Introduction
Colorectal cancer (CRC) ranks as the third-leading
cause of cancer-associated mortalities in Taiwan (1). The majority of Taiwanese patients with
CRC (60–70%) present with stage II–IV disease at initial diagnosis
(2,3).
Among newly diagnosed cases, ~20–25% of patients have advanced
disease with distant metastases (2,3).
Recurrence occurs in ~30% of patients with advanced CRC, even
following curative resection (2).
Patients with stage I and II CRC have an 86–95% five-year survival
rate, whereas those with stage III and IV metastatic diseases have
five-year survival rates of ~67 and ~12%, respectively (3). In a previous study by the present
authors, it was reported that the genetic background of Taiwanese
patients with CRC was different from that of Caucasian patients
with CRC (4). In the study, ~33.8% of
tumor tissues in Taiwanese patients with CRC contained Adenomatous
polyposis coli (APC) mutations. This figure is close to that
reported in Asia but significantly lower compared with the values
reported in western countries (70–80%) (5). Therefore, studies of the dynamic nature
of molecular signatures in CRC are required to determine the
prognosis of patients.
Ribonucleotide reductase (RR) is a highly regulated
rate-limiting enzyme that is used in the conversion of
ribonucleoside diphosphate to 2′-deoxyribonucleoside diphosphate
(6), and it is essential for DNA
synthesis (6,7). In humans, one large subunit (M1) and two
small subunits (RRM2 and p53R2) of RR have been identified
(6,7).
Although the protein sequence of the two small RR subunits, p53R2
and RRM2, show 80% similarity, their biological function is
markedly different (7). Previous
reports demonstrated that the regulation of RRM2 and
p53R2 might play a critical role in the invasion potential
of cancer cells and the establishment of the metastatic phenotype
(8,9).
Research also suggested that RRM2 might potentially serve as
a biomarker for predicting aggressive CRC, with poor survivability
and progression-free survival (10).
Inhibition of RR activity has been tested as a potential
therapy in anticancer settings (11).
Therefore, the overexpression of RRM2 has an important role
in the pathogenesis of CRC.
Research has suggested that microRNAs (miRNAs),
which are small, noncoding RNAs, modulate gene expression by
degrading mRNA and/or inhibiting protein translation (12). The dysregulation of miRNA has been
implicated in numerous processes during tumoral progression
(12). In previous reports by the
present authors, it was demonstrated that different miRNAs were
involved in the pathogenesis in CRC, depending on the presence or
absence of an APC gene mutation (5,13–15). For example, miR-224 suppressed
the migration of CRC cells by targeting cell division cycle 42 in
patients with CRC and an APC gene mutation (13). It was also demonstrated by the present
authors that the downregulation of let-7a-5p in sera and
tumor tissues of patients with CRC could be used to predict lymph
node metastasis and disease prognosis (14). In addition, the present authors
demonstrated that let-7a appeared to regulate the expression
of miR-21 (15). miR-21 has
oncogene-like activity and is highly expressed in several types of
cancer (16). In a recent study, it
was also found that patients with APC mutations and high
miR-21 expression had lower APC gene expression and
exhibited poorer overall survival (OS) rates compared with patients
with APC mutations and low miR-21 expression,
APC wild-type and high miR-21 expression, and
APC wild-type and high miR-21 expression (5). The same study demonstrated that the
downregulation of APC gene expression was associated not
only with expression of the APC gene mutation but also with
upregulation of miR-21 (5).
Based on these findings, the present authors speculated that the
expression of the p53, APC and k-ras genes may be
associated with miRNA expression in patients with CRC.
The k-ras gene is a member of the ras gene
family (H-, K- and N-ras) (17).
Oncogenic k-ras mutations have been detected in several
types of cancer (e.g., lung, colon and pancreatic) (17). Additionally, ~20–50% of primary
colorectal tumors contain oncogenic k-ras mutations
(18). Recent clinical trials
verified that the k-ras gene mutation was associated with
cetuximab resistance in patients with metastatic CRC (19). However, whether mutant k-ras
genes affect survival rates, tumoral recurrence and drug resistance
in patients with CRC and who receive adjuvant chemotherapy is
unclear. Previous research identified a positive association
between RRM2 and k-ras genes, showing that
re-expressed k-ras genes in a HKe3 colon cancer cell line
induced RRM2 expression (20).
Based on these findings, we suggest that the interaction of
k-ras with RRM2 may play a role in survival rates and
drug resistance in CRC patients.
In the present study, it was hypothesized that the
downregulation of miR-211 would induce RRM2
expression and promote tumorigenesis in CRC and that the expression
levels of miR-211, p53R2 and RRM2 may be used as
biomarkers to predict clinical outcomes and tumoral recurrence in
CRC. It was also hypothesized that p53/APC/k-ras gene
mutations would result in overexpression of RRM2 and have a
role in disease progression and clinical outcomes of patients with
CRC. Therefore, the associations between miR-211, p53R2 and
RRM2 gene expression and clinical outcomes in CRC patients
with and without p53/APC/k-ras mutations were analyzed.
Patients and methods
Study population
CRC tumor tissue samples were collected from 192
non-selected patients who underwent surgical resection for CRC at
the Department of Surgery, Taipei Medical University Hospital
(Taipei, Taiwan) between December 2011 and December 2013 (14). The acquisition of the samples and
their subsequent examination were approved by the Institutional
Review Board of Taipei Medical University (Taipei, Taiwan).
Informed written consent was obtained from all the patients and/or
guardians prior to the use of the resected specimens.
None of the participants had a previous history of
cancer. The clinical stages and pathological features of primary
tumors were defined according to the criteria of the American Joint
Commission on Cancer (https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx).
A total of 33 of the 192 patients with stage IV disease and distant
metastasis were enrolled in the present study, of which 5 had lung
metastasis, 16 had liver metastasis, 9 had peritoneum metastasis
and 3 had para-aortic lymph nodes metastasis. All of the patients
had oligometastatic disease and had undergone surgery but not
chemotherapy. Postoperative follow-up visits were scheduled every
three months thereafter during the first two years, then every six
months thereafter, or more frequently if needed. Survival and
recurrence were followed up in all the patients in the present
study.
The CRC tissues and paired non-tumor tissues from
the aforementioned patients were obtained from the Tissue Bank of
Taipei Medical University (Taipei. Taiwan) between December 2011
and December 2013. In the present study, a total 192 patients were
enrolled, including 90 females and 102 males, age ranging from 40
to 90 years old. A pathologist confirmed that >95% of the cells
were tumor cells based on H&E-stained frozen sections. The
normal tissues were used as a control. These tissue samples were
obtained from the same patient and were checked by a
pathologist.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) -based detection of
miR-211
Total RNA was extracted from the tumor tissue
samples or sera of the patients with CRC using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The expression
of mature miRNA was detected by a TaqMan miRNA assay (Applied
Biosystems, Thermo Fisher Scientific, Inc; catalog no. 4427975;
sequence; UUCCCUUUGUCAUCCUUCGCCU) and normalized relative to U6B
using the 2−ΔΔCq method (21). All TaqMan PCRs were performed in
triplicate. The PCR reaction was conducted with the following
conditions: starting with 95°C for 10 min, followed by 40 cycles of
amplification (95°C for 15 sec and 60°C for 1 min). The definition
of high and low expression of miR-211 was dependent on the
mean value of the expression of these genes in the normal tissues
of the patients. High expression was defined as expression levels
higher than the mean expression in non-tumor tissues. Low
expression was defined as expression levels lower than he mean
expression in normal tissues. The mean expression level of
miR-211 in normal colon tissue samples was 20.21±10.81. The
PCR-based detection of miR-211 was conducted as described in
a previous report by the present authors (14).
