Introduction
The microRNA (miR)-30 family contains five members
and six mature miRs, namely miR-30a, miR-30b, miR-30c-1, mIR-30c-2,
miR-30d and miR-30e (1). The majority
of previous studies have shown that miR-30 family members act as
tumor suppressors in various types of cancer (2–4). In
addition, miR-30 family members are frequently found to be
downregulated in these types of tumor tissue. Several studies have
revealed that miR-30d and miR-30a are antimetastatic factors in
tumors (5,6), and interfere with the
epithelial-mesenchymal transition (EMT) (7). Certain miR-30 family members have been
identified to abrogate chemoresistance and promote cancer cell
apoptosis (7–12). In a previous study on lung cancer,
Chan et al established the potential role of the miR-30
family in the modulation of the phosphoinositide 3-kinase-seven in
absentia homolog 2 interaction in non-small cell lung cancer
(NSCLC) (13). In other
investigations, miR-30b and miR-30c were found to inhibit sarcoma
viral oncogene homolog, which affected gefitinib-induced apoptosis
and the EMT of NSCLC cells (14).
miR-30a was also found to be negatively associated with the
expression of N-cadherin, a mesenchymal marker (2), and miR-30d was demonstrated to inhibit
tumor growth and invasion in NSCLC (15). However, the possible role of miR-30a
in lung cancer remains to be elucidated. In the present study, it
was found that miR-30a was underexpressed in lung cancer tissue,
compared with that in non-cancerous tissue. Furthermore, the
expression of miR-30a was inversely correlated with the mRNA and
protein expression levels of myocyte enhancer factor 2D (MEF2D) in
these tissue samples. The data showed that miR-30a suppressed lung
cancer cell proliferation via directly targeting the 3′untranslated
region (UTR) of MEF2D mRNA, inducing tumor cell apoptosis.
Materials and methods
Tissues and cell culture
The present study involved the collection of eight
NSCLC specimens with the approval of the Ethical Committee of
Qingdao Municipal Hospital (Qingdao, China) and each patient
provided written informed consent. All procedures performed in
investigations involving human participants were in accordance with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The tumor specimens were homogenized
in tissue homogenization buffer immediately following resection and
stored at −80°C until use. Patient information is available in
Table I.
 | Table I.Patient information. |
Table I.
Patient information.
| No. | Gender | Age | Dates of
collection | Method of
collection |
|---|
| 1 | Male | 42 | 2015.4.5 | Puncture surgery |
| 2 | Male | 44 | 2015.4.12 | Puncture surgery |
| 3 | Male | 57 | 2015.4.19 | Puncture surgery |
| 4 | Male | 60 | 2015.4.22 | Puncture surgery |
| 5 | Male | 38 | 2015.4.25 | Puncture surgery |
| 6 | Male | 51 | 2015.5.30 | Puncture surgery |
| 7 | Male | 59 | 2015.6.4 | Puncture surgery |
| 8 | Male | 65 | 2015.6.5 | Puncture surgery |
The A549 human lung cancer cell line (American Type
Culture Collection, Manassas, VA, USA) was cultured in Dulbecco's
modifed Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific,
Inc.) with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA, Carlsbad, CA, USA) and
penicillin (100 U/ml) RPMI 1640 at 37°C with 5% CO2.
Immunohistochemistry and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The formalin-fixed, paraffin-embedded tissue
sections underwent immunohistochemical staining using the
streptavidin-biotin-peroxidase complex method. For antigen
retrieval, ultrathin sections (5 µm) were heated in 0.5 M Tris-HCl
(pH 9.0), for 1–2 h at 95°C. Rabbit anti-human MEF2D antibody (cat.
no. HPA004807; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
used to detect the expression of MEF2D. Primary antibody was
diluted to 1:500 and incubated at 4°C overnight. The slides were
counterstained using hematoxylin. The images were captured using a
Leica Aperio VERSA system (Leica Microsystems, Inc., Buffalo Grove,
IL, USA), at magnification, ×200. The 3′UTR of MEF2D mRNA was
predicted to be targeted by miR-30a in the TargetScan database
(www.targetscan.org). The processing of
all cell lines and tissue samples for RNA extraction were performed
using an mRNA and miRNA isolation kit [RNeasy Mini Kit (50), cat.
no. 74104; Qiagen China Co., Ltd., Shanghai, China]. According to
the manufacturer's protocol The stem-loop RT-qPCR primers were as
follows: miR-30a, RT primer
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCTGCA-3′, forward
primer 5′-CGACGACTTTCA-GTCGGATGTT-3′ and reverse primer
5′-GTGCAGGGTCCGAGGT-3′; U6, RT and reverse primer
5′-GTGCA-GGGTCCGAGGT-3′ and forward primer, 5′-CTCGCTTCGGCAGCACA-3′
as previously described (2). The
method of quantification was the 2−ΔΔCq method (16).
