Introduction
Colorectal cancer is a malignant tumor with
morbidity ranking as the third highest globally; patients exhibited
a 5-year survival rate of ~50% in 2014 (1). The primary causes of mortality are
cancer metastasis to the liver and the lungs (2). The primary lesion size, differentiation
degree and degree of lymphatic metastasis of the tumor may
influence disease prognosis (2). The
genesis and development of colorectal cancer is a complex process
with multiple stages, including carcinogenic gene activation and
cancer suppressor gene inactivation, causing the tissue to progress
from normal colon mucosa to adenoma and then to adenocarcinoma
(3). In spite of the numerous
different clinical treatment strategies, including surgical
resection, chemotherapy and radiotherapy, treatment-resistance,
relapse and metastasis remain the leading cause of mortality for
patients with splenoma (2,4). Hence, a worthwhile focus for the
prophylaxis and treatment of colorectal cancer is the investigation
of its underlying molecular mechanism, which is of notable
practical significance (5).
Enhancer of zeste 2 polycomb repressive complex 2
subunit (EZH2) is a novel polycomb-group gene identified in
Drosophila melanogaster; in 2000, its chromosomal locus was
determined to be 7q35 in humans (6).
Previous studies revealed that the EZH2 gene participated in
cellular growth regulation (6,7). The
mechanism underlying the function of EZH2 function was the
inhibition of the Wnt signaling pathway in chromatin and the
promotion of cell proliferation (7,8).
Furthermore, as a transcription inhibition factor, EZH2 influenced
the activity of multiple genes at the gene level; most notably, it
inhibited tumor metastasis genes (including phosphoinositide,
protein kinase B and matrix metalloproteinases), aiding the
promotion of infiltration and migration of cancer cells (7,8).
Wnt signaling pathway serves notable functions in
numerous different life events, including biological development,
cell transportation and apoptosis (9). A previous study suggested that the Wnt
pathway has at least three branches, including the canonical Wnt
pathway, termed the Wnt/β-catenin pathway (10). At present, the majority of research is
focused on the Wnt/β-catenin signaling pathway (11). Numerous clinical and experimental
studies confirmed that the abnormal activation of the Wnt/β-catenin
signaling pathway is closely associated with the occurrence and
development of a tumor, including colorectal, breast, lung,
endothelial-like ovarian, prostrate, endometrial, primary liver and
thyroid cancer, and melanoma (11,12).
Methyl jasmonate is a salicylic acid isolated from
jasmine (13–15). Research revealed that methyl jasmonate
could be used to treat skin fungal infection, in addition to
possessing antitumor and anti-angiogenesis effects, which may
inhibit the proliferation of numerous different types of cancer
cells and induce cell apoptosis, including in gastric carcinoma,
lung cancer, colon cancer, cervical cancer and melanoma (13–15). The
mechanism of action of methyl jasmonate may be associated with the
inactivation of free radicals, the activation of cyclin and the
activation of cyclin-dependent kinase (16). Additionally, methyl jasmonate may
influence the tumor protein p53 pathway, caspase activity and
B-cell lymphoma-2-associated X protein signal transduction pathway
closely associated with tumorigenesis (16). In the present study, the anticancer
effect of methyl jasmonate via induction of apoptosis in human
colorectal cancer cells via the downregulation of EZH2 expression
was examined.
Materials and methods
Cell culture
The human bladder cancer T24 cell line was obtained
from the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
(v/v) fetal bovine serum (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a humidified incubator under 5%
CO2. GSK343, an EZH2 inhibitor (1 nM; MedChemExpress
LLC, Monmouth Junction, NJ, USA) and 2.0 mM methyl jasmonate were
added to the cells for 48 h at 37°C.
