Introduction
Oral squamous cell carcinoma (OSCC) is the most
common type of cancer in the oral and maxillofacial region.
Although substantial efforts have been made to improve the
diagnosis and treatment of patients with OSCC, the clinical outcome
of patients with OSCC remains poor, with a 5-year survival rate
50–60%, and is even lower in patients with locally advanced lesions
(1,2).
At present, the recommended treatment strategies for patients with
resectable OSCC at late clinical stages are surgery and
post-operative radiotherapy or chemoradiotherapy (3).
The aim of induction chemotherapy is to reduce or
downstage locally advanced or aggressive tumors, in order to
improve the likelihood of primary lesion eradication and to
preserve the organs (4). At present,
the role of induction chemotherapy in OSCC management remains
unclear.
Previously, we conducted a phase 3 trial of TPF
(docetaxel, cisplatin and 5-fluorouracil; registration ID,
NCT01542931) induction chemotherapy in resectable locally advanced
OSCC (5). However, neither short-term
nor long-term results from the initial trial revealed improvement
in clinical outcomes. Only a proportion of the patients benefited
from induction chemotherapy. It is considered that there may be a
specific patient population defined by clinical and/or molecular
criteria that would benefit from induction chemotherapy (5,6).
Therefore, identifying candidate biomarkers to predict the response
to a particular treatment strategy may be useful in improving
overall survival (OS).
Growth differentiation factor 15 (GDF15) is a member
of transforming growth factor-β superfamily, involved in a wide
variety of physiological and pathological processes (7,8). Although
the function of GDF15 in oncogenesis remains unclear,
overexpression of GDF15 has been reported in prostate, colon,
pancreas, thyroid and breast carcinomas, as well as in OSCC
(9–12). Additionally, an elevated serum GDF15
concentration has been reported to be associated with disease
progression, shorter survival times and recurrence (7,13–18). In our previous studies, elevated GDF15
mRNA and protein expression was correlated with the malignancy of
OSCC tissues (12), and the patients
with low GDF15 expression exhibited a 3-year survival advantage
(19). It is likely that GDF15
expression may be a candidate prognostic/predictive biomarker for
patients with OSCC.
The present study identified the long-term
prognostic value of GDF15 expression in patients with stage III/IVA
OSCC, as well as its long-term predictive value for TPF induction
chemotherapy, compared with the standard treatment alone in the
phase 3 trial.
Materials and methods
Patients
A total of 256 patients (179 men and 77 women; aged
from 26 to 75 years, with a mean of 55.4 years) with resectable
stage III and IVA OSCC (T1-2N1-2M0 or T3-4N0-2M0) using the
Tumor-Node-Metastasis staging system (20), who came from a prospective,
randomized, phase 3 trial at Ninth People's Hospital, Shanghai Jiao
Tong University School of Medicine (Shanghai, China; registration
ID: NCT01542931), were enrolled in the present study. The aim of
the trial was to test the hypothesis that TPF induction
chemotherapy administered prior to surgery and post-operative
radiotherapy improves survival in patients with locally advanced
OSCC. The experimental group received TPF induction chemotherapy
followed by surgery and post-operative radiotherapy; the control
group underwent surgery and post-operative radiotherapy; and there
were 128 patients in each group. The detailed treatment protocol
was as previously described (5). In
patients assigned to the experimental group, the palpable edges of
the primary lesion (both the longest and shortest axis) were marked
prior induction chemotherapy by at least four points that were 0.5
cm away from the lesion. Chemotherapy consisted of docetaxel (75
mg/m2), cisplatin (75 mg/m2) and
5-fluorouracil (750 mg/m2/day) as a 120-h infusion for 5
days. Induction chemotherapy was administered every 3 weeks for 2
cycles. Surgery was performed ≥2 weeks after completion of
induction chemotherapy. Radical resection of the primary lesion and
full neck dissection with appropriate reconstruction was performed.
The safety margins of the primary lesion were 1.5 cm away from the
palpable margins; for patients who received induction chemotherapy,
the safety margins were 1.0 cm away from the marks that had been
placed prior to induction chemotherapy. Frozen sections of the
surgical margins, including one or more of the anterior, posterior,
upper and lower margins, which are at the mucosal surfaces and
bottom margins, which are the deepest muscle layers, towards the
muscle layers of the surgical bed, which is the remaining surface
following tumor removal, were removed during the surgery. If cancer
cells were identified in the margins, wider resection for at least
1.0 cm away from the positive margins was performed as possible as
the surgeons can. Frozen section of the new surgical margins would
be performed again to ensure the margins to be negative.
Post-operative radiotherapy was initiated 4–6 weeks after surgery.
Standard conformal or intensity-modulated radiotherapy was
utilized, at a dose of 1.8–2 Gy/day, 5 days/week for 6 weeks,
totaling 54–60 Gy. In patients with high-risk features (defined if
two or more regional lymph nodes were involved, if there was an
extracapsular spread of disease, or a microscopically involved
mucosal margin of resection), a total dose of 66 Gy was
recommended.
