Introduction
Laryngeal cancer is a common malignant tumor of the
head and neck, with the main pathological type being squamous cell
carcinoma. It accounts for 2.4% of new occurrences of malignant
tumors worldwide (1). Due to
environmental changes and the role of carcinogenic factors, the
incidence of laryngeal cancer has increased in recent years
(2). At present, laryngeal cancer
treatment predominantly involves surgery and radiation therapy, but
may also involve antitumor drug therapy and gene therapy. Although
early laryngeal cancer treatment is effective, clinical treatment
failure and poor prognosis are frequent in cases of advanced
laryngeal cancer involving invasion and metastasis (3). Therefore, it is essential to improve the
treatment of patients with laryngeal cancer by exploring effective
detection and prognostic indicators and determining optimal
therapeutic strategies.
Pituitary tumor-transforming 1 (PTTG1) was first
isolated from rat pituitary tumor cells in 1997 (4). The study found that PTTG1 is a
cancer-causing gene (4). Furthermore,
its overexpression has been shown to be involved in inducing cell
transformation in nude mice and tumor formation in vitro,
and PTTG1 expression levels are associated with tumor invasion
(5). PTTG1 is an important biological
marker of cancer cells and may be considered an independent
predictor of overall survival as well as tumor differentiation,
metastasis, and progression. It is considered to be a key gene
associated with tumor metastasis (6)
and with the prognosis of malignant head and neck tumors (7).
Matrix metalloproteinase (MMP)-2 and MMP-9 of matrix
metalloproteinase were closely associated with the occurrence,
development, infiltration and metastasis of laryngeal cancer
(8). In the present study,
immunohistochemistry and western blotting were used to detect
PTTG1, MMP-2 and MMP-9 expression in laryngeal cancer tissues. In
addition, the correlation of PTTG1 with MMP-2 and MMP-9 expression
in laryngeal cancer, as well as its association with laryngeal
cancer occurrence, development and metastasis, were investigated in
order to provide a novel basis for prognostic judgment and targeted
therapy for patients with this malignancy.
Materials and methods
Materials
A total of 210 patients with laryngeal squamous cell
carcinoma, who were treated between January 2004 and December 2008
in the Otolaryngology Department of the Affiliated Hospital Weifang
Medical University (Weifang, China), were included. This comprised
a total of 199 men and 11 women, with an age range of 39–80 years
and a mean age of 62.3 years. There were 48 patients with
well-differentiated tumors, 66 patients with moderately
differentiated tumors, and 96 patients with poorly differentiated
tumors. Lymph node metastasis was present in 117 patients, while 93
patients had no metastasis. According to the tumor-node-metastasis
staging system (Union for International Cancer Control, 2010)
(9), 95 cases were stage I–II and 115
cases were stage III–IV. There were 82 cases of supraglottic
tumors, 117 cases of glottic tumors, and 11 cases of subglottic
tumors. None of the patients received radiotherapy or chemotherapy
prior to surgery. Patient characteristics are summarized in
Table I. In addition to the laryngeal
cancer tissues, 210 samples of normal laryngeal tissues adjacent to
the carcinoma were taken from the mucosa 5 cm from the tumor edge
for use as the control group. Laryngeal cancer tissues, normal
laryngeal tissues and lymph node metastasis were all confirmed by
hematoxylin and eosin staining. Survival time was calculated from
the date of surgery to the date of the last follow-up or the date
of mortality of patients who succumbed to tumor recurrence or
metastasis. The follow-up period ranged from 7 to 60 months, with
an average of 39.5 months. The study was designed so as to comply
with the ethical standards outlined by the Declaration of Helsinki
(1975).
 | Table I.Expression of PTTG1, MMP-2 and MMP-9
and their associations with clinicopathological features. |
Table I.
Expression of PTTG1, MMP-2 and MMP-9
and their associations with clinicopathological features.
|
|
| PTTG1 | MMP-2 | MMP-9 |
|---|
|
|
|
|
|
|
|---|
| Baseline
characteristic | Total cases | Positive, n (%) | P-value | Positive, n (%) | P-value | Positive, n (%) | P-value |
|---|
| Patient sex |
|
| 0.855 |
| 0.255 |
| 1.000 |
| Male | 199 | 176 (88.44) |
| 177 (88.94) |
| 175 (87.94) |
|
|
Female | 11 | 9 (81.82) |
| 8 (72.73) |
| 10 (90.91) |
|
| Patient age,
years |
|
| 0.673 |
| 0.190 |
| 0.642 |
| ≥55 | 134 | 119 (88.81) |
| 121 (90.30) |
| 117 (87.31) |
|
|
<55 | 76 | 66 (86.84) |
| 64 (84.21) |
| 68 (89.47) |
|
| Lymph node
metastasis |
|
| <0.001 |
| <0.001 |
| <0.001 |
|
Present | 117 | 112 (95.73) |
| 114 (97.44) |
| 115 (98.29) |
|
|
Absent | 93 | 73 (78.49) |
| 71 (76.34) |
| 70 (75.27) |
|
|
Tumor-node-metastasis stage |
|
| <0.001 |
| <0.001 |
| 0.001 |
|
I/II | 95 | 74 (77.89) |
| 71 (74.74) |
| 76 (80.00) |
|
|
III/IV | 115 | 111 (96.52) |
| 114 (99.13) |
| 109 (94.78) |
|
| Tumor
differentiation |
|
| <0.001 |
| <0.001 |
| 0.001 |
|
Well/moderately
differentiated | 114 | 91 (79.82) |
| 92 (80.70) |
| 93 (81.58) |
|
| Poorly
differentiated | 96 | 94 (97.92) |
| 93 (96.88) |
| 92 (95.83) |
|
| Tumor location |
|
| 0.325 |
| 1.000 |
| 0.948 |
|
Supraglottic/glottic | 198 | 176 (88.89) |
| 174 (87.87) |
| 175 (88.38) |
|
|
Subglottic | 12 | 9 (75.00) |
| 11 (91.66) |
| 10 (83.33) |
|
The study protocol was approved by the Ethics
Committees of the Affiliated Hospital of Weifang Medical
University, and all participants provided written informed
consent.
