Introduction
Colon cancer is a common malignant tumor of the
gastrointestinal tract and is the fourth leading cause of
cancer-associated mortality (1). The
5-year survival rate of patients with colon cancer is 50% (1,2). The onset
of colon cancer is associated with genetic and environmental
factors (e.g., exposure to carcinogens and smoking) (3). Inactivation of tumor suppressor genes
and mutation of oncogenes lead to the development of malignant
tumors (3). Previous studies
investigated the effect of drugs on the proliferation, adhesion,
invasion and migration of colon cancer cells, and the underlying
molecular mechanisms of drug resistance in colon cancer (4–9).
The Wnt signaling pathway regulates diverse
developmental processes, including cell adhesion, proliferation,
differentiation, migration and apoptosis (10). Previous studies have demonstrated that
numerous types of cancer, including melanoma, hepatocarcinoma,
gastrointestinal, breast and ovarian cancer (11), are associated with abnormal Wnt
signaling pathway. Abnormal activation of Wnt signaling pathway has
been reported in colorectal cancer (12,13). Wnt
family of proteins includes at least 19 secreted-type glycoproteins
with conserved 22–24 cysteine residues, and serves vital functions
in carcinogenesis and embryogenesis (14). Previous studies have reported that
Wnt6 is upregulated in gastrointestinal cancer and cervical cancer,
and overexpression of Wnt6 promotes physiological or pathological
processes via activation of Wnt/β-catenin signaling pathway in
various cancer cell lines (15–18). Wnt6
is highly expressed in colorectal adenoma and may be associated
with increased risk of colorectal cancer (19). However, the role of Wnt6 in
occurrence, progression and metastasis of colon cancer remains
unclear.
In the present study, the expression of Wnt6 was
evaluated in colon cancer cell lines (LoVo, SW480, HCT116, SW620
and HT29). The effects of overexpression and knockdown of Wnt6 on
proliferation, cell cycle and apoptosis of colon cancer cells were
investigated. The aim of the present study was to investigate the
function of Wnt6 in tumorigenesis and progression of colon cancer
and provide the basis for a novel therapeutic target in the
treatment of colon cancer.
Materials and methods
Cell culture
Wnt6 high expression cell lines were selected from
five human colon cancer cell lines (LoVo, SW480, HCT116, SW620 and
HT29). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml of penicillin and 100 µg/ml of
streptomycin, and were cultured at 37°C in a humidified atmosphere
containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Ambion; Thermo Fisher Scientific, Inc.). RNA was
reverse-transcribed into cDNA using PrimeScript™ 1st Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China), and
qPCR was performed using a KAPA SYBR Green qPCR kit (Kapa
Biosystems, Inc., Wilmington, MA, USA). The primer sequences for
Wnt6 were as follows: 5′-CGGAAGTGGTGGCAGAG-3′ (forward) and
5′-CAGGATGCGTCCAAAGG-3′ (reverse). The primer sequences for β-actin
were as follows: 5′-ACACTGTGCCCATCTACG-3′ (forward) and
5′-TGTCACGCACGATTTCC-3′ (reverse). Each reaction (20 µl total
volume) contained 10 µl SYBR, 0.40 µmol/l each primer and 0.2±0.02
µg cDNA template. The thermocycling conditions were as follows:
Pre-denaturation at 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 20 sec and
elongation at 72°C for 20 sec. The threshold cycle (Ct) was
determined for each reaction by using the 2−ΔΔCq method,
which generated Ct values for each gene of interest normalized to
the endogenous control gene (β-actin) (20). For each group, three replicates of
each measurement were performed.
Cell transfection
Cells (HTC116 or SW480) in 1×105 cells/ml
were seeded in 6-well plates and cultured until cells reached
confluency (70–80%). Cells were then transfected with 800 ng/well
of plasmids using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The primers for Wnt6 were as follows:
5′-CACCCTGCCGCCCTTACCCTCC-3′ (forward) and
5′-GATCCGGGTCACAGGCAGAGGC-3′ (reverse). The corresponding cDNAs
were inserted into the pGPU6/green fluorescent protein (GFP)/Neo
vector to construct the recombinant pGPU6/GFP/Neo-Wnt6-Homo-1
plasmid, to overexpress Wnt6 (GenePharma Shanghai, China). An empty
vector (EV) was used as a negative control. Additionally, specific
short hairpin (sh)RNA-expressing vectors were employed to knockdown
Wnt6. Negative control (NC) pGPU6/GFP/Neo-shNC (target sequence,
GTTCTCCGAACGTGTCACGT) and pGPU6/GFP/Neo-Wnt6-Homo-A (target
sequence, AAGTGGTGGCAGAGCTAGCTC) vectors were obtained from
Shanghai GenePharma Co., Ltd. (Shanghai, China). Untransfected
HCT116 or SW480 cells consider as control group.
MTT assay
Cell proliferation was evaluated using an MTT assay.
HCT116 and SW480 cells were seeded in 96-well plates and
transfected with expression or empty vector with 1×104
cells in each well. At indicated timepoints (24, 48 and 72 h), 20
µl MTT (5 mg/ml; Bioswamp, Wuhan, China) was added to each culture
prior to incubation at 37°C for an additional 4 h. Then, DMEM
medium was removed and 150 µl dimethylsulfoxide was added to each
well and mixed for 10 min. Absorbance was read at a wavelength of
490 nm.
Flow cytometric analysis of
apoptosis
Apoptosis was detected by double staining with
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
(BD Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. At 48 h post-transfection, HCT116 and
SW480 cells (1×106 cells/ml) were washed three times
with ice-cold PBS and incubated for 30 min at 4°C in the dark in
200 µl binding buffer containing 10 µl Annexin V-FITC and 10 µl PI.
Apoptotic cells were analyzed using a flow cytometer (Beckman
Coulter, Inc., Brea, CA, USA) and Cytomics FC 500 MCL with CXP
software 5.0 network (Beckman Coulter, Inc.).