Cell viability assay and
cytotoxicity
T24 cells were seeded in a 96-well plate at a
density of 1×104 cells per well and cultured with 5 mM
dimethyl sulfoxide (DMSO) or methyl jasmonate (0.5, 0.75, 1.5 and
2.0 mM, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
different periods of time (0, 24, 48 and 72 h). A total of 10 µl
Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) solution was added into every well and incubated
for additional 2 h at 37°C in the dark. Cell viability was detected
at 450 nm using a microplate reader. Lactate dehydrogenase (LDH;
Beyotime Institute of Biotechnology, Jiangsu, China) was added into
every well and incubated for additional 2 h at 37°C in the dark.
Cytotoxicity was detected at 490 nm using a microplate reader.
Quantification of apoptosis rates
T24 cells were seeded in a 96-well plate at a
density of 2×106 cells per well and cultured with DMSO
(5 mM) or methyl jasmonate (0.75, 1.5 and 2.0 mM) for 48 h.
Apoptotic rates were assessed using flow cytometry following
fluorescein isothiocyanate-conjugated annexin V and propidium
iodide staining (BD Pharmingen™ FITC Annexin V; cat. no.
556420; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) at
room temperature for 15 min, according to the manufacturer's
protocol. Apoptosis rates were analyzed using flow cytometry
(FACScan; BD Biosciences) and FlowJo version 7.6.1 (FlowJo LLC,
Ashland, OR, USA).
Quantification of caspase-3
activity
T24 cells were seeded in a 96-well plate at a
density of 1×104 cells per well and cultured with DMSO
(5 mM) or methyl jasmonate (0.75, 1.5 and 2.0 mM) for 48 h.
Caspase-3 activity was analyzed using a Caspase-3 Activity Assay
kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol.
Western blot analysis
T24 cells were seeded in a 96-well plate at a
density of 1×104 cells per well and cultured with DMSO
(5 mM) or methyl jasmonate (0.75, 1.5 and 2.0 mM) for 48 h at 37°C.
T24 cells were collected, and lysed with an RIPA buffer (Promega
Corporation, Madison, WI, USA). Protein concentration was
determined using a BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). A total of 50 µg total protein were separated using 10–12%
SDS-PAGE and were transferred onto polyvinylidene difluoride
membranes. Alternative immunoblot analysis was performed using
anti-EZH2 antibodies (sc-25383; 1:3,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and β-actin antibodies (sc-7210; 1:2,000;
Santa Cruz Biotechnology, Inc.) at 4°C overnight after blocking
with 5% non-fat in TBST for 1 h at 37°C. Immunoreactive bands were
washed with 0.1% Tween TBST for 15 min, visualized by using the
goat anti-rabbit IgG specific horseradish peroxidase-conjugated
secondary antibody (sc-2004, 1:5,000; Santa Cruz Biotechnology,
Inc.) for 1 h at 37°C and an electrochemiluminesence system
(BeyoECL Moon; Beyotime Institute of Biotechnology). Protein
expression was quantified from the band density using Image Lab 3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical
analyses were performed using SPSS version 17.0 (SPSS, Inc.).