Main reagents
The rabbit polyclonal antibodies against PTTG1 was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA;
cat. no. SC-56207). The goat anti-rabbit IgG (cat. no. ZB-2010), Ra
(cat. no. TA-09), MMP-2 (cat. no. ZA-0331), MMP-9 (cat. no.
ZA-0562) and bovine serum albumin (cat. no. ZLI-9027) were
purchased from Beijing ZhongShan Golden Bridge Biological
Technology Co., Ltd. (Beijing, China). The SP-kit, containing
endogenous peroxidase blocker, normal goat serum, biotin-labeled
goat anti-mouse/rabbit IgG polymer and horseradish peroxidase
working solution was purchased from Beijing ZhongShan Golden Bridge
Biological Technology Co., Ltd. (Beijing, China; cat. no. SP-9000).
The ECL kit was purchased from Beyotime Institute of Biotechnology,
Haimen, China. (cat. no. P1008).
Immunohistochemical analysis
The expression of PTTG1, MMP-2, and MMP-9 in
laryngeal cancer normal cancer-adjacent laryngeal tissue were
determined using an immunohistochemical avidin-biotin peroxidase
complex method, according to the manufacturer's protocols (Santa
Cruz Biotechnology, Inc. and Beijing ZhongShan Golden Bridge
Biological Technology Co., Ltd., Beijing, China). Specimens of
laryngeal cancer tissues and normal cancer-adjacent laryngeal
tissues were fixed with formalin, embedded in paraffin, and cut
into 4-µm-thick slices. Samples were baked at 68°C for 20 min. A
conventional xylene dewax for 25 min and dehydration with graded
alcohol for 10 min was performed. Endogenous peroxidase activity
was blocked using 3% H2O2 for 10 min. Antigen
retrieval was carried out by boiling samples in 0.01 M citric acid
buffer (pH 6.0) at 95°C for 15–20 min, cooling for >20 min and
washing with PBS. Blocking was performed with goat serum at 37°C
for 20 min. Primary antibodies were incubated at 4°C overnight.
PTTG1 (SC-56207; dilution, 1:200; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), MMP-2 (cat. no. ZA-0331; dilution, 1:50) and
MMP-9 (cat. no. ZA-0562; dilution, 1:50) were purchased from
Beijing ZhongShan Golden Bridge Biological Technology Co., Ltd.
This was followed by 3 PBS washes for 5 min each. As a negative
control, PBS was used in place of primary antibodies. The
biotin-labeled secondary antibody was used at a dilution of 1:100,
added dropwise and incubated at 37°C for 30 min, followed by three
PBS washes lasting 5 min each. Samples were incubated with
streptomycin ovalbumin solution labeled with horseradish peroxidase
for 30 min at 37°C, followed by three PBS washes each lasting 5
min. Following this, the samples were stained with DABcolor (1:100
dilution) at room temperature for 5–10 min, washed with PBS or tap
water for 10 min, counterstained with hematoxylin for 2 min, washed
with tap water for 10–15 min, conventionally dehydrated,
transparent, mounted and examined with a light microscope
(magnification, ×200 and ×400). Known positive laryngeal squamous
cell carcinoma sections were used as the positive control, and PBS
buffer instead of primary antibody was used as the negative
control.
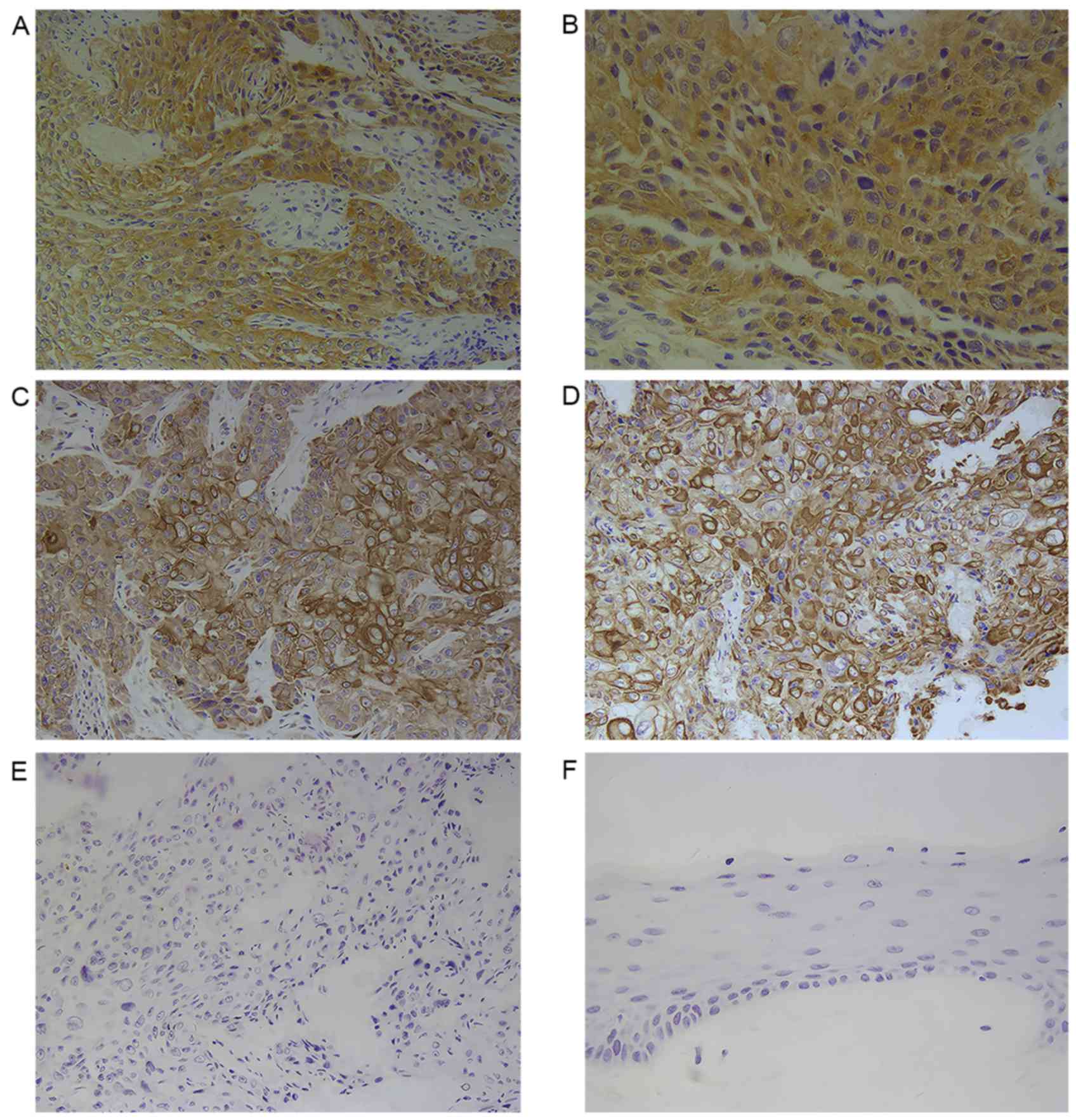
For evaluation, tissues were viewed under a light
microscope (Olympus BX46; Olympus Corporation, Tokyo, Japan).
Evaluation was performed by a pathologist (Department of Pathology,