Immunohistochemistry
Formalin-fixed (at room temperature for 12–18 h for
5 µm sections) and paraffin-embedded biopsies were collected for
immunohistochemical staining against GDF15. The methodology and
assessment were as described previously (19). Following deparaffinization with
xylene, the sections (5 µm) were rehydrated using an ethanol series
of 100, 95, 85 and 5% ethanol, then distilled water. Prior to
incubation with antibody solutions, the sections were heated by a
water bath at 98°C with 0.01M citrate buffer solution (pH=6.0) for
20 min for antigen retrieval and cooled at room temperature, then
washed with phosphate buffer solution 3 times for 5 min each. Then,
the sections were incubated with the primary rabbit polyclonal
antibody against GDF15 (cat no. ab82569; dilution, 1:100; Abcam,
Cambridge, UK) overnight at 4°C and the secondary goat polyclonal
to rabbit IgG antibody for 1 h at room temperature (cat no.
ab150077; dilution, 1:3,000; Abcam), visualized using a
3,3′-diaminobenzidine detection kit (cat no. K067311; Dako; Agilent
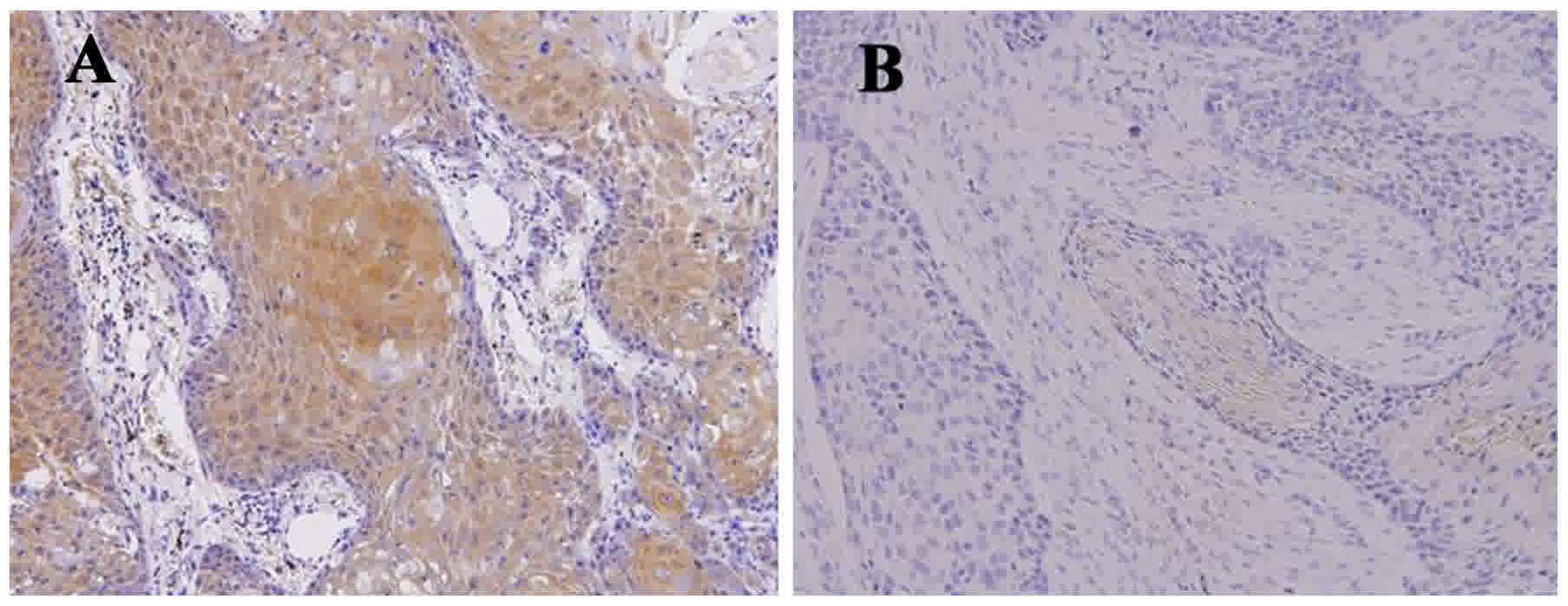
Technologies, Inc., Santa Clara, CA, USA). Positive staining for
GDF15 expression was observed in the cytoplasm. Two pathologists
performed blind examination using a light microscope at a
magnification of ×200. The GDF15 expression level was determined
using the immunoreactive score (IRS) system, including a proportion
score (PS) and an intensity score (IS). The PS was calculated as
the percentage ratio of positive GDF15-stained tumor cells to the
total number of tumor cells, classified as follows: 0, 0%; 1,
1–10%; 2, 11–50%; 3, 51–80%; and 4, >80%. The IS was calculated
as the staining intensity by visual assessment and was scored as
follows: 0, negative; 1+, weak; 2+, moderate; and 3+, strong. The
final GDF15 expression score was calculated using the PS and the IS
(IRS=PSxIS), which ranged between 0 and 12. GDF15 expression was
classified as low when IRS≤3 and as high when IRS≥4 (Fig. 1) (19,21).
Follow-up and outcomes
Following initial treatment, patients were monitored
every 3 months in the first 2 years, every 6 months in the
subsequent 3–5 years, and once a year thereafter until mortality or
data censoring. OS was counted from the date of random assignment
to the date of mortality. Disease-free survival (DFS), locoregional
recurrence-free survival (LRFS) and distant metastasis-free
survival (DMFS) were counted from the date of random assignment to
the date of recurrence, locoregional recurrence, distant metastasis
or mortality, respectively.