Analysis of cell cycle
Cell cycle was analyzed using flow cytometry. At 48
h post-transfection, HCT116 and SW480 cells in 1×106
cells/ml were washed with ice-cold PBS and then fixed with 70%
ethanol at 4°C for 1 h. Following washing with PBS, cells were
stained with PI [10 mM Tris (pH 7.0), 0.1% NP-40, 1 mM NaCl, 0.7
µg/ml ribonuclease A and 5 µg/ml PI] for 30 min in the dark. Cell
cycle analysis was performed using a flow cytometer and Cytomics FC
500 MCL with CXP 5.0 software.
Cell migration assay
Cell migration were assessed using Transwell assays.
HCT116 and SW480 cells (1×105 cells/ml) were starved in
serum-free DMEM medium for 24 At 48 h, cells were fixed for 10 min
using 4% paraformaldehyde (Bioswamp; Wuhan Beinglay Biological
Technology Co., Wuhan, China) and incubated with 0.5% crystal
violet (Bioswamp; Wuhan Beinglay Biological Technology Co.) for 30
min. A total of 100 µl cell suspension was seeded into transwell
chambers with 8 µm prore polycarbonate membrane insert (Corning
Incorporated, Corning, NY, USA). A total of 600 µl RPMI-1640 medium
(Beijing Solarbio Science & Technology, Co., Ltd., Beijing,
China) containing 20% FBS was added in lower chambers. Following
incubation for 24 h in transwell chamber, cells remaining on the
upper membrane were removed carefully with a cotton swab. Stained
cells were counted in three fields using a light microscope (Nikon
Corporation, Tokyo, Japan; magnification, ×200).
Western blot analysis
Western blot analysis was performed as previously
described (21). Antibodies against
Wnt6 (cat. no. ab50030, 1:500 dilution; Abcam, Cambridge, UK),
B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) (cat. no.
ab32503, 1:2,000 dilution; Abcam), caspase-3 (cat. no. ab32351,
1:2,000 dilution; Abcam), matrix metalloproteinase (MMP)2 (cat. no.
ab37150, 1:1,000 dilution; Abcam) and β-actin (cat. no. 49675,
1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA,
USA) were used. HCT116 and SW480 cells were washed twice with PBS
and homogenized in radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) containing
protease inhibitor (cat. no. 58715; Cell Signaling Technology) and
centrifuged at 12,000 × g for 15 min at 4°C. The concentration of
the proteins was measured using a bicinchoninic acid assay kit
(cat. no. P0011; Beyotime Institute of Biotechnology). A total of
30 µg proteins were separated by 10% SDS-PAGE and transferred onto
a polyvinylidene difluoride membrane (Merck KGaA, Darmstadt,
Germany). The membranes were blocked with 5% skim milk for 2 h at
room temperature in Tris-buffered saline. Then, the membranes were
incubated with primary antibodies overnight at 4°C. Anti-β-actin
antibody was selected as internal reference. Then, the membranes
