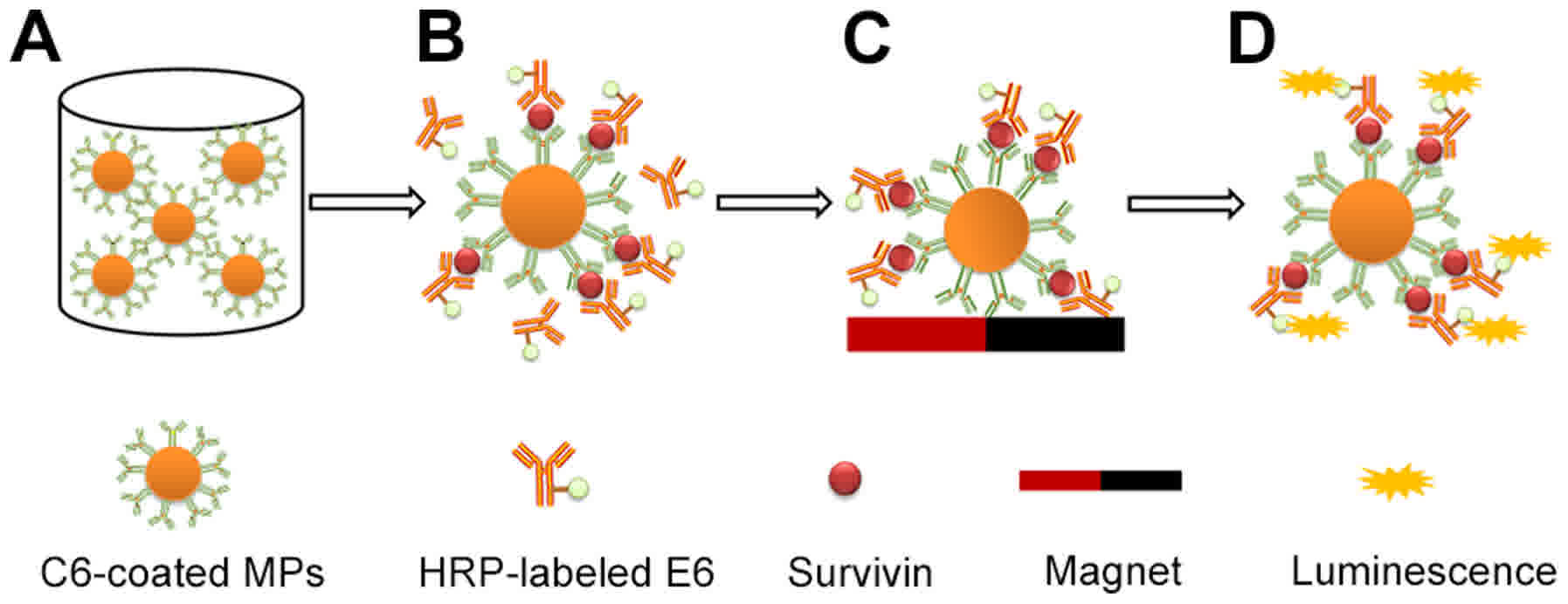
Introduction
Bladder cancer (BC) and renal cell carcinoma (RCC)
are two of the major tumors in urinary system. BC is the ninth most
common cancer for both men and women (1) and kidney cancer is also among the ten
most common malignant diseases in both sexes combined (2), of which the histological type is usually
RCC. The urinary protein analysis could help discover non-invasive
biomarkers for urinary system diseases (3,4).
Urinary BTA and NMP22 are recognized as putative
biomarkers for diagnosis of BC (5),
but their applications are limited due to the relatively low
sensitivity or specificity (6,7). There is
no definitively validated biomarker for diagnosis of RCC
recommended in clinical practice so far (8).
Survivin is an important member of the Inhibitor of
Apoptosis Protein family and the overexpression of survivin can be
found in various tumors but barely in normal tissues (9). Urinary survivin has been proven to be a
diagnostic marker in BC (10) but the
diagnostic value of urinary survivin in RCC has not been fully
elucidated. For now, the detection of survivin mainly employs
quantitative sandwich enzyme immunoassay technique which requires
multiple procedures and a relatively long period. Therefore,
establishing a simple step method for the detection of urinary
survivin is in urgent need.
Lysosome-associated protein transmembrane-4β
(LAPTM4B) is a novel oncogene that was initially identified in
hepatocellular carcinoma and LAPTM4B-35 protein was found to be
overexpressed in various malignant tumors (11). But the expression of the protein in
urine has not been studied yet.
In this study, we established a one-step magnetic
particles (MPs)-based chemiluminescence enzyme immunoassay (CLEIA)
for the detection of urinary survivin and applied it to the
preliminary diagnosis of BC and RCC. The urine LAPTM4B level was
also measured in BC and RCC patients. We explored the combined
diagnostic value of survivin and LAPTM4B for these two tumors in
order to find a novel biological marker for the preliminary
diagnosis of BC and RCC.
Materials and methods
Chemicals, reagents and apparatus
Incomplete freund's adjuvant (IFA), PEG, horseradish
peroxidase (HRP; H1759), sodium borohydride (NaBH4; 10H3440),
sodium m-periodate (NaIO4) (38F-0860) and
(+)-biotin-N-hydroxysuccinimide (NHSB; HMBD0595 V) were from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Hypoxanthine-aminopterin-thymidine and hypoxanthine-thymidine were
from Corning Incorporated (Corning, NY, USA). MPs (Dynabeads M-280
Tosylactivated) with a average diameter of 2.8 µm and the
DynaMag-96 Side Skirted Magnet were purchased from Invitrogen Dynal
AS (Oslo, Norway). Chemiluminescent substrates were purchased from
Ke Yue Zhong Kai Co., Ltd. (Beijing, China). Protein-A/G sepharose
(HiTrap Protein G HP) was from GE Healthcare Life Sciences
(Buckinghamshire, UK). The human LAPTM4B enzyme-linked
immunosorbent assay (ELISA) kit was purchased from Lifespan
Biosciences (Seattle, WA, USA). BCA protein assay kit was obtained
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Phosphate-buffered saline (PBS) buffer and bovine serum albumin
(BSA) were from ZSGB-Bio (Beijing, China). Tween-20, oxalic acid,
glucose, vitamin C, galactose, creatinine, urea, albumin were
purchased from Solarbio (Beijing, China). Livin peptide was from
Bioss (Beijing, China). The washing buffer was 0.01M PBS containing
0.05% (v/v) Tween-20 (PBST). The antibody diluent was 0.1M PBST
with 1% BSA and 4% PEG 6000, and it was used to dilute mAb-coated
MPs and HRP-labeled mAb. SpectraMax L microplate reader from
Molecular Devices, LLC (Sunnyvale, CA, USA) was employed to detect
the chemiluminescence. The IKA® MS3 Digital (IKA,
Staufen, Germany) was used for the shaking of the microplates. The
white opaque 96-well flat-bottomed microplates were from Nunc
(Roskilde, Denmark).
Experimental animals
Female Balb/c mice weighing 18–22 g were purchased
from the Laboratory Animal Centre of Chinese Academy of Medical
Sciences. The animal experiments were approved by the Animal Care
Committee of Peking University (Beijing, China). The animal studies
were performed in accordance with the Experimental Animal
Management Ordinance approved by the Scientific and Technological
Committee of China.
Preparation and purification of
monoclonal antibody (mAb) against survivin and preparation of
standard series of survivin
The immunization and cell fusion procedure were
carried out according to the methods described by Chang et
al (12). Hybridoma cell lines
(C6 and E6) were selected for ascites production in vitro
and the details were carried out as described previously (12). Both the mAbs (C6 and E6) against
survivin were first purified by the ammonium sulfate precipitation
method to remove most of the hybrid protein and then purified by
the protein G affinity chromatography columns. The purified mAbs
were then subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) analysis and commassie blue staining.
Human recombinant sequence survivin protein MS2-survivin
was produced by our laboratory (13).
The standard series of survivin were prepared by diluting
MS2-survivin stock with 0.01M PBS to reach the desired
concentration of 200, 100, 50, 25, 12.5, 6.25, 3.125, 0 ng/ml,
assigning to S1, S2, S3,
S4, S5, S6, S7 and
S0, respectively.