Statistical analysis
For descriptive analysis, categorical data are
expressed as the number and percentage. Survival analyses was
conducted using the Kaplan-Meier method, followed by the log-rank
test. Hazard ratios (HR) were calculated using the Cox proportional
hazards model. Subgroup survival analyses according to the baseline
characteristics (including the following subgroups: Sex, male and
female; age, <60 years and ≥60 years; site, tongue and
non-tongue; T stage, cT1/2N1-2M0 and cT3/4N0-2M0; N stage,
cT3/T4N0M0 and cT1-4N1-2M0; clinical stage, clinical III and IV;
pathological differentiation grade, well and moderately/poorly
(22); smoke status, never smokers
and current/former smokers; alcohol use, positive and negative use)
were performed in patients with low or high GDF15 expression. All
hypothesis-generating tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference. Data
analyses were performed using the statistical software SPSS 18.0
for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics and long-term
treatment outcomes
Among the 256 patients, pretreatment biopsy samples
were available from 230 patients for assessing GDF15 expression,
including 104 patients in the experiment group and 126 patients in
the control group. The difference in distribution of baseline
characteristics, including sex, age, primary tumor site, clinical
Tumor-Node-Metastasis stage or pathological differentiation grade
between the patients with low and high GDF15 expression was not
statistically significant (P>0.05) (19). At the time of data cut-off in December
2014, the median follow-up period was 67 months. Although patients
in the experimental group had a slight survival advantage in OS,
DFS, LRFS and DMFS, this difference was not significant (Fig. 2).
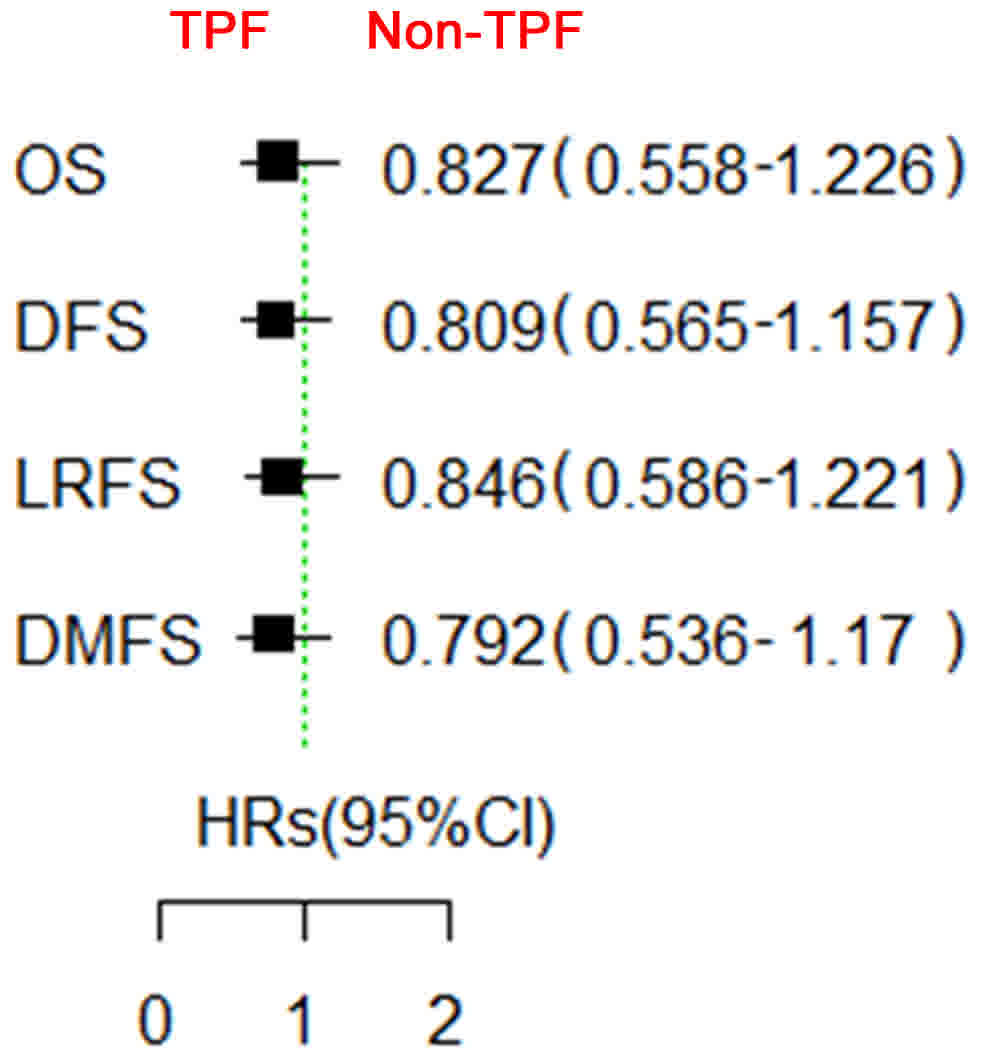 | Figure 2.Survival comparison between the
patients treated with and without TPF induction chemotherapy. In
general, the patients did not benefit from TPF induction
chemotherapy when added prior to standard surgery and postoperative
radiotherapy. TPF, docetaxel, cisplatin and 5-fluorouracil; GDF15,
growth differentiation factor 15; OS, overall survival; DFS,
disease-free survival; LRFS, locoregional recurrence-free survival;
DMFS, distant metastasis-free survival; HR, hazards ratio; CI,
confidence interval. The green line is a reference line for
HR=1. |
Low GDF15 expression indicates better
long-term outcomes in patients with OSCC
Among the 230 patients with OSCC with biopsy
samples, low GDF15 expression was observed in 68 patients (31 in
the experimental group and 37 in the control group) and high GDF15
expression in 162 patients (73 in the experimental group and 89 in
the control group), with similar distribution of GDF15 expression
between the two groups (P=0.942).