were washed with Tris-buffered saline and incubated in biotinylated
goat IgG conjugated to horseradish peroxidase (HRP) secondary
antibody (cat. no. ab7090, Abcam) for 2 h at room temperature.
Immunoreactivity was visualized by colorimetric reaction using an
enhanced chemiluminesence substrate buffer (Merck KgaA). Membranes
were scanned with Gel Doz EZ imager (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Bans were quantified using Quantity One 5.0
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analyzed using SPSS software (version
18.0; SPSS, Inc., Chicago, IL, USA). The relevant data are
expressed as the mean ± standard error of the mean. Statistical
analysis was performed using one-way analysis of variance followed
by Duncan's multiple range test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of Wnt6 in human colon
cancer cell lines
RT-qPCR was employed to evaluate the expression
levels of Wnt6 in human colon cancer cells. The results
demonstrated that HCT116 and SW480 cells exhibited increased
expression levels of Wnt6 (Fig. 1A).
Therefore, we selected HCT116 and SW480 cells lines in the further
studies, to explore the effect of Wnt6 expression on colon cancer.
Additionally, pGPU6/GFP/Neo-Wnt6-Homo-1 plasmid was constructed to
assess the effects of overexpression of Wnt6, whereas specific
shRNA-expressing vectors were employed to knockdown Wnt6. Fig. 1B and C demonstrate the transfection
efficiency of vectors using HCT116 and SW480 cells. As presented in
Fig. 1D and E, shWnt6 (a plasmid
carrying shRNA targeting Wnt6) significantly reduced the expression
of Wnt6, whereas pWnt6 (Wnt6 overexpression plasmid) significantly
increased the expression of Wnt6 in HCT116 and SW480 cells.
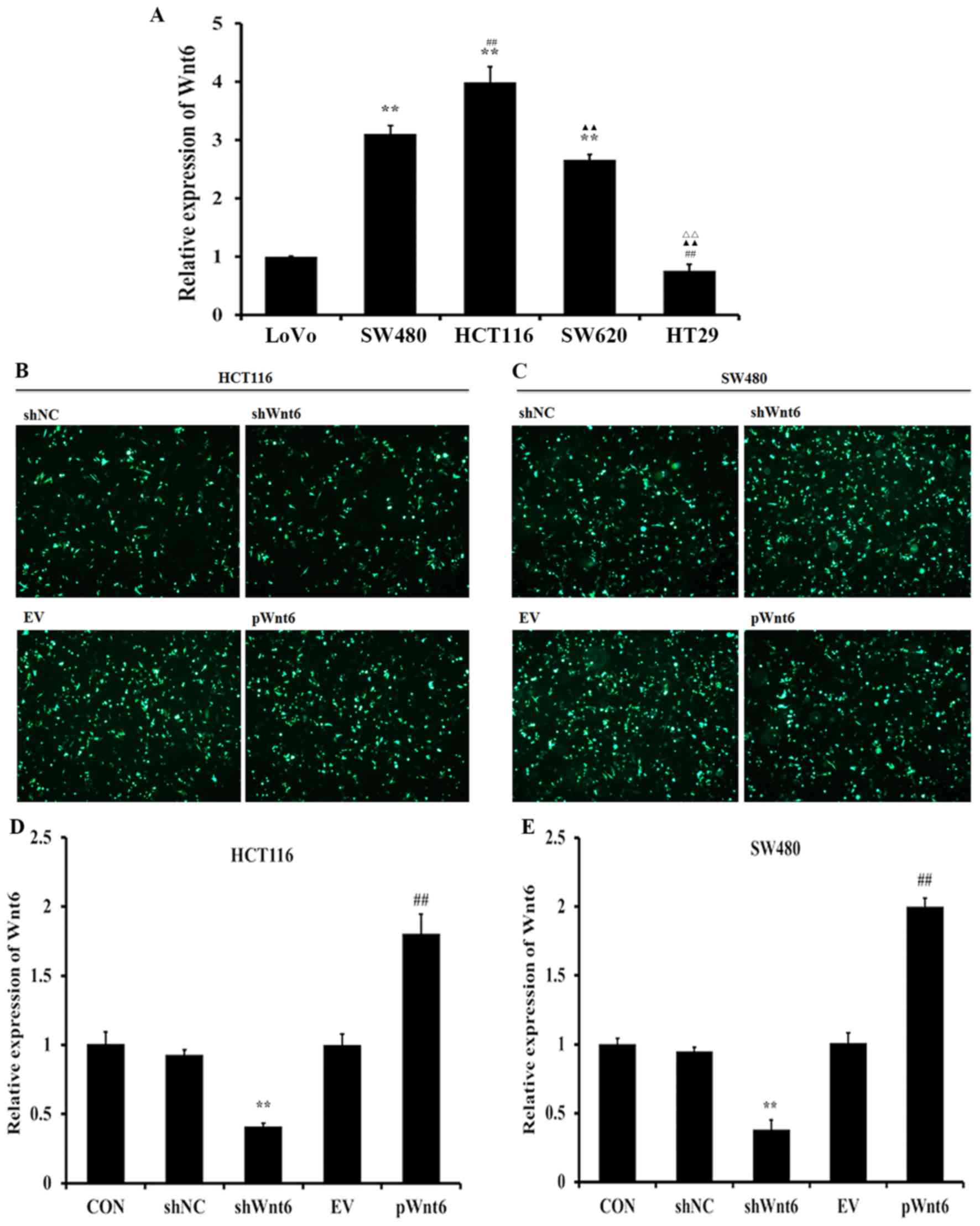 | Figure 1.Expression of Wnt6 in human colon
cancer cell lines. (A) RT-qPCR analysis of the expression levels of
Wnt6 in colon cancer cells (LoVo, SW480, HCT116, SW620 and HT29).
**P<0.01 vs. LoVo; ##P<0.01 vs. SW480;
▲▲P<0.01 vs. HCT116; rrP<0.01 vs.
SW620. HCT116 cells and SW480 cell were transfected with The shNC,
shWnt6, EV or pWnt6 were successfully transfected into (B) HCT116
cells and (C) SW480 cell and detected using fluorescence
microscope. The expression levels of Wnt6 in (D) HCT116 cells and
(E) SW480 cells transfected with shNC, shWnt6, EV or pWnt6 as
detected using RT-qPCR. **P<0.01 vs. shNC;
##P<0.01 vs. shWnt6. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; sh, short
hairpin; NC, negative control; EV, empty vector; p, plasmid; CON,
control. |
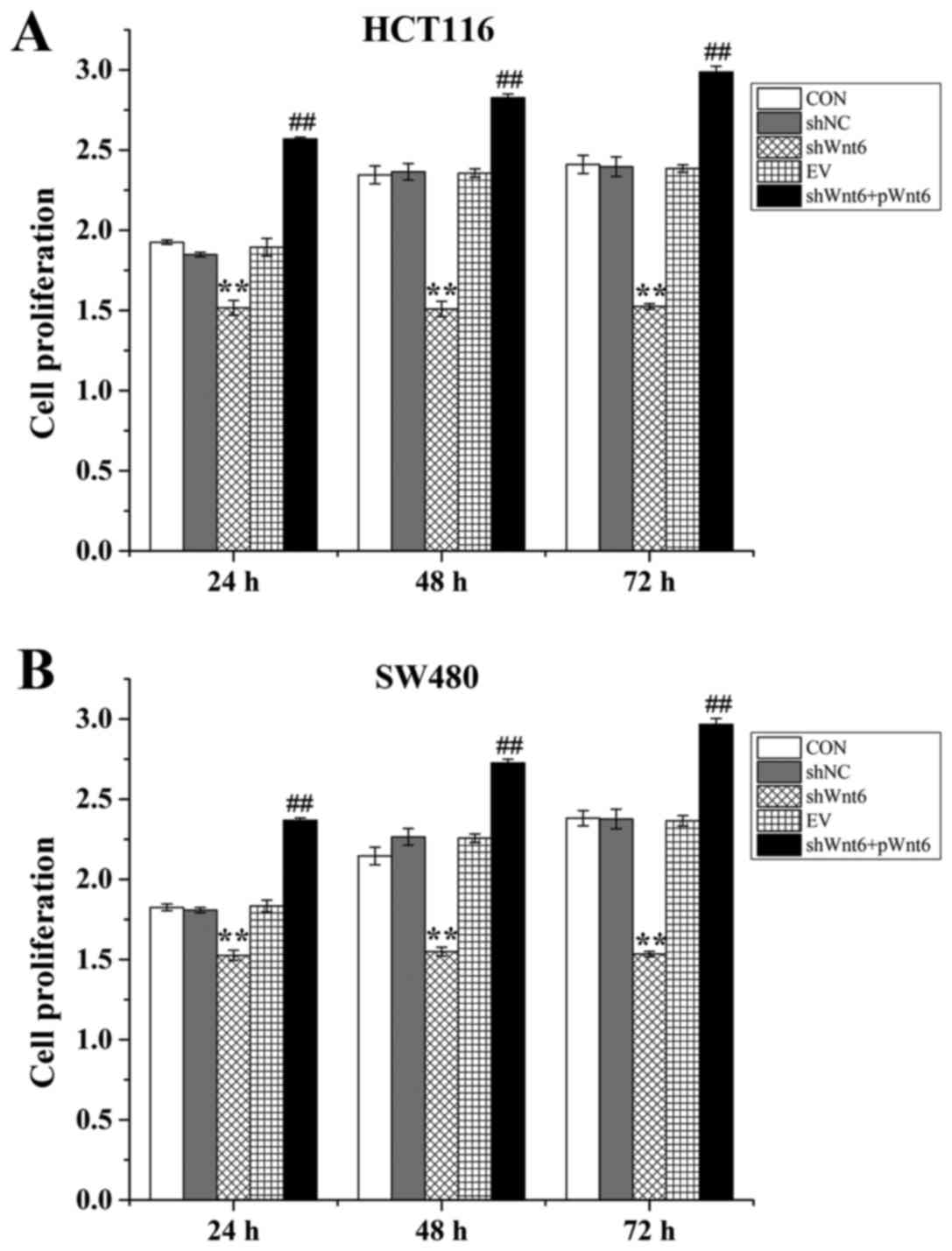
Overexpression of Wnt6 induced colon
cancer cell proliferation
Cell proliferation was evaluated using an MTT assay.