Preparation of HRP-labeled mAb and
anti-survivin mAb-coated MPs
The mAb E6 was selected as detecting antibody and
the procedure of labeling it with HRP was performed as described
previously (12). The MPs were coated
with C6 according to the manufacturer's protocols (Dynal
Biotech).
Human urine specimens collection
The urine samples were obtained from Peking
University Cancer Hospital (Beijing, China) in 2017. All the cancer
patients were histopathologically diagnosed as urothelial carcinoma
of bladder or RCC. The staging was determined according to the
tumor-node-metastasis (TNM) classification released by the American
Joint Committee on Cancer (AJCC; 7th edition, 2010). The grading
for BC was made in accordance with the 2004 World Health
Organization (WHO)/International Society of Urologic Pathology
(ISUP) grading system. Healthy controls were chosen at the Medical
Examination Center of Peking University Cancer Hospital. A total of
200 BC patients and 81 RCC were enrolled in the study and the
urinary survivin levels of them were detected. The urine survivin
levels of 114 age- and sex-matched healthy donors were also
measured. The urinary LAPTM4B levels of them were detected
simultaneously. Clean catch midstream urine samples were obtained
and centrifuged immediately at 3,000 rpm for 5 min. The supernatant
was aliquoted and stored at −40°C until detection. This study was
approved by the ethics committee of the Peking University Cancer
Hospital. The entire study was conducted according to the
Declaration of Helsinki. All of the patients and healthy controls
provided written informed consent for participation in the
study.
MPs-based CLEIA of urinary survivin
and the detection of urine LAPTM4B levels
The procedures of the MPs-based CLEIA are displayed
in Fig. 1, and the detailed steps
were as follows. First, 50 µl C6-coated MPs diluted in the antibody
diluent was added into each well of the microplates. Then, a
mixture of 50 µl of either survivin standard series or sample and
50 µl of the HRP-labeled E6 (diluted in the antibody diluent) was
added into each well of the microplates and incubated in the shaker
for 60 min at room temperature with 300 rpm. After that, the MPs
which formed sandwich immunocomplexes were magnetically separated
for 2 min and the supernatant containing excess HRP-labeled E6 was
removed. Subsequently, the MPs were resuspended in 150 µl washing
buffer and washing was performed four times. Finally, the
chemiluminescent substrate was added into each well and the
relative light units (RLU) value was measured.
The detection of urine LAPTM4B was performed using
sandwich ELISA following the manufacturer's instructions (Lifespan
Biosciences). The detection wavelength of LAPTM4B was 450 nm by
microplate reader.
Development of MPs based CLEIA
We optimized different experimental parameters to
gain the widest detection range (RLUS1/RLUS0)
and the highest sensitivity (RLUS7/RLUS0).
The best dilutions of C6-MPs and HRP-labeled E6 were 1:200 and
1:1,200, respectively. The best reaction condition was at room
temperature shaking (400 rpm) for 1 h. The optimal substrate volume
was 100 µl per well and the maximal RLUs were observed immediately
after adding the chemiluminescent substrate (avoiding light).
Statistical analysis
Statistical analysis was carried out using SPSS
v19.0 software (SPSS, Inc., Chicago, IL, USA). The measurement data
were expressed in the form of median (interquartile range).
Comparisons of different groups were analyzed by Mann-Whitney
U-test for continuous variables. Receiver operating characteristic
(ROC) curves were created to evaluate the diagnostic efficiency.
MedCalc statistical software was employed for the comparison of ROC
curves. We employed bivariate logistic regression to obtain the
predictive P-values and used the P-values to build a new ROC curve
for the two biomarkers combined. Cut-off value was determined by
the optimal Youden's index (sensitivity + specifcity-1). P<0.05
was considered to indicate a statistically significant
difference.
Results
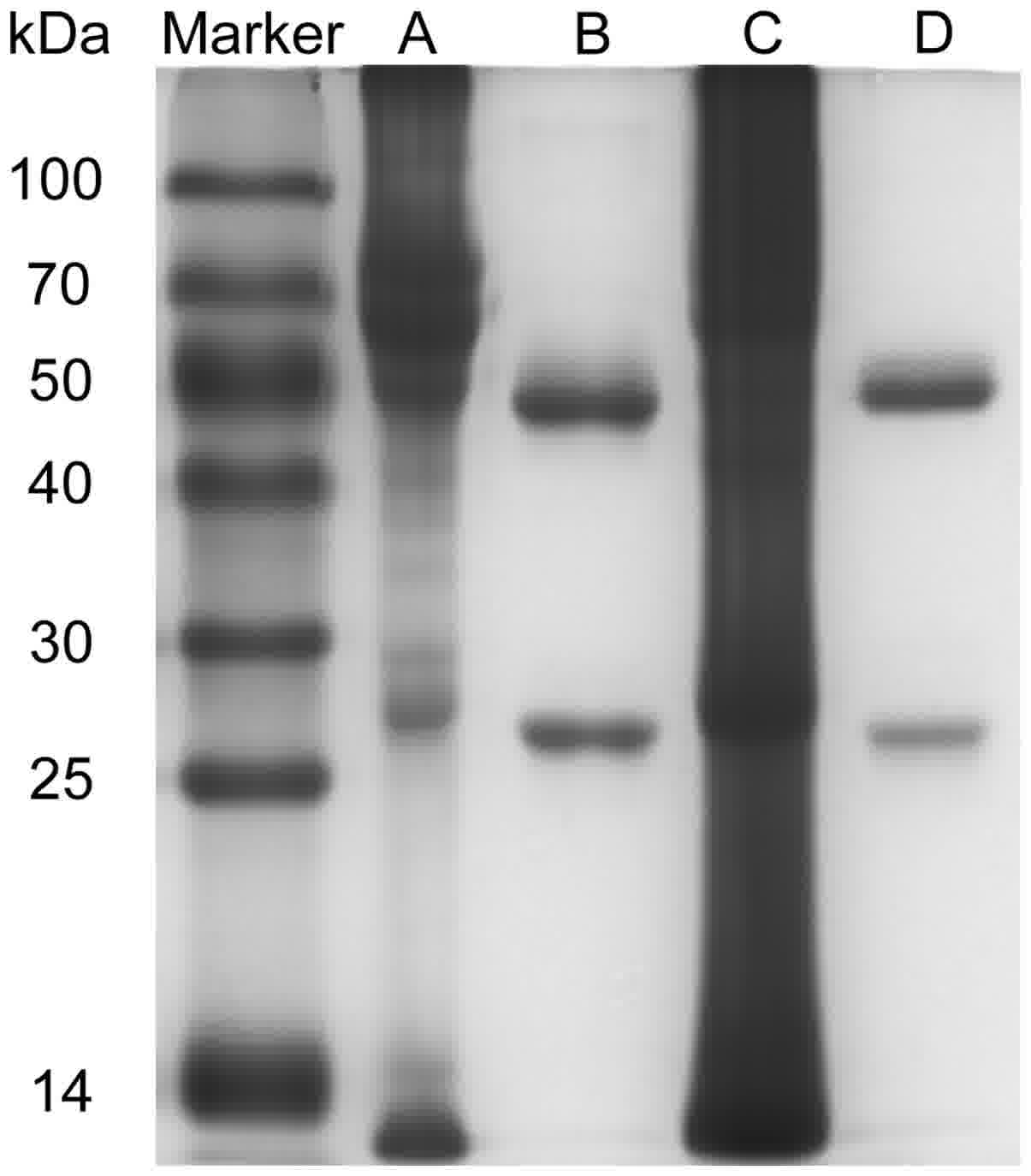
Purification of mAbs against
survivin
The mAbs (C6, E6) recognizing different isotopes of
survivin were purified. This antibody pair has been tested in a
sandwiched ELISA assay. C6 was used as capturing antibody and E6
was detecting antibody. The purity of them would affect the
sensitivity and specificity of the proposed method. The
purification effect is shown in Fig.
2. There were two straps (heavy and light chains) for each mAb
and almost all of the other proteins were removed.