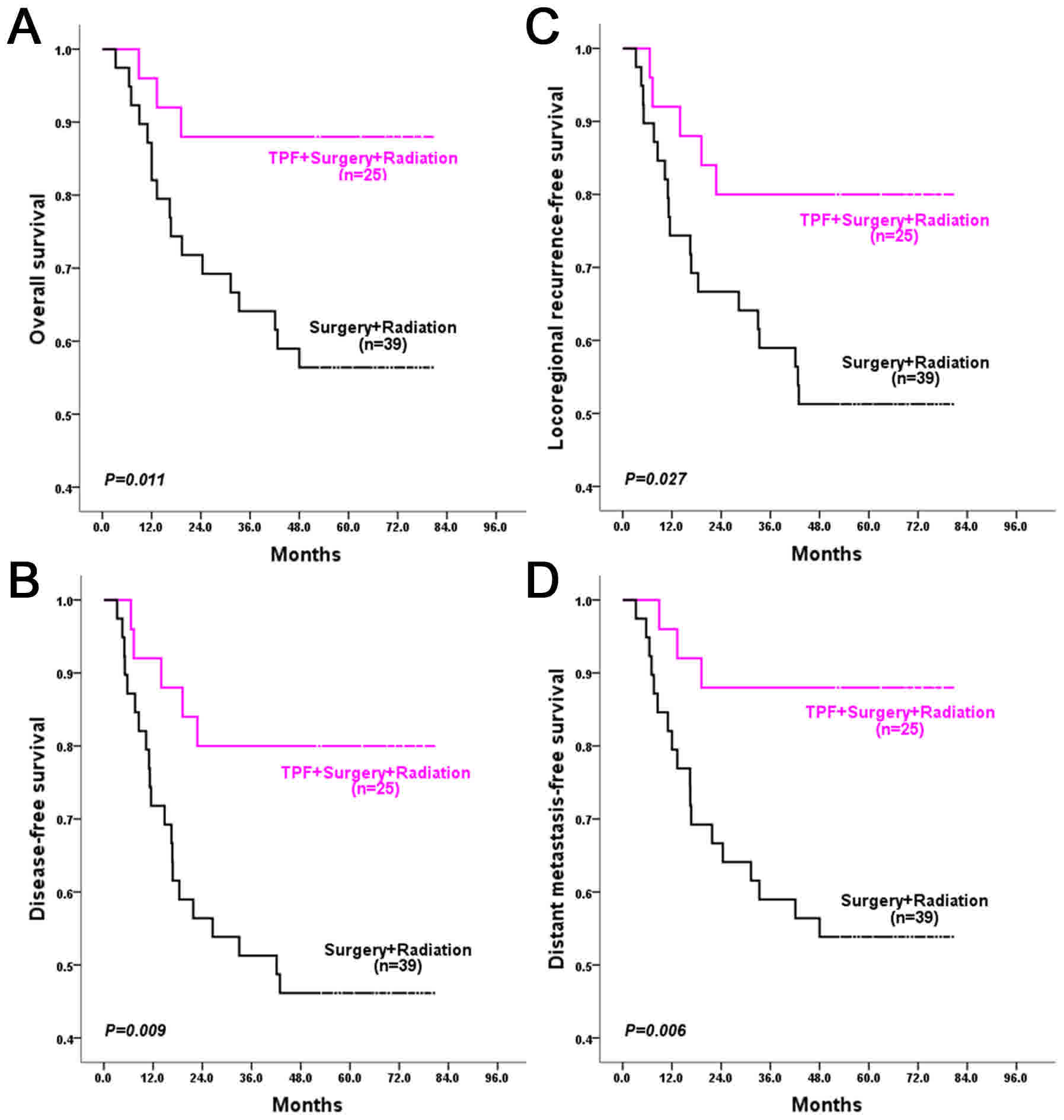
In the patients with low GDF15 expression, the
5-year OS, DFS, LRFS and DMFS rates were 73.4, 64.5, 66.0 and
73.4%, respectively, the locoregional recurrence rate was 27.9% and
the distant metastasis rate was 5.9%. In the patients with high
GDF15 expression, the 5-year OS, DFS, LRFS and DMFS rates were
57.7, 49.2, 51.5 and 56.6%, respectively, the locoregional
recurrence rate was 39.5% and the distant metastasis rate was
9.3%
Patients with low GDF15 expression exhibited
significantly better long-term outcomes, with regards to DFS
(P=0.033), LRFS (P=0.043) and DMFS (P=0.038) rates, compared with
those with high GDF15 expression. Although the difference in the OS
rate was not significant, there was a trend towards a better OS
rate (P=0.059) for patients with low GDF15 expression than those
with high GDF15 expression (Fig.
3).
Analysis of the impact of baseline characteristics
on the time-to-event end points was performed using a univariate
Cox proportional hazards model according to the method used
previously (19,23). Risk factors for OS, DFS, LRFS and DMFS
included GDF15 expression (low vs. high), lymph node status (cN-
vs. cN+) and clinical stage (stage III vs. stage IV). The risk
factors for GDF15 expression and clinical stage were used in
subsequent multivariate Cox model analysis, lymph node status was
not included due to the direct association between clinical stage
and lymph node status. GDF15 expression and clinical stage were
independent risk factors for OS (P=0.022 and P<0.001), DFS
(P=0.01 and P<0.001), LRFS (P=0.012 and P<0.001) and DMFS
(P=0.014 and P<0.001) rates.