Transfection and vectors
The A549 cells were plated in 6-well plates
(6×105 cells/well) and 100 nm of miR-30a mimics or
negative control of miR mimics were transfected into the A549 cells
using Lipofectamine™ RNAiMAX transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The above mimics were purchased from Shanghai GenePharma
Co., Ltd. (Shanghai, China).
Cell proliferation assay
At 24 h post-transfection, the medium was replaced
with 100 µl fresh medium, and 20 µl of CellTiter 96 AQueous One
solution (Promega Corporation, Madison, WI, USA; cat. no. G3582).
The plate was incubated at 37°C for 1 h, and 60 µl of medium in
each well was transferred into an ELISA 96-well plate, following
which the absorbance at 490 nm (450–540 nm) was recorded with a
96-well plate reader.
Colony formation assay
In a glass bottle, 4% agar was melted in a microwave
and maintained in a 56°C water bath. The bottom layer was prepared
from 5 ml of 10% FBS DMEM containing 0.75% agar. The top layer was
prepared from 3 ml of 10% FBS DMEM-containing 3×104
cells and 0.36% agar. The dish was cultured in a 37°C incubator for
15 days.
Analysis of apoptosis
The cells were collected and washed twice with PBS.
Each pellet was resuspended in PBS (400 µl). Subsequently, 100 µl
of incubation buffer was added to the cells with 2 µl of Annexin (1