One-way analysis of variance followed by Dunnett's test was used to
conduct multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
Methyl jasmonate suppresses the
viability of human colorectal cancer cells
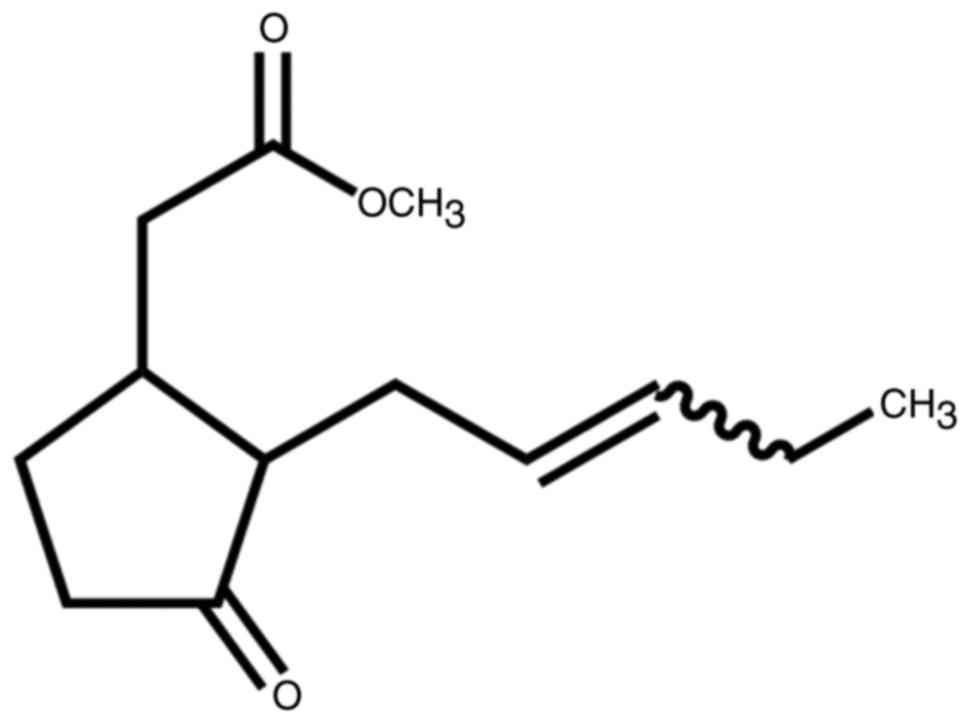
The chemical structure of methyl jasmonate is
presented in Fig. 1. To identify the
effect of methyl jasmonate on the viability of human colorectal
cancer cells, a CCK-8 assay was used to analyze the anticancer
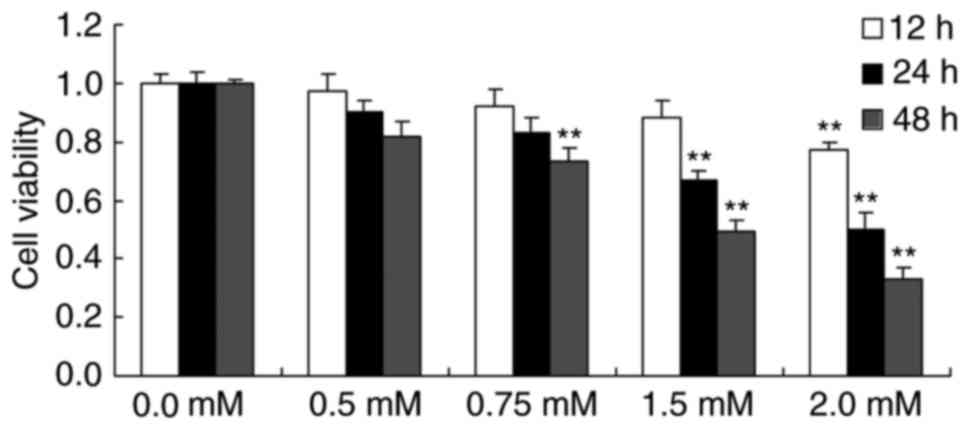
effects. As presented in Fig. 2,
treatment with 0.75, 1.5 or 2.0 mM methyl jasmonate for 48 h, 1.5
or 2.0 mM methyl jasmonate for 24 h and 2.0 mM methyl jasmonate for
24 h significantly decreased the viability of T24 cells compared
with the control group (P<0.01).