Cells were divided into the following groups: Control, shNC
(transfection with negative control), shWnt6 (transfection with
shWnt6), EV (transfection with empty vector) and shWnt6+pWnt6
(combined transfection with shWnt6 and pWnt6). The results
demonstrated that cell proliferation was decreased in the shWnt6
group compared with that of the shNC group at 24, 48 and 72 h
(Fig. 2A and B), indicating
downregulation of Wnt6 inhibited the proliferation of HTC116 and
SW480. Cell viability was significantly increased in the
shWnt6+pWnt6 group in a time-dependent manner compared with the
shWnt6 group (Fig. 2A and B).
Therefore, knockdown of Wnt6 decreased cell proliferation and
transfection with pWnt6 reversed this effect in HCT116 and SW480
cells.
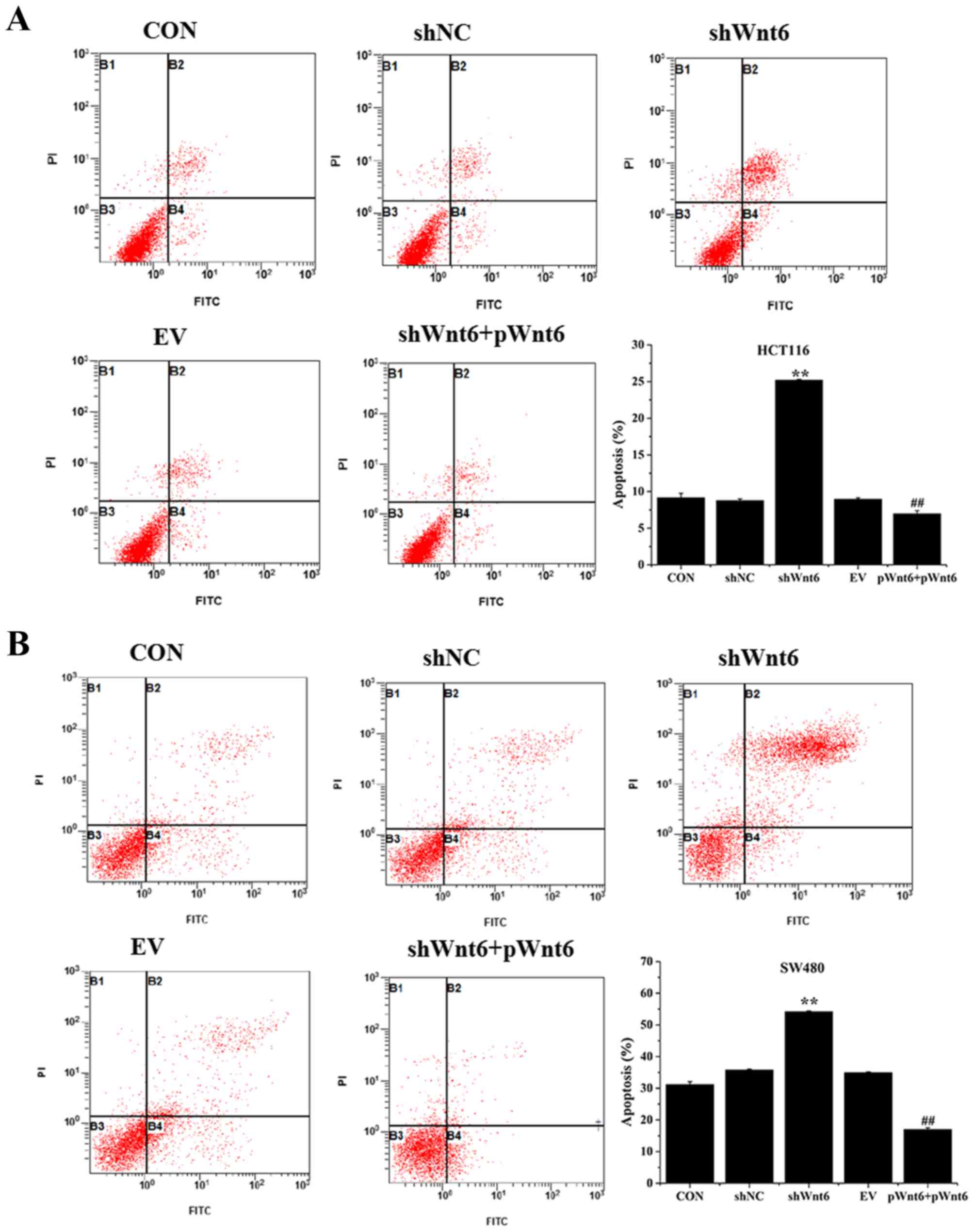
Overexpression of Wnt6 inhibits the
apoptosis of colon cancer cell
Cell apoptosis was evaluated using Annexin V-FITC/PI
staining and flow cytometry. As presented in Fig. 3A and B, the percentage of apoptotic
cells was significantly increased in the shWnt6 group compared with
that of the shNC group. However, shWnt6+pWnt6 group exhibited
decreased apoptosis compared with that of shWnt6 group. Knockdown
of Wnt6 increased apoptosis and transfection with pWnt6 reversed
this effect in HCT116 and SW480 cells.