mg/ml) and 2 µl of propidium iodide (PI; 1 mg/ml). The cells were
then subjected to flow cytometry (BD Accuri™ C6; BD Biosciences,
Franklin Lakes, NJ, USA).
Western blot analysis
Western blot analyses were performed as described by
Ma et al (17). Total protein
was extracted and quantified using Beyotime protein systems
(pre-chilled lysis buffer, cat. no. P0013; bicinchoninic acid
assay, cat. no. P0012; Beyotime Institute of Biotechnology, Haimen,
China) according to the manufacturer's protocol. Proteins were
separated using 10% SDS-PAGE. Gels were transferred to
nitrocellulose membranes, which were blocked in 5% fetal bovine
serum (FBS; Sigma-Aldrich; Merck KGaA) in Tris-buffered saline with
Tween-20 for 1 h. The membrane was subsequently incubated with
MEF2D antibody (1:1,000; cat. no. 610774, BD Biosciences) at 4°C,
overnight. The horseradish peroxidase-conjugated secondary antibody
(1:3,000; cat. no. 7074/7076; Cell Signaling Technology, Inc.,
Danvers, MA, USA) was incubated with the membranes at room
temperature for 1 h. Chemiluminescent horseradish peroxidase
substrate was purchased from Merck KGaA (cat. no. WBKLS0500).
Quantification of blots bands was performed using ImageJ software
(version 1.8.0; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
The data obtained for RT-qPCR analysis, cell growth
rate and colony formation are expressed as the mean ± standard