Methyl jasmonate induces the
cytotoxicity of human colorectal cancer cells
To confirm that methyl jasmonate induces
cytotoxicity in human colorectal cancer cells, an LDH assay was
performed to measure the effect of methyl jasmonate on the
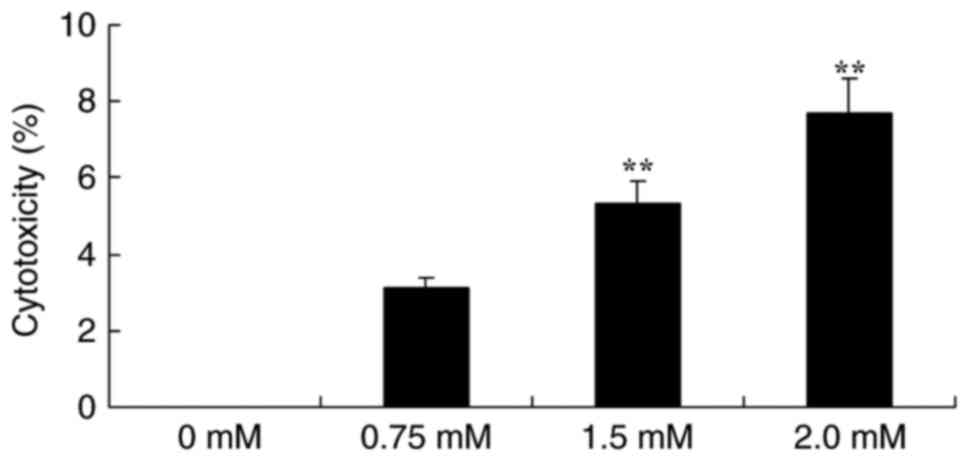
cytotoxicity of T24 cells. As presented in Fig. 3, treatment with 0.75, 1.5 or 2.0 mM
methyl jasmonate induced the cytotoxicity of T24 cells in a
dose-dependent manner for 48 h compared with the control group.
Methyl jasmonate induces the apoptosis
of human colorectal cancer cells
To elucidate the effect of methyl jasmonate on the
induction of apoptosis of human colorectal cancer cells, flow
cytometry was performed to analyze the apoptotic rate of T24 cells.
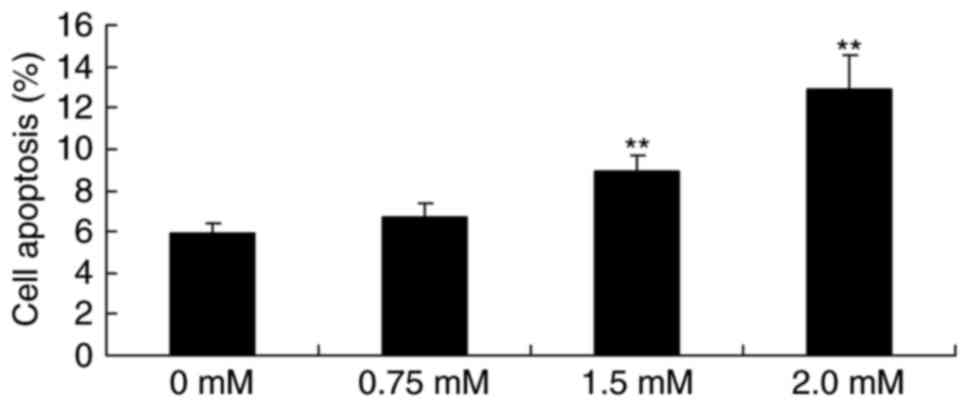
As presented in Fig. 4, treatment
with 1.5 or 2.0 mM methyl jasmonate significantly induced the
apoptosis rate of T24 cell in a dose-dependent manner compared with
the control cells (P<0.01).