Affiliated Hospital of Weifang Medical University) with 5 slices in
each group, randomly selecting 4 fields of view at a magnification
of ×200 and ×400. PTTG1-positive laryngeal cancer cells were those
that exhibited light brown staining in the cytoplasm and cell
membrane. MMP-2 and MMP-9 were expressed in the cytoplasm cells
that exhibited tan staining were positive for MMP-2 or −9. A
previously published semi-quantitative evaluation method was used
(10). The positive staining
percentage was divided into 5 levels, assigned a score of 0–4: 0,
<10% of cells positively stained; 1, 11–25% stained; 2, 26–50%
stained; 3, 51–75% stained; and 4, >75% stained. The intensity
of positive staining was assigned a score of 0–3: 0, negative; 1,
weak; 2, medium; and 3, strong.
The product of the two staining variable scores
represented the PTTG1 expression score for each sample: A score of
≥6 was considered high expression, while a score of <6 was
considered low expression.
Western blot analysis
PTTG1 expression was detected by western blotting of
proteins extracted from laryngeal tissues, with reference to the
methods reported by Towbin et al (11). Laryngeal cancer and cancer-adjacent
normal laryngeal tissues (50 mg) were processed with
radioimmunoprecipitation assay buffer (Shanghai HuaYi Bi-technology
Co., Ltd.) in order to extract total protein. Protein concentration
was determined using a bicinchoninic acid assay. Samples of total
protein (20 µg) were mixed with 5 µl of SDS-PAGE loading buffer
(Beyotime Institute of Biotechnology) and heated at 100°C for 5
min. The samples were then separated by 8% SDS-PAGE. Polyvinylidene
difluoride membranes were blocked for 2 h at room temperature with
TBST containing 5% bovine serum albumin, and proteins were
electrotransferred onto the membranes at 100 V for 90 min at room
temperature. The membranes were incubated with primary antibodies
[anti-PTTG1 (dilution 1:1,000) and anti-β-actin (dilution,
1:2,000)] overnight at 4°C, then with the horseradish
peroxidase-labeled secondary antibody (dilution, 1:1,000)] at 37°C
for 2 h prior development with the ECL kit. ImageJ software
(version 1.38; National Institutes of Health, Bethesda, MA, USA)
was used to read the image reversal pattern of the grey value of
the protein bands. The experiment was repeated three times.
Statistical methods
Statistical tests were processed with SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). A
χ2 test was used to analyze the associations between the
PTTG1 positive rate and the clinicopathological parameters. A
Spearman's rank correlation coefficient (rs) analysis
was used to analyze the correlations between PTTG1, MMP-2 and MMP-9
expressions levels. P<0.05 was considered to indicate a
statistically significant difference.
Results
PTTG1 expression differs between
laryngeal cancer tissues and cancer-adjacent normal laryngeal
tissues
Immunohistochemistry revealed that PTTG1 was
predominantly expressed in the cytoplasm of laryngeal cancer cells
(Fig. 1). The rate of positive
expression of PTTG1 among the laryngeal cancer tissue samples was
88.09% (185/210 patients) (10),
whereas the rate among the normal cancer-adjacent tissues was
17.14% (36/210); this difference was statistically significant
(χ2=212.020, P<0.001).