Method evaluation
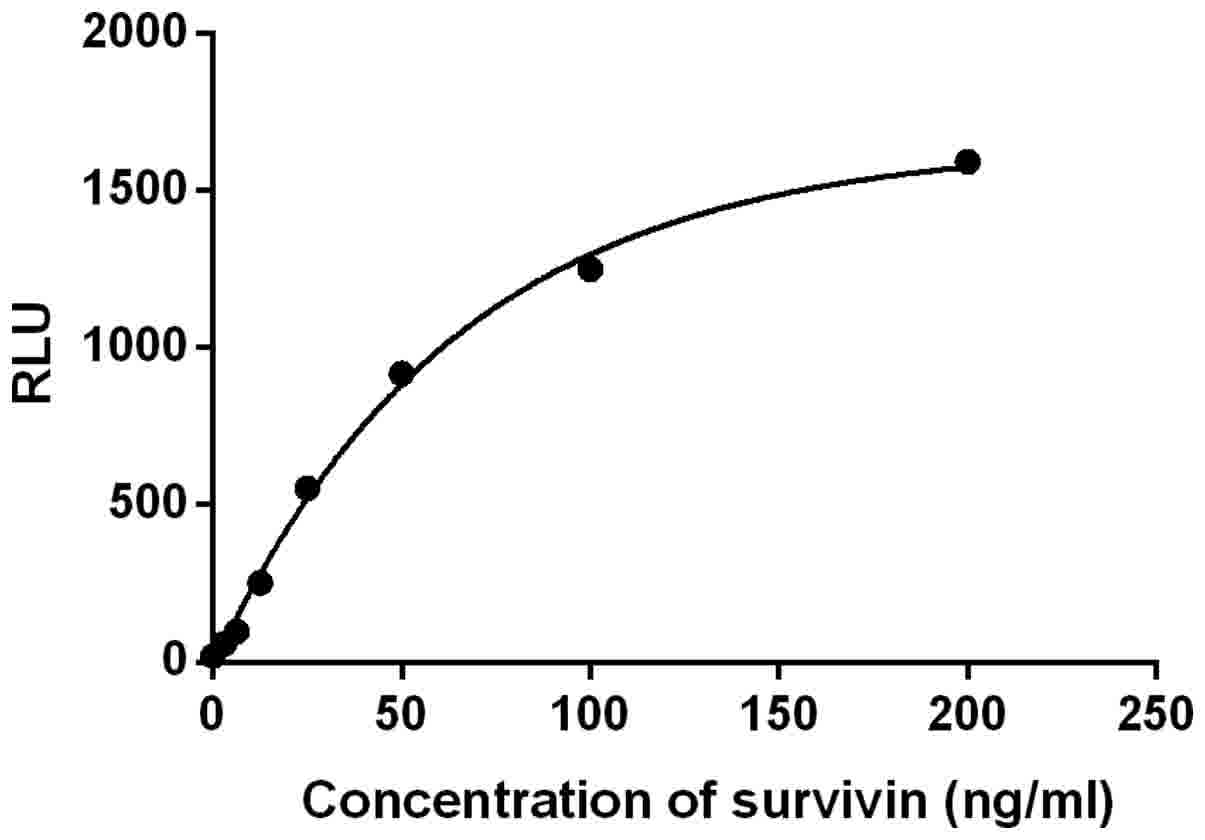
Standard curve and sensitivity
Under the optimal conditions, a standard curve was
obtained by four-parameter logistic curve fitting (Fig. 3) with a correlation coefficient of
0.9983. The sum of the average RLU and 2 standard deviations (SDs)
of 10 replicates of S0 was considered as the detection
limit. According to the standard curve, the concentration of the
detection limit was 0.949 ng/ml.
Stability
The stability of the C6-coated MPs was also
investigated, C6-coated MPs were stored at 4°C for 14 days. During
this period, C6-coated MPs were taken out on day 1, 3, 5, 7, 14 to
detect S1 and S0. As shown in Fig. 4A, the RLU value showed no obvious
decrease and the proposed method retained more than 90% of its
initial response after the storage of C6-coated MPs for 14 days at
4°C. Therefore, the result indicated that the stability of
C6-coated MPs was acceptable within 2 weeks.
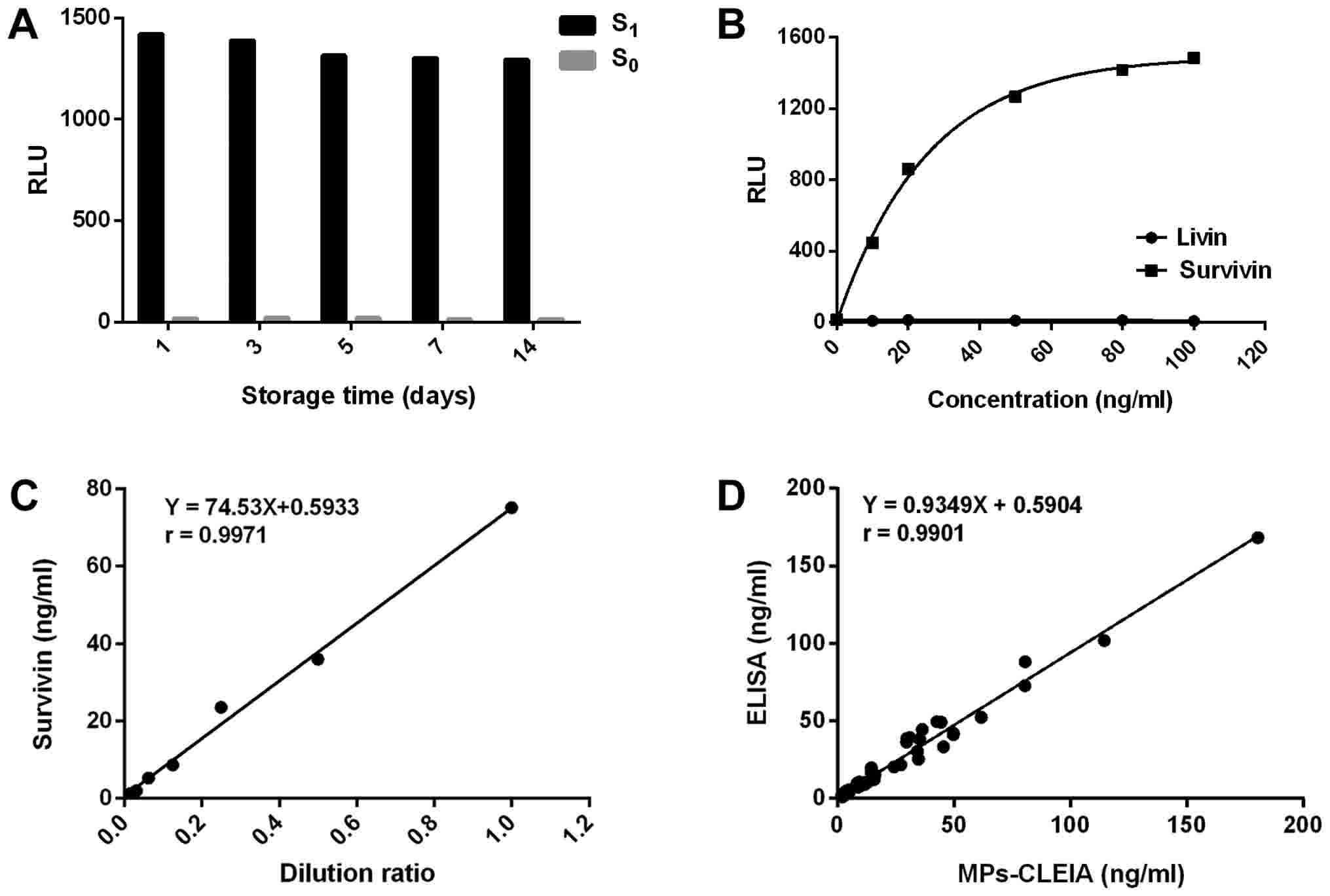 | Figure 4.Method evaluation of the MPs-based
CLEIA. (A) Detection results of the average RLU of S1
and S0 at different storage times (1, 3, 5, 7 and 14
days). In total, >90% of the initial response was obtained
following MPs storage for 14 days at 4°C. (B) The average RLU of
the assay for survivin and livin over the concentration range 0 to
100 ng/ml. The response of survivin was dependent on its
concentration, while livin was not detected. (C) The
linearity-dilution effect of the high concentration urine. The
association between the concentration of the diluted survivin and
the dilution ratios produced a good linear correlation coefficient
of 0.9971. (D) Correlation between the results measured by the
proposed MPs-CLEIA method and ELISA. The survivin levels in 90
urine samples were determined simultaneously using the proposed
MPs-CLEIA method and ELISA. The correlation coefficient between the
2 methods was 0.9901. RLU, relative light units; MPs, magnetic
particles; CLEIA, chemiluminescence enzyme immunoassay;
S1 and S0, MS2-survivin at 200 and
0 ng/ml, respectively. |
Hook effect
As the immunoassay was a one-step procedure, the
hook effect was studied. A sample spiked with survivin at extremely
high concentration (1,000 ng/ml) was tested using the MPs-based
CLEIA. The RLU value turned out to exceed the upper detection limit
and showed no decline. Thus, the pseudo-negative can be avoided
successfully in the case of high concentration urine samples.
