Introduction
Lung cancer is the primary cause of
cancer-associated mortality worldwide. Approximately 85% of these
fatalities are accounted for by non-small cell lung cancer (NSCLC)
(1), of which the most common
histopathological type in recent decades has been adenocarcinoma
(2). The majority of patients with
NSCLC are not amenable to curative resection with locally advanced
or advanced disease (3). The standard
treatment for these patients is platinum-based combination
chemotherapy with concurrent radiotherapy (RT), which can improve
survival for certain patients (4).
However, the 5-year overall survival (OS) rate for lung cancer
remains at 15% (5), as not all
patients respond to the chemoradiotherapy (CRT) due to high levels
of toxicity. Therefore, early predictions of therapy response and
patient outcome are particularly important, such that patients who
are be likely to benefit from treatment may be identified.
In light of this, it has been suggested that the
non-invasive molecular imaging tool,
18F-fluorodeoxyglucose positron emission tomography
(18F-FDG PET), may aid in alleviating these problems.
Over time, 18F-FDG PET has become an accepted method for
staging and evaluating therapeutic response in NSCLC patients
(6,7).
In a prospective study, Mac Manus et al (8) demonstrated that the post-treatment
18F-FDG PET response was more significantly associated
with survival compared with the post-treatment computed tomography
(CT) in patients treated with determinate radiation or CRT. The
major advantage of 18F-FDG PET when compared with
structural imaging techniques is the detection of a change in
cellular metabolism earlier than any change in the tumor size.
Therefore, 18F-FDG PET could be used as a sensitive tool
to predict treatment response (9).
The purpose of the present study was to evaluate different
18F-FDG PET/CT parameters, including maximum
standardized uptake values (SUVmax), mean standardized uptake
values (SUVmean), metabolic tumor volume (MTV) and total lesion
glycolysis (TLG), as survival prognostic factors in patients with
stage III NSCLC. The prognoses were determined by
18F-FDG PET/CT prior to and following 60-Gy radiotherapy
with a concurrent cisplatin/pemetrexed chemotherapy regimen.
Patients and methods
Patients
The present study is a prospective study of 14
consecutive patients with locally advanced NSCLC (stage IIIA/IIIB)
who were treated with concurrent CRT in Shandong Cancer Hospital
Affiliated to Shandong University (Jinan, Shandong, China) between
May 2015 and May 2016. The following inclusion criteria were used:
Stage III NSCLC [using American Joint Committee on Cancer (7th
edition)] (10); histopathologically
confirmed primary adenocarcinoma; an Eastern Cooperative Oncology
Group (ECOG) Performance Status (11)
of 0 or 1; and complete physical examination, serum tumor marker
determination, blood count and serum biochemistry. The exclusion
criteria were as follows: RT or surgery of the chest within 3
months prior to entering the study; and a previous history of
cancer or diabetes mellitus. This study was approved by the
Institutional Review Boards and Ethics Committees of Shandong
Cancer Hospital Affiliated to Shandong University. All procedures
involving human participants were in accordance with the ethical
standards of the institution and/or national research committee,
and with the 1964 Declaration of Helsinki and its later amendments.
Informed consent was obtained from all participants included in the
study.
CRT
All patients were treated with conventionally
fractioned RT at 5 doses of 2 Gy per week up to a total dose of
60–66 Gy, based on a 18F-FDG PET/CT scans. RT was
delivered by three-dimensional conformal RT or intensity modulated
RT techniques with 6-MV photons (based on lesion size and fitness
of target volume). The primary tumor and any clinically involved
lymph nodes were included in the gross tumor volume, and planning
target volume included the gross tumor volume with a margin of 0.8
cm. All patients were treated with 2-cycles of concurrent
chemotherapy with cisplatin (25 mg/m2 on days 1–3 iv.
drip) and pemetrexed (500 mg/m2 on day 1 iv. drip). This
was repeated every 21 days for a total of four to six cycles.
Standard premedication of dexamethasone and antihistaminergic drugs
was provided for the pemetrexed treatment.