High GDF15 expression predicts
significant long-term survival benefit from TPF induction
chemotherapy in patients with cT3/4N0M0 OSCC
In order to analyze whether the patients with low or
high GDF15 expression may benefit from TPF induction chemotherapy,
survival analysis was performed to determine the difference in the
outcomes of patients with different expression levels of GDF15 and
with differing characteristics. In general, the patients did not
benefit from TPF induction chemotherapy, neither those with low
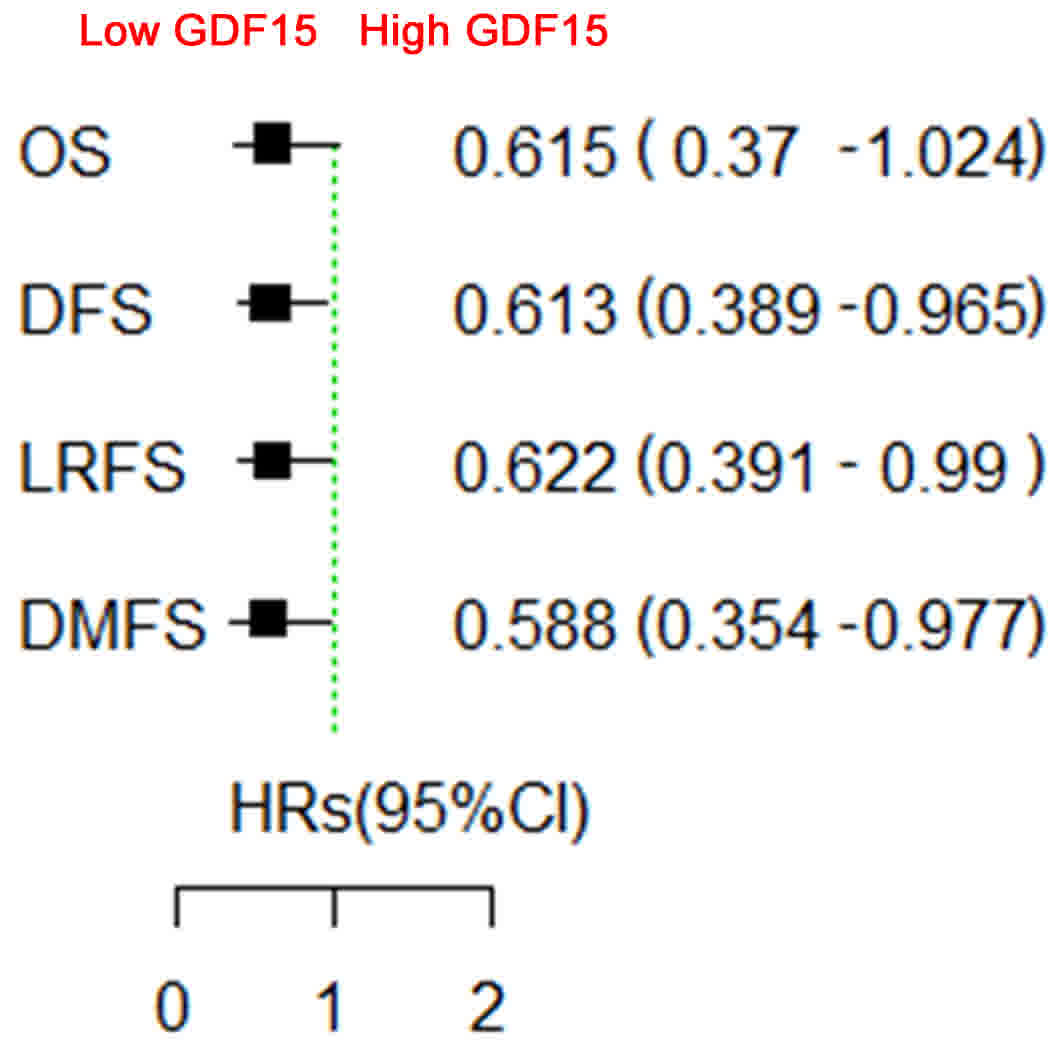
GDF15 expression nor those with high GDF15 expression (Fig. 4). Subgroup survival analysis according
to baseline characteristics revealed that only the patients with
high GDF15 expression and cN0 benefited from TPF induction
chemotherapy with respect to OS (HR=0.233; 95% CI=0.068–0.795;
P=0.02), DFS (HR=0.296; 95% CI=0.111–0.785; P=0.014), LRFS
(HR=0.347; 95% CI=0.129–0.929; P=0.035) and DMFS (HR=0.212; 95%
CI=0.062–0.72; P=0.013; Fig. 5);
while the patients with low GDF15 expression and cN0 did not
benefit from TPF induction chemotherapy. No significant difference
was identified between any other subgroups.