deviation, and were compared at a given time point using one-way
analysis of variance followed by Fisher's LSD test. Analysis was
performed using SPSS software (version 19.0.1; IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
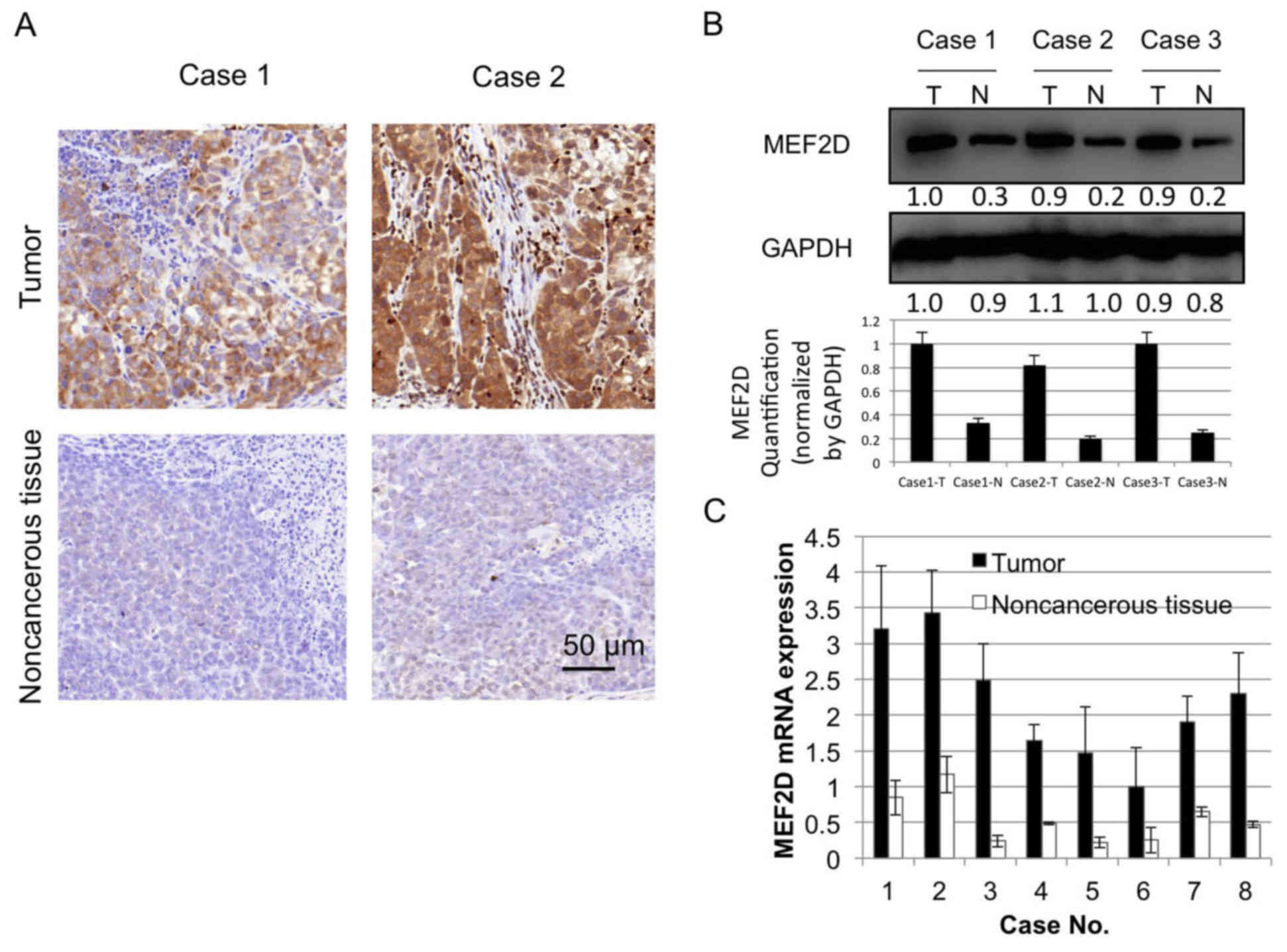
MEF2D is overexpressed in lung
cancer
MEF2D has been identified as an oncogene in liver
cancer (17), however, the expression
level of MEF2D in lung cancer has not to been elucidated. To
investigate whether MEF2D is aberrantly expressed in lung cancer
tissue, immunohistochemical staining for MEF2D was performed in the
present study to evaluate differential staining in cancer tissue,
vs. non-cancerous tissue. Eight cancer tissue samples were stained
for the expression of MEF2D, of which six were strongly positive
and two were moderately positive. By contrast, the paired
non-cancerous tissues were negative or weakly positive (Fig. 1A). The western blot and RT-qPCR
analyses of the protein and mRNA expression levels of MEF2D in
paired tumor and non-cancerous tissues showed similar results
(Fig. 1B and C).
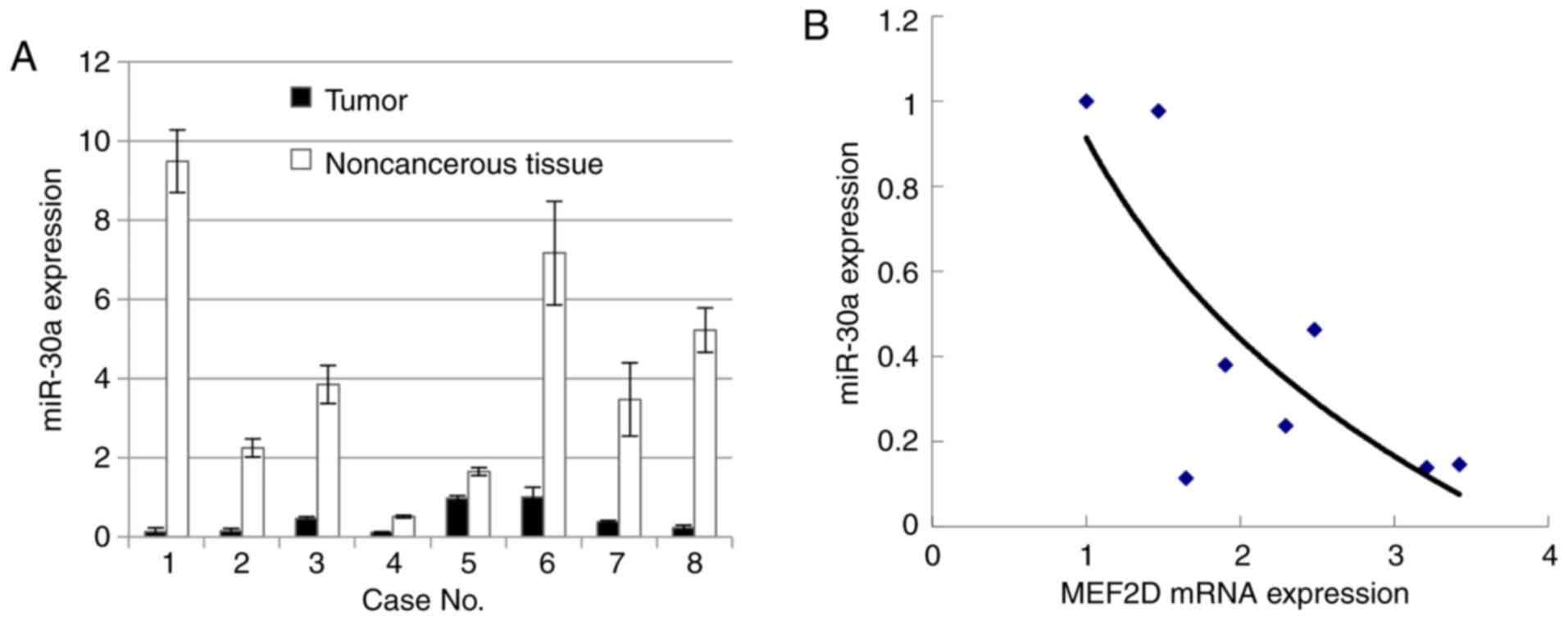
Expression of MEF2D is regulated by
miR-30a in lung cancer
miR-30c was previously reported to be poorly
expressed in lung cancer as a marker of EMT (18). To investigate whether miR-30a is
underexpressed in cancer tissues, vs. non-cancerous tissues,
