3
|
Gao KT and Lian D: Long non-coding RNA
MALAT1 is an independent prognostic factor of osteosarcoma. Eur Rev
Med Pharmacol Sci. 20:3561–3565. 2016.PubMed/NCBI
|
|
4
|
Tang B, Liu C, Zhang QM and Ni M:
Decreased expression of miR-490-3p in osteosarcoma and its clinical
significance. Eur Rev Med Pharmacol Sci. 21:246–251.
2017.PubMed/NCBI
|
|
5
|
Chang CC, Hung CM, Yang YR, Lee MJ and Hsu
YC: Sulforaphane induced cell cycle arrest in the G2/M phase via
the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J
Ovarian Res. 6:412013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi HJ, Fukui M and Zhu BT: Role of
cyclin B1/Cdc2 up-regulation in the development of mitotic
prometaphase arrest in human breast cancer cells treated with
nocodazole. PLoS One. 6:e243122011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Liao Y, Long D, Yu T, Shen F and
Lin X: The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the
cytotoxic effects of taxol through Op18/stathmin in non-small cell
lung cancer cells in vitro. Int J Mol Med. 40:235–242. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu D, Chen K, Bai Y, Zhu X, Chen Z, Wang
C, Zhao Y and Li M: Screening of diagnostic markers for
osteosarcoma. Mol Med Rep. 10:2415–2420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Leng C, Wu C, Zhang Z, Dou L, Luo
X, Zhang B and Chen X: Smad4 sensitizes colorectal cancer to
5-fluorouracil through cell cycle arrest by inhibiting the
PI3K/Akt/CDC2/survivin cascade. Oncol Rep. 35:1807–1815. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motoyama K, Inoue H, Mimori K, Tanaka F,
Kojima K, Uetake H, Sugihara K and Mori M: Clinicopathological and
prognostic significance of PDCD4 and microRNA-21 in human gastric
cancer. Int J Oncol. 36:1089–1095. 2010.PubMed/NCBI
|
|
11
|
Zhang J, Wang D, Xiong J, Chen L and Huang
J: MicroRNA-33a-5p suppresses growth of osteosarcoma cells and is
downregulated in human osteosarcoma. Oncol Lett. 10:2135–2141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao XF, Zhao MY, Chai L, Kukuruga D, Tan
M and Stass SA: Amplified RPS6KB1 and CDC2 genes are potential
biomarkers for aggressive HIV+/EBV+diffuse large B-cell lymphomas.
Int J Clin Exp Pathol. 6:148–154. 2013.PubMed/NCBI
|
|
13
|
Mou S, Wang G, Ding D, Yu D, Pei Y, Teng S
and Fu Q: Expression and function of PIM kinases in osteosarcoma.
Int J Oncol. 49:2116–2126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma YC, Su N, Shi XJ, Zhao W, Ke Y, Zi X,
Zhao NM, Qin YH, Zhao HW and Liu HM: Jaridonin-induced G2/M phase
arrest in human esophageal cancer cells is caused by reactive
oxygen species-dependent Cdc2-tyr15 phosphorylation via
ATM-Chk1/2-Cdc25C pathway. Toxicol Appl Pharmacol. 282:227–236.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Connell MJ, Lavery I, Yothers G, Paik S,
Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J,
et al: Relationship between tumor gene expression and recurrence in
four independent studies of patients with stage II/III colon cancer
treated with surgery alone or surgery plus adjuvant fluorouracil
plus leucovorin. J Clin Oncol. 28:3937–3944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma C, Zhang Z, Cui Y, Yuan H and Wang F:
Silencing FAT10 inhibits metastasis of osteosarcoma. Int J Oncol.
49:666–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiao Q, Jiang Y and Li G: Curcumin
enhances the response of non-Hodgkin's lymphoma cells to ionizing
radiation through further induction of cell cycle arrest at the
G2/M phase and inhibition of mTOR phosphorylation. Oncol Rep.
29:380–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jansen MP, Reijm EA, Sieuwerts AM,
Ruigrok-Ritstier K, Look MP, Rodríguez-González FG, Heine AA,
Martens JW, Sleijfer S, Foekens JA, et al: High miR-26a and low
CDC2 levels associate with decreased EZH2 expression and with
favorable outcome on tamoxifen in metastatic breast cancer. Breast
Cancer Res Treat. 133:937–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fletcher CDM, Bridge JA, Hogendoorn PCW,
Hogendoorn PC, Merterns F and Hogendoom P: WTO classification of
tumours of soft tissue and bone. IARC Press. 46:95–104. 2013.
|
|
20
|
Okita Y, Narita Y, Miyakita Y, Ohno M,
Matsushita Y, Fukushima S, Sumi M, Ichimura K, Kayama T and Shibui
S: IDH1/2 mutation is a prognostic marker for survival and predicts
response to chemotherapy for grade II gliomas concomitantly treated
with radiation therapy. Int J Oncol. 41:1325–1336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leijen S, Beijnen JH and Schellens JH:
Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase
results in sensitization of p53-deficient tumor cells to
DNA-damaging agents. Curr Clin Pharmacol. 5:186–191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong BS, Cho JH, Kim H, Choi EJ, Rho S,
Kim J, Kim JH, Choi DS, Kim YK, Hwang D and Gho YS: Colorectal
cancer cell-derived microvesicles are enriched in cell
cycle-related mRNAs that promote proliferation of endothelial
cells. BMC Genomics. 10:5562009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malumbres M: Physiological relevance of
cell cycle kinases. Physiol Rev. 91:973–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilkinson S, Croft DR, O'Prey J,
Meedendorp A, O'Prey M, Dufès C and Ryan KM: The cyclin-dependent
kinase PITSLRE/CDK11 is required for successful autophagy.
Autophagy. 7:1295–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv TZ and Wang GS: Antiproliferation
potential of withaferin A on human osteosarcoma cells via the
inhibition of G2/M checkpoint proteins. Exp Ther Med. 10:323–329.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chae SW, Sohn JH, Kim DH, Choi YJ, Park
YL, Kim K, Cho YH, Pyo JS and Kim JH: Overexpressions of Cyclin B1,
cdc2, p16 and p53 in human breast cancer: The clinicopathologic
correlations and prognostic implications. Yonsei Med J. 52:445–453.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JQ, Liu HX, Liang Z, Sun YM and Wu M:
Over-expression of p53, p21 and Cdc2 in histologically negative
surgical margins is correlated with local recurrence of laryngeal
squamous cell carcinoma. Int J Clin Exp Pathol. 7:4295–4302.
2014.PubMed/NCBI
|