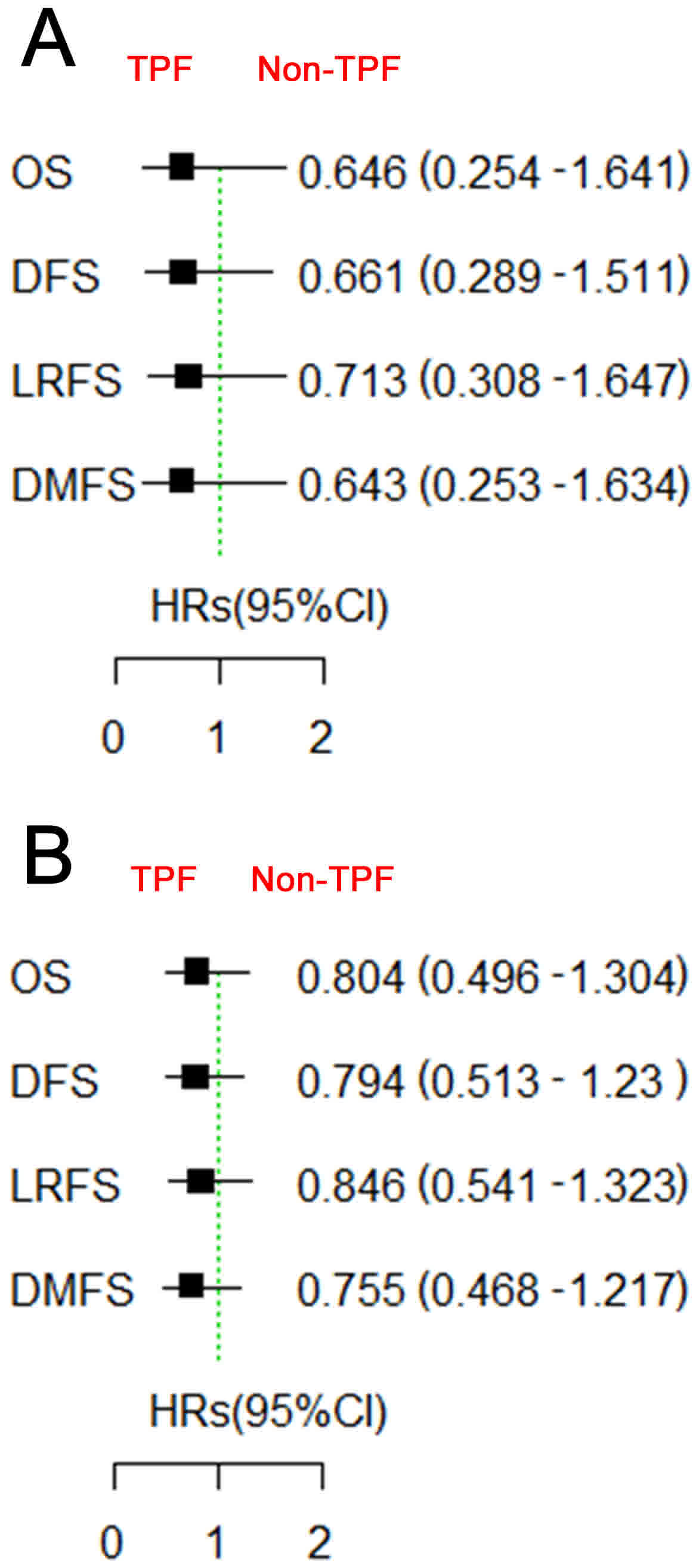 | Figure 4.Survival comparison between patients
treated with and without TPF induction chemotherapy, based on GDF15
expression. Patients with (A) low or (B) high GDF15 expression did
not benefit from TPF induction chemotherapy. TPF, docetaxel,
cisplatin and 5-fluorouracil; GDF15, growth differentiation factor
15; OS, overall survival; DFS, disease-free survival; LRFS,
locoregional recurrence-free survival; DMFS, distant
metastasis-free survival; HR, hazards ratio; CI, confidence
interval. |
Discussion
The present study revealed that elevated GDF15
expression may be used as a long-term prognostic biomarker for poor
clinical outcomes in patients with locally advanced OSCC. Elevated
GDF15 expression in patients with cT3/4N0M0 disease predicted
significant long-term benefit of survival from the addition of TPF
induction chemotherapy prior to standard treatment of surgery and
post-operative radiotherapy, compared with the standard treatment
alone in OSCC. The results of the present study suggested a
personalized treatment regimen in which patients with cT3/4N0M0
disease exhibiting high GDF15 expression would receive TPF
induction chemotherapy prior to surgery for long-term benefit of
clinical outcomes while the others would not receive TPF induction
chemotherapy prior to surgery, a situation that would avoid the
excessive toxicities of chemotherapy and the delay of definitive
treatment. As such, we previously designed and performed a
randomized clinical study of TPF induction chemotherapy in patients
with cT3/4N0M0 OSCC, prospectively embedding GDF15 expression as a
predictive biomarker (registration ID, NCT02285530).
GDF15 is involved in the regulation of various
physiological and pathological processes, including inflammation,
cellular stress, immune response, tissue repair and oncogenesis
(7,8).
Elevated GDF15 expression has been reported to be correlated with
neoplasm progression in several types of cancer, including
prostate, colon, pancreas, thyroid and breast carcinomas (9–11). In our
previous study, elevated GDF15 expression was revealed to promote
the tumorigenesis and progression of OSCC through Akt and
extracellular signal-regulated kinase 1/2 phosphorylation (12). In the present study with long-term
follow-up, the patients with OSCC exhibiting elevated GDF15
expression would have a poorer prognosis than those with low GDF15
expression. This is similar to other types of cancer, including
prostate, ovarian and colorectal cancers (10,24,25).
However, to the best of our knowledge, the present study is the
first to report the long-term results of GDF15 expression as a
predictive biomarker for survival benefit from TPF induction
chemotherapy in patients with OSCC. The patients with cT3/4N0M0
disease exhibiting high GDF15 expression benefited from TPF
induction chemotherapy with respect to OS, DFS, LRFS and DMFS;
while the other patients did not benefit from TPF induction
chemotherapy.