18F-FDG PET/CT image
acquisition
18F-FDG PET/CT scans were performed with
a Discovery LS PET-CT system (GE Healthcare Life Sciences, Little
Chelfont, UK). All patients had been resting and fasting for at
least 6 h prior to the scan and their serum glucose levels were
measured to ensure a value of <6.6 mmol/l. Following intravenous
injection with 5.0 MBq/kg 18F-FDG, patients rested for
45–60 min. Emission scans were obtained from the skull to the
thighs for 5 min per field of view, each covering 14.5 cm, at an
axial sampling thickness of 4.25 mm per slice. CT data were also
collected. During 18F-FDG PET/CT scanning, quiet
respiration was required to ensure the quality of images. The total
time varied between 25 and 30 min per patient. PET images were
reconstructed with CT-derived attenuation correction using an
ordered subset expectation maximization algorithm. Pre-treatment
18F-FDG PET/CT scans were conducted 1–3 days prior to
the start of RT as part of the initial staging. Repeat whole-body
18F-FDG PET/CT scans were performed once the total RT
dose had reached 60 Gy in order to evaluate the therapeutic
effect.
Interpretation of
18F-FDG PET/CT images
Interpretation of 18F-FDG PET/CT imaging
was performed by nuclear medicine physicians, who were affiliated
to our hospital and were blinded to patient histories, using
consensus criteria. SUVmean and SUVmax were acquired using
attenuation-corrected images. SUVmax of the primary tumor was
obtained in the transaxial view. An SUV cutoff of 2.5 was used to
measure MTV and thus, MTV was defined as the sum of voxels
exhibiting an SUV of 2.5 or more. When all the hypermetabolic tumor
foci were segmented, the EBW workstation (Philips Healthcare,
Andover, MA, USA) calculated MTV automatically, defined as the
total volume of all tumors in the body. The SUVmean was obtained by
the same method as the SUVmax. TLG was calculated as the product of
the MTV and the SUVmean. The percentage change (Δ) in each of the
parameters (P) between pre- and post-treatment was calculated using
the following formula: ΔP=[(Ppre-Ppost)/Ppre] ×100
Response evaluation
PERCIST was used to evaluate the effect of CRT
treatment, while RECIST (12) was
used to evaluate the short-term outcome at 4 weeks after
termination of CRT. Evaluations were made blinded to the
18F-FDG PET/CT scans. The responders were defined as
exhibiting a complete response (CR) or a partial response (PR)
according to RECIST. Patients with an outcome of stable disease
(SD) or progressive disease (PD) were subsequently classified as
non-responders.
Statistical analysis
The Statistical Package for SSPS v.17.0 (SPSS, Inc.,
Chicago, IL, USA) was used. Quantitative data, including SUVmean,
SUVmax, MTV and TLG, are expressed as mean ± standard deviation.
Statistically significant differences between unpaired quantitative
parameters were analyzed using Student's t-test. The difference in
response evaluated by RECIST and PERCIST was analyzed using the
χ2 test. All P-values were two-sided, and statistical
significance was indicated by P<0.05.
Results
Patient characteristics
Table I presents the
clinical characteristics of all patients involved in the present
study. 18F-FDG PET/CT images were available for 14
patients. The median age of the study population was 64 years
(range, 55–82 years), 71.4% of the participants were male and the
proportion of never-smokers was 21.4%. All patients had
histologically confirmed adenocarcinoma. According to the RECIST
criterion (12), a total of 6
patients (1 with CR and 5 with PR) were assessable for response,
and the overall response rate was 42.9%. By contrast, 7 patients
exhibited SD while only 1 patient exhibited PD.
 | Table I.Clinicopathological features of 14
patients with non-small cell lung cancer. |
Table I.