Precision
Three different concentrations of the urine samples
were measured 8 times in one assay to evaluate the intra-assay
precision. The same samples were analyzed on different days (5
days) using the same protocol (3 replicates per run) to obtain the
inter-assay precision. The urine samples were patient pools. The
results showed that the intra-assay CV was <7% and the
inter-assay CV was <10% (Table
I).
 | Table I.Intra- and inter-assay variability
for survivin. |
Table I.
Intra- and inter-assay variability
for survivin.
|
| Intra-assay | Inter-assay |
|---|
|
|
|
|
|---|
| Sample no. | No. of
replications | Concentration
(ng/ml) | CV (%) | Days of
replication | Concentration
(ng/ml) | CV (%) |
|---|
| 1 | 8 | 7.07 | 6.80 | 5 | 6.52 | 8.28 |
| 2 | 8 | 33.63 | 4.23 | 5 | 32.78 | 9.84 |
| 3 | 8 | 86.71 | 3.08 | 5 | 86.42 | 6.48 |
Recovery
The accuracy was studied through a recovery test.
Different amounts of survivin were added to three human urine
samples to obtain high, middle and low distributions and these were
detected in triplicate. As shown in Table II, the average recoveries were
between 95 and 105%, indicating that the accuracy of the proposed
method was satisfactory.
 | Table II.Recovery of survivin in human
urine. |
Table II.
Recovery of survivin in human
urine.
| Survivin
concentration in human urine (ng/ml) | Amount of survivin
added (ng/ml) | Mean measured
concentration (ng/ml) | Mean recovery
(%) |
|---|
| 1.12 | 5 | 5.89 | 95.39 |
|
| 30 | 30.15 | 96.78 |
|
| 80 | 79.52 | 98.00 |
| 1.46 | 5 | 6.39 | 98.43 |
|
| 30 | 32.96 | 105.00 |
|
| 80 | 82.89 | 101.78 |
| 2.41 | 5 | 7.48 | 101.50 |
|
| 30 | 31.00 | 95.31 |
|
| 80 | 78.62 | 95.27 |
Interferences
The interference of potential endogenous
interferents was assessed by overloading three urine samples
(survivin=10.96, 59.33 and 93.50 ng/ml). And the results showed
that the interferences of a final concentration of 1.6 mmol/l
oxalic acid, 2.4 mmol/l glucose, 2.3 mmol/l vitamin C, 350 µmol/l
galactose, 1,000 µmol/l creatinine, 500 mmol/l urea, and 600 mg/l
albumin were all <10% on the survivin MPs-based CLEIA.
Cross-reactivity (CR)
The inhibitor of apoptosis (IAP) protein family
shows similar structural features and different degrees of
homology. Survivin and livin are both members of the family and the
specificity of the proposed method was evaluated using livin
peptide. The average CR for livin over 0–100 ng/ml was assessed
(Fig. 4B). The CR was calculated as
follows: CR=100 × Csurvivin/Ccross-reactant,
where Csurvivin refers to the concentration of survivin
determined by applying the tested cross-reactant signal to the
dose-response curve and the Ccross-reactant refers to
the concentration of livin. The results showed that C6 and E6 did
not cross-react with livin.
Linearity-dilution effect
The linearity-dilution effect was studied by
selecting a human urine sample with a relatively high concentration
of 75.14 ng/ml. It was diluted by S0 to obtain a series
of concentrations 1/2, 1/4, 1/8, 1/16, 1/32 and 1/64 of the
original concentration. Each sample was measured in duplicate. The
results were shown in Fig. 4C. As can
be seen, the relationship between the concentration of the diluted
survivin and the dilution ratios had a favorable linear correlation
coefficient of 0.9971.
Reference interval
We collected the urine samples from 114 healthy
individuals. Reference interval was determined by nonparametric
analysis at 95% confidence level and the upper reference limit was
2.73 ng/ml for survivin.
Comparison with ELISA
The ELISA procedure was performed as described
previously (12). The proposed method
was applied for the determination of survivin in 90 clinical urine
samples and the results were compared with those obtained by ELISA
(Fig. 4D). There was a good
correlation coeffcient of 0.9901.
Sample analysis
Urinary survivin in BC and RCC
compared with healthy controls
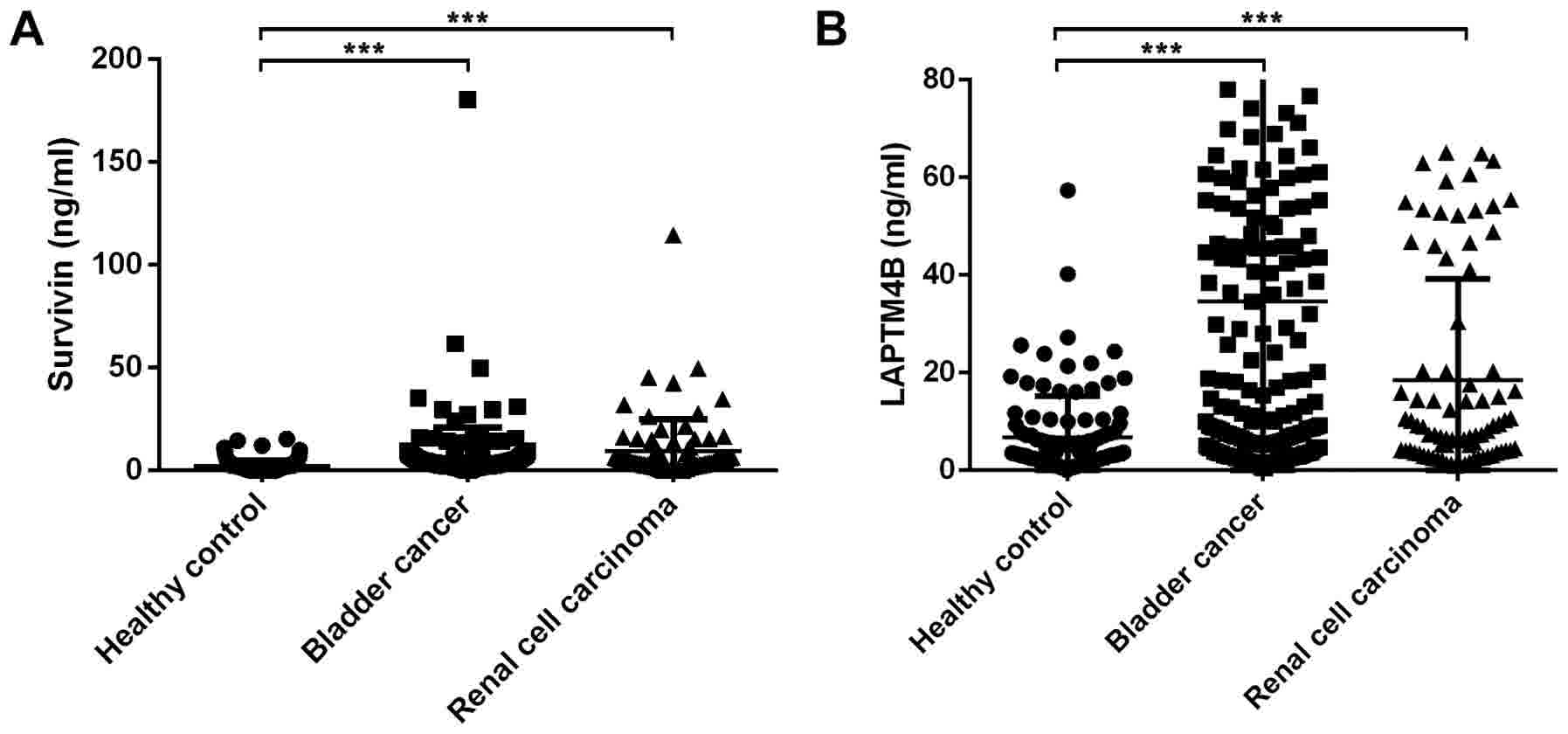
As can be seen in Fig.