Although the detailed beneficial mechanism of TPF
inductive chemotherapy agents on the patients with cT3/4N0M0
disease exhibiting high GDF15 expression with regards to long-term
outcomes, potential associations between GDF15 expression and
chemotherapy agents have been investigated with inconsistent
results. For example, in colorectal cancer, GDF15 knockdown with
small interfering RNA has been reported to prevent
docetaxel-induced cell death in the wild-type p53 HCT-116 cell line
(26). By contrast, GDF15 silencing
prior to application of oxaliplatin and 5-fluorouracil has also
been reported to sensitize wild-type p53 colorectal cancer cells to
drug-induced apoptosis (27). In
prostate cancer, overexpression of GDF15 has been reported to be
associated with resistance to docetaxel, which suggests the
potential utility of GDF15 as a predictive biomarker of response to
docetaxel in prostate cancer (10,28,29).
However, in the present study, the patients with OSCC exhibiting a
high GDF15 expression had a poorer prognosis compared with those
exhibiting a low GDF15 expression. The subgroup patients with large
tumor size only (cT3/4N0M0) and high GDF15 expression benefited
from TPF induction chemotherapy, indicating that the patients with
OSCC overexpressing GDF15 may be sensitive to docetaxel, cisplatin
and 5-fluorouracil. It may be possible to control local primary
tumors with GDF15 overexpression using TPF induction chemotherapy
agents. However, when lymph node metastasis occurs in those
patients, induction chemotherapy agents may be unable to control
the disease. It has been reported by previous clinical trials that
the patients with head neck squamous cell carcinoma and advanced
lymph node metastasis do not benefit from TPF induction
chemotherapy (30–32). Therefore, induction chemotherapy may
be unable to control the patients with lymph node metastasis.
However, further studies are required to reveal the detailed
mechanisms of this.
The results of the present study suggested that
elevated GDF15 expression may be used as a long-term prognostic
biomarker for poor clinical outcomes in patients with locally
advanced OSCC. Elevated GDF15 expression in patients with cT3/4N0M0
disease predicted significant long-term benefit of survival from
the addition of TPF induction chemotherapy ahead of standard
treatment of surgery and post-operative radiotherapy, compared with
the standard treatment alone in OSCC. Detailed mechanism studies
are required to explain the clinical phenomenon and to provide
basic support to optimize the personalized treatment strategies in
OSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81672660 and
81472519), the Shuguang Program of Shanghai Municipal Education
Commission (grant no. 17SG18) and by the Shanghai Jiao Tong
University School of Medicine (grant no. 15ZH2008 and
17XJ12004).
Availability of data and materials
The data and materials are available from the
corresponding authors if necessary.
Authors' contributions
LZ and ZZ were responsible for the study design,
interpretation of the data and revision of the manuscript. XT and
YH were responsible for data acquisition, analysis of the work
presented and the preparation of the manuscript. WJ, YF, WS, YL,
YT, LW, JL, YT and CZ participated in the clinical management of
the patients. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Ninth People's Hospital, Shanghai Jiao Tong
University School of Medicine (IRB approval documents of 2008–12
and 2014-75) and written informed consent was obtained from all
participants.
Consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®) Head and Neck Cancers. (Version 1.2016).
http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdfAugust
5–2016
|
|
4
|
Zhong LP and Zhang ZY: Reply to K.
Devisetty et al. J Clin Oncol. 31:2972–2973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong LP, Zhang CP, Ren GX, Guo W, William
WN Jr, Sun J, Zhu HG, Tu WY, Li J, Cai YL, et al: Randomized phase
III trial of induction chemotherapy with docetaxel, cisplatin, and
fluorouracil followed by surgery versus up-front surgery in locally
advanced resectable oral squamous cell carcinoma. J Clin Oncol.
31:744–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong LP, Zhang CP, Ren GX, Guo W, William
WN Jr, Hong CS, Sun J, Zhu HG, Tu WY, Li J, et al: Long-term
results of a randomized phase III trial of TPF induction
chemotherapy followed by surgery and radiotherapy in locally
advanced oral squamous cell carcinoma. Oncotarget. 6:18707–18714.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Breit SN, Johnen H, Cook AD, Tsai VW,
Mohammad MG, Kuffner T, Zhang HP, Marquis CP, Jiang L, Lockwood G,
et al: The TGF-β superfamily cytokine, MIC-1/GDF15: A pleotrophic
cytokine with roles in inflammation, cancer and metabolism. Growth
Factors. 29:187–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corre J, Hebraud B and Bourin P: Concise
review: Growth differentiation factor 15 in pathology: A clinical
role? Stem Cells Transl Med. 2:946–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khaled YS, Elkord E and Ammori BJ:
Macrophage inhibitory cytokine-1: A review of its pleiotropic
actions in cancer. Cancer Biomark. 11:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown DA, Lindmark F, Stattin P, Bälter K,
Adami HO, Zheng SL, Xu J, Isaacs WB, Grönberg H, Breit SN and
Wiklund FE: Macrophage inhibitory cytokine 1: A new prognostic
marker in prostate cancer. Clin Cancer Res. 15:6658–6664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallin U, Glimelius B, Jirstrom K,
Darmanis S, Nong RY, Pontén F, Johansson C, Påhlman L and Birgisson
H: Growth differentiation factor 15: A prognostic marker for
recurrence in colorectal cancer. Br J Cancer. 104:1619–1627. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Yang X, Pan HY, Zhou XJ, Li J,
Chen WT, Zhong LP and Zhang ZY: Expression of growth
differentiation factor 15 is positively correlated with
histopathological malignant grade and in vitro cell proliferation
in oral squamous cell carcinoma. Oral Oncol. 45:627–632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown DA, Stephan C, Ward RL, Law M,
Hunter M, Bauskin AR, Amin J, Jung K, Diamandis EP, Hampton GM, et
al: Measurement of serum levels of macrophage inhibitory cytokine 1
combined with prostate-specific antigen improves prostate cancer
diagnosis. Clin Cancer Res. 12:89–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Selander KS, Brown DA, Sequeiros GB,
Hunter M, Desmond R, Parpala T, Risteli J, Breit SN and
Jukkola-Vuorinen A: Serum macrophage inhibitory cytokine-1
concentrations correlate with the presence of prostate cancer bone
metastases. Cancer Epidemiol Biomarkers Prev. 16:532–537. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown DA, Ward RL, Buckhaults P, Liu T,
Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B and
Breit SN: MIC-1 serum level and genotype: Associations with
progress and prognosis of colorectal carcinoma. Clin Cancer Res.