Clinicopathological features of 14
patients with non-small cell lung cancer.
|
Characteristics | Value |
|---|
| Age,
yearsa | 64±8.91 |
| Sex, n (%) |
|
|
Male | 10 (71.4) |
|
Female | 4 (28.6) |
| Smoking status, n
(%) |
|
|
Non-smoker | 3 (21.4) |
|
Smoker | 11 (78.6) |
| Stage, n (%) |
|
|
IIIA | 9 (64.3) |
|
IIIB | 5 (35.7) |
| RECIST, n (%) |
|
|
Complete response | 1 (7.1) |
| Partial
response | 5 (35.7) |
| Stable
disease | 7 (50.0) |
|
Progressive disease | 1 (7.1) |
| PERCIST, n (%) |
|
|
Complete metabolic
response | 1 (7.15) |
| Partial
metabolic response | 7 (50.0) |
| Stable
metabolic response | 5 (35.7) |
|
Progressive metabolic
response | 1 (7.1) |
18F-FDG metabolic
changes
The pre- and post-treatment 18F-FDG
uptake parameters, listed in Table
II, were determined by two nuclear medicine physicians. SUVmax,
SUVmean, MTV and TLG at baseline 18F-FDG PET/CT scans
exhibited no statistically significant differences between the
responders and the non-responders (P>0.05). In the responders
(42.9%), SUVmax fell from 11.7±4.3 to 5.0±3.9 following initial
CRT. In the non-responders (57.1%), the SUVmax increased from
12.8±4.9 to 12.9±3.8. There was a mean reduction in SUVmax of 51.9%
and an increase of 0.5% in responders and non-responders,
respectively. The ΔSUVmax, ΔMTV and ΔTLG differed significantly
between responders and non-responders (P=0.015, P=0.006 and
P=0.004, respectively). Similarly, post-CRT SUVmax and CEA levels
were significantly higher in responders compared with those in
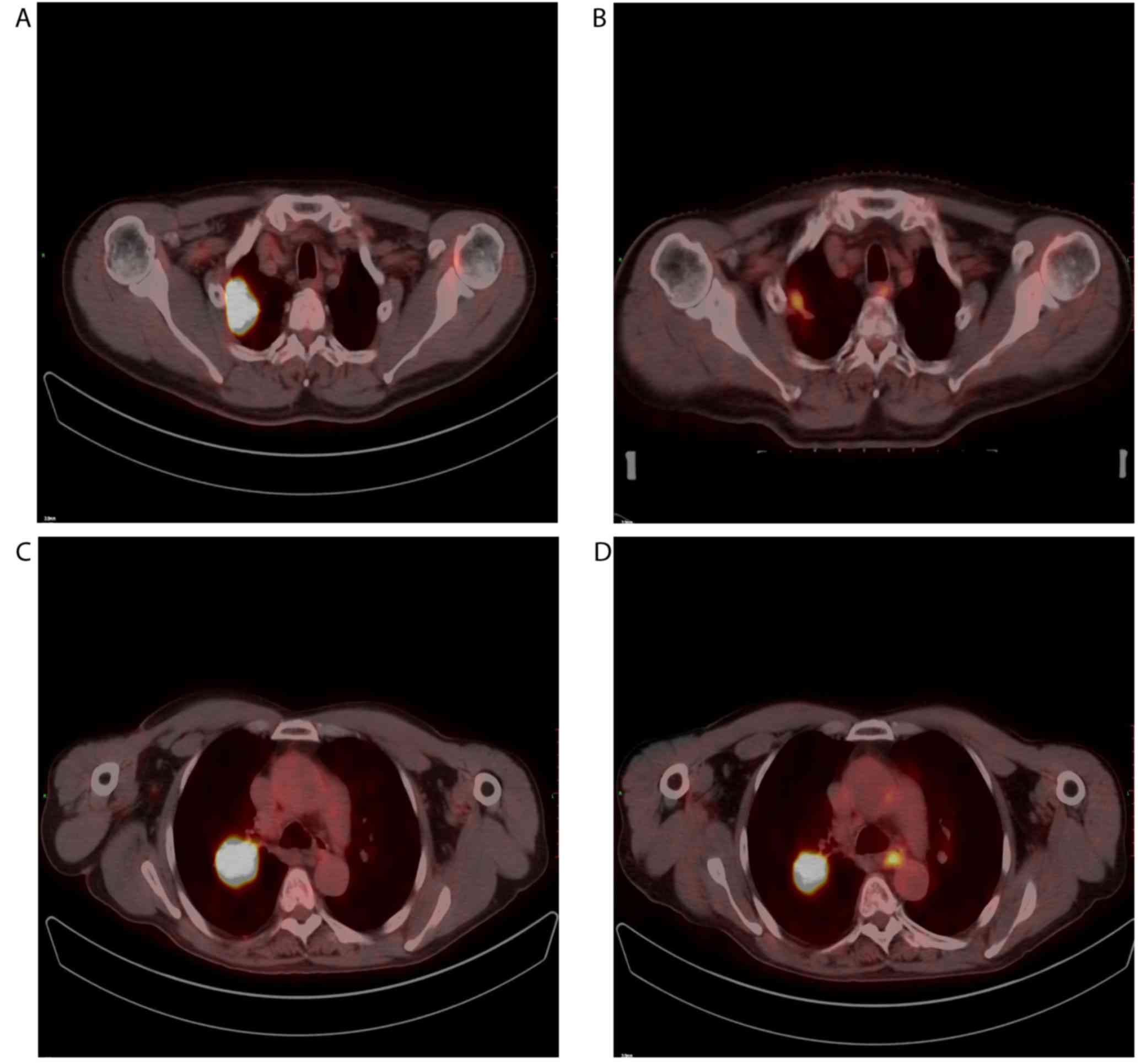