RT-qPCR analysis was used to evaluate the level of miR-30a in
cancer tissues and the paired non-cancerous tissues. The results
showed that miR-30a was underexpressed in the lung cancer samples,
compared with that in the paired non-cancerous samples.
Subsequently, a correlation curve was established between the mRNA
level of MEF2D and expression level of miR-30a (Fig. 2A). The data showed that the mRNA level
of MEF2D was negatively correlated with that of miR-30a (Fig. 2B). The 3′UTR of MEF2D mRNA was
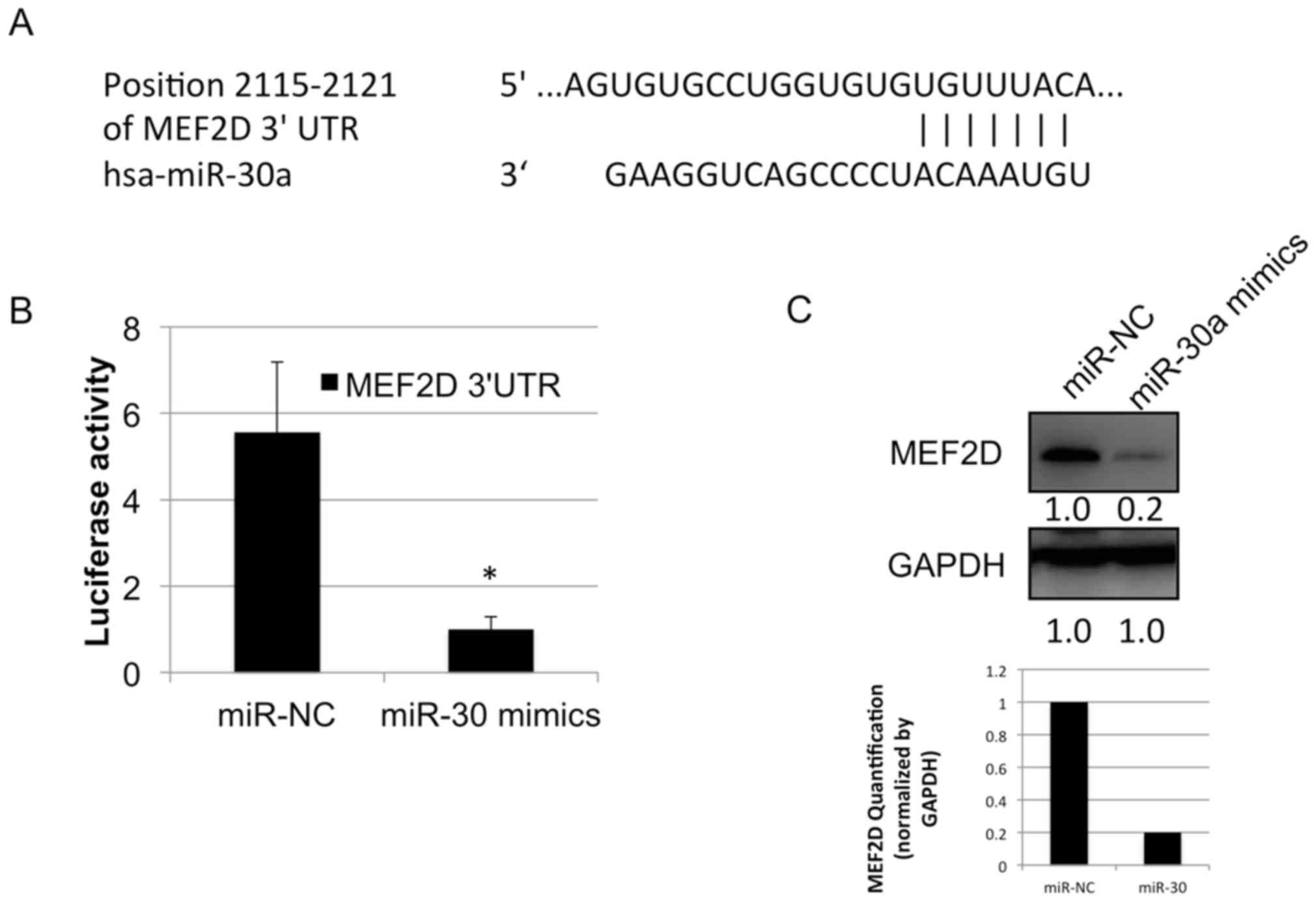
predicted to be targeted by miR-30a in the TargetScan database
(www.targetscan.org) (Fig. 3A). To investigate whether miR-30a
directly targets the MEF2D 3′ UTR, a luciferase reporter vector was
constructed which carried the MEF2D 3′UTR containing the specific
seed sequence of miR-30a. This vector was transfected with or
without miR-30a mimics into the A549 cell line. The results showed
that the luciferase activity was significantly inhibited by
transfection with the miR-30a mimics, compared with that in cells
transfected with the miR-negative control (Fig. 3B). The protein level of MEF2D was
markedly downregulated following the transfection of miR-30a mimics
in the A549 cell line (Fig. 3C).
These data indicated that miR-30a regulated the protein expression
of MEF2D in the human lung cancer cell line.