Detection of p53R2 and RRM2 protein
expression by immunohistochemistry
Formalin-fixed and paraffin-embedded specimens were
sectioned at a thickness of 3 µm. All the sections were
deparaffinized in xylene, sequentially rehydrated through serial
dilutions of alcohol, and washed in phosphate-buffered saline. The
sections used for p53R2 and RRM2 detection were
immersed in a citrate buffer (pH 6.0) and heated in a microwave
oven twice for 5 min. Mouse anti-p53R2 (catalog no. SC-137174) and
RRM2 (catalog no. SC-81850) monoclonal antibodies (all 1:100; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) were used as the primary
antibodies. The conventional streptavidin peroxidase method (DAKO;
Agilent Technologies, Inc., Santa Clara, CA, USA; LSAB Kit K675)
was performed to develop signals according to the manufacturer's
protocol and the cells were counter-stained with hematoxylin. The
details of the protocol used have been described previously
(22). Negative controls that did not
include the primary antibodies were also prepared.
A total of three observers independently evaluated
the results and scored the percentage of positive expression in the
samples. In each specimen, the cells that positively stained for
anti-p53R2 and RRM2 antibodies were recorded as a percentage (%)
using a labeling index, and the measurements were calculated. The
scores were as follows: 0, no positive staining; +, from 1 to 10%
positive cells; ++, from 11 to 50% positive cells; and +++, >50%
positive cells. Among the 192 CRC patients, 99 samples were
negative for p53R2 protein expression. None of the patients were
scored +, 57 were scored ++ and 36 were scored +++. For RRM2
protein expression, 96 patients were negative. None of the patients
were scored +, 35 were scored ++ and 61 were +++. The scores of ++
and +++ were considered to represent high immunostaining, and
scores of 0 and + were classified as low immunostaining.
Mutation analysis of APC, k-ras and
p53 genes
Genomic DNA was prepared from 192 frozen CRC tissues
using standard proteinase K digestion and phenol/chloroform
extraction following homogenization. Mutations in the APC,
p53 and k-ras genes were determined by direct sequencing
of PCR products. The detailed protocol used has been described
previously (23). The target
sequences were amplified in a 50 µl reaction mixture containing 20
pmol of each primer, 2.5 U Taq polymerase (Takara Bio, Inc., Otsu,
Japan), 0.5 mmol/l dNTPs, 5 µl PCR reaction buffer and 1 µl genomic
DNA as the template. Oligonucleotide primers were used to amplify
the mutation cluster region of the APC, p53 and k-ras
genes. A total of four sets of oligonucleotide primers were used
for APC: Forward, 5′-CAGACTTATTGTGTAGAAGA-3′ and reverse,
5′-CTCCTGAAGAAAATTCAACA-3′; forward, 5′-AGGGTTCTAGTTTATCTTCA-3′ and
reverse, 5′-TCTGCTTGGTGGCATGGTTT-3′; forward,
5′-GGCATTATAAGCCCCAGTGA-3′ and reverse, 5′-AAATGGCTCATCGAGGCTCA-3′;
forward, 5′-ACTCCAGATGGATTTTCTTG-3′ and reverse,
5′-GGCTGGCTTTTTTGCTTTAC-3′. A total of three sets of
oligonucleotide primers were used for p53: Forward,
5′-TGCCCTGACTTTCAACTCTG-3′ and reverse:
5′-AGTTGCAAACCAGACCTCAGG-3′; forward, 5′-CCTGTGTTATCTCCTAGGTTG-3′
and reverse, 5′-TCTCCTCCACCGCTTCTTGT-3′; forward,
5′-AAGGCGCACTGGCCTCATCTT-3′ and reverse,
5′-GAATCTGAGGCATAACTGCAC-3′. One set of oligonucleotide primers was
used for k-ras: Forward, 5′-AGGCCTGCTGAAAATGACTGAA-3′ and
reverse, 5′-AAAGAATGGTCCTGCACCAG-3′.
Ingenuity Pathways Analysis (IPA)
The Ingenuity Pathways Analysis (IPA) platform was
used (http://www.ingenuity.com/) to
investigate associations between RRM2, p53R2 (RRM2B), KRAS, and
miR-211. The platform can reveal molecular interactions according
to records contained in its Ingenuity Knowledge Base. The Path
Explore tool in the platform was used to identify interactions
between the molecules. In total, 13 interacting proteins were
identified, including 4 transcription regulators, 3 enzymes, 1
transporter, and 5 proteins with other functions. Among the
proteins, BCL2, an apoptosis regulator, serves a central role by
interacting with RRM2, KRAS, and miR-211.
Statistical analysis
All data were analyzed using the Statistical Package
for the Social Sciences, software (version 13.0; SPSS, Inc.,
Chicago, IL, USA). A chi-square test (χ2 test) was used
to compare the association of p53R2 and RRM2 gene
expression with clinical parameters in CRC. A total of two
independent tests and k-independent nonparametric tests were used
to compare the expression level of miR-211 in the presence
of different clinical parameters in CRC. A probability value of
P<0.05 was considered statistically significant. Kaplan-Meier
survival curves were constructed for overall survival (OS) and
disease-free survival (DFS), and the log-rank test was used to
evaluate the differences in survival curves of patients with and
without p53R2 and RRM2 expression. In the present
study, survival was defined as the time from the date of the
surgical intervention until 31st July 2016.
Results
RRM2/p53R2 expression is associated
with tumoral metastasis in CRC
The p53R2 gene was expressed in 93 of 192
(48.4%) patients, and hRRM2 was expressed in 96 of 192
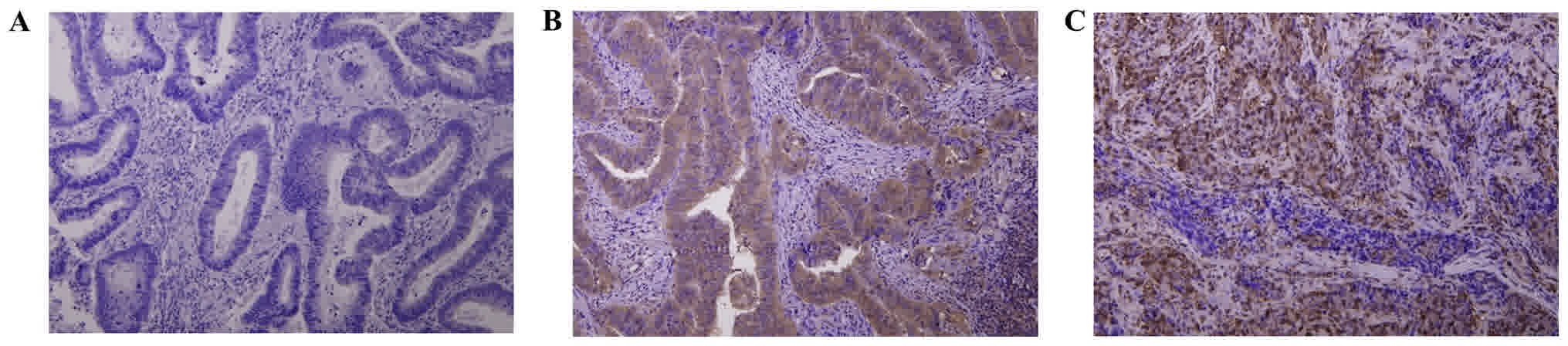
(50.0%) patients. Both proteins were expressed in CRC tumor cells
(Fig. 1). As shown in Table I, the expression level of p53R2
was significantly lower in patients with distant metastasis and
late-stage CRC compared with patients with no metastasis and
early-stage CRC (M factor, distant metastasis, P=0.002; stage,
P=0.008; Table I). No additional
associations were identified between p53R2 expression and
other clinical parameters (Table I).
In addition, RRM2 expression was significantly higher in
patients with lymph node metastasis and late-stage CRC compared
with patients with no metastasis and early-stage CRC (N factor,
lymph node metastasis, P=0.001; stage, P=0.001). No additional
associations were detected between RRM2 and other clinical
parameters (Table I).
 | Table I.Association of p53R2 and
RRM2 expression and clinical parameters in tumor tissues of
patients with colorectal cancer. |
Table I.