non-responders (P=0.009 and P=0.019, respectively). Fig. 1 depicts typical examples of
18F-FDG PET/CT scans in patients with responding and
non-responding tumors.
 | Table II.Changes in the parameters of
pre-treatment and post-treatment FDG PET/CT scans. |
Table II.
Changes in the parameters of
pre-treatment and post-treatment FDG PET/CT scans.
| Parameters | All patients | Non-responders | Responders | P-value |
|---|
| SUVmax |
|
|
|
|
| PET-1 | 12.16±4.34 | 12.81±4.94 | 11.72±4.32 | 0.721 |
| PET-2 | 8.19±5.31 | 12.96±3.85 | 5.01±3.39 | 0.009 |
| ΔP | 28.92±40.10 | −5.53±21.03 | 51.89±32.34 | 0.015 |
| SUVmean |
|
|
|
|
| PET-1 | 4.82±1.22 | 4.99±0.89 | 4.69±1.47 | 0.729 |
| PET-2 | 3.81±1.42 | 4.74±1.35 | 3.19±1.17 | 0.088 |
| ΔP | 19.91±22.03 | 5.71±14.51 | 29.38±21.87 | 0.096 |
| MTV |
|
|
|
|
| PET-1 | 79.85±90.83 | 111.46±132.55 | 58.78±54.54 | 0.401 |
| PET-2 | 50.53±93.29 | 114.54±129.91 | 7.86±8.77 | 0.072 |
| ΔP | 36.45±57.25 | −16.69±35.69 | 71.88±37.01 | 0.006 |
| TLG |
|
|
|
|
| PET-1 | 405.76±503.81 | 612.96±765.01 | 267.62±220.20 | 0.316 |
| PET-2 | 240.08±450.52 | 551.04±624.27 | 32.76±50.69 | 0.070 |
| ΔP | 42.48±52.71 | −7.33±24.39 | 75.69±36.57 | 0.004 |
When applying the PERCIST at post-CRT
18F-FDG PET/CT, 8/14 patients (57.1%) exhibited a CR or
a PR, while 6/14 patients (42.9%) exhibited SD. The differences in
outcome between the PERCIST and the RECIST evaluations were
statistically significant (P=0.01) and the overall response rate
was higher when evaluated by the former. This is consistent with
the previous observation that cellular metabolism changes more
rapidly than tumor size.