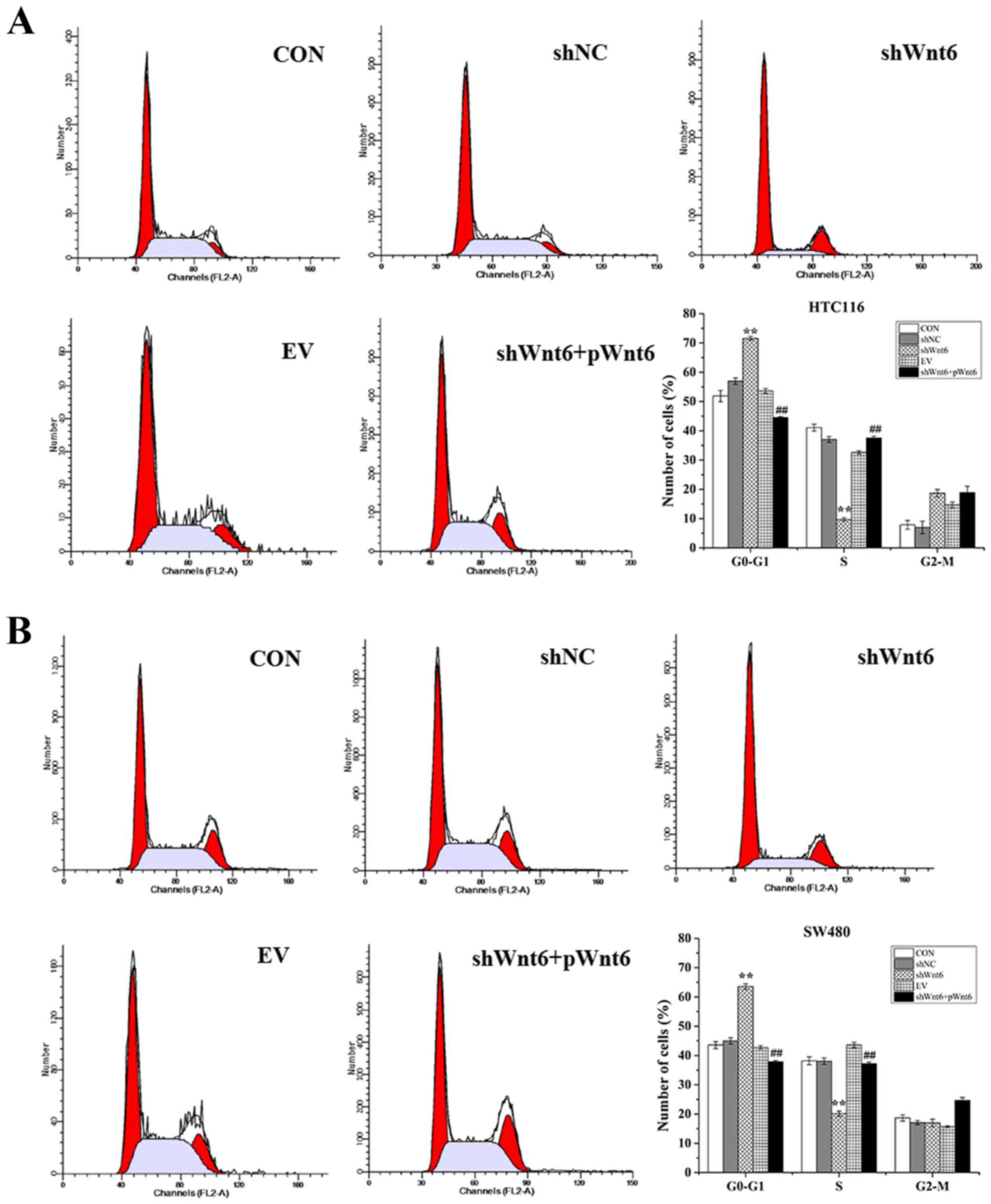
Overexpression of Wnt6 promotes colon
cancer cell cycle
The effect of Wnt6 on cell cycle was evaluated using
flow cytometry. As presented in Fig. 4A
and B, cells transfected with shWnt6 exhibited a significant
G0-G1 cell cycle arrest accompanied with a
reduction of cell numbers in S-phase, which was reversed in
response to transfection with pWnt6. These results suggest that
knockdown of Wnt6 may induce cell cycle arrest in
G0-G1 phase in colon cancer cell lines and
transfection with pWnt6 reversed this effect.
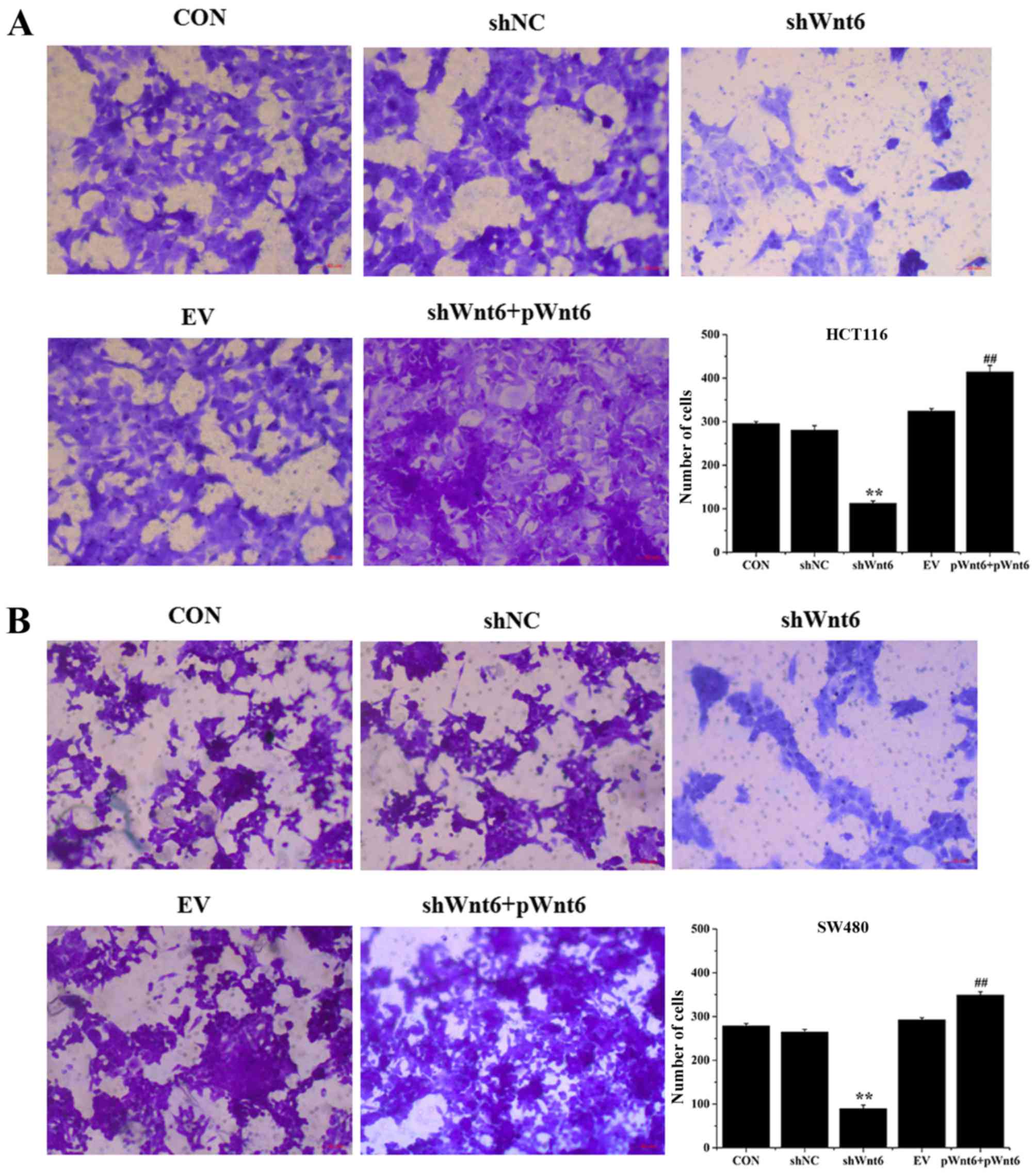
Overexpression of Wnt6 induces the
migration of colon cancer cell
Cell migration was evaluated using Transwell assays.