Association of p53R2 and
RRM2 expression and clinical parameters in tumor tissues of
patients with colorectal cancer.
| A, p53R2
expression |
|---|
|
|---|
| Parameters | Low (n=99) | High (n=93) |
|---|
| Age, years |
|
|
|
≤65 | 44 | 50 |
|
>65 | 55 | 43 |
|
P-value | 0.248 |
|
| Sex |
|
|
|
Female | 43 | 47 |
|
Male | 56 | 46 |
|
P-value | 0.386 |
|
| T factor |
|
|
| 1 | 5 | 2 |
| 2 | 12 | 16 |
| 3 | 57 | 53 |
| 4 | 25 | 22 |
|
P-value | 0.562 |
|
| N factor |
|
|
| 0 | 43 | 40 |
|
1+2 | 56 | 53 |
|
P-value | 1.000 |
|
| M factor |
|
|
| 0 | 74 | 85 |
| 1 | 25 | 8 |
|
P-value | 0.002 |
|
| Stage |
|
|
| I | 13 | 10 |
| II | 24 | 29 |
|
III | 36 | 46 |
| IV | 26 | 8 |
|
P-value | 0.008 |
|
|
| B, RRM2
expression |
|
|
Parameters | Low
(n=96) | High
(n=96) |
|
| Age, years |
|
|
|
≤65 | 47 | 47 |
|
>65 | 49 | 49 |
|
P-value | 1.000 |
|
| Sex |
|
|
|
Female | 40 | 50 |
|
Male | 56 | 46 |
|
P-value | 0.193 |
|
| T factor |
|
|
| 1 | 4 | 3 |
| 2 | 19 | 9 |
| 3 | 51 | 59 |
| 4 | 22 | 25 |
|
P-value | 0.206 |
|
| N factor |
|
|
| 0 | 53 | 30 |
|
1+2 | 43 | 66 |
|
P-value | 0.001 |
|
| M factor |
|
|
| 0 | 82 | 77 |
| 1 | 14 | 19 |
|
P-value | 0.445 |
|
| Stage |
|
|
| I | 18 | 5 |
| II | 33 | 20 |
|
III | 30 | 52 |
| IV | 15 | 19 |
|
P-value | 0.001 |
|
Effect of RRM2 and p53R2 expression on
OS and DFS in CRC
We hypothesized that the expression levels of
p53R2 and RRM2 would contribute to tumoral
progression and metastasis in CRC and that the expression of
p53R2 and RRM2 would be associated with OS and DFS in
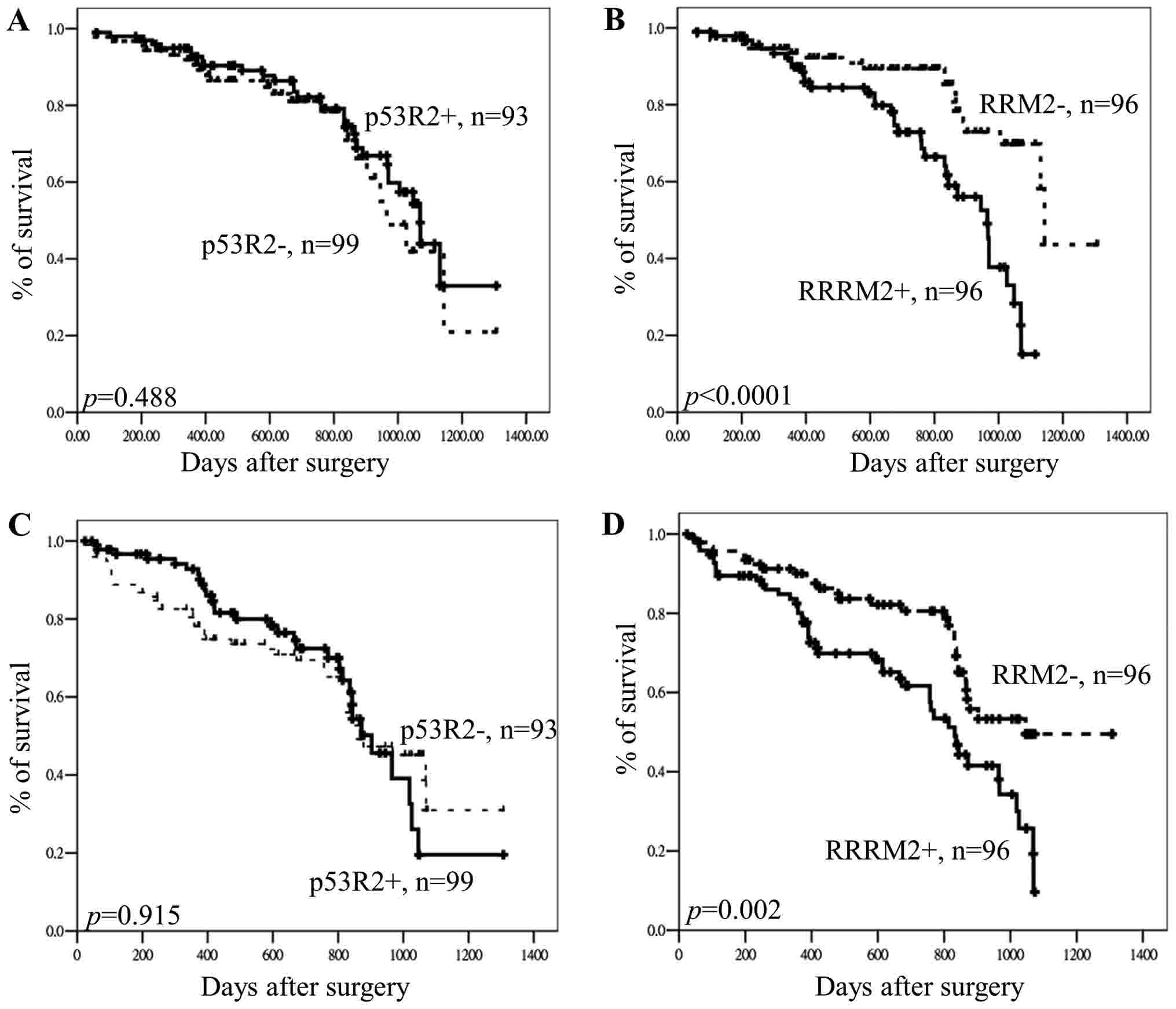
CRC. As indicated by the results of the Kaplan-Meier analysis,
p53R2 protein expression levels exhibited no association
with OS or DFS in CRC (OS, P=0.488; DFS, P=0.915 for; Fig. 2). However, the results of the
Kaplan-Meier analysis demonstrated that RRM2 protein
expression was associated with OS and DFS in patients with CRC (OS,
P<0.0001; DFS, P=0.002; Fig. 2).
Patients with low RRM2 expression had longer OS and DFS
compared patients with high RRM2 expression. Therefore, it
is suggested that RRM2 expression has potential to be a
prognostic and tumoral recurrence biomarker in patients with
CRC.
RRM2 expression is associated with the
k-ras gene mutation but not with p53/APC gene mutations in patients
with CRC
The associations between p53R2 and
RRM2 expression and gene mutations (APC, p53 and
k-ras) in patients with CRC were further analyzed. As
indicated in Table II, the
expression of p53R2 and RRM2 was not associated with
APC or P53 gene mutations in tumors of patients with
CRC. A positive association was observed between RRM2
protein expression and the k-ras gene mutation. However,
there was no association between p53R2 expression and the
k-ras mutation. In addition, the expression of RRM2
was higher in patients with the k-ras gene mutation compared
with patients with wild-type k-ras (P=0.008; Table II).
 | Table II.Association between p53R2 and
RRM2 expression and APC, p53, and k-ras gene
mutations in patients with colorectal cancer. |
Table II.