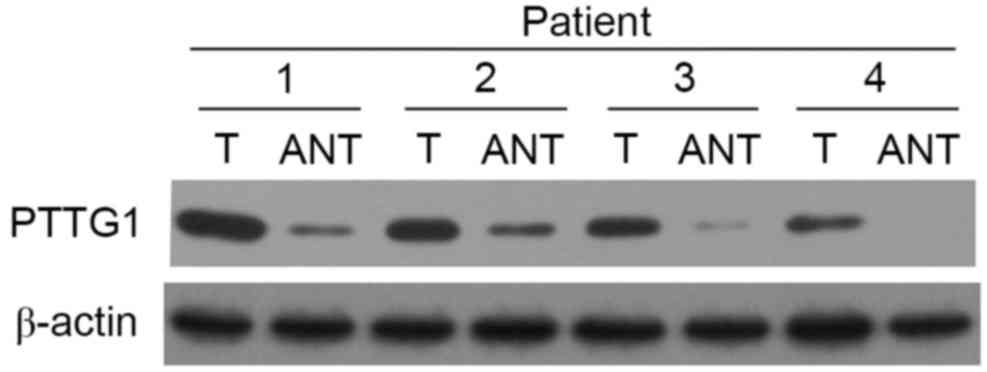
The western blot analysis revealed a clear band
corresponding to a 68-kD protein. Among the 210 cases, 179
laryngeal cancer tissues exhibited PTTG1 protein expression
(85.23%), while 53 tumor-adjacent tissues exhibited PTTG1
expression (24.76%); statistical analysis indicated that PTTG1
protein expression was increased in laryngeal cancer compared with
tumor-adjacent normal tissues (χ2=46.829, P<0.001;
Fig. 2). β-actin was used as an
internal control.
Association of PTTG1, MMP-2 and MMP-9
expression with clinicopathological variables in laryngeal
cancer
The PTTG1 positive expression rate in the group of
patients with lymph node metastasis (95.73%, 112/117) was
significantly higher than that in the group without lymph node
metastasis (78.49%, 73/93; χ2=14.670, P<0.001). In
addition, the PTTG1 positive rate was higher in the stage III–IV
group compared with the stage I–II group (χ2=5.265,
P<0.001), and was higher in the poorly differentiated group
compared with the group with well/moderately differentiated tumors
(χ2=5.476, P<0.001). PTTG1 expression in laryngeal
cancer demonstrated no association with patient age or sex, or with
tumor site (P>0.05; Table I).
The χ2 test indicated that MMP-2
expression was significantly associated with lymph node metastasis,
tumor differentiation degree, and clinical stage (P<0.001), but
was no correlated with patient age or sex, or with tumor site
(P>0.05). Similarly, MMP-9 expression level was significantly
associated with lymph node metastasis, tumor differentiation
degree, and clinical stage (P≤0.001), but not with the other
variables assessed (P>0.05; Table
I).
PTTG1 expression is correlated with
the expression of MMP-2 and MMP-9 in laryngeal carcinoma
Of the 185 laryngeal cancer cases with positive
PTTG1 expression, there were 167 cases with positive MMP-2
expression and 163 cases with positive MMP-9 expression. The
Spearman rank correlation analysis indicated that the expression
level of PTTG1 was positively correlated with the expression levels
of MMP-2 (rs=0.622, P<0.05) and MMP-9
(rs=0.818, P<0.05) in laryngeal cancer cells
(Table II).
 | Table II.Correlation of PTTG1 expression with