Discussion
Unresectable, locally advanced, stage III NSCLC
remains a therapeutic challenge for oncologists. Despite the
development of numerous treatment modalities the 5-year survival
rate of patients with NSCLC remain unsatisfactory. Patients at the
same stage of cancer and receiving the same treatment, may
experience different outcomes (13).
Therefore, prognostic tools are required in order to determine
optimum treatment strategies. The present study investigated the
role of 18F-FDG PET/CT parameters in the prognosis
prediction of NSCLC, such that any required dose-escalation or
treatment addition could be put into effect without a break from
therapy.
Observations of increased 18F-FDG uptake
in the majority of lung cancer types and subsequently reduced
uptake following successful treatment have led to increased
enthusiasm for the use of 18F-FDG PET/CT in assessing
therapeutic response (14,15). 18F-FDG PET/CT is known to
provide more time-efficient and more accurate results than those
provided by standard morphological imaging (16). In addition, 18F-FDG uptake
can be used to predict the pathological response of residual
metabolic activity in tumors following RT (17), and is a reliable prognostic factor for
survival rate in patients with NSCLC (17–19). A
number of researchers recommend a delay of 6–8 weeks or longer
following RT prior to performing the post-treatment
18F-FDG PET/CT scan (20).
However, this would result in a break in therapy if the treatment
regime were to be changed based on the findings of the scan. In the
present study, 18F-FDG PET/CT scans were repeated using
60-Gy RT, which reflects the final dose most frequently used
clinically. Undertaking this analysis toward the end of treatment
may be useful for determining whether or not additional dose
escalation is required, and would allow adequate time to
incorporate a boost treatment without requiring a break in
therapy.
The SUV is currently the most widely used
semi-quantitative parameter of 18F-FDG uptake to
evaluate therapeutic response in tumors. The RTOG 0235 trial
(21) demonstrated that higher
survival was significantly associated with a lower post-CRT peak
SUV (P=0.02). Furthermore, Xu et al (22) and Bollineni et al (23) concluded that a lower post-CRT SUVmax
was associated with higher regional and distant control rates
(P=0.003 and P=0.002, respectively) in patients with NSCLC. It was
also reported by the M.D. Anderson Cancer Center that the
disease-free survival and OS time were associated with the post-RT
SUVmax in patients with NSCLC (24).
A meta-analysis of 18 trials revealed that a lower post-RT SUVmax
was significantly associated with an improved local control rate
and overall survival time (25). In
line with this, the present study identified that the post-CRT
SUVmax and ΔSUVmax differed significantly between responders and
non-responders (P=0.009 and P=0.015, respectively).
However, SUVmax is a single-pixel value reflecting
the maximum intensity of 18F-FDG activity in tumors, and
we therefore suggest that it does not account for changes in the
distribution of a tracer within a lesion and in the extent of
metabolic abnormality. Therefore, the alternative use of SUVmean,
MTV and TLG, which incorporate the tumor size and its metabolism,
is proposed. The MTV is assessed using semi-automatic analysis,
which configures the volume of the metabolically active areas of
the tumor with an SUV value of 2.5. TLG is then calculated as the
product of SUVmean and MTV. A study undertaken by Satoh et
al (26) revealed that MTV and
TLG were reliable predictive parameters of disease-free survival,
while SUVmax was not, suggesting that MTV and TLG may be more
reliable variables than SUVmax for predicting outcomes in NSCLC.
The same study also speculated that as tumors become larger, the
single-voxel-based SUVmax is less likely to reflect the overall
aggressiveness of the tumor, as it does not consider the volume of
the metabolically active areas. A study undertaken by Lee et
al (27) reported that, when used
in isolation, high MTV values reflecting tumor burden are poor
prognostic variables for disease progression and survival in
patients with stage I–IV NSCLC. An increase of 25 cm3 in
the MTV value resulted in a 5.4-fold increase in the risk of
disease progression and a 7.6-fold increase in the risk of
mortality. Therefore, the present study also incorporated these
parameters. Furthermore, it was concluded that ΔSUVmax, ΔMTV and
ΔTLG all differed significantly between responders and
non-responders (P=0.015, P=0.006 and P=0.004, respectively), while
no significant difference was observed in either the pre- or
post-treatment parameters between responders and non-responders.