As presented in Fig. 5A and B,
transfection with shWnt6 significantly suppressed the migration of
cells compared with that of the shNC group, whereas shWnt6+pWnt6
group exhibited increased numbers of migrated cells. Knockdown of
Wnt6 decreased the migratory ability of cells and transfection with
pWnt6 reversed this effect in HCT116 and SW480 cells.
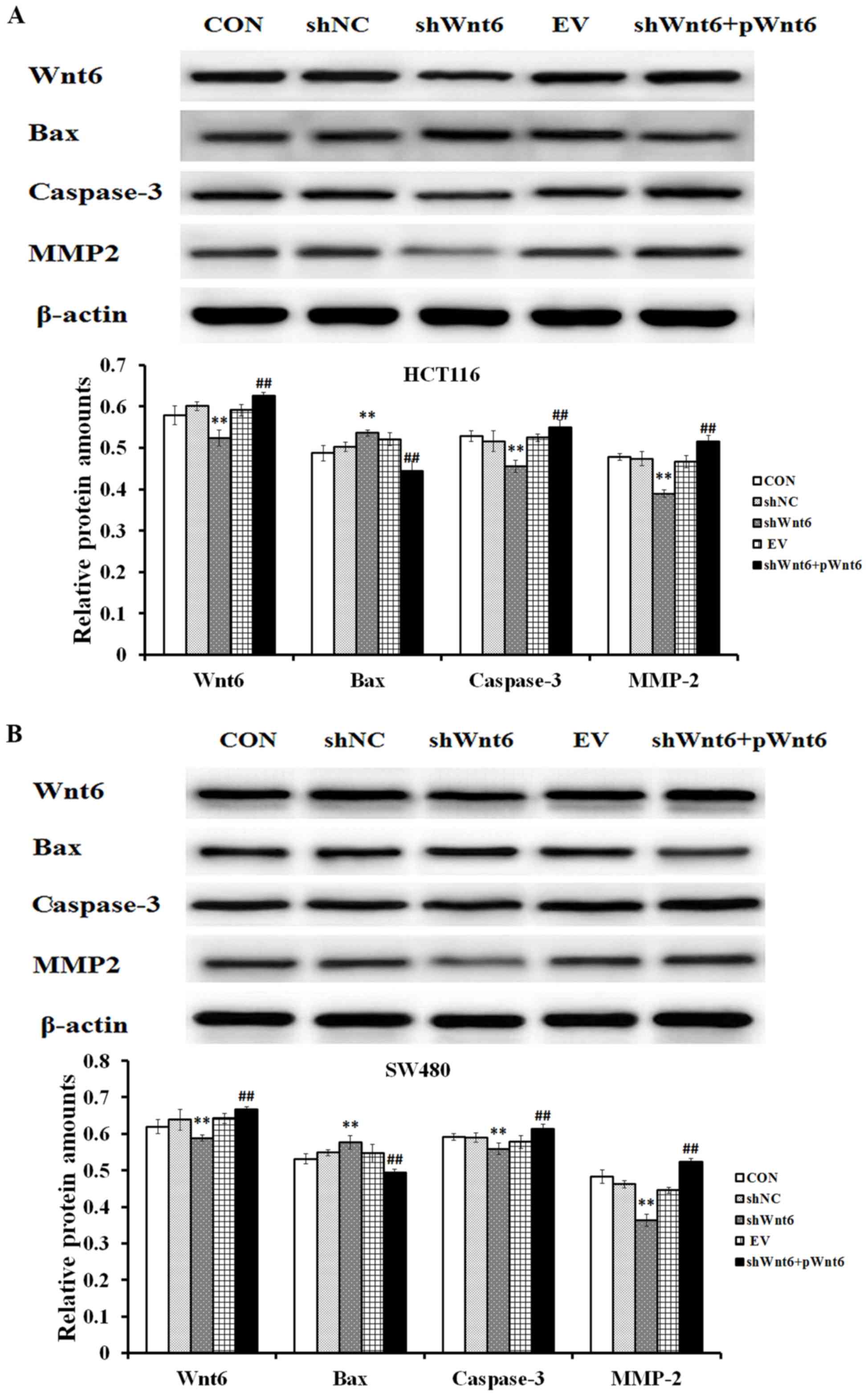
Overexpression of Wnt6 effects on the
expression of apoptosis-associated proteins
The expression of Bax, caspase-3 and MMP2 was
assessed using western blot analysis. The results confirmed that
shWnt6-transfected cells exhibited decreased expression levels of
Wnt6 compared with that of the shNC group, whereas the shWnt6+pWnt6
group exhibited increased expression levels of Wnt6 compared with
that of the shNC group (Fig. 6A and
B). Additionally, the expression levels of caspase-3 and MMP2
were decreased, whereas the expression levels of Bax were increased
in response to shWnt6 (Fig. 6). The
expression of caspase-3 and MMP2 was increased, whereas the
expression of Bax was decreased following overexpression of Wnt6
(achieved by pWnt6) (Fig. 6A and
B).