MMP-2 and MMP-9 levels. |
Table II.
Correlation of PTTG1 expression with
MMP-2 and MMP-9 levels.
|
| MMP-2 | MMP-9 |
|---|
|
|
|
|
|---|
| PTTG1 level | Level | rs
value | P-value | Level | rs
value | P-value |
|---|
|
| 0 | 1 | 2 | 3 |
|
| 0 | 1 | 2 | 3 |
|
|
| 1 | 1 | 17 | 21 | 17 | 0.622 | <0.05 | 9 | 13 | 23 | 11 | 0.818 | <0.05 |
| 2 | 13 | 25 | 29 | 18 |
|
| 9 | 27 | 26 | 23 |
|
|
| 3 | 4 | 13 | 20 | 7 |
|
| 4 | 12 | 22 | 6 |
|
|
PTTG1 expression level is associated
with postoperative survival rate in laryngeal cancer
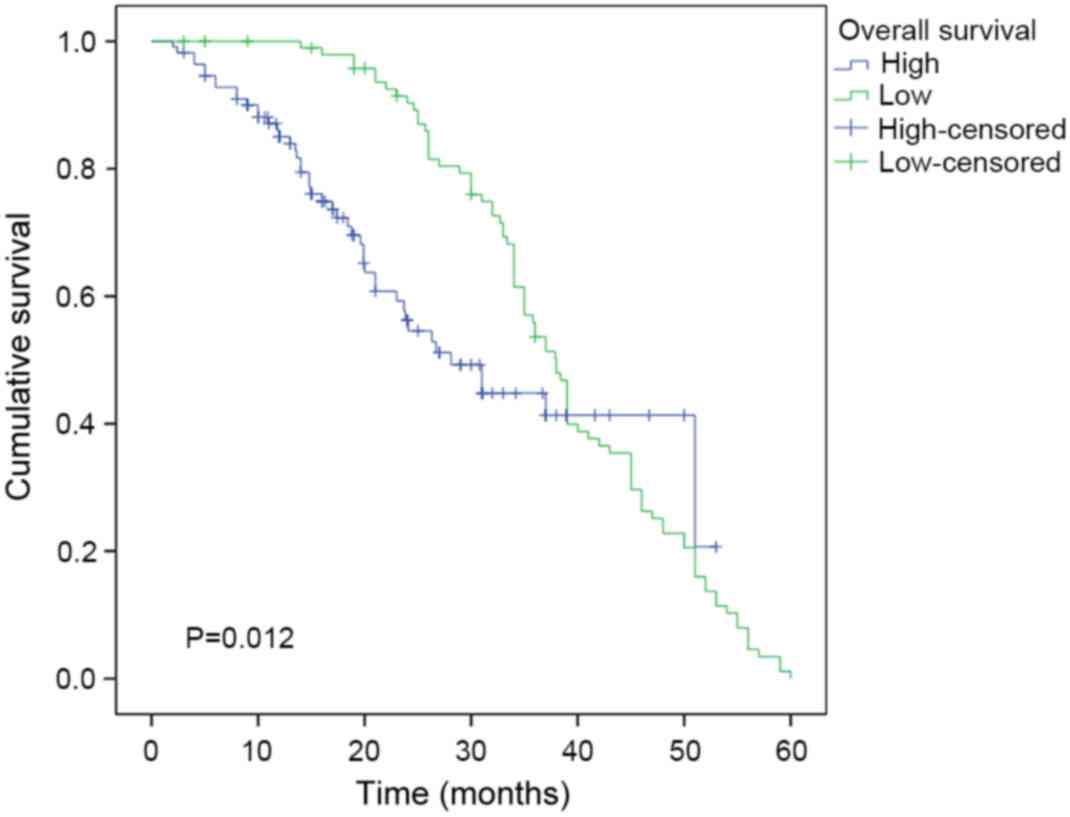
Kaplan-Meier analysis and log-rank test results
showed that the median survival time in the group of patients with
low PTTG1 expression was 38 months, whereas it was 28.1 months in
the group of patients with high PTTG1 expression (P<0.05;
Fig. 3). The survival rate of
patients with low PTTG1 expression was significantly higher than
that of patients with high PTTG1 expression; thus, the expression
of PTTG1 appears to be negatively correlated with the overall
survival rate of patients with laryngeal cancer.
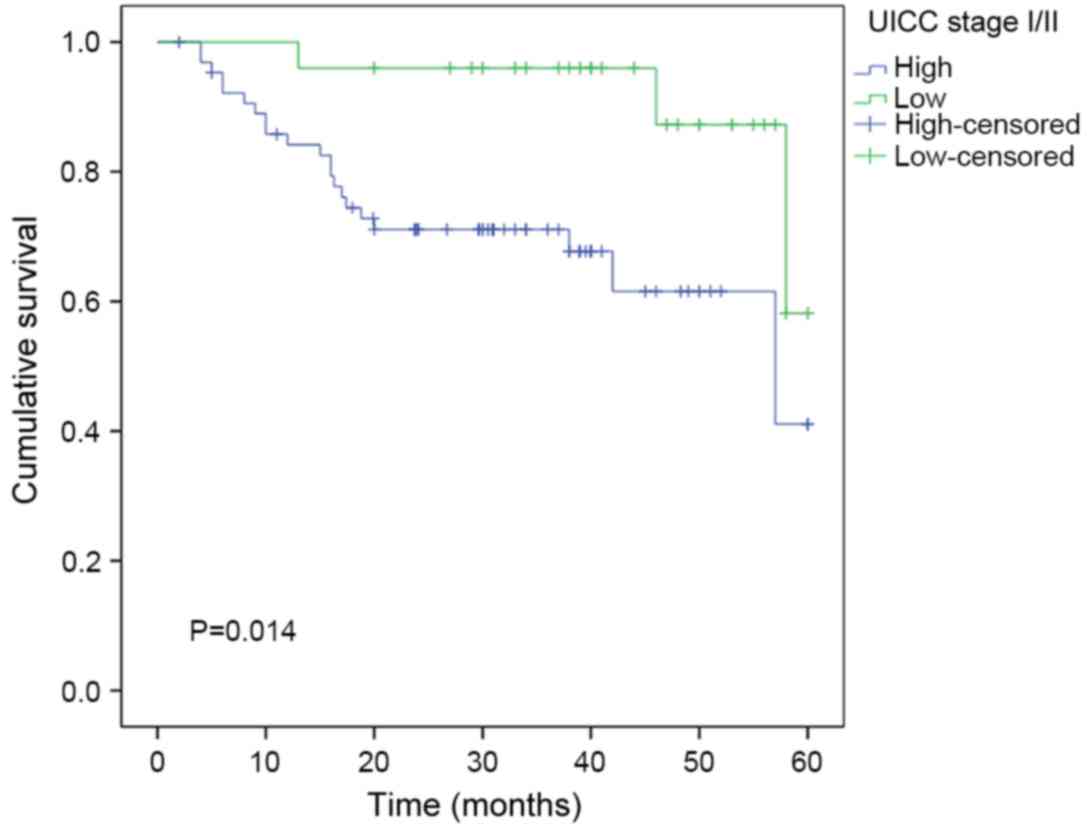
In addition, when patients were subgrouped according
to stage of laryngeal cancer, the overall survival rates were
significantly shorter in patients with high vs. low PTTG1
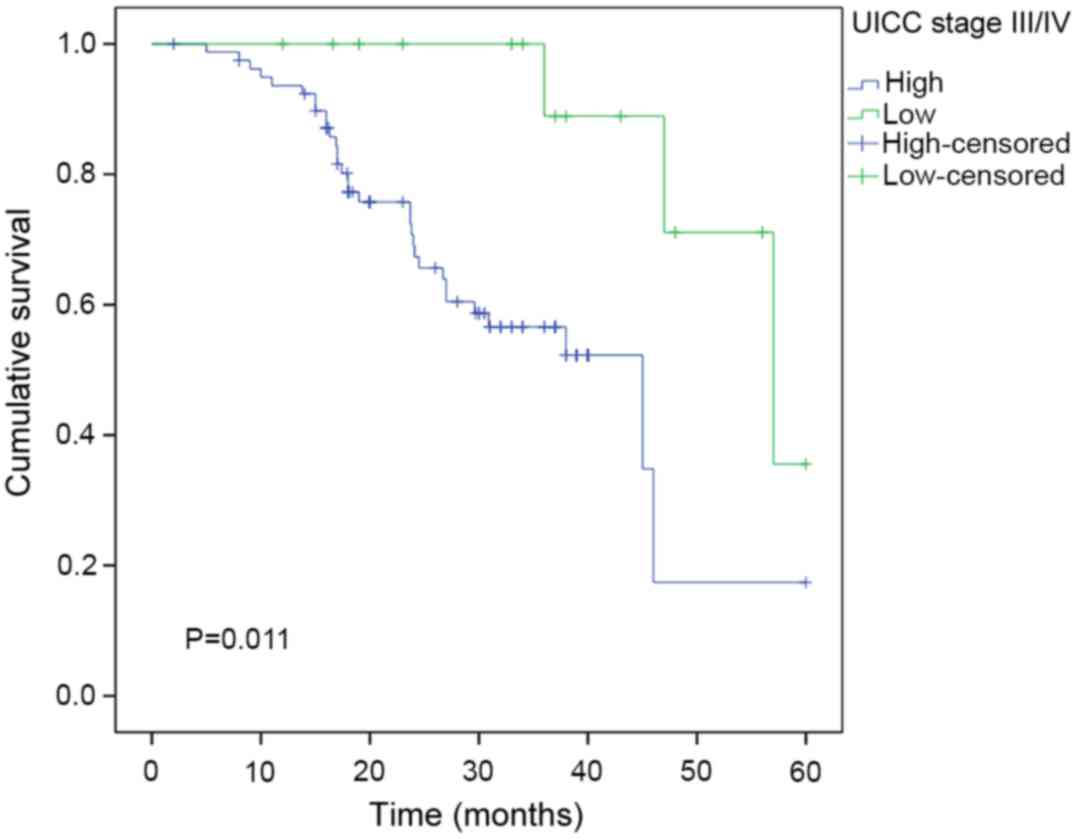
expression in the stage I/II subgroup (n=95; P=0.014; Fig. 4) and in the stage III/IV subgroup
(n=115; P=0.011; Fig. 5).
Discussion
In the present study, PTTG1 was identified as a
cancer-promoting gene that appears to serve an important role in
laryngeal cancer development. The occurrence and development of
Laryngeal cancer is considered to be a result of the interaction of
multiple factors, and one of the most important mechanisms
underlying laryngeal cancer is proto-carcinogenic gene regulation
causing abnormal cell apoptosis and cell proliferation (12). Although substantial progress has been
made in the diagnosis and treatment of laryngeal cancer, invasion,
metastasis, and recurrence remain the main causes of mortality in
affected patients. Worldwide, the survival rate did not
significantly increased from the mid-1980s to the mid-1990s. The