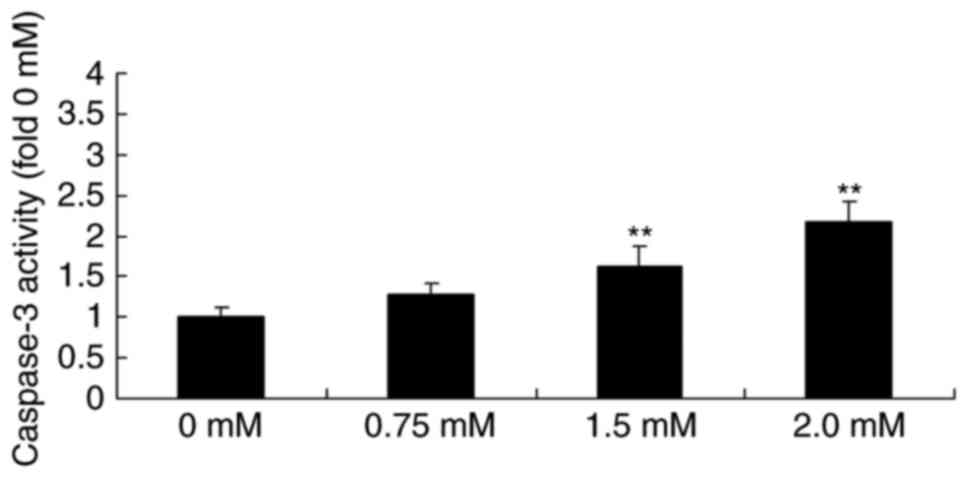
Methyl jasmonate induces caspase-3
activity in human colorectal cancer cells
Next, the effect of methyl jasmonate on the
caspase-3 activity of T24 cells was investigated. As presented in
Fig. 5, treatment with 1.5 or 2.0 mM
methyl jasmonate significantly induced the caspase-3 activity of
T24 cell in a dose-dependent manner compared with the control cells
(P<0.01).
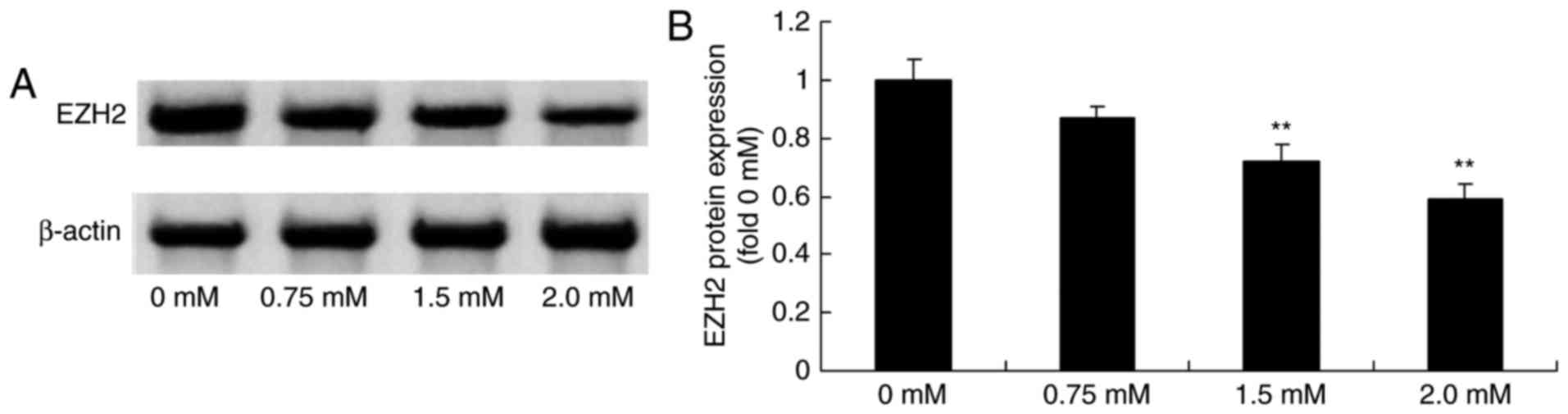
Methyl jasmonate downregulates EZH2
expression levels in human colorectal cancer cells
Next, to test the anticancer effect of methyl
jasmonate on EZH2 expression in human colorectal cancer cells, the
EZH2 protein expression levels in T24 cells was detected using a
western blot analysis. As presented in Fig. 6, treatment with 1.5 or 2.0 mM methyl
jasmonate significantly downregulated EZH2 protein expression in
T24 cell in a dose-dependent manner compared with the control cells
(P<0.01).