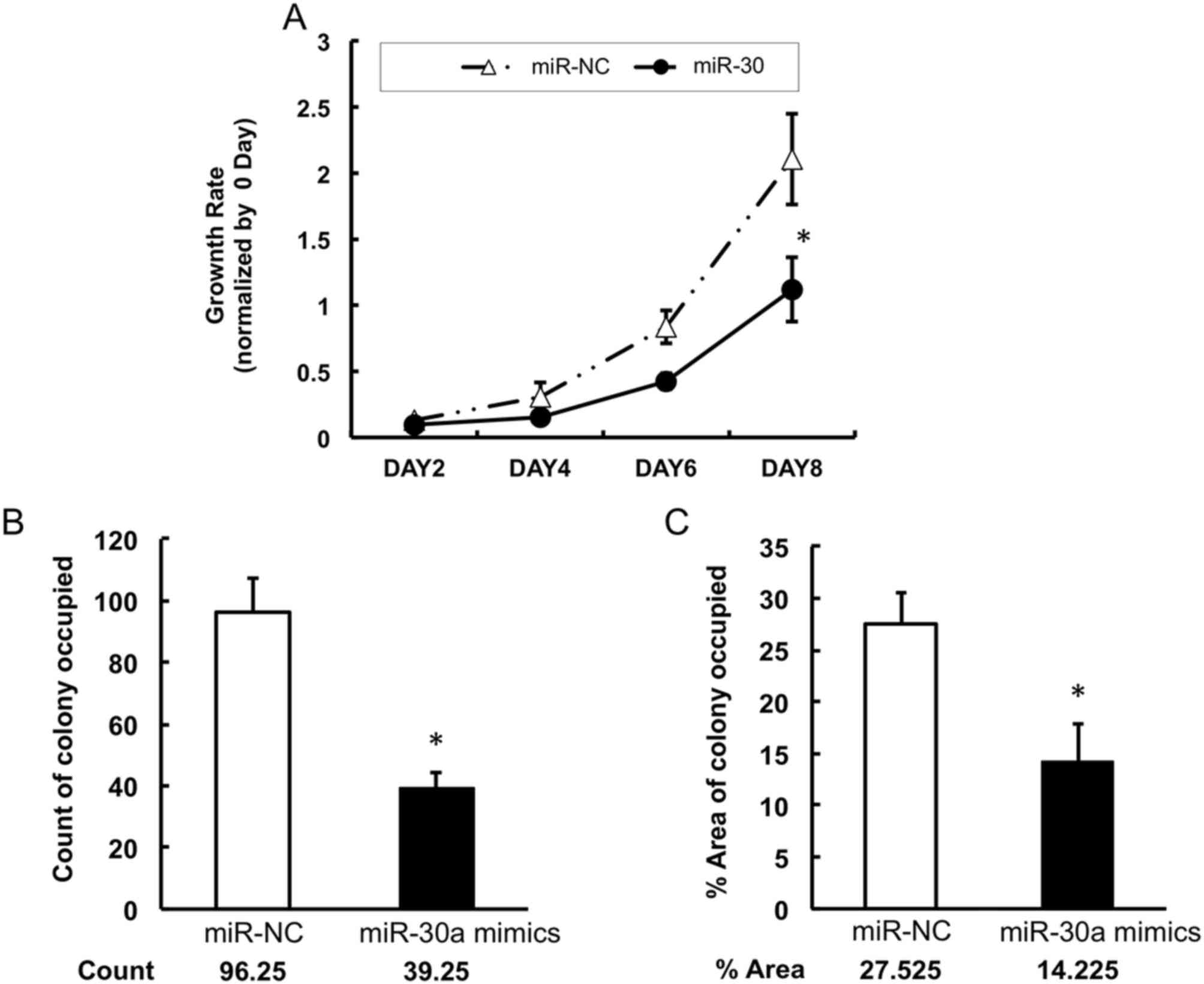
Low expression of miR-30a promotes the
growth of lung cancer cells
To investigate whether miR-30a can inhibit lung
cancer cell growth, the present study measured the proliferation of
A549 cells following transfection with miR-30a mimics or negative
control mimics. It was found that the proliferation of A549 cells
was significantly suppressed by the restoration of miR-30a from day
6, compared with that in the negative control group (Fig. 4A). Similarly, the colony formation
assays showed that the miR-30a-transfected group had fewer colonies
of A549 cells, compared with the control group (Fig. 4B and 4C). To determine the mechanisms
by which miR-30a reduced the proliferation of lung cancer cells,
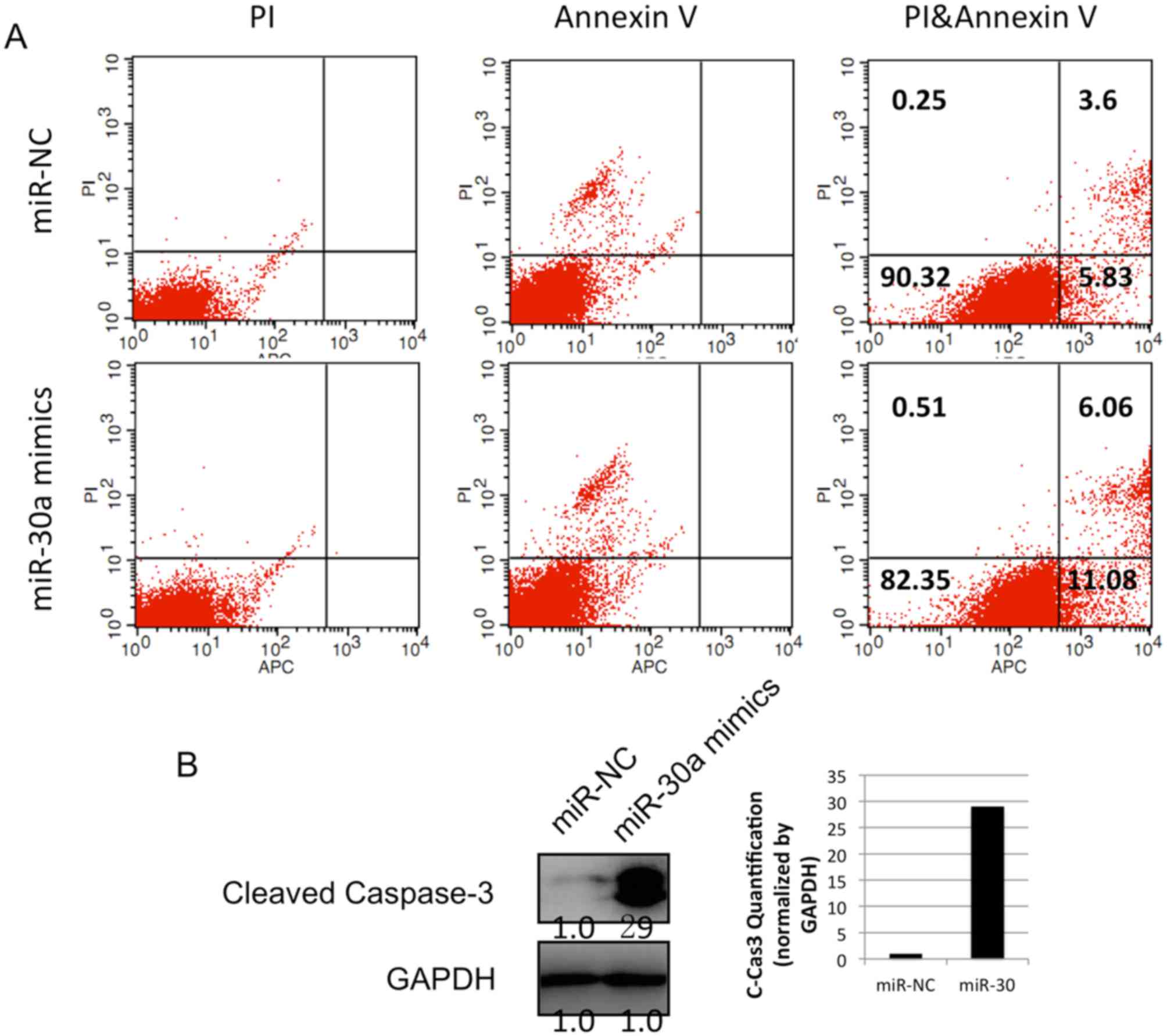
the apoptosis of the A549 cell line was examined using an Annexin
V/PI kit and flow cytometry. The data showed that, following
transfection with miR-30a mimics, the percentage of Annexin
V+/PI− cells in the population was increased
from 5.83 to 11.08% in the lung cancer cells (Fig. 5A). This result revealed that miR-30a
induced the apoptosis of lung cancer cells. In addition, the level
of cleaved caspase 3 was detected using western blot analysis. The
protein level of cleaved caspase 3 was increased following the
infection of miR-30a mimics (Fig.