Association between p53R2 and
RRM2 expression and APC, p53, and k-ras gene
mutations in patients with colorectal cancer.
| A,
p53R2 |
|---|
|
|---|
| APC, n | − (n=99) | + (n=93) |
|---|
|
Wild-type | 63 | 49 |
|
Mutant | 36 | 44 |
|
P-value | 0.144 |
|
| P53, n |
|
|
|
Wild-type | 73 | 73 |
|
Mutant | 26 | 19 |
|
P-value | 0.397 |
|
| K-ras,
n |
|
|
|
Wild-type | 74 | 69 |
|
Mutant | 25 | 24 |
|
P-value | 1.000 |
|
|
| B,
RRM2 |
|
| APC,
n | -
(n=96) | +
(n=96) |
|
|
Wild-type | 55 | 57 |
|
Mutant | 41 | 39 |
|
P-value | 0.884 |
|
| P53, n |
|
|
|
Wild-type | 70 | 76 |
|
Mutant | 25 | 20 |
|
P-value | 0.398 |
|
| K-ras,
n |
|
|
|
Wild-type | 80 | 63 |
|
Mutant | 16 | 33 |
|
P-value | 0.008 |
|
Effect of RRM2 expression on OS and
DFS of patients with CRC according to k-ras status
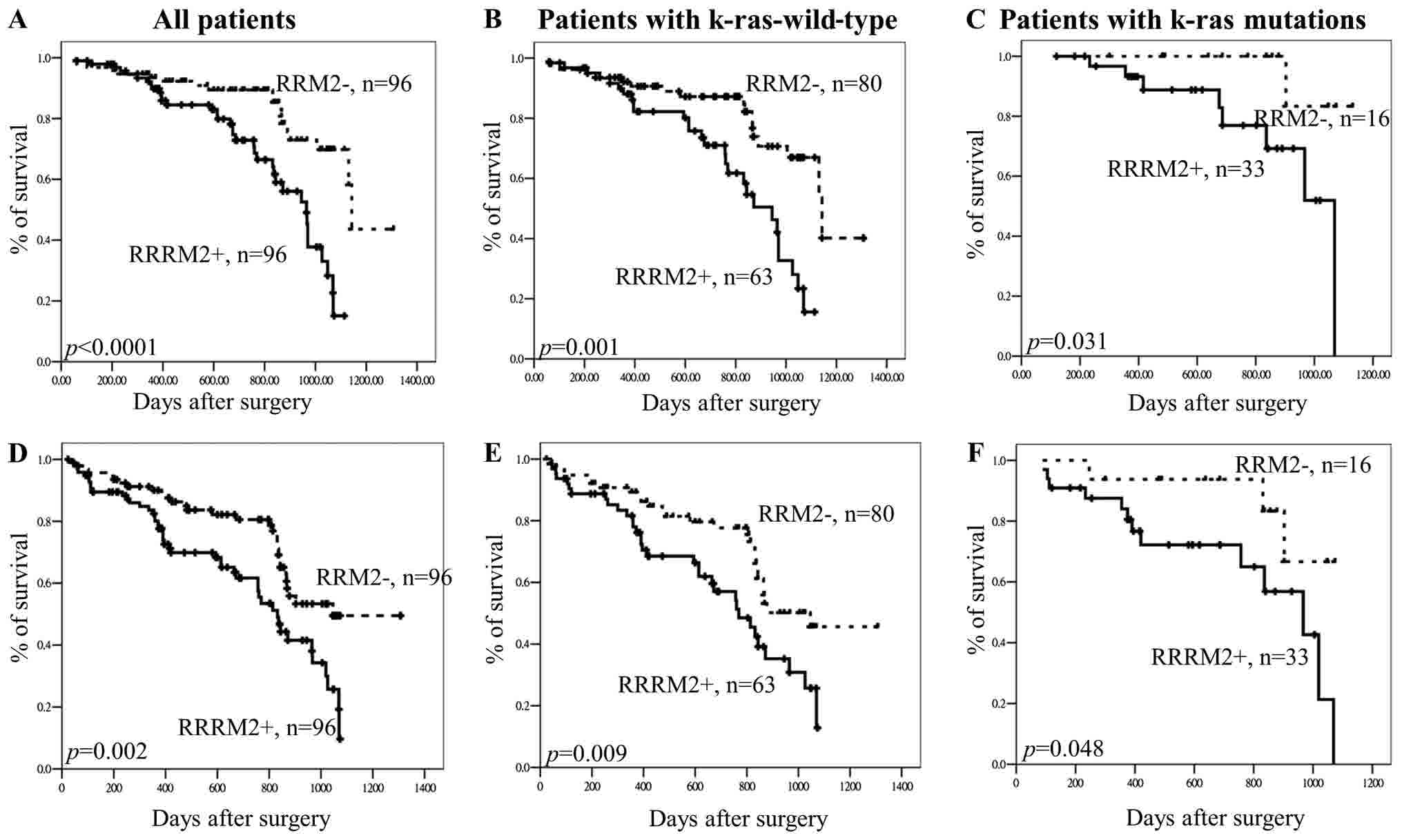
The results indicated that RRM2 expression
was associated with the k-ras gene mutation. Therefore, it
was hypothesized that the effects of RRM2 expression on OS
or DFS in CRC would differ among patients, depending on the
presence or absence of k-ras mutations. The results of the
Kaplan-Meier analysis indicated that RRM2 expression was
associated with clinical outcomes in patients with wild-type
k-ras (P=0.001) and those with k-ras gene mutations
(P=0.031) (all patients, P<0.0001; Fig. 3A-C). The association between
RRM2 expression and DFS in patients with and without
k-ras mutations was also analyzed. The results revealed that
hRRM2 expression was associated with overall DFS in patients
with wild-type k-ras (P=0.009) and in those with the
k-ras gene mutation (P=0.048) (all patients, P=0.002;
Fig. 3D-F). Therefore, it is
suggested that RRM2 is not associated with the k-ras
gene status but that RRM2 expression may have potential as a
prognostic and recurrence marker in patients with CRC.
miR-211 expression negatively
regulates RRM2 expression in patients with CRC and k-ras gene
mutations
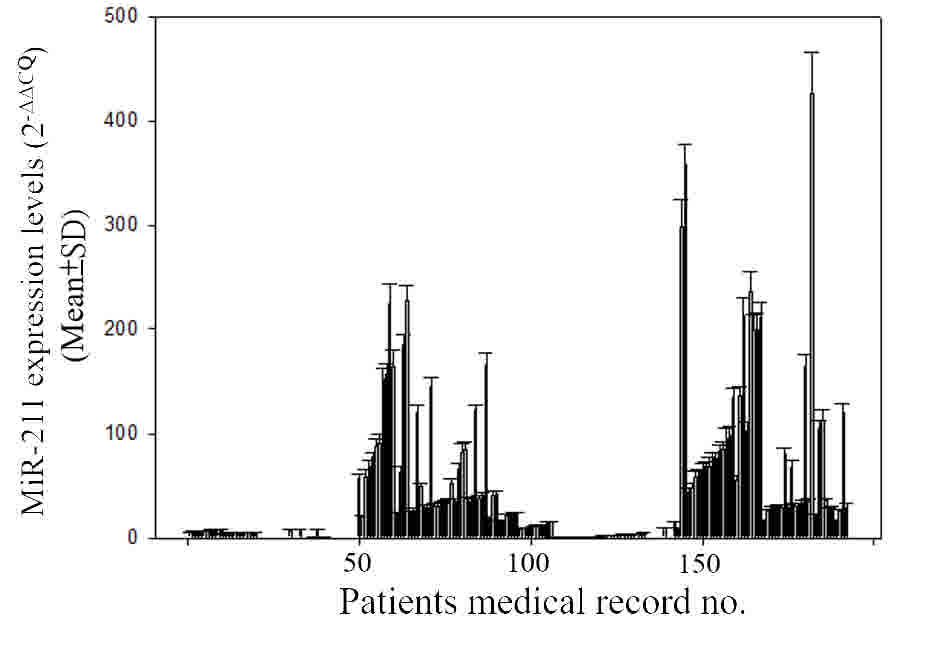
The association of miR-211 with RRM2
expression in tumor tissue samples of patients with CRC was
analyzed. The levels of miR-211 expression in tumor tissue
samples from patients with CRC are illustrated in Fig. 4. As indicated in Table III, the level of miR-211 was
significantly negatively associated with RRM2 protein
expression in patients with CRC and k-ras gene mutation
(P<0.0001) but not in patients with CRC and wild-type
k-ras gene (P=0.634). In addition, miR-211 expression
was negatively associated with lymph node metastasis, distant
metastasis, and cancer stage (all P<0.0001; Table III). Patients with lymph node
metastasis, distant metastasis, and late-stage disease had lower
miR-211 expression compared with those with early-stage
disease and without metastasis (Table
III).