5A, urinary survivin in BC [3.65 ng/ml (3.42 ng/ml),
P<0.001] and RCC [4.23 ng/ml (7.07 ng/ml), P<0.001] displayed
significantly higher levels than the healthy controls [1.54 ng/ml
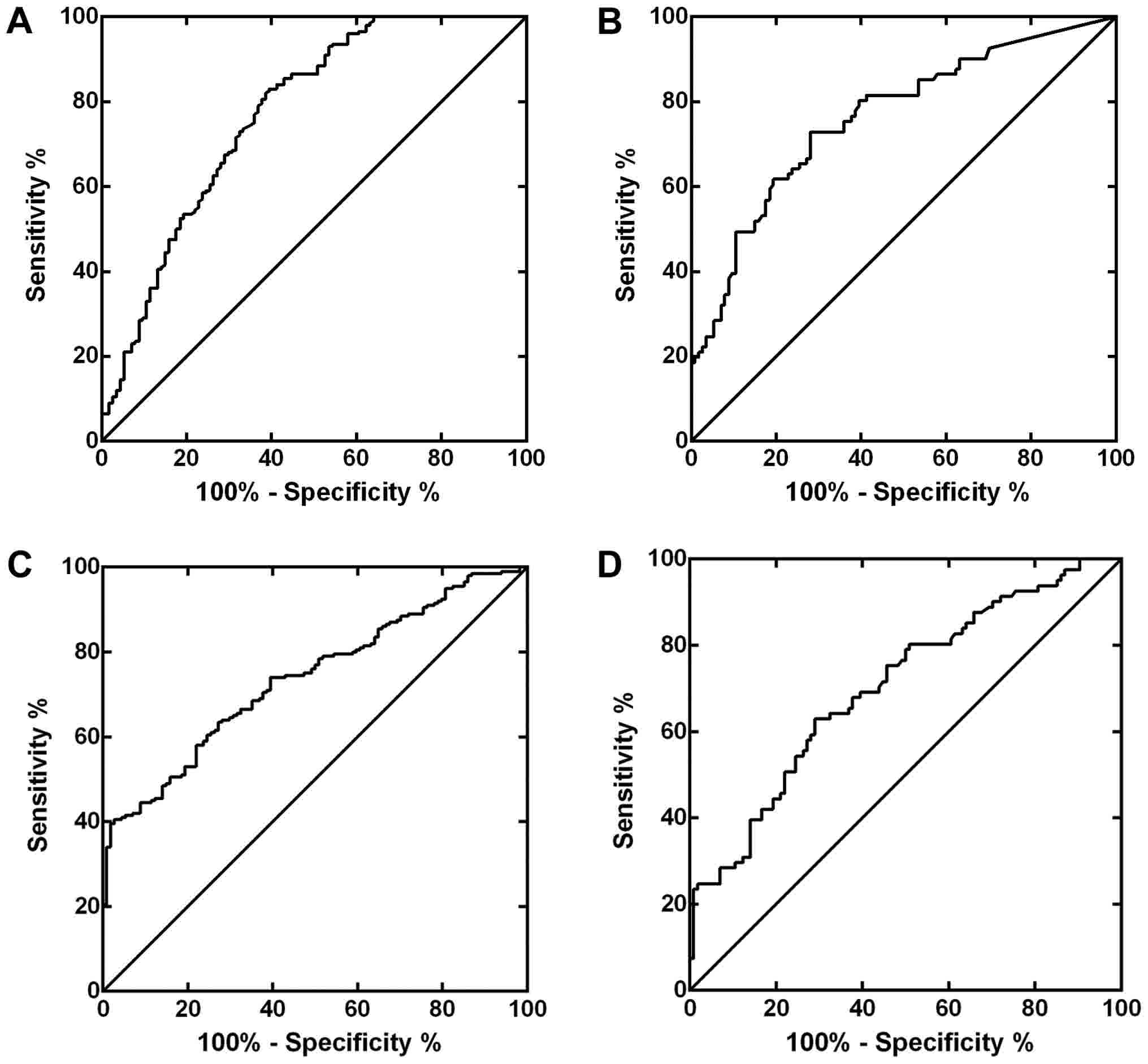
(3.10 ng/ml)]. ROC curves were constructed for BC and RCC (Fig. 6A and B). The area under the curve
(AUC) was 0.771 for BC and 0.763 for RCC, respectively.
Relationships between urine survivin
and clinicopathological characteristics
We analyzed urine survivin levels between different
clinicopathological parameters in BC and RCC. The results were
shown in Tables III and IV. In BC patients, urine survivin levels
were related to the tumor stage (P=0.002), lymph node metastasis
(P=0.017), distant metastasis (P=0.005) and tumor size (P=0.02). In
patients with RCC, urinary survivin showed no correlations with any
clinicopathological parameters listed in Table IV, which was probably due to the
limited number of RCC patients recruited in this study.
 | Table III.Associations between urinary survivin
and clinicopathological characteristics in bladder cancer. |
Table III.
Associations between urinary survivin
and clinicopathological characteristics in bladder cancer.
| Clinicopathological
characteristic | No. of
patients | Survivin
(ng/ml) | P-value |
|---|
| Sex |
|
|
|
|
Male | 150 | 3.23 (2.91) | 0.268 |
|
Female | 50 | 3.27 (3.00) |
|
| Age (years) |
|
|
|
|
≤60 | 81 | 3.89 (3.45) | 0.064 |
|
>60 | 119 | 3.14 (2.66) |
|
| Stage |
|
|
|
|
I+II | 103 | 3.02 (2.81) | 0.002 |
|
III+IV | 97 | 2.81 (3.00) |
|
| Lymph node
metastasis |
|
|
|
| No | 137 | 3.14 (2.71) | 0.017 |
|
Yes | 63 | 4.00 (3.31) |
|
| Distant
metastasis |
|
|
|
| No | 144 | 3.11 (2.73) | 0.005 |
|
Yes | 56 | 4.13 (4.52) |
|
| Tumor size
(cm) |
|
|
|
|
<2 | 75 | 2.83 (2.53) | 0.020 |
| ≥2 | 125 | 2.53 (2.53) |
|
| Grade |
|
|
|
| Low
grade | 43 | 3.03 (2.50) | 0.187 |
| High
grade | 148 | 3.60 (3.16) |
|
| Muscle
invasion |
|
|
|
| No | 93 | 3.49 (2.72) | 0.612 |
|
Yes | 97 | 2.72 (2.72) |
|
| Vascular tumor
thrombus |
|
|
|
|
Invisible | 32 | 3.81 (4.10) | 0.441 |
|
Visible | 28 | 2.90 (3.57) |
|
| NMP22 |
|
|
|
| − | 77 | 3.14 (2.57) | 0.493 |
| + | 33 | 2.57 (3.58) |
|
| Urine cytology |
|
|
|
| − | 23 | 3.28 (2.18) | 0.564 |
| + | 28 | 3.64 (2.50) |
|
 | Table IV.Relationships between urinary
survivin and clinicopathological characteristics in renal cell
carcinoma. |
Table IV.