Due to the fact that TLG represents a combination of SUV and MTV,
and MTV represents the degree of 18F-FDG uptake and the
volume of metabolically active tumors, these parameters may offer
an improved metabolic index of tumor burden.
The present study only included cases of
histopathologically confirmed primary adenocarcinoma, as previous
literature had reported that the type of pathology may affect the
uptake of 18F-FDG. For example, Casali et al
(28) reported that the SUVmax was
significantly associated with histological subtypes; the median
SUVmax was 5.1 in patients with adenocarcinoma and 8.3 in those
with other types of NSCLC. Adenocarcinomas also exhibited a
significantly lower SUVmax than that observed in the other tumor
types (P<0.001). Additionally, Vesselle et al (29) reported that adenocarcinomas exhibited
reduced 18F-FDG uptake and lower Ki-67 scores than those
of squamous cell carcinomas or large cell undifferentiated
carcinomas. Therefore, only patients with adenocarcinoma were
included in the present study in order to minimize the effects of
different histological subtypes.
As it has been reported that the assessment of the
predictive value of SUVmax for NSCLC requires consideration of
primary tumor size, the evidence acquired here is not sufficient to
suggest that 18F-FDG uptake could provide prognostic
information in a primary NSCLC (30).
Furthermore, tumor size has been revealed to be a significant
factor in prognosis, and thus survival in NSCLC. Notably, a number
of studies have demonstrated that SUVmax increases with increasing
tumor size (31). Clinical studies
undertaken by Takeda et al (32) demonstrated that a tumor size >5 cm
was markedly associated with a poor prognosis at 5-year survival
rate in patients with NSCLC. For the aforementioned reasons, tumor
size was also considered in the present study, but was identified
to not significantly impact SUVmax and SUVmean (P=0.216 and
P=0.349, respectively; data not shown).
In conclusion, the present study demonstrated that
18F-FDG PET/CT scans could differentiate responders from
non-responders in advanced NSCLC patients following CRT, as
post-CRT SUVmax, ΔSUVmax, ΔMTV and ΔTLG were all significantly
associated with the response of the lesion. This finding may aid in
deciding upon future treatment options, including changes in
dosages and the incorporation of boost treatments. Furthermore, the
relative speed with which the results can be obtained would remove
the requirement for a break in therapy. However, further clinical
studies including larger patient populations are required to fully
establish the potential of this approach.
Acknowledgements
Not applicable.
Funding
The present study was supported by Science and
Technology Development Plans of Shandong Province (grant no.
2014GSF118176) and Shandong Province Natural Science Foundation
(ZR2015HM041).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ performed the research and drafted the
manuscript. YZ participated in the design of the study and
performed the statistical analysis. YY conceived the study,
participated in its design and coordination, and assisted in
drafting the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Boards and Ethics Committees of Shandong Cancer Hospital Affiliated
to Shandong University. Informed consent was obtained from all
participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
RT
|
radiotherapy
|
|
CRT
|
chemoradiotherapy
|
|
18F-FDG PET
|
18F-fluorodeoxyglucose
positron emission tomography
|
|
CT
|
computed tomography
|
|
SUVmax
|
maximum standardized uptake value
|
|
SUVmean
|
mean standardized uptake value
|
|
MTV
|
metabolic tumor volume
|
|
TLG
|
total lesion glycolysis
|
|
PERCIST
|
PET Response Criteria In Solid
Tumors
|
|
RECIST
|
Response Evaluation Criteria In Solid
Tumors
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
OS
|
overall survival
|
References
|
1
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ladanyi M and Pao W: Lung adenocarcinoma:
Guiding EGFR-targeted therapy and beyond. Mod Pathol. 21(Suppl 2):
S16–S22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin J, Ginsberg RJ, Venkatraman ES,
Bains MS, Downey RJ, Korst RJ, Kris MG and Rusch VW: Long-term
results of combined-modality therapy in resectable non-small-cell
lung cancer. J Clin Oncol. 20:1989–1995. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strauss LT, Herndon J, Chang J, Parker WY,
Levy DA, Bowens SB, Zane SB and Berg CJ; CDC: Abortion
surveillance-United States, 2001. MMWR Surveill Summ. 53:1–32.