9:2642–2650. 2003.PubMed/NCBI
|
|
16
|
Schiegnitz E, Kämmerer PW, Koch FP, Kruger
M, Berres M and Al-Nawas B: GDF 15 as an anti-apoptotic, diagnostic
and prognostic marker in oral squamous cell carcinoma. Oral Oncol.
48:608–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schiegnitz E, Kämmerer PW, Rode K, Schorn
T, Brieger J and Al-Nawas B: Growth differentiation factor 15 as a
radiation-induced marker in oral carcinoma increasing radiation
resistance. J Oral Pathol Med. 45:63–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shnaper S, Desbaillets I, Brown DA, Murat
A, Migliavacca E, Schluep M, Ostermann S, Hamou MF, Stupp R, Breit
SN, et al: Elevated levels of MIC-1/GDF15 in the cerebrospinal
fluid of patients are associated with glioblastoma and worse
outcome. Int J Cancer. 125:2624–2630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CZ, Ma J, Zhu DW, Montgomery B, Wang
LZ, Li J, Zhang ZY, Zhang CP and Zhong LP: GDF15 is a potential
predictive biomarker for TPF induction chemotherapy and promotes
tumorigenesis and progression in oral squamous cell carcinoma. Ann
Oncol. 25:1215–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH and Wittekind CH: International
Union Against Cancer (UICC): TNM classification of malignant
tumours. 6th edition. Wiley; New York, NY: 2002
|
|
21
|
Kaemmerer D, Peter L, Lupp A, Schulz S,
Sänger J, Baum RP, Prasad V and Hommann M: Comparing of IRS and
Her2 as immunohistochemical scoring schemes in
gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp
Pathol. 5:187–194. 2012.PubMed/NCBI
|
|
22
|
Arduino PG, Carrozzo M, Chiecchio A,
Broccoletti R, Tirone F, Borra E, Bertolusso G and Gandolfo S:
Clinical and histopathologic independent prognostic factors in oral
squamous cell carcinoma: A retrospective study of 334 cases. J Oral
Maxillofac Surg. 66:1570–1579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong LP, Zhu DW, William WN Jr, Liu Y, Ma
J, Yang CZ, Yang X, Wang LZ, Li J, Myers JN, et al: Elevated cyclin
D1 expression is predictive for a benefit from TPF induction
chemotherapy in oral squamous cell carcinoma patients with advanced
nodal disease. Mol Cancer Ther. 12:1112–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Staff AC, Bock AJ, Becker C, Kempf T,
Wollert KC and Davidson B: Growth differentiation factor-15 as a
prognostic biomarker in ovarian cancer. Gynecol Oncol. 118:237–243.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Lee BY, Brown DA, Molloy MP, Marx
GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN, et al:
Identification of candidate biomarkers of therapeutic response to
docetaxel by proteomic profiling. Cancer Res. 69:7696–7703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim IY, Park SY, Kang Y, Thapa D, Choi HG
and Kim JA: Role of nonsteroidal anti-inflammatory drug-activated
gene-1 in docetaxel-induced cell death of human colorectal cancer
cells with different p53 status. Arch Pharm Res. 34:323–330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Proutski I, Stevenson L, Allen WL, McCulla
A, Boyer J, McLean EG, Longley DB and Johnston PG: Prostate-derived
factor-a novel inhibitor of drug-induced cell death in colon cancer
cells. Mol Cancer Ther. 8:2566–2574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Magadoux L, Isambert N, Plenchette S,
Jeannin JF and Laurens V: Emerging targets to monitor and overcome
docetaxel resistance in castration resistant prostate cancer
(review). Int J Oncol. 45:919–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mahon KL, Lin HM, Castillo L, Lee BY,
Lee-Ng M, Chatfield MD, Chiam K, Breit SN, Brown DA, Molloy MP, et
al: Cytokine profiling of docetaxel-resistant castration-resistant
prostate cancer. Br J Cancer. 112:1340–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hitt R, Grau JJ, López-Pousa A, Berrocal
A, García-Girón C, Irigoyen A, Sastre J, Martínez-Trufero J,
Castelo Brandariz JA, Verger E, et al: A randomized phase III trial
comparing induction chemotherapy followed by chemoradiotherapy
versus chemoradiotherapy alone as treatment of unresectable head
and neck cancer. Ann Oncol. 25:216–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. J Clin Oncol. 32:2735–2743. 2014. View Article : Google Scholar : PubMed/NCBI
|