Relationships between urinary
survivin and clinicopathological characteristics in renal cell
carcinoma.
| Clinicopathological
characteristic | No. of
patients | Survivin
(ng/ml) | P-value |
|---|
| Sex |
|
|
|
|
Male | 59 | 4.91 (5.60) | 0.222 |
|
Female | 22 | 3.52 (9.25) |
|
| Age (years) |
|
|
|
|
≤60 | 46 | 4.15 (10.24) | 0.630 |
|
>60 | 35 | 4.90 (4.49) |
|
| Stage |
|
|
|
|
I+II | 58 | 3.97 (6.68) | 0.530 |
|
III+IV | 23 | 5.84 (7.29) |
|
| Lymph node
metastasis |
|
|
|
| No | 66 | 3.84 (5.09) | 0.128 |
|
Yes | 15 | 6.33 (12.05) |
|
| Distant
metastasis |
|
|
|
| No | 70 | 3.84 (5.09) | 0.238 |
|
Yes | 11 | 6.33 (12.05) |
|
| Tumor size
(cm) |
|
|
|
| ≤3 | 33 | 3.16 (10.13) | 0.273 |
|
>3 | 48 | 5.02 (5.22) |
|
| Fuhrman grade |
|
|
|
|
1-2 | 56 | 4.15 (6.18) | 0.790 |
|
3-4 | 18 | 4.16 (18.29) |
|
| Vascular tumor
thrombus |
|
|
|
|
Invisible | 70 | 3.97 (6.81) | 0.316 |
|
Visible | 6 | 5.88 (7.97) |
|
| Depth of
infiltration |
|
|
|
| T1 | 64 | 4.56 (7.58) | 0.685 |
|
T2-T4 | 17 | 3.57 (8.74) |
|
Urinary survivin and LAPTM4B in BC and
RCC
As shown in Fig. 5B,
urine LAPTM4B levels were significantly higher in BC [11.20 ng/ml
(46.70 ng/ml), P<0.001] and RCC patients [8.08 ng/ml (21.47
ng/ml), P<0.001] than in healthy controls [3.90 ng/ml (7.91
ng/ml)]. Fig. 6C and D displayed the
ROC curves of LAPTM4B for BC and RCC. The AUCs were 0.738 for BC
and 0.704 for RCC, which showed no significant differences from
survivin (P>0.05). There was no significant correlation between
urinary survivin and LAPTM4B (P=0.790). We combined urinary
survivin with LAPTM4B in the preliminary diagnosis of BC and RCC.
The ROC curves were shown in Fig. 7,
of which the AUCs were 0.842 for BC and 0.920 for RCC. There were
statistical differences between the AUCs of LAPTM4B and the
combined evaluation (P<0.001 for BC, P=0.0017 for RCC,
respectively), statistical differences were also found between the
combination and survivin (P<0.001 for BC, P=0.0405 for RCC,
respectively). Table V gave the
sensitivities and specificities of the two markers evaluated
separately and jointly. Taken the parameters above into account,
the combination of urinary survivin and LAPTM4B could provide a
better diagnosis performance for BC and RCC.
 | Table V.Cut-off values, sensitivities and
specificities of urinary survivin and lysosome-associated protein
transmembrane-4β evaluated separately and jointly in BC and
RCC. |
Table V.
Cut-off values, sensitivities and
specificities of urinary survivin and lysosome-associated protein
transmembrane-4β evaluated separately and jointly in BC and
RCC.
|
| Cut-off value
(ng/ml) | Sensitivity
(%) | Specificity
(%) |
|---|
|
|
|
|
|
|---|
| Factor
detected | BC | RCC | BC | RCC | BC | RCC |
|---|
| Survivin | 1.97 | 2.71 | 83.00 | 72.84 | 60.53 | 71.93 |
| LAPTM4B | 25.69 | 6.16 | 40.50 | 62.96 | 97.37 | 71.05 |
| Combined |
|
|
|
|
|
|
|
Survivin | 2.39 | 6.26 | 87.50 | 85.19 | 69.3 | 85.96 |
|
LAPTM4B | 5.32 | 3.39 |
|
|
|
|
Discussion
In this study, we presented a simple step MPs-based
CLEIA for the detection of urine survivin. As urine has very
complex components and the pH of urine varies, the matrix effect
would be a thorny problem (14,15). Some
commercial ELISA kits employ sample diluent to solve the problem,
but it would take a lot of effort to dilute every single sample.
Normally, ELISA costs about 3–4 h and requires multiple procedures
including dilution, incubation, aspirating and washing while the
MPs based CLEIA could be done within one and a half hours. The MPs
based CLEIA needs only one-step shaking and the magnetic separation
makes solid-liquid phase separation quite convenient. Compared with
ELISA, the proposed method provided simplified procedures and
rapidity. A recent study by our colleagues presented a streptavidin
MPs based immunoassay (12). Though
this method has greatly reduced the consuming time of the whole
assay, it still needed multiple procedures and the matrix effect of
urine remained inevitable. Compared to that, the proposed method in
the present study was a one-step process which made the detection
of urinary survivin much more convenient. The relatively small
proportion of sample in the whole reaction system volume and the
higher ionic strength of the antibody diluent which resulted to
better buffering capacity greatly lowered the impact of matrix.
Consequently, the method evaluation demonstrated its accuracy and
precision. One-step immunoassay is normally faced with hook effect
(16,17), but the pseudo-negative result did not
appear when testing the extremely high concentration of survivin.
This is probably due to the sufficient antibodies coated on the
large specific surface of MPs (16,17). In
addition, because of less spatial hindrance underwent by antibodies
on the MPs surface, the antibody's biological activity could be
reserved efficiently (18).
Survivin mRNA measurement (19) and immunohistochemical (IHC) staining
of survivin (20) demonstrated the
higher expression of survivin in BC. The diagnostic value of urine
survivin for BC has been validated (21–23), which
is accorded with the findings of our study. Employing the proposed
method, we also found that urinary survivin was related to the
tumor stage, lymph node metastasis, distant metastasis and tumor
size in BC, which indicated that urinary survivin could also
monitor the development and metastasis state of BC. These results
are partially consistent with the studies of Chang et al
(12) and Gogalic et al
(21). The expression of survivin in
RCC by IHC technique has been investigeted (24–26), but
there were few researches about the diagnostic value of urinary
survivin for RCC. In this study, we proposed the possibility of
urine survivin serving as a potential biological marker for the
preliminary diagnosis of RCC. Some researches indicated that in RCC
survivin expression by IHC staining was associated with tumor stage
and grade (25,27), and another study found that survivin
expression was also correlated with lymph node metastasis (26). But in our study, no associations were
found between urinary survivin and clinicopathological features in
RCC patients. One reason might be the limited number of RCC
patients enrolled and the very complex urine composition (28) would probably impose serious impacts on
the results. In addition, IHC staining is a direct measurement of
suvivin expression of the tumor while the urinary survivin is
secreted by the tumor and the protein needs to pass through the
glomerular filtration barrier. Thus, the relationships between
urine survivin and the clinicopathological features would not be
the same as the results of IHC staining of RCC. Interestingly,
Baytekin et al (29) found no
correlation between survivin expression and stage, but they
indicated an inverse correlation between survivin expression and
tumor grade. Hence, the relationship between survivin and
clinicopathological parameters of RCC should be further
investigated.
LAPTM4B was found to be overexpressed in various
solid tumors and was associated with the prognosis of the tumors
(30–33). But the potential diagnostic value of
LAPTM4B levels in urine has not been studied yet. In this study, we
tested the urinary LAPTM4B in BC and RCC. The results showed that
the urine LAPTM4B levels were elevated in the patients compared
with healthy controls, and the ROC curves demonstrated urinary
LAPTM4B was of certain value for the preliminary diagnosis of BC
and RCC. It is the first time to investigate the possibility of
urinary LAPTM4B as a potential biomarker for the preliminary
diagnosis of BC and RCC.
We also found that the combination of urinary
survivin and LAPTM4B could give a better diagnostic performance.