Discussion
The Wnt/β-catenin signaling pathway is triggered by
a series of signaling cascade reactions, thus leading to
transcription of target genes in the nucleus (10). The Wnt signaling pathway is involved
in cell proliferation, apoptosis and epithelial-mesenchymal
transition (EMT) (22). Additionally,
previous studies demonstrated that aberrant Wnt signaling pathway
promotes cell proliferation and tumorigenesis (23) in various types of cancer, including
gastrointestinal (24), breast
(25), kidney (26), pancreatic (27), prostate cancer (28), melanoma (29) and osteosarcoma (30). Wnt6 is a member of the Wnt protein
family that has been reported to be involved various types of
cancer (19,31). However, the function of Wnt6 in colon
cancer remains unclear.
In the present study, the expression of Wnt6 was
increased in HCT116 and SW480 cells compared with LoVo and HT29
cells. Therefore, HCT116 and SW480 cells were selected for
subsequent experiments. The expression pattern of Wnt6 may differ
in colon cell lines due to differences in histological
differentiation, tumor staging and biological characteristics.
Kirikoshi et al (15) reported
that Wnt6 is strongly expressed in SW480 cells. Wnt proteins have
been reported to promote various types of cancer. Wnt1 regulates
the progression of breast cancer by promoting cell proliferation
and migration (32). Overexpression
of Wnt2 contributes to tumorigenesis of colorectal and lung cancer
(33,34). Wnt10b expression serves an important
function in the development of endometrial cancer (35). In the present study, it was
demonstrated that inhibition of Wnt6 may inhibit cell
proliferation, cell cycle process and migration, and promote cell
apoptosis, and overexpression of Wnt6 reversed this effect, thus
upregulation of Wnt6 may contribute to tumorigenesis and
development of malignant colon tumor.
The results demonstrated that the expression of
caspase-3 and MMP2 was increased, whereas the expression of Bax was
decreased following overexpression of Wnt6. Caspase-3 has been
demonstrated to be cleaved in apoptotic cells and the expression of
caspase-3 precursor decreased (36).
The results of the present study demonstrated that overexpression
of Wnt6 increased the expression of caspase-3 precursor, indicating
that Wnt6 may inhibit cell apoptosis. MMP2 is involved in the
breakdown of extracellular matrix. MMP2 is associated with the
development of various malignant tumors and may promote EMT, a key
process involved in cancer metastasis (37). Wnt6 increased the expression of MMP2
indicating that Wnt6 may promote cell migration. Bax is an
apoptosis-promoting member of the Bcl-2 family (38). The results of the present study
demonstrated that overexpression of Wnt6 decreased the expression
of Bax, indicating that Wnt6 may inhibit cell apoptosis. Wnt1, Wnt3
and Wnt8 have been reported to activate Wnt/β-catenin signaling
pathway (39,40). However, whether Wnt6 may activate the
Wnt signaling pathway in colon cancer requires further
investigation.
The present study demonstrated that HCT116 and SW480
cells exhibit increased expression levels of Wnt6. Downregulation
of Wnt6 by shWnt6 inhibited cell proliferation and migration and
induced cell apoptosis of HCT16 and SW480 cells. Additionally,
overexpression of Wnt6 may promote the proliferation, cell cycle
and migration of HCT116 and SW480 cells, but inhibit cell apoptosis
by upregulation of the expression of caspase-3 and MMP2, and
downregulation of the expression of Bax. These results indicated
that Wnt6 may serve a vital function in the progression of colon
cancer and may be utilized as a potential therapeutic target.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National High
Technology Research and Development Program 863 (grant no.
20151127D2811).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ was responsible for all the experiments and data
analyses and editing of the manuscript; HY was responsible for the
overall design of the study and responsible for providing the
materials. All the authors approved the final submission.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed S, Johnson K, Ahmed O and Iqbal N:
Advances in the management of colorectal cancer: From biology to
treatment. Int J Colore Dis. 29:1031–1042. 2014. View Article : Google Scholar
|
|
2
|
László L: Predictive and prognostic
factors in the complex treatment of patients with colorectal
cancer. Magy Onkol. 54:383–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung JJ, Lau JY, Goh KL and Leung WK: Asia
Pacific Working Group on Colorectal Cancer: Increasing incidence of
colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu T, Li Z, Gao CY and Cho CH: Mechanisms
of drug resistance in colon cancer and its therapeutic strategies.
World J Gastroenterol. 22:6876–6889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi W, Ye Z, Zhuang L, Li Y, Shuai W, Zuo
Z, Mao X, Liu R, Wu J, Chen S and Huang W: Olfactomedin 1
negatively regulates NF-kB signalling and suppresses the growth and
metastasis of colorectal cancer cells. J Pathol. 240:352–365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan H, Hu K, Wu W, Li Y, Tian H, Chu Z,
Koeffler HP and Yin D: Low expression of DYRK2 (Dual specificity
tyrosine phosphorylation regulated kinase 2) correlates with poor
prognosis in colorectal cancer. PLoS One. 11:e01599542016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Lin C, Liao G, Liu S, Ding J,
Tang F, Wang Z, Liang X, Li B, Wei Y, et al: MicroRNA-506
suppresses tumor proliferation and metastasis in colon cancer by
directly targeting the oncogene EZH2. Oncotarget. 6:32586–32601.
2015.PubMed/NCBI
|
|
8
|
Zhou FQ, Qi YM, Xu H, Wang QY, Gao XS and
Guo HG: Expression of EpCAM and Wnt/β-catenin in human colon
cancer. Genet Mol Res. 14:4485–4494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phipps AI, Shi Q, Newcomb PA, Nelson GD,
Sargent DJ, Alberts SR and Limburg PJ: Associations between
cigarette smoking status and colon cancer prognosis among
participants in North central cancer treatment group phase III
trial N0147. J Clin Oncol. 31:2016–2023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo J, Chen J, Deng ZL, Luo X, Song WX,
Sharff KA, Tang N, Haydon RC, Luu HH and He TC: Wnt signaling and
human diseases: What are the therapeutic implications? Lab Invest.