 | Table III.Association of miR-211
expression levels and clinical parameters in tumor tissues of
patients with colorectal cancer. |
Table III.
Association of miR-211
expression levels and clinical parameters in tumor tissues of
patients with colorectal cancer.
|
| miR-211
expression |
|---|
|
|
|
|---|
| Parameters | (Mean ± SD) | P-value |
|---|
| Age, years |
|
|
|
≤65 | 54.18±68.41 | 0.501 |
|
>65 | 48.23±71.53 |
|
| Sex |
|
|
|
Female | 53.63±77.48 | 0.769 |
|
Male | 48.94±62.76 |
|
| T factor |
|
|
| 1 | 52.89±65.55 | 0.055 |
| 2 | 84.07±93.65 |
|
| 3 | 44.29±62.90 |
|
| 4 | 47.29±66.46 |
|
| N factor |
|
|
| 0 | 70.58±77.04 | <0.0001 |
|
1+2 | 36.34±60.20 |
|
| M factor |
|
|
| 0 | 58.26±73.51 | <0.0001 |
| 1 | 16.83±31.86 |
|
| Stage |
|
|
| I | 95.22±102.20 | <0.0001 |
| II | 75.37±76.91 |
|
|
III | 37.55±53.40 |
|
| IV | 16.33±31.50 |
|
| RRM2
expression |
|
|
|
Overall |
|
|
| − | 56.39±69.67 | 0.087 |
| + | 45.90±70.10 |
|
| K-ras
wild-type |
|
|
| − | 43.45±60.84 | 0.634 |
| + | 52.91±74.54 |
|
| K-ras mutation |
|
|
| − | 121.08±76.77 | <0.0001 |
| + | 32.50±59.50 |
|
Effect of miR-211 expression on OS and
DFS in CRC
It was hypothesized that miR-211 expression
would be associated with clinical outcomes in patients with CRC,
depending on the k-ras gene status of the patient. As
indicated by the results of the Kaplan-Meier analysis, there was no
association between OS and miR-211 expression in overall
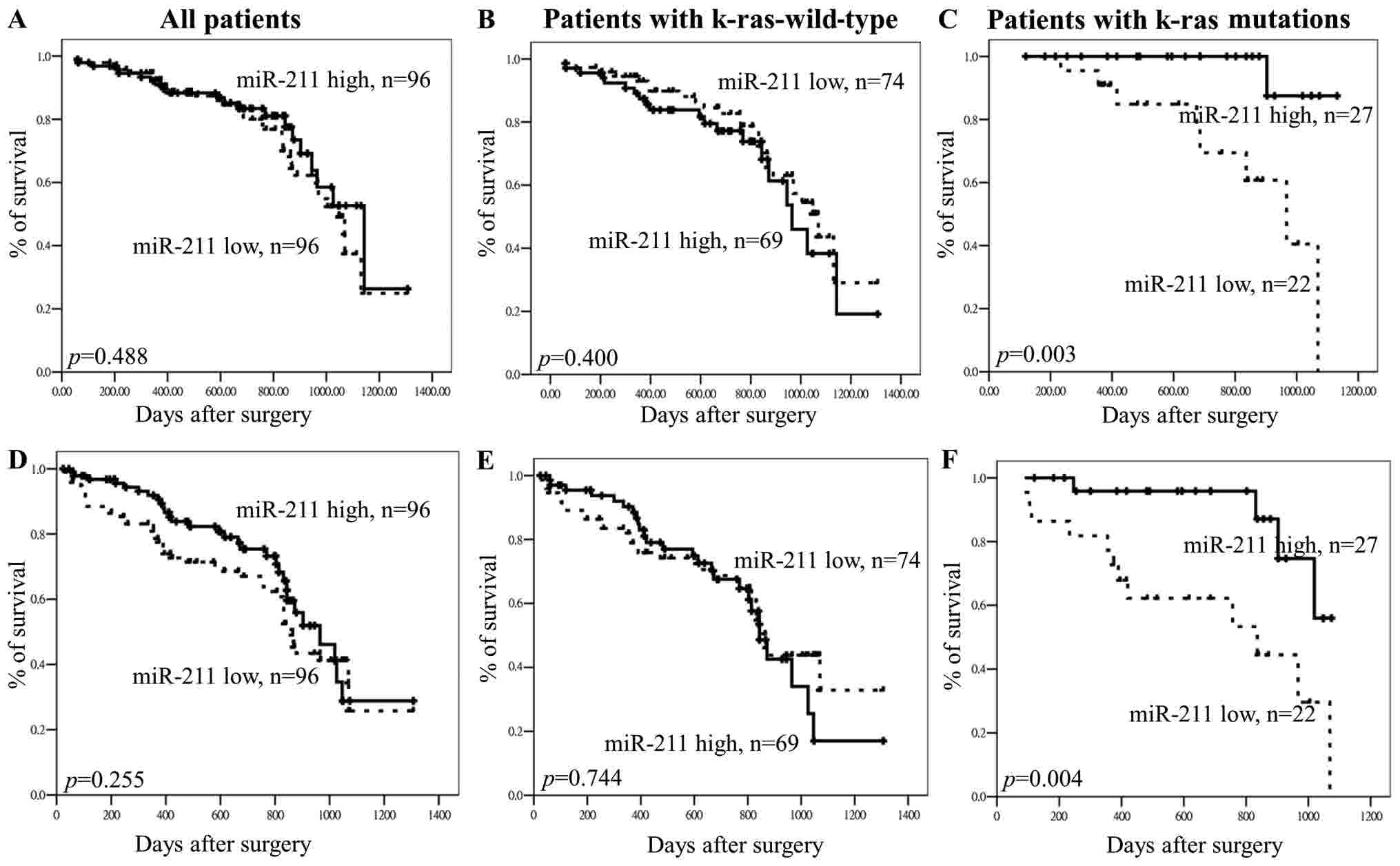
patients (P=0.488; Fig. 5A) and
patients with the wild-type k-ras gene (P=0.400; Fig. 5B), but had effects in patients with
k-ras gene mutations (P=0.003, Fig.
5C). In addition, there was no association between
miR-211 and DFS in all patients (P=0.255; Fig. 5D) and patients with the wild-type
k-ras gene (P=0.744; Fig. 5E).
However, miR-211 expression had effects on OS and DFS in
patients with CRC and k-ras gene mutations (Fig. 5C and F), patients with high
miR-211 expression having longer OS and DFS compared with
patients with low miR-211 expression (OS, P=0.003; Fig. 5C; DFS, P=0.004; Fig. 5F).