The AUCs of the combined evaluation was bigger than LAPTM4B or
survivin alone for both malignant diseases. Thecombined evaluation
showed better sensitivities and specificities. A recent study by
Sha Li et al (34)
demonstrated that nuclear survivin protein expression were linearly
correlated with LAPTM4B in breast cancer. We investigated the
urinary survivin and LAPTM4B simultaneously in BC and RCC for the
first time. But in our study, urinary survivin did not have
correlation with LAPTM4B. Survivin is a 16.5 kDa protein and the
molecular weight of LAPTM4B is 35 kDa, which makes them small
enough to pass through the glomerular filtration barrier and then
could be detected in urine. But there are also a lot of exogenous
and endogenous interferences along with the process of urine
formation and sample collection (3,35). As a
result, the urinary survivin and LAPTM4B could not directly reflect
the expression of survivin and LAPTM4B in the tumor tissue, which
may explain the inconsistency between the study of Sha Li et
al (34) and our findings. This
inconsistency might also due to the heterogeneity in different
organs. For example, another study in human liver cell line did not
indicate the relationship between suvivin and LAPTM4B (36). Therefore, the relationships between
urinary survivin and LAPTM4B in BC and RCC need further
investigation.
There are three major molecular sub-types of
urothelial carcinoma: Urobasal, Genomically Unstable and SCC-like
(37). Urobasal tumors show frequent
expression of FGFR3, CCND1, KRT5, CDH3 (P-cadherin), CDH1
(E-cadherin) and maintain a urothelial differentiation axis
consisting of PPARG/RXRA, FOXA1/GATA3 and anterior HOXA and HOXB
genes. Genomically Unstable tumors are characterized by RB1
deletions, TP53 mutations and high ERBB2, CDH1 (E-cadherin)
expression. SCC-like tumors demonstrate enhanced expression of
KRT5, EGFR and CDH3 (P-cadherin). Both Genomically Unstable and
SCC-like tumors show increased proliferative activity via the
PLK1-FOXM1 axis (37,38). CCND1 could bind to the survivin
promoter region and activate it (39,40). The
expression levels of CCND1 and survivin have proven to be elevated
in various tumors (41,42). Survivin is a downstream target
molecule of Wnt/β-catenin signaling and inhibition of CDH3
(P-cadherin) could induce the down-regulation of β-catenin
(43), which suggests a positive
correlation between CDH3 (P-cadherin) and survivin expression.
Previous research showed abberrant CDH1 (E-cadherin) staining was
associated with overexpression of survivin in pTa urothelial
bladder carcinoma (44). Survivin
promoter interferes with the binding of GATA3 and GATA3 might play
a role in survivin expression (45).
Tumor suppressor protein pRB can repress survivin transcription
through interacting with survivin promoter, which indicates RB1
deletions could lead to elevated survivin expression (46). Wild-type p53 is known to repress
survivin expression at both mRNA and protein levels while mutant
p53 had little effect on survivin repression (47). It has been proven that EGFR could
upregulate survivin expression via the PI-3 kinase pathway
(48). LAPTM4B overexpression was
found to correlate with EGFR activation (49) and LAPTM4B could enhance and prolong
EGFR signaling through inhibiting EGF-induced EGFR intraluminal
sorting and lysosomal degradation (50). PLK1-FOXM1 axis regulates the
expression of survivin which is one of its down-stream targets
(51). Thus, survivin and LAPTM4B are
closely related to the molecular sub-types of BC.
In conclusion, we established a novel simple step
MPs-based CLEIA for the detection of urinary survivin and applied
it to the preliminary diagnosis of BC and RCC. Urinary survivin was
proved to be related to the tumor stage, lymph node metastasis,
distant metastasis and tumor size in BC, which indicated that
urinary survivin could monitor the tumor development and metastasis
to a certain degree in BC. Our findings also demonstrated that
urinary survivin and LAPTM4B could be of certain value for the
preliminary diagnosis of BC and RCC. Additionally, the combination
of them would present a better diagnostic performance. However,
this study is limited by the sample size and the lack of external
quality assessment of the proposed method. A larger sample size is
needed to further validate our findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81572910), The
Capital Healthy Development Special Fund (grant no. 2011-1009-03)
and The Capital Laboratory Medicine Clinical Characteristic Fund
(grant no. Z121107005112004).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY acquired the data and drafted the manuscript. JX
analyzed and interpreted the patient data. QYZ was a major
contributor in conception and design, and gave final approval of
the version to be published. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Care Committee of Peking University (Beijing, China). The
experiments involving patient samples were approved by the Ethics
Committee of the Peking University Cancer Hospital (Beijing,
China). All of the patients and healthy controls provided written
informed consent for participation in the study.
Consent for publication
All of the patients and healthy controls provided
written informed.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiedler GM, Baumann S, Leichtle A, Oltmann
A, Kase J, Thiery J and Ceglarek U: Standardized peptidome
profiling of human urine by magnetic bead separation and
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry. Clin Chem. 53:421–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito M, Kimoto M, Araki T, Shimada Y,
Fujii R, Oofusa K, Hide M, Usui T and Yoshizato K: Proteome
analysis of gelatin-bound urinary proteins from patients with
bladder cancers. Eur Urol. 48:865–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou R, Gore JL, Buckley D, Fu R,
Gustafson K, Griffin JC, Grusing S and Selph S: Urinary biomarkers
for diagnosis of bladder cancer: A systematic review and
meta-analysis. Ann Intern Med. 163:922–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo A, Wang X, Gao L, Shi J, Sun C and Wan
Z: Bladder tumour antigen (BTA stat) test compared to the urine
cytology in the diagnosis of bladder cancer: A meta-analysis. Can
Urol Assoc J. 8:E347–E352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pichler R, Tulchiner G, Fritz J, Schaefer
G, Horninger W and Heidegger I: Urinary UBC rapid and NMP22 test
for bladder cancer surveillance in comparison to urinary cytology:
Results from a prospective single-center study. Int J Med Sci.
14:811–819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papale M, Vocino G, Lucarelli G,
Rutigliano M, Gigante M, Rocchetti MT, Pesce F, Sanguedolce F, Bufo
P, Battaglia M, et al: Urinary RKIP/p-RKIP is a potential
diagnostic and prognostic marker of clear cell renal cell
carcinoma. Oncotarget. 8:40412–40424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altieri DC: Survivin-The inconvenient IAP.
Semin Cell Dev Biol. 39:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Hakim Abd TF, El-Shafie MK, Abdou AG,
Azmy RM, El-Naidany SS and El-Din Badr MO: Value of urinary
survivin as a diagnostic marker in bladder cancer. Anal Quant
Cytopathol Histpathol. 36:121–127. 2014.PubMed/NCBI
|
|
11
|
Meng Y, Wang L, Chen D, Chang Y, Zhang M,
Xu JJ, Zhou R and Zhang QY: LAPTM4B: An oncogene in various solid
tumors and its functions. Oncogene. 35:6359–6365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang Y, Xu J and Zhang Q: Microplate
magnetic chemiluminescence immunoassay for detecting urinary
survivin in bladder cancer. Oncol Lett. 14:4043–4052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Zhang QY and Wang YM: Cloning of
survivin gene and preparation its monoclonal antibodies as well as
checking survivin expression in liver carcinoma cells. Chin J Lab
Med. 29:258–262. 2006.(In Chinese).
|
|
14
|
Chatziharalambous D, Lygirou V, Latosinska
A, Stravodimos K, Vlahou A, Jankowski V and Zoidakis J: Analytical
performance of ELISA assays in urine: One more bottleneck towards
biomarker validation and clinical implementation. PLoS One.
11:e01494712016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thway T and Salimi-Moosavi H: Evaluating
the impact of matrix effects on biomarker assay sensitivity.
Bioanalysis. 6:1081–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Lin JM and Ying X: Evaluation of
carbohydrate antigen 50 in human serum using magnetic
particle-based chemiluminescence enzyme immunoassay. Anal Chim
Acta. 598:261–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Wang X, Li Z and Lin JM:
Evaluation of alpha-fetoprotein (AFP) in human serum by
chemiluminescence enzyme immunoassay with magnetic particles and
coated tubes as solid phases. Anal Chim Acta. 631:212–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Zhang QY, Li ZJ, Ying XT and Lin
JM: Development of high-performance magnetic chemiluminescence
enzyme immunoassay for alpha-fetoprotein (AFP) in human serum. Clin
Chim Acta. 393:90–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horstmann M, Bontrup H, Hennenlotter J,
Taeger D, Weber A, Pesch B, Feil G, Patschan O, Johnen G, Stenzl A
and Brüning T: Clinical experience with survivin as a biomarker for
urothelial bladder cancer. World J Urol. 28:399–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HA, Su CM, Hsieh HY, Tung CL, Hsu CD,
Wang YH and Shen CH: Clinical significance of survivin expression
in patients with urothelial carcinoma. Dis Markers.