87:97–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bienz M and Clevers H: Linking colorectal
cancer to Wnt signaling. Cell. 103:311–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basu S, Haase G and Ben-Ze'ev A: Wnt
signaling in cancer stem cells and colon cancer metastasis.
F1000Res. 5:pii: F1000 Faculty Rev. –699. 2016. View Article : Google Scholar
|
|
14
|
Kikuchi A: Canonical Wnt signaling pathway
and cellular responses. Clin Calcium. 23:799–807. 2013.(In
Japanese). PubMed/NCBI
|
|
15
|
Kirikoshi H, Sekihara H and Katoh M:
WNT10A and WNT6, clustered in human chromosome 2q35 region with
head-to-tail manner, are strongly coexpressed in SW480 cells.
Biochem Biophys Res Commun. 283:798–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan G, Regel I, Lian F, Friedrich T,
Hitkova I, Hofheinz RD, Ströbel P, Langer R, Keller G, Röcken C, et
al: WNT6 is a novel target gene of caveolin-1 promoting
chemoresistance to epirubicin in human gastric cancer cells.
Oncogene. 32:375–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katoh M: WNT and FGF gene clusters
(review). Int J Oncol. 21:1269–1273. 2002.PubMed/NCBI
|
|
18
|
Lavery DL, Davenport IR, Turnbull YD,
Wheeler GN and Hoppler S: Wnt6 expression in epidermis and
epithelial tissues during Xenopus organogenesis. Dev Dyn.
237:768–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galbraith RL, Poole EM, Duggan D, Muehling
J, Hsu L, Makar K, Xiao L, Potter JD and Ulrich CM: Polymorphisms
in WNT6 and WNT10A and colorectal adenoma risk. Nutr Cancer.
63:558–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Na YJ, Jeon YJ, Suh JH, Kang JS, Yang KH
and Kim HM: Suppression of IL-8 gene expression by radicicol is
mediated through the inhibition of ERK1/2 and p38 signaling and
negative regulation of NF-kappaB and AP-1. Int Immunopharmacol.
1:1877–1887. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T,
Liu Y, Li X, Xiang R and Li N: SOX2 promotes tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
WNT/β-catenin signal network. Cancer Lett. 336:379–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
24
|
Kirikoshi H, Sekihara H and Katoh M:
Up-regulation of WNT10A by tumor necrosis factor alpha and
Helicobacter pylori in gastric cancer. Int J Oncol. 19:533–536.
2001.PubMed/NCBI
|
|
25
|
Mukherjee N, Bhattacharya N, Alam N, Roy
A, Roychoudhury S and Panda CK: Subtype-specific alterations of the
Wnt signaling pathway in breast cancer: Clinical and prognostic
significance. Cancer Sci. 103:210–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guillen-Ahlers H: Wnt signaling in renal
cancer. Curr Drug Targets. 9:591–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen GAJ, Wang M, Farley S, Lee LY, Lee LC
and Sawicki MP: Menin promotes the Wnt signaling pathway in
pancreatic endocrine cells. Mol Cancer Res. 6:1894–1907.
2008.PubMed/NCBI
|
|
28
|
Robinson DR, Zylstra CR and Williams BO:
Wnt signaling and prostate cancer. Curr Drug Targets. 9:571–580.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larue L and Delmas V: The WNT/Beta-catenin
pathway in melanoma. Front Biosci. 11:733–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Modder UI, Oursler MJ, Khosla S and Monroe
DG: Wnt10b activates the Wnt, notch, and NFkB pathways in U2OS
osteosarcoma cells. J Cell Biochem. 112:1392–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan G, Regel I, Lian F, Friedrich T,
Hitkova I, Hofheinz RD, Ströbel P, Langer R, Keller G, Röcken C, et
al: WNT6 is a novel target gene of caveolin-1 promoting
chemoresistance to epirubicin in human gastric cancer cells.
Oncogene. 32:375–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wieczorek M, Paczkowska A, Guzenda P,
Majorek M, Bednarek AK and Lamparska-Przybysz M: Silencing of Wnt-1
by siRNA induces apoptosis of MCF-7 human breast cancer cells.
Cancer Biol Ther. 7:268–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung YS, Jun S, Lee SH, Sharma A and Park
JI: Wnt2 complements Wnt/β-catenin signaling in colorectal cancer.
Oncotarget. 6:37257–37268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, Li
H, Guo Y and Qi D: Wnt2 promotes non-small cell lung cancer
progression by activating WNT/β-catenin pathway. Am J Cancer Res.
5:1032–1046. 2015.PubMed/NCBI
|
|
35
|
Chen H, Wang Y and Xue F: Expression and
the clinical significance of Wnt10a and Wnt10b in endometrial
cancer are associated with the Wnt/β-catenin pathway. Oncol Rep.
29:507–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao LH, Li HT, Lin WQ, Tan HY, Xie L,
Zhong ZJ and Zhou JH: Morphine, a potential antagonist of cisplatin
cytotoxicity, inhibits cisplatin-induced apoptosis and suppression
of tumor growth in nasopharyngeal carcinoma xenografts. Sci Rep.
6:187062016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sugimoto C, Fujieda S, Seki M, Sunaga H,
Fan GK, Tsuzuki H, Borner C, Saito H and Matsukawa S:
Apoptosis-promoting gene (bax) transfer potentiates sensitivity of
squamous cell carcinoma to cisplatin in vitro and in vivo. Int J
Cancer. 82:860–867. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee EH, Chari R, Lam A, Ng RT, Yee J,
English J, Evans KG, Macaulay C, Lam S and Lam WL: Disruption of
the Non-canonical WNT pathway in lung squamous cell carcinoma. Clin
Med Oncol. 2:169–179. 2008.
|
|
40
|
Ramel MC and Lekven AC: Repression of the
vertebrate organizer by Wnt8 is mediated by Vent and Vox.
Development. 131:3991–4000. 2004. View Article : Google Scholar : PubMed/NCBI
|