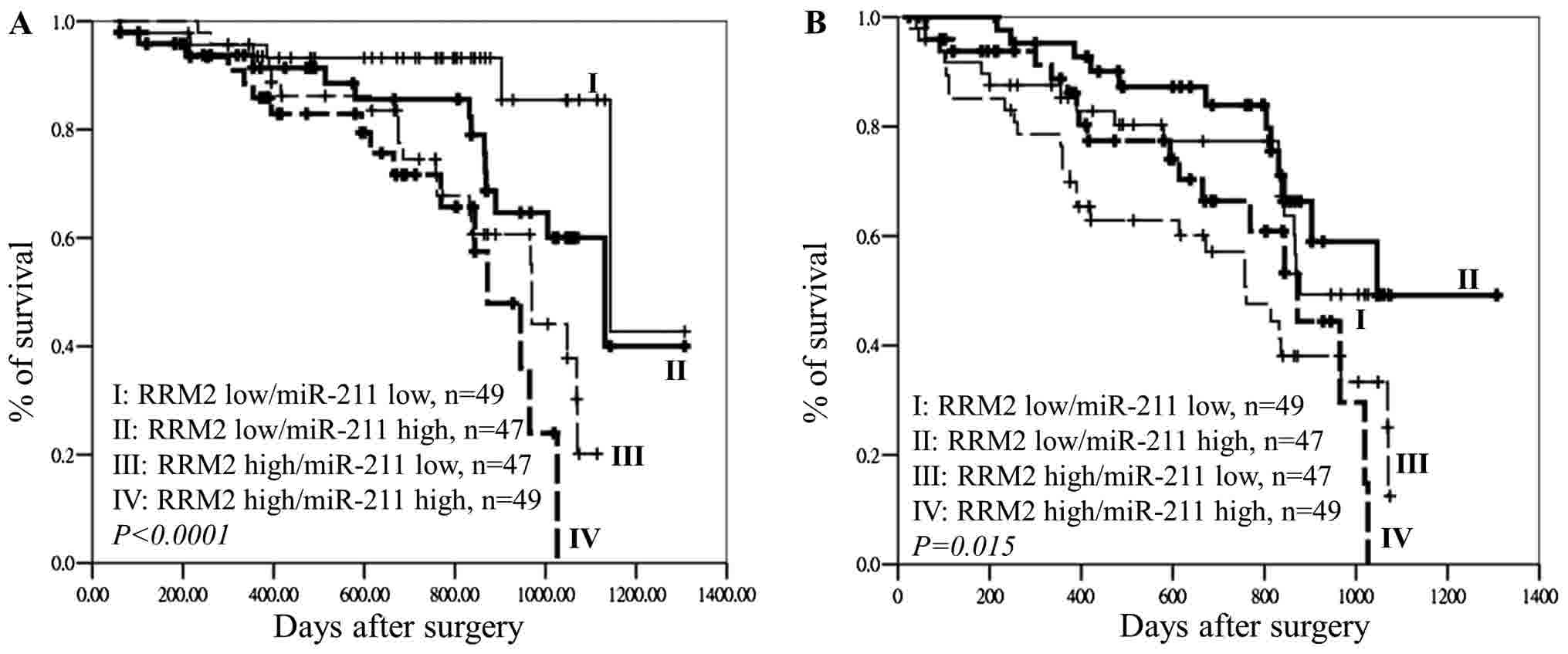
The effects of miR-211 and RRM2 expression on OS
and DFS were analyzed using the Kaplan-Meier method is presented in
Fig. 6. The results indicated that
patients with CRC and high RRM2/low miR-211
expression and high RRM2/high miR-211 expression had
significantly poorer OS (P<0.0001; Fig. 6A) and DFS (P=0.015; Fig. 6B) compared with those with low
RRM2/high miR-211 expression and low RRM2/low
miR-211 expression. A shown by the results of the Cox
regression analysis, patients with late-stage disease and high
RRM2 expression had a higher hazard ratio compared with
patients with early-stage disease and low RRM2 expression
(Table IV). miR-211
expression and the presence of the k-ras mutation showed no
significant different with overall survival of patients with CRC.
These observations demonstrated that the expression of RRM2
in tumor tissues rather than the expression of miR-211 could
be used as an independent biomarker to predict OS in CRC (Table IV). It is suggested that a
combination of miR-211 and RRM2 expression could be
used as a prognostic and tumoral recurrence marker only in patients
with CRC and the k-ras gene mutation.
 | Table IV.Multivariate Cox regression analysis
of the combined effects of RRM2, miR-211, distant
metastasis, tumor stage and k-ras gene mutation on the
overall survival of patients with colorectal cancer. |
Table IV.
Multivariate Cox regression analysis
of the combined effects of RRM2, miR-211, distant
metastasis, tumor stage and k-ras gene mutation on the
overall survival of patients with colorectal cancer.
| Parameters | HR | 95% CI | P-value |
|---|
| Tumor stage |
|
|
|
|
Early/late | 3.008 | 1.358–6.662 | 0.007 |
| Distant
metastasis |
|
|
|
|
No/yes | 1.233 | 0.669–2.273 | 0.501 |
| K-ras
mutation |
|
|
|
|
No/yes | 0.503 | 0.242–1.043 | 0.065 |
| RRM2 |
|
|
|
|
Low/high | 2.175 | 1.186–3.987 | 0.012 |
| miR-211 |
|
|
|
|
High/low | 1.063 | 0.607–1.864 | 0.830 |
Discussion
Previous reports demonstrated that patients with
high RRM2 expression had poor prognoses and tumoral
recurrence in several types of cancer, including hepatocellular
cancer, prostate cancer, pancreatic cancer, CRC and lung cancer
(9,22,24–26). In
the present study, it was detected that RRM2 expression in
tumor tissues of patients with CRC and lymph node metastasis was
significantly higher compared with patients without lymph node
metastasis (Table I). In addition,
patients with high RRM2 expression in tumors had poor DFS
(Fig. 2). A previous study by Maftouh
et al (27) on SUIT2-007 and
SUIT2-028, subclones of a human pancreatic adenocarcinoma cell line
(SUIT-2), reported that miR-211 targeted RRM2 and
modulated its sensitivity to gemcitabine. In the present study, the
expression of miR-211 was negatively associated with
RRM2 expression in tumor tissues of patients with CRC and
k-ras gene mutation (Table
III). Tumoral recurrence was lower in patients with CRC and
k-ras mutation and high miR-211 expression compared
with patients with the k-ras mutation and low miR-211
expression. Therefore, it was proposed that the level of
RRM2 expression and upstream expression of miR-211 in
tumor tissues of patients with CRC may be useful biomarkers to
predict tumoral metastasis and tumoral recurrence, particularly in
patients with the k-ras gene mutation.
A previous study reported that RRM2
cooperated with a variety of oncogenes to promote cell
transformation and tumorigenesis in cell model experiments
(28). In addition, human and mouse
cell models demonstrated that RRM2 played a critical role in
enhancing the invasive potential of tumor cells (28–31). In
the present study, RRM2 expression was higher in lymph nodes
invasion groups compared with the group without invasion (Table I). Therefore, it was suggested that
RRM2 expression in patients with CRC may be associated with
increased metastasis of tumor cells.
As shown in a previous study, miR-211
modulated gemcitabine activity and inhibited the invasive ability
of cancer cells by negatively regulating the expression of
RRM2 (27). The downregulation
of let-7a and miR-211 was associated with the
overexpression of k-ras in 9, 10-dimethyl-1,2-benz
[a]anthracene-induced mouse skin tumorigenesis (32). In the present study, the expression of
miR-211 was negatively associated with RRM2
expression in tumor tissues of patients with CRC and k-ras
mutation. Patients with low miR-211 expression and high
RRM2 expression had poor DFS, particularly those with the
k-ras mutation. Therefore, it was suggested that the
k-ras gene mutation may be associated with downregulation of
miR-211 and that this results in the overexpression of
RRM2 and the induction of CRC tumorigenesis.