2004.PubMed/NCBI
|
|
5
|
Spiro SG and Silvestri GA: One hundred
years of lung cancer. Am J Respir Crit Care Med. 172:523–529. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dwamena BA, Sonnad SS, Angobaldo JO and
Wahl RL: Metastases from non-small cell lung cancer: Mediastinal
staging in the 1990s-meta-analytic comparison of PET and CT.
Radiology. 213:530–536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cerfolio RJ, Ojha B, Bryant AS, Raghuveer
V, Mountz JM and Bartolucci AA: The accuracy of integrated PET-CT
compared with dedicated PET alone for the staging of patients with
nonsmall cell lung cancer. Ann Thorac Surg. 78:1017–1023. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mac MMP, Hicks RJ, Matthews JP, McKenzie
A, Rischin D, Salminen EK and Ball DL: Positron emission tomography
is superior to computed tomography scanning for response-assessment
after radical radiotherapy or chemoradiotherapy in patients with
non-small-cell lung cancer. J Clin Oncol. 21:1285–1292. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novello S, Vavala T, Levra MG, Solitro F,
Pelosi E, Veltri A and Scagliotti GV: Early response to
chemotherapy in patients with non-small-cell lung cancer assessed
by [18F]-fluoro-deoxy-D-glucose positron emission tomography and
computed tomography. Clin Lung Cancer. 14:230–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on Cancer (AJCC)
From the AJCC Cancer Staging Manual. 7th edition. Springer-Verlag;
New York, NY; 2010
|
|
11
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Birim O, Kappetein AP, van Klaveren RJ and
Bogers AJ: Prognostic factors in non-small cell lung cancer
surgery. Eur J Surg Oncol. 32:12–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weber WA, Petersen V, Schmidt B,
Tyndale-Hines L, Link T, Peschel C and Schwaiger M: Positron
emission tomography in non-small-cell lung cancer: Prediction of
response to chemotherapy by quantitative assessment of glucose use.
J Clin Oncol. 21:2651–2657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoekstra CJ, Stroobants SG, Smit EF,
Vansteenkiste J, van Tinteren H, Postmus PE, Golding RP, Biesma B,
Schramel FJ, van Zandwijk N, et al: Prognostic relevance of
response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron
emission tomography in patients with locally advanced
non-small-cell lung cancer. J Clin Oncol. 23:8362–8370. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiraishi K, Nomori H, Ohba Y, Kaji M,
Mori T, Shibata H, Oya N and Sasaki J: Repeat FDG-PET for
predicting pathological tumor response and prognosis after
neoadjuvant treatment in nonsmall cell lung cancer: Comparison with
computed tomography. Ann Thorac Cardiovasc Surg. 16:394–400.
2010.PubMed/NCBI
|
|
17
|
Yamamoto Y, Nishiyama Y, Monden T,
Sasakawa Y, Ohkawa M, Gotoh M, Kameyama K and Haba R: Correlation
of FDG-PET findings with histopathology in the assessment of
response to induction chemoradiotherapy in non-small cell lung
cancer. Eur J Nucl Med Mol Imaging. 33:140–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eschmann SM, Friedel G, Paulsen F, Reimold
M, Hehr T, Budach W, Dittmann H, Langen HJ and Bares R: Repeat
18F-FDG PET for monitoring neoadjuvant chemotherapy in patients
with stage III non-small cell lung cancer. Lung Cancer. 55:165–171.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mac MMP, Hicks RJ, Matthews JP, Wirth A,
Rischin D and Ball DL: Metabolic (FDG-PET) response after radical
radiotherapy/chemoradiotherapy for non-small cell lung cancer
correlates with patterns of failure. Lung Cancer. 49:95–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Avril NE and Weber WA: Monitoring response
to treatment in patients utilizing PET. Radiol Clin North Am.