overall 5-year survival rate of laryngeal carcinoma was ~60%
(13,14), which is partly due to a lack of
effective biological markers.
PTTG1 is a recently discovered gene with
carcinogenic characteristics which may be independently expressed
in pituitary tumor cells (4). PTTG
mRNA and protein are expressed in a variety of cancer types
(15–17), suggesting the participation of PTTG in
tumorigenesis. Overexpression of PTTG promote cell proliferation,
cell-induced transformation, and sister chromatids separation
inhibition mechanisms involved in tumor formation (18), plays an important role in regulating
cell growth (19). Furthermore,
studies by Heaney et al (20,21) and
Kim et al (22) suggested that
PTTG may also induce the secretion of basic fibroblast growth
factor (bFGF), implicating PTTG in the promotion of tumor
angiogenesis, which is necessary for tumor growth. Ramaswamy et
al (6) proposed that PTTG is also
closely associated with tumor metastasis, and reported that high
expression of PTTG may be associated with tumor invasion,
metastasis, and angiogenic ability.
In this study, PTTG1 expression was detected in
laryngeal cancer tissues by immunohistochemistry. The results
revealed that the rate of positive PTTG1 expression in the
laryngeal cancer group was 88.09% (185 out of 210 patients),
whereas, in normal laryngeal tissues adjacent to the carcinoma, it
was 17.14% (36 out of 210 patients). Thus, PTTG1 expression in
laryngeal cancer tissues was significantly higher than that in
normal carcinoma-adjacent laryngeal tissues
(χ2=212.0198, P<0.0001). Additionally, the positive
expression rate of PTTG1 protein was significantly higher in the
lymph node metastasis group compared with the group without lymph
node metastasis, in the stage III–IV group compared with the stage
I–II group, and in the poorly differentiated group compared with
the highly/moderately differentiated group. These results indicated
that PTTG1 expression is associated with lymph node metastasis,
tumor stage and degree of malignancy. It may be ascertained from
these results that PTTG1 plays an important role in the processes
of laryngeal cancer occurrence, development and metastasis, and may
be a risk factor for laryngeal cancer.