A meta-analysis indicated that the k-ras
mutation was present in 1,364 of 4,687 (29.10%) patients with CRC
(33). This result is similar to the
results found in the present study (25.5%; Table II). Previous studies reported an
association between k-ras gene mutation and
clinicopathological characteristics (34–37).
However, the association remains unclear, where some reports
demonstrated that the k-ras gene mutation appeared to be
associated with clinical outcomes, while other studies found no
evidence to suggest that it could be used to predict clinical
outcomes (34–37). The present study demonstrated that
patients with low miR-211 expression had poor DFS, as did
patients with the k-ras gene mutation. By contrast, the
wild-type k-ras gene was not associated with poor DFS. A
negative association was detected between RRM2 and
miR-211 expression in patients with CRC and k-ras
gene mutation (Table III). These
findings indicate that a combination of k-ras gene mutation
and miR-211 and RRM2 expression, may be a useful
biomarker to monitor tumoral recurrence in CRC. However,
k-ras alone cannot be used as a biomarker.
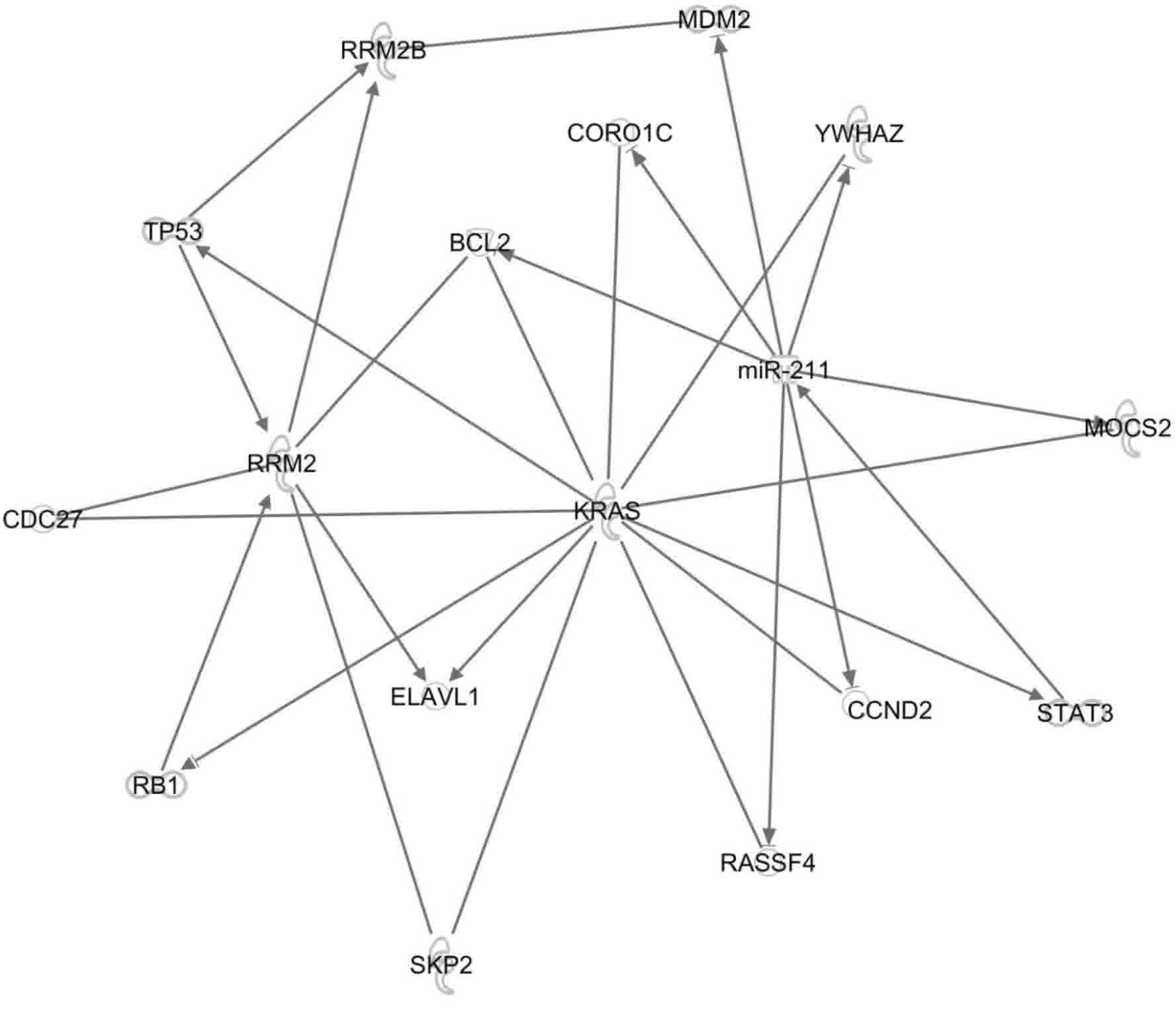
In the present study, the IPA platform was used to
investigate the associations between RRM2, p53R2,
(RRM2B), k-ras and miR-211. The platform can
reveal molecular interactions between these genes based on data
recorded in the Ingenuity Knowledge Base. The Path Explore tool in
IPA was used to identify interactions between the molecules
(Fig. 7). The following 13
interacting proteins were identified: Transcription regulators
(n=4), enzymes (n=3), transporters (n=1) and proteins (n=5) with
other functions. One of the proteins identified, Bcl2 is an
apoptosis regulator, and it plays a central role by interacting
with RRM2, k-ras and miR-211. The associations of
these 13 target genes (Fig. 7) and
the clinical application of these genes need to be examined in
future studies.
In conclusion, it was detected that RRM2 and
p53R2 protein expression was associated with lymph node and
distant metastasis in CRC. Additionally, the expression level of
RRM2 was regulated by miR-211. The protein expression
of miR-211 and RRM2 but not that of p53R2
could be used to monitor metastasis, OS, and DFS in CRC,
particularly in patients with CRC and k-ras gene mutation.
The present authors propose that the activation of the k-ras
gene may downregulate the expression of mir-211, resulting
in RRM2 overexpression and the induction of tumoral
recurrence in patients with CRC and k-ras gene mutation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Health and Welfare Surcharge of Tobacco Products (grant no.
MOHW105-TDU-B-212-134001) and the Ministry of Health and Welfare of
Taiwan (grant nos. MOHW103-TD-B-111-01 and
MOHW103-TDU-B-212-113001).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YWC designed the study and wrote the paper. YWC,
CCC, KTY, CCH and NYH designed the experiments, wrote the paper,
and prepared the figures. CCL, CHW, KTY, PLW and CCH collected the
colorectal tumor samples and clinical data. TWK evaluated the
immunohistochemistry results. KCH analyzed the molecular pathway
using the Ingenuity Pathways Analysis platform. All the authors
gave their approval for the manuscript to be submitted for
publication.
Ethics approval and consent to
participate
The acquisition of the samples and their subsequent
examination were approved by the Institutional Review Board of
Taipei Medical University (Taipei, Taiwan). Informed written
consent was obtained from all the patients and/or guardians prior
to the use of the resected specimens.
Consent for publication
All identifying information is removed.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Su SY, Huang JY, Jian ZH, Ho CC, Lung CC
and Liaw YP: Mortality of colorectal cancer in Taiwan, 1971–2010:
Temporal changes and age-period-cohort analysis. Int J Colorectal
Dis. 27:1665–1672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taiwan Cancer Registry. http://tcr.cph.ntu.edu.tw/main.php?Page=N1
|
|
3
|
Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ
and Lai MS: Incidence and survival of adult cancer patients in
Taiwan, 2002–2012. J Formos Med Assoc. 115:1076–1088. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen TH, Chang SW, Huang CC, Wang KL, Yeh
KT, Liu CN, Lee H, Lin CC and Cheng YW: The prognostic significance
of APC gene mutation and miR-21 expression in advanced-stage
colorectal cancer. Colorectal Dis. 15:1367–1374.. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fodde R, Smits R and Clevers H: APC,
signal transduction and genetic instability in colorectal cancer.