43:189–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Machtay M, Duan F, Siegel BA, Snyder BS,
Gorelick JJ, Reddin JS, Munden R, Johnson DW, Wilf LH, DeNittis A,
et al: Prediction of survival by [18F]fluorodeoxyglucose positron
emission tomography in patients with locally advanced
non-small-cell lung cancer undergoing definitive chemoradiation
therapy: Results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol.
31:3823–3830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu X, Yu J, Sun X, Yang G, Li K, Fu Z, Han
A and Zheng J: The prognostic value of 18F-fluorodeoxyglucose
uptake by using serial positron emission tomography and computed
tomography in patients with stage III nonsmall cell lung cancer. Am
J Clin Oncol. 31:470–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bollineni VR, Widder J, Pruim J,
Langendijk JA and Wiegman EM: Residual 18F-FDG-PET
uptake 12 weeks after stereotactic ablative radiotherapy for stage
I non-small-cell lung cancer predicts local control. Int J Radiat
Oncol Biol Phys. 83:e551–e555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lopez Guerra JL, Gladish G, Komaki R,
Gomez D, Zhuang Y and Liao Z: Large decreases in standardized
uptake values after definitive radiation are associated with better
survival of patients with locally advanced non-small cell lung
cancer. J Nucl Med. 53:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Na F, Wang J, Li C, Deng L, Xue J and Lu
Y: Primary tumor standardized uptake value measured on
F18-Fluorodeoxyglucose positron emission tomography is of
prediction value for survival and local control in non-small-cell
lung cancer receiving radiotherapy: Meta-analysis. J Thorac Oncol.
9:834–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Satoh Y, Onishi H, Nambu A and Araki T:
Volume-based parameters measured by using FDG PET/CT in patients
with stage I NSCLC treated with stereotactic body radiation
therapy: Prognostic value. Radiology. 270:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee P, Weerasuriya DK, Lavori PW, Quon A,
Hara W, Maxim PG, Le QT, Wakelee HA, Donington JS, Graves EE and
Loo BW Jr: Metabolic tumor burden predicts for disease progression
and death in lung cancer. Int J Radiat Oncol Biol Phys. 69:328–333.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Casali C, Cucca M, Rossi G, Barbieri F,
Iacuzio L, Bagni B and Uliano M: The variation of prognostic
significance of Maximum Standardized Uptake Value of
[18F]-fluoro-2-deoxy-glucose positron emission tomography in
different histological subtypes and pathological stages of
surgically resected non-small cell lung carcinoma. Lung Cancer.
69:187–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vesselle H, Salskov A, Turcotte E, Wiens
L, Schmidt R, Jordan CD, Vallières E and Wood DE: Relationship
between non-small cell lung cancer FDG uptake at PET, tumor
histology, and Ki-67 proliferation index. J Thorac Oncol.
3:971–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikushima H, Dong L, Erasmus J, Allen P,
McAleer MF, Zhuang Y, Sasaki R and Komaki R: Predictive value of
18F-fluorodeoxyglucose uptake by positron emission tomography for
non-small cell lung cancer patients treated with radical
radiotherapy. J Radiat Res. 51:465–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mery CM, Pappas AN, Burt BM, Bueno R,
Linden PA, Sugarbaker DJ and Jaklitsch MT: Diameter of non-small
cell lung cancer correlates with long-term survival: Implications
for T stage. Chest. 128:3255–3260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeda S, Fukai S, Komatsu H, Nemoto E,
Nakamura K and Murakami M; Japanese National Chest Hospital Study
Group: Impact of large tumor size on survival after resection of
pathologically node negative (pN0) non-small cell lung cancer. Ann
Thorac Surg. 79:1142–1146. 2005. View Article : Google Scholar : PubMed/NCBI
|