In the process of invasion and metastasis, the
degradation of the extracellular matrix (ECM) and basement membrane
is a key step. MMPs are a family of highly conserved zinc-dependent
incision proteolytic enzymes. MMPs can degrade the ECM into various
protein components, and also degrade the basement membrane,
regulate tumor cell growth, promote new blood vessel formation, and
regulate cell adhesion. These processes are associated with
malignant tumor invasion and metastases (23–25). The
roles of MMPs in the development, invasion and metastasis of tumors
are widely recognized (26). MMP-2
and MMP-9 are gelatinase enzymes, and mainly act to hydrolyze and
degrade type IV collagen. They play important roles in the
processes of tumor angiogenesis, tumor cell invasion and metastasis
(27). Through the detection of MMP-2
and MMP-9 expression, it is possible to predict metastasis and
prognosis in certain types of cancer.
Existing research shows that MMP-2 and MMP-9 are
highly expressed in laryngeal cancer, and are associated with lymph
node metastasis and poor prognosis (28,29). In
the present study, using the immunohistochemical SP method to
detect the positive expression rate of the proteins, it was
demonstrated that MMP-2 and MMP-9 were more highly expressed in
laryngeal cancer tissues than in normal tissues adjacent to the
carcinoma, and were positively associated with lymph node
metastasis, degree of tumor differentiation, and clinical stage
(P<0.05), but were not associated with age, sex, or tumor
location (P>0.05). This is consistent with the results of
previous studies (28,29) and indicates that high levels of MMP-2
and MMP-9 in laryngeal cancer tissues are closely associated with
the incidence of laryngeal cancer. Combined with the above
description, we hypothesize that the expression of MMP-2 and MMP-9
may promote lymph node metastasis and serve important roles in the
invasion and metastasis of laryngeal cancer.
Analysis of the present experimental data revealed
that the positive expression rates of PTTG1, MMP-2 and MMP-9 in
laryngeal cancer tissues were significantly higher than those in
normal tissues adjacent to the carcinoma, with the lymph node
metastasis subgroup exhibiting higher levels than the subgroup
without lymph node metastasis (P<0.05). In addition, the
expression rates of these three proteins in laryngeal cancer
tissues were positively correlated with one another (Table II). The results suggest that there is
a significant relationship between PTTG1, MMP-9 and MMP-2.
PTTG1 causes cancer cells to infiltrate the
surrounding area, and this mechanism may involve altering the
activity and secretion of MMP-2 (30). High expression of MMP-2 and MMP-9 can
promote the invasion of non-small cell lung cancer (31), and interference with PTTG1 expression
can reduce the expression of MMP-2 and MMP-9, reducing the invasion
and metastasis of non-small cell lung cancer (32), This may be explained in that PTTG1 is
a important regulatory factor of MMP-2 and MMP-9, and these three
proteins serve a crucial role in cancer occurrence, invasion and
metastasis.
In the present study, single-factor survival
analysis results showed that patient age and sex, and tumor
location had no significant effect on the outcomes of 210 cases of
laryngeal cancer, whereas expression of PTTG1, lymph node
metastasis, tumor stage, and degree of differentiation had marked
influences on prognosis. The cumulative survival rates at 3 and 5
years after surgery in the PTTG1-negative subgroup were 85.19 and
73.68%, while in the PTTG1-positive subgroup they were 41.34 and
3.52%, respectively. The survival rates at 3 and 5 years in the
PTTG1-positive group were significantly lower than those in the
PTTG1-negative group (P<0.0001). Thus, PTTG1 is negatively
correlated with overall survival, and the higher the PTTG1
expression, the shorter the patient survival time. This indicates
that PTTG1 is an important factor in the occurrence and development
of laryngeal cancer and acts to promote the invasion and metastasis
of laryngeal cancer cells. Thus, it may be used as a marker of
prognosis in patients with laryngeal cancer. The Cox model results
showed that the positive expression of PTTG1 is an independent
factor in evaluating the prognosis of patients with laryngeal
cancer.
In summary, the present study demonstrated that
PTTG1 expression in laryngeal cancer cells serves a key role in
tumor development and could be used as an independent biological
marker for the evaluation of prognosis in patients with laryngeal
cancer. PTTG1 can promote the proliferation and metastasis of
laryngeal cancer cells, and the levels of PTTG1, MMP-2 and MMP-9
are significantly positively correlated. Studying the effect of
PTTG1 may provide novel targets for the prevention and treatment of
laryngeal cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Soft Science
Project of Weifang Science and Technology Bureau (grant no.
2015RKX040).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KM conceived and designed the present study,
collected data, performed data interpretation and drafted and
revised the manuscript. LM performed experiments and analyzed the
data. ZJ performed experiments and analysis.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the Affiliated Hospital of Weifang Medical University,
and all participants provided written informed consent.
Consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Papadas TA, Alexopoulos EC, Mallis A,
Jelastopulu E, Mastronikolis NS and Goumas P: Survival after
laryngectomy: A review of 133 patients with laryngeal carcinoma.
Eur Arch Otorhinolaryngol. 267:1095–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah JP, Karnell LH, Hoffman HT, Ariyan S,
Brown GS, Fee WE, Glass AG, Goepfert H, Ossoff RH and Fremgen A:
Patterns of care for cancer of the larynx in the United States.
Arch Otolaryngol Head Neck Surg. 123:475–483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jose J, Coatesworth AP, Johnston C and
MacLennan K: Cervical node metastases in squamous cell carcinoma of
the of upper aerodigestive tract: The significance of extracapsular
spread and soft tissue deposits. Head Neck. 25:451–456. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei L and Melmed S: Isolation and
characterization of a pituitary tumor-transforming gene (PTTG). Mol
Endocrinol. 11:433–441. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Demeure MJ, Coan KE, Grant CS, Komorowski
RA, Stephan E, Sinari S, Mount D and Bussey KJ: PTTG1
overexpression in adrenocortical cancer is associated with poor
survival and represents a potential therapeutic target. Surgery.