Nat Rev Cancer. 1:55–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reichard P: Ribonucleotide reductases: The
evolution of allosteric regulation. Arch Biochem Biophy.
397:149–155. 2002. View Article : Google Scholar
|
|
7
|
Tanaka H, Arakawa H, Yamaguchi T,
Shiraishi K, Fukuda S, Matsui K, Takei Y and Nakamura Y:
Ribonucleotide reductase gene involved in a p53-dependent
cell-cycle checkpoint for DNA damage. Nature. 404:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan H, Villegas C and Wright JA:
Ribonucleotide reductase R2 component is a novel malignancy
determinant that cooperates with activated oncogenes to determine
transformation and malignant potential. Proc Natl Acad Sci USA.
93:14036–14040. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Zhou B, Xue L, Shih J, Tye K, Lin
W, Qi C, Chu P, Un F, Wen W and Yen Y: Metastasis-suppressing
potential of ribonucleotide reductase small subunit p53R2 in human
cancer cells. Clin Cancer Res. 12:6337–6344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Zhang H, Lai L, Wang X, Loera S,
Xue L, He H, Zhang K, Hu S, Huang Y, et al: Ribouncleotide
reductase small subunit M2 serves as a prognostic biomarker and
predicts poor survival of colorectal cancers. Clin Sci (Lond).
124:567–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cerqueira NM, Pereira S, Fernandes PA and
Ramos MJ: Overview of ribonucleotide reductase inhibitors: An
appealing target in anti-tumour therapy. Curr Med Chem.
12:1283–1294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slack FJ and Weidhaas JB: MicroRNA in
cancer prognosis. N Engl J Med. 359:2720–2722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ke TW, Hsu HL, Wu YH, Chen WT, Cheng YW
and Cheng CW: MicroRNA-224 suppresses colorectal cancer cell
migration by targeting Cdc42. Dis Markers. 2014:6171502014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu TP, Huang CC, Yeh KT, Ke TW, Wei PL
and Cheng YW: Down-regulation of let-7a-5p predicts lymph node
metastasis and prognosis impact on chemotherapy of colorectal
cancer. Surg Oncol. 25:429–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X and Qin Z: Post-transcriptional
regulation by microRNA-21 and let-7a microRNA in paediatric
cholesteatoma. J Int Med Res. 39:2110–2118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfeffer SR, Yang CH and Pfeffer LM: The
role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang F and Cheong JK: The renewed battle
against RAS-mutant cancers. Cell Mol Life Sci. 73:1845–1858. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rui YY, Zhang D, Zhou ZG, Wang C, Yang L,
Yu YY and Chen HN: Can K-ras Gene mutation be utilized as
prognostic biomarker for colorectal cancer patients receiving
chemotherapy? A meta-analysis and systematic review. PLoS One.
8:e779012013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Normanno N, Tejpar S, Morgillo F, De Luca
A, Van Cutsem E and Ciardiello F: Implications for KRAS status and
EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol.
6:519–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida Y, Tsunoda T, Doi K, Tanaka Y,
Fujimoto T, Machida T, Ota T, Koyanagi M, Takashima Y, Sasazuki T,
et al: KRAS-mediated up-regulation of RRM2 expression is essential
for the proliferation of colorectal cancer cell lines. Anticancer
Res. 31:2535–2539. 2011.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu NY, Wu JY, Liu X, Yen Y, Chen CY, Chou
MC, Lin CH, Lee H and Cheng YW: Expression status of ribonucleotide
reductase small subunits hRRM2/p53R2 as prognostic biomarkers in
stage i and ii non-small cell lung cancer. Anticancer Res.
31:3475–3481. 2011.PubMed/NCBI
|
|
23
|
Chen IC, Lee KH, Hsu YH, Wang WR, Chen CM
and Cheng YW: Expression pattern and clinicopathological relevance
of the indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase
protein in colorectal cancer. Dis Markers. 2016:81697242016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Y, Liu X, Wang YH, Yeh SD, Chen CL,
Nelson RA, Chu P, Wilson T and Yen Y: The prognostic value of
ribonucleotide reductase small subunit M2 in predicting recurrence
for prostate cancers. Urol Oncol. 32:51.e9–e19. 2014. View Article : Google Scholar
|
|
25
|
Wei CH, Gorgan TR, Elashoff DA, Hines OJ,
Farrell JJ and Donahue TR: A meta-analysis of gemcitabine
biomarkers in patients with pancreaticobiliary cancers. Pancreas.
42:1303–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Huang J and Jiang M: RRM2
computational phosphoprotein network construction and analysis
between no-tumor hepatitis/cirrhotic liver tissues and human
hepatocellular carcinoma (HCC). Cell Physiol Biochem. 26:303–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maftouh M, Avan A, Funel N, Frampton AE,
Fiuji H, Pelliccioni S, Castellano L, Galla V, Peters GJ and
Giovannetti E: miR-211 modulates gemcitabine activity through
downregulation of ribonucleotide reductase and inhibits the
invasive behavior of pancreatic cancer cells. Nucleosides
Nucleotides Nucleic Acids. 33:384–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong Z, Cao Y, Yang S and Zhang S:
Overexpression of RRM2 in gastric cancer cell promotes their
invasiveness via AKT/NF-κB signaling pathway. Pharmazie.
71:280–284. 2016.PubMed/NCBI
|
|
29
|
Fang Z, Gong C, Liu H, Zhang X, Mei L,
Song M, Qiu L, Luo S, Zhu Z, Zhang R, et al: E2F1 promote the
aggressiveness of human colorectal cancer by activating the
ribonucleotide reductase small subunit M2. Biochem Biophys Res
Commun. 464:407–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou BS, Tsai P, Ker R, Tsai J, Ho R, Yu
J, Shih J and Yen Y: Overexpression of transfected human
ribonucleotide reductase M2 subunit in human cancer cells enhances
their invasive potential. Clin Exp Metastasis. 16:43–49. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duxbury MS and Whang EE: RRM2 induces
NF-kB-dependent MMP-9 activation and enhances cellular
invasiveness. Biochem Biophys Res Commun. 354:190–196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tiwari P, Sahay S, Pandey M, Qadri SS and
Gupta KP: Preventive effects of butyric acid, nicotinamide, calcium
glucarate alone or in combination during the 7, 12-dimethylbenz
anthracene induced mouse skin tumorigenesis via modulation of
K-Ras-PI3K-AKTpathway and associated micro RNAs. Biochimie.
121:112–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren J, Li G, Ge J, Li X and Zhao Y: Is
K-ras gene mutation a prognostic factor for colorectal cancer: A
systematic review and meta-analysis. Dis Colon Rectum. 55:913–923.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bazan V, Migliavacca M, Zanna I, Tubiolo
C, Grassi N, Latteri MA, La Farina M, Albanese I, Dardanoni G,
Salerno S, et al: Specific codon 13 K-ras mutations are predictive
of clinical outcome in colorectal cancer patients, whereas codon 12
K-ras mutations are associated with mucinous histotype. Ann Oncol.
13:1438–1446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conlin A, Smith G, Carey FA, Wolf CR and
Steele RJ: The prognostic significance of K-ras, p53 and APC
mutations in colorectal carcinoma. Gut. 54:1283–1286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pricolo VE, Finkelstein SD, Wu TT, Keller
G, Bakker A, Swalsky PA and Bland KI: Prognostic value of TP53 and
K-ras-2 mutational analysis in stage III carcinoma of the colon. Am
J Surg. 171:41–46. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Andersen SN, Lovig T, Breivik J, Lund E,
Gaudernack G, Meling GI and Rognum TO: K-ras mutations and
prognosis in large-bowel carcinomas. Scand J Gastroenterol.
32:62–69. 1997. View Article : Google Scholar : PubMed/NCBI
|