5B). Together, these data indicated that the overexpression of
miR-30a in lung cancer cells induced cancer cell apoptosis by
stimulating the apoptotic pathway involving caspase 3.
Discussion
MEF2D is a member of a family of four myocyte
enhancer factor transcription factors, (MEF2A, 2B, 2C and 2D), with
an important role in muscle differentiation (19,20). The
transcription factor MEF2D promotes the survival of various types
of cells and functions as an oncogene in liver cancer (17,21,22).
Zhao et al reported that oleanolic acid (OA)
suppressed the proliferation of lung carcinoma cells (23). Furthermore, their data revealed that
OA induced the expression of miR-122. Cyclin G1 and MEF2D, two
putative miR-122 targets, were found to be downregulated by OA
treatment. However, the MEF2D expression profile in lung cancer and
its regulatory mechanism remained to be elucidated.
In the present study, the mRNA and protein levels of
MEF2D were measured in lung cancer using immunohistochemistry,
western blot analysis and RT-qPCR analysis (Fig. 1A-C). The data showed that MEF2D was
overexpressed in the lung cancer tissues, compared with the
non-cancerous tissues. Of note, it was observed that miR-30a was
downregulated in lung cancer samples, compared with control samples
(Fig. 2A). In addition, the levels of
MEF2D mRNA and miR-30a were inversely correlated (Fig. 2B).
To investigate whether miR-30a regulated the
expression of MEF2D in lung cancer, mimics of miR-30a and its
comparative control were synthesized and transfected into A549 lung
cancer cells. The data showed that the increased expression of
miR-30a suppressed the protein level of MEF2D (Fig. 3C). These results led to the
investigation of whether miR-30a regulated MEF2D by directly
targeting its 3′UTR. The present study analyzed miR-30a and MEF2D
mRNA 3′UTR sequences using the online miRNA database, TargetScan.
It was found that the targeted sequence sites in the 3′UTR of MEF2D
by miR-30a were conserved among species, which indicated that these
sites may be functional for physiological processes. In addition, a
luciferase reporter vector was constructed, which carried specific
miR-30a seed sequences within the MEF2D mRNA 3′UTR. The results
showed that the activity of luciferase was markedly inhibited by
transfection with miR-30a mimics in the lung cancer cell line
(Fig. 3B). Therefore, it was
hypothesized that the suppression of MEF2D was responsible, at
least in part, for the antitumor effect of miR-30a. In accordance,
Song et al (24) demonstrated
that the expression of MEF2D is decreased by another miR (miR-218),
in lung carcinoma. One possible explanation is that the same miR
may have different targets and the same mRNA may be targeted by
different miRs in diverse cell types (22,24).
In the present study, the data also showed that the
upregulation of miR-30a inhibited the proliferation of lung cancer
cells by inducing apoptosis. In addition, an increase in the level
of cleaved-caspase 3 was detected, which is a critical executioner
of apoptosis. Taken together, the results indicated that the
upregulation of miR-30a induced the apoptosis of lung cancer
cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NL performed functional assays. YW collected
specimens. XL performed data analyses and wrote the manuscript. All
authors participated in discussions and interpretation of the data
and results.
Ethics approval and consent to
participate
The present study involved the collection of eight
NSCLC specimens with the approval of the Ethical Committee of
Qingdao Municipal Hospital (Qingdao, China) and each patient
provided written informed consent.
Consent for publication
Each patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang TC, Yu D, Lee YS, Wentzel EA, Arking
DE, West KM, Dang CV, Thomas-Tikhonenko A and Mendell JT:
Widespread microRNA repression by Myc contributes to tumorigenesis.
Nat Genet. 40:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Z, Tu K and Liu Q: Effects of
microRNA-30a on migration, invasion and prognosis of hepatocellular
carcinoma. FEBS Lett. 588:3089–3097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sousa JF, Nam KT, Petersen CP, Lee HJ,
Yang HK, Kim WH and Goldenring JR: miR-30-HNF4γ and miR-194-NR2F2
regulatory networks contribute to the upregulation of metaplasia
markers in the stomach. Gut. 65:914–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu K, Guo L, Guo Y, Zhou B, Li T, Yang H,
Yin R and Xi T: AEG-1 3′-untranslated region functions as a ceRNA
in inducing epithelial-mesenchymal transition of human non-small
cell lung cancer by regulating miR-30a activity. Eur J Cell Biol.