154:1405–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solbach C, Roller M, Eckerdt F, Peters S
and Knecht R: Pituitary tumor-transforming gene expression is a
prognostic marker for tumor recurrence in squamous cell carcinoma
of the head and neck. BMC Cancer. 6:2422006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langenskiold M, Holmdahl L, Falk P and
Ivarsson ML: Increased plsama MMP-2 protein expression in lymph
node-positive patients with colorectal cancer. Int J Colorectal
Dis. 20:245–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB and Compton CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SS, Kim JE, Kim YA, Kim YC and Kim
SW: Caveolin-1 is down-regulated and inversely correlated with HER2
and EGFR expression status in invasive ductal carcinoma of the
breast. Histopathology. 47:625–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Starska K, Forma E, Jóźwiak P, Bryś M,
Lewy-Trenda I, Brzezińska-Błaszczyk E and Krześlak A: Gene and
protein expression of glucose transporter 1 and glucose transporter
3 in human laryngeal cancer-the relationship with regulatory
hypoxia-inducible factor-1α expression, tumor invasiveness, and
patient prognosis. Tumor Biol. 36:2309–2321. 2015. View Article : Google Scholar
|
|
13
|
Hoffman HT, Porter K, Karnell LH, Cooper
JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A and Robinson RA:
Laryngeal cancer in the United States: Changes in demographics,
patterns of care, and survival. Laryngoscope. 116(Suppl 111):
S1–S13. 2006. View Article : Google Scholar
|
|
14
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: A site-specific analysis of the SEER
database. Int J cancer. 114:806–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramos-Morales F, Dominguez A, Romero F,
Luna R, Multon MC, Pintor-Toro JA and Tortolero M: Cell cycle
regulated expression and phosphorylation of hpttg proto-oncogene
product. Oncogene. 19:403–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solbach C, Roller M, Fellbaum C, Nicoletti
M and Kaufmann M: PTTG mRNA expression in primary breast cancer: A
prognostic marker for lymph node invasion and tumor recurrence.
Breast. 13:80–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rehfeld N, Geddert H, Atamna A, Rohrbeck
A, Garcia G, Kliszewski S, Neukirchen J, Bruns I, Steidl U, Fenk R,
et al: The influence of the pituitary tumor transforming gene-1
(PTTG-1) on survival of patients with small cell lung cancer and
non-small cell lung cancer. J Carcinog. 5:42006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith VE, Franklyn JA and McCabe CJ:
Pituitary tumor-transforming gene and its binding factor in
endocrine cancer. Expert Rev Mol Med. 12:e382010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pei L: Activation of mitogen-activated
protein kinase cascade regulates pituitary tumor-transforming gene
transactivation function. J Biol Chem. 275:31191–31198. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heaney AP, Singson R, McCabe CJ, Nelson V,
Nakashima M and Melmed S: Expression of pituitary-tumour
transforming gene in colorectal tumours. Lancet. 355:716–719. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heaney AP, Horwitz GA, Wang Z, Singson R
and Melmed S: Early involvement of estrogen-induced pituitary tumor
transforming gene and fibroblast growth factor expression in
prolactinoma pathogenesis. Nat Med. 5:1317–1321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim DS, Franklyn JA, Stratford AL,
Boelaert K, Watkinson JC, Eggo MC and McCabe CJ: Pituitary
tumor-transforming gene regulates multiple downstream angiogenic
genes in thyroid cancer. J Clin Endocrinol Metab. 91:1119–1128.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curran S and Murray GI: Matrix
metalloproteinases: Molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunér S, Lopatko Lindman J, Ansari D,
Gundewar C and Andersson R: Pancreatic cancer: The role of
pancreatic stellate cells in tumor progression. Pancreatology.
10:673–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parsons SL, Watson SA, Collins HM, Griffin
NR, Clarke PA and Steele RJ: Gelatinase (MMP-2 and −9) expression
in gastrointestinal malignancy. Br J Cancer. 78:1495–1502. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christopoulos TA, Papageorgakopoulou N,
Ravazoula P, Mastronikolis NS, Papadas TA, Theocharis DA and Vynios
DH: Expression of metalloproteinases and their tissue inhibitors in
squamous cell laryngeal carcinoma. Oncol Rep. 18:855–860.
2007.PubMed/NCBI
|
|
29
|
Saussez S, Cludts S, Capouillez A,
Mortuaire G, Smetana K Jr, Kaltner H, André S, Leroy X, Gabius HJ
and Decaestecker C: Identification of matrix metalloproteinase-9 as
an independent prognostic marker in laryngeal and hypopharyngeal
cancer with opposite correlations to adhesion/growth-regulatory
galectins-1 and −7. Int J Oncol. 34:433–439. 2009.PubMed/NCBI
|
|
30
|
Malik MT and Kakar SS: Regulation of
angiogenesis and invasion by human pituitary tumor transforming
gene (PTTG) through increased expression and secretion of matrix
metalloproteinases-2 (MMP-2). Mol Cancer. 5:612006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim BJ, Jung SS, Choi SY and Lee CS:
Expression of metastasis associated molecules in non-small cell
lung cancer and their prognostic significance. Mol Med Rep.
3:43–49. 2010.PubMed/NCBI
|
|
32
|
Li H, Yin C, Zhan B, Sun Y, Shi L, Liu N,
Liang S, Lu S, Liu Y, Zhang J, et al: PTTG1 promotes migration and
invasion of human non-small cell lung cancer cells and is modulated
by miR-186. Carcinogenesis. 34:2145–2155. 2013. View Article : Google Scholar : PubMed/NCBI
|