2014:5749852014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gogalic S, Sauer U, Doppler S and
Preininger C: Bladder cancer biomarker array to detect aberrant
levels of proteins in urine. Analyst. 140:724–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt J, Propping C, Siow WY,
Lohse-Fischer A, Toma M, Baldauf-Twelker A, Hakenberg OW, Wirth MP
and Fuessel S: Diagnostic and prognostic value of bladder
cancer-related transcript markers in urine. J Cancer Res Clin
Oncol. 142:401–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sah NK and Seniya C: Survivin splice
variants and their diagnostic significance. Tumour Biol.
36:6623–6631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zamparese R, Pannone G, Santoro A, Lo
Muzio L, Corsi F, Pedicillo MC, Scillitani EL, Tortorella S,
Staibano S, Piscuoglio S, et al: Survivin expression in renal cell
carcinoma. Cancer Invest. 26:929–935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Byun SS, Yeo WG, Lee SE and Lee E:
Expression of survivin in renal cell carcinomas: Association with
pathologic features and clinical outcome. Urology. 69:34–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lei Y, Geng Z, Guo-Jun W, He W and
Jian-Lin Y: Prognostic significance of survivin expression in renal
cell cancer and its correlation with radioresistance. Mol Cell
Biochem. 344:23–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi ZG, Li SQ, Li ZJ, Zhu XJ, Xu P and Liu
G: Expression of vimentin and survivin in clear cell renal cell
carcinoma and correlation with p53. Clin Transl Oncol. 17:65–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adachi J, Kumar C, Zhang Y, Olsen JV and
Mann M: The human urinary proteome contains more than 1500
proteins, including a large proportion of membrane proteins. Genome
Biol. 7:R802006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baytekin F, Tuna B, Mungan U, Aslan G and
Yorukoglu K: Significance of P-glycoprotein, p53, and survivin
expression in renal cell carcinoma. Urol Oncol. 29:502–507. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Yang H, McNutt MA, Xiong F, Nie X,
Li L and Zhou R: LAPTM4B overexpression is an independent
prognostic marker in ovarian carcinoma. Oncol Rep. 20:1077–1083.
2008.PubMed/NCBI
|
|
31
|
Zhou L, He XD, Cui QC, Zhou WX, Qu Q, Zhou
RL, Rui JA and Yu JC: Expression of LAPTM4B-35: A novel marker of
progression, invasiveness and poor prognosis of extrahepatic
cholangiocarcinoma. Cancer Lett. 264:209–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang Y, Yin M, Jiang W, Zhang H, Xia B,
Xue Y and Huang Y: Overexpression of LAPTM4B-35 is associated with
poor prognosis in colorectal carcinoma. Am J Surg. 204:677–683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang H, Xiong FX, Lin M, Yang Y, Nie X and
Zhou RL: LAPTM4B-35 overexpression is a risk factor for tumor
recurrence and poor prognosis in hepatocellular carcinoma. J Cancer
Res Clin Oncol. 136:275–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li S, Wang L, Meng Y, Chang Y, Xu J and
Zhang Q: Increased levels of LAPTM4B, VEGF and survivin are
correlated with tumor progression and poor prognosis in breast
cancer patients. Oncotarget. 8:41282–41293. 2017.PubMed/NCBI
|
|
35
|
Zerefos PG and Vlahou A: Urine sample
preparation and protein profiling by two-dimensional
electrophoresis and matrix-assisted laser desorption ionization
time of flight mass spectroscopy. Methods Mol Biol. 428:141–157.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Shan Y, Yang H, Zhang S, Lin M, Zhu
P, Chen XY, Yi J, McNutt MA, Shao GZ and Zhou RL: Upregulation of
LAPTM4B-35 promotes malignant transformation and tumorigenesis in
L02 human liver cell line. Anat Rec (Hoboken). 294:1135–1142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sjödahl G, Lövgren K, Lauss M, Patschan O,
Gudjonsson S, Chebil G, Aine M, Eriksson P, Månsson W, Lindgren D,
et al: Toward a molecular pathologic classification of urothelial
carcinoma. Am J Pathol. 183:681–691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eriksson P, Aine M, Veerla S, Liedberg F,
Sjödahl G and Höglund M: Molecular subtypes of urothelial carcinoma
are defined by specific gene regulatory systems. BMC Med Genomics.
8:252015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Z, Li X, Li C, Su Y, Fang W, Zhong C,
Ji W, Zhang Q and Su C: Transcription factor OCT4 promotes cell
cycle progression by regulating CCND1 expression in esophageal
carcinoma. Cancer Lett. 354:77–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao L, Li C, Shen S, Yan Y, Ji W, Wang J,
Qian H, Jiang X, Li Z, Wu M, et al: OCT4 increases BIRC5 and CCND1
expression and promotes cancer progression in hepatocellular
carcinoma. BMC Cancer. 13:822013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elmegeed GA, Yahya SM, Abd-Elhalim MM,
Mohamed MS, Mohareb RM and Elsayed GH: Evaluation of heterocyclic
steroids and curcumin derivatives as anti-breast cancer agents:
Studying the effect on apoptosis in MCF-7 breast cancer cells.
Steroids. 115:80–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diaz A, Valera A, Carrera C, Hakim S,
Aguilera P, García A, Palou J, Puig S, Malvehy J and Alos L:
Pigmented spindle cell nevus: Clues for differentiating it from
spindle cell malignant melanoma. A comprehensive survey including
clinicopathologic, immunohistochemical, and FISH studies. Am J Surg
Pathol. 35:1733–1742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun L, Hu H, Peng L, Zhou Z, Zhao X, Pan
J, Sun L, Yang Z and Ran Y: P-cadherin promotes liver metastasis
and is associated with poor prognosis in colon cancer. Am J Pathol.
179:380–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Breyer J, Gierth M, Shalekenov S, Aziz A,
Schäfer J, Burger M, Denzinger S, Hofstädter F, Giedl C and Otto W:
Epithelial-mesenchymal transformation markers E-cadherin and
survivin predict progression of stage pTa urothelial bladder
carcinoma. World J Urol. 34:709–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rodriguez L, Villalobos X, Dakhel S,
Padilla L, Hervas R, Hernández JL, Ciudad CJ and Noé V: Polypurine
reverse Hoogsteen hairpins as a gene therapy tool against survivin
in human prostate cancer PC3 cells in vitro and in vivo. Biochem
Pharmacol. 86:1541–1554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang Y, Saavedra HI, Holloway MP, Leone G
and Altura RA: Aberrant regulation of survivin by the RB/E2F family
of proteins. J Biol Chem. 279:40511–40520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mirza A, McGuirk M, Hockenberry TN, Wu Q,
Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, et al:
Human survivin is negatively regulated by wild-type p53 and
participates in p53-dependent apoptotic pathway. Oncogene.
21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Q and Greene MI: EGFR enhances
Survivin expression through the phosphoinositide 3 (PI-3) kinase
signaling pathway. Exp Mol Pathol. 79:100–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tian M, Chen Y, Tian D, Qiao X, Ma Z and
Li J: Beclin1 antagonizes LAPTM4B-mediated EGFR overactivation in
gastric cancer cells. Gene. 626:48–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tan X, Sun Y, Thapa N, Liao Y, Hedman AC
and Anderson RA: LAPTM4B is a PtdIns(4,5)P2 effector that regulates
EGFR signaling, lysosomal sorting, and degradation. EMBO J.
34:475–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Momeny M, Zarrinrad G, Moghaddaskho F,
Poursheikhani A, Sankanian G, Zaghal A, Mirshahvaladi S, Esmaeili
F, Eyvani H, Barghi F, et al: Dacomitinib, a pan-inhibitor of ErbB
receptors, suppresses growth and invasive capacity of
chemoresistant ovarian carcinoma cells. Sci Rep. 7:42042017.
View Article : Google Scholar : PubMed/NCBI
|