94:22–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao J, Liang L, Huang S, Ding J, Tan N,
Zhao Y, Yan M, Ge C, Zhang Z, Chen T, et al: MicroRNA-30d promotes
tumor invasion and metastasis by targeting Galphai2 in
hepatocellular carcinoma. Hepatology. 51:846–856. 2010.PubMed/NCBI
|
|
7
|
Kao CJ, Martiniez A, Shi XB, Yang J, Evans
CP, Dobi A, deVere White RW and Kung HJ: miR-30 as a tumor
suppressor connects EGF/Src signal to ERG and EMT. Oncogene.
33:2495–2503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hershkovitz-Rokah O, Modai S,
Pasmanik-Chor M, Toren A, Shomron N, Raanani P, Shpilberg O and
Granot G: MiR-30e induces apoptosis and sensitizes K562 cells to
imatinib treatment via regulation of the BCR-ABL protein. Cancer
Lett. 356:597–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen
X, Chen X, Zhang CY, Zhang Q and Zen K: MicroRNA-30a sensitizes
tumor cells to cis-platinum via suppressing beclin 1-mediated
autophagy. J Biol Chem. 287:4148–4156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ouzounova M, Vuong T, Ancey PB, Ferrand M,
Durand G, Le-Calvez Kelm F, Croce C, Matar C, Herceg Z and
Hernandez-Vargas H: MicroRNA miR-30 family regulates non-attachment
growth of breast cancer cells. BMC Genomics. 14:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heinzelmann J, Unrein A, Wickmann U,
Baumgart S, Stapf M, Szendroi A, Grimm MO, Gajda MR, Wunderlich H
and Junker K: MicroRNAs with prognostic potential for metastasis in
clear cell renal cell carcinoma: A comparison of primary tumors and
distant metastases. Ann Surg Oncol. 21:1046–1054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fuster O, Llop M, Dolz S, García P, Such
E, Ibáñez M, Luna I, Gómez I, López M, Cervera J, et al: Adverse
prognostic value of MYBL2 overexpression and association with
microRNA-30 family in acute myeloid leukemia patients. Leuk Res.
37:1690–1696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan LW, Wang F, Meng F, Wang L, Wong SC,
Au JS, Yang S and Cho WC: MiR-30 family potentially targeting
PI3K-SIAH2 predicted interaction network represents a novel
putative theranostic panel in non-small cell lung cancer. Front
Genet. 8:82017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen D, Guo W, Qiu Z, Wang Q, Li Y, Liang
L, Liu L, Huang S, Zhao Y and He X: MicroRNA-30d-5p inhibits tumour
cell proliferation and motility by directly targeting CCNE2 in
non-small cell lung cancer. Cancer Lett. 362:208–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Liu J, Liu L, Duan G, Wang Q, Xu Y,
Xia F, Shan J, Shen J, Yang Z, et al: Overexpression of the
transcription factor MEF2D in hepatocellular carcinoma sustains
malignant character by suppressing G2-M transition genes. Cancer
Res. 74:1452–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong Z, Xia Y, Wang P, Liu B and Chen Y:
Low expression of microRNA-30c promotes invasion by inducing
epithelial mesenchymal transition in non-small cell lung cancer.
Mol Med Rep. 10:2575–2579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molkentin JD and Olson EN: Combinatorial
control of muscle development by basic helix-loop-helix and
MADS-box transcription factors. Proc Natl Acad Sci USA.
93:9366–9373. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McKinsey TA, Zhang CL and Olson EN: MEF2:
A calcium-dependent regulator of cell division, differentiation and
death. Trends Biochem Sci. 27:40–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong X, Hong XY, Li T and He CY: Pokemon
and MEF2D co-operationally promote invasion of hepatocellular
carcinoma. Tumour Biol. 36:9885–9893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su L, Luo Y, Yang Z, Yang J, Yao C, Cheng
F, Shan J, Chen J, Li F, Liu L, et al: MEF2D transduces
microenvironment stimuli to ZEB1 to promote epithelial-mesenchymal
transition and metastasis in colorectal cancer. Cancer Res.
76:5054–5067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Liu M and Li D: Oleanolic acid
suppresses the proliferation of lung carcinoma cells by
miR-122/Cyclin G1/MEF2D axis. Mol Cell Biochem. 400:1–7. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song L, Li D, Zhao Y, Gu Y, Zhao D, Li X,
Bai X, Sun Y, Zhang X, Sun H, et al: miR-218 suppressed the growth
of lung carcinoma by reducing MEF2D expression. Tumour Biol.
37:2891–900. 2016. View Article : Google Scholar : PubMed/NCBI
|