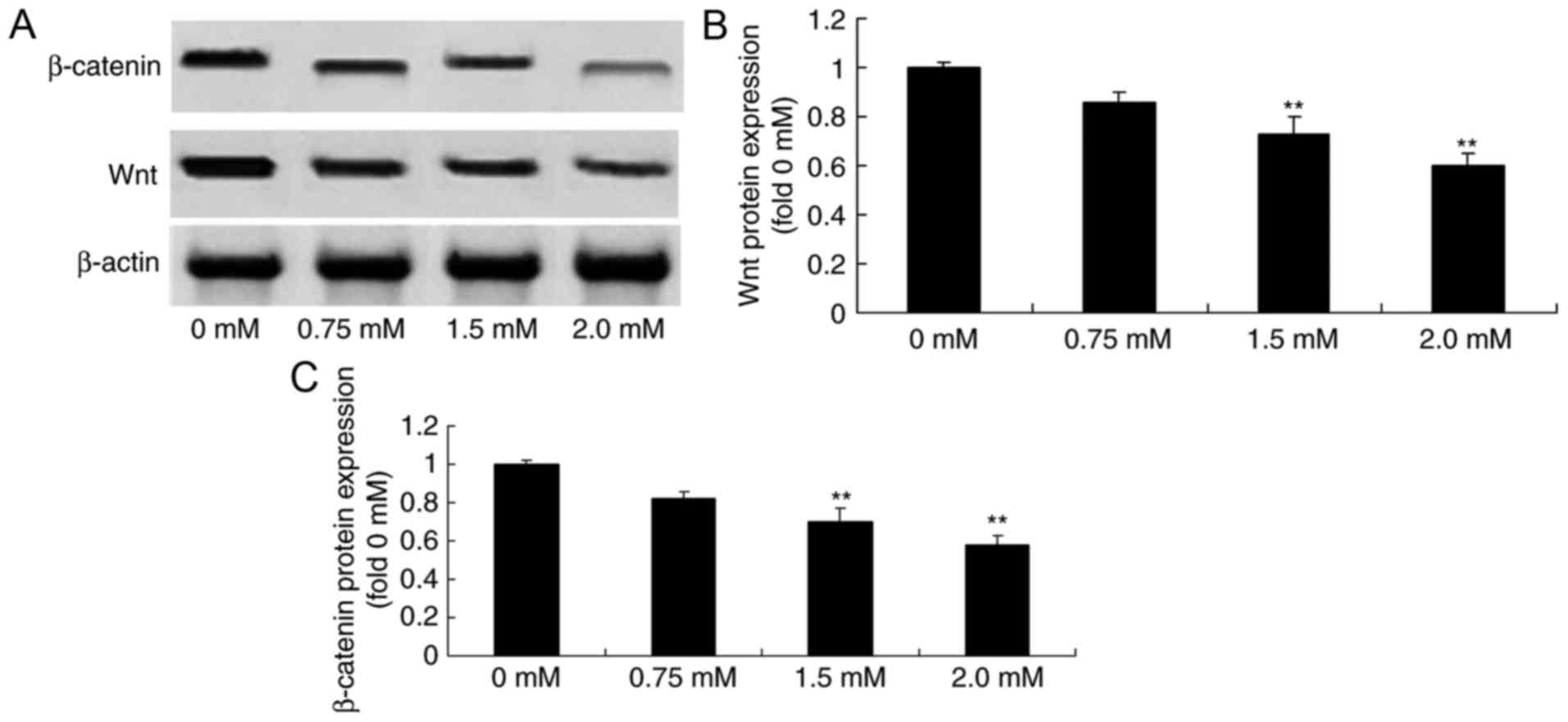
Methyl jasmonate downregulates
Wnt/β-catenin protein expression levels in human colorectal cancer
cells
To determine whether methyl jasmonate affected Wnt
and β-catenin expression in human colorectal cancer cells, the
expression levels of Wnt and β-catenin of T24 cell were detected
using western blot analysis. The results of this analysis revealed
that Wnt and β-catenin protein expression levels were significantly
suppressed by 1.5 or 2.0 mM methyl jasmonate in T24 cells in a
dose-dependent manner compared with the control group (P<0.01;
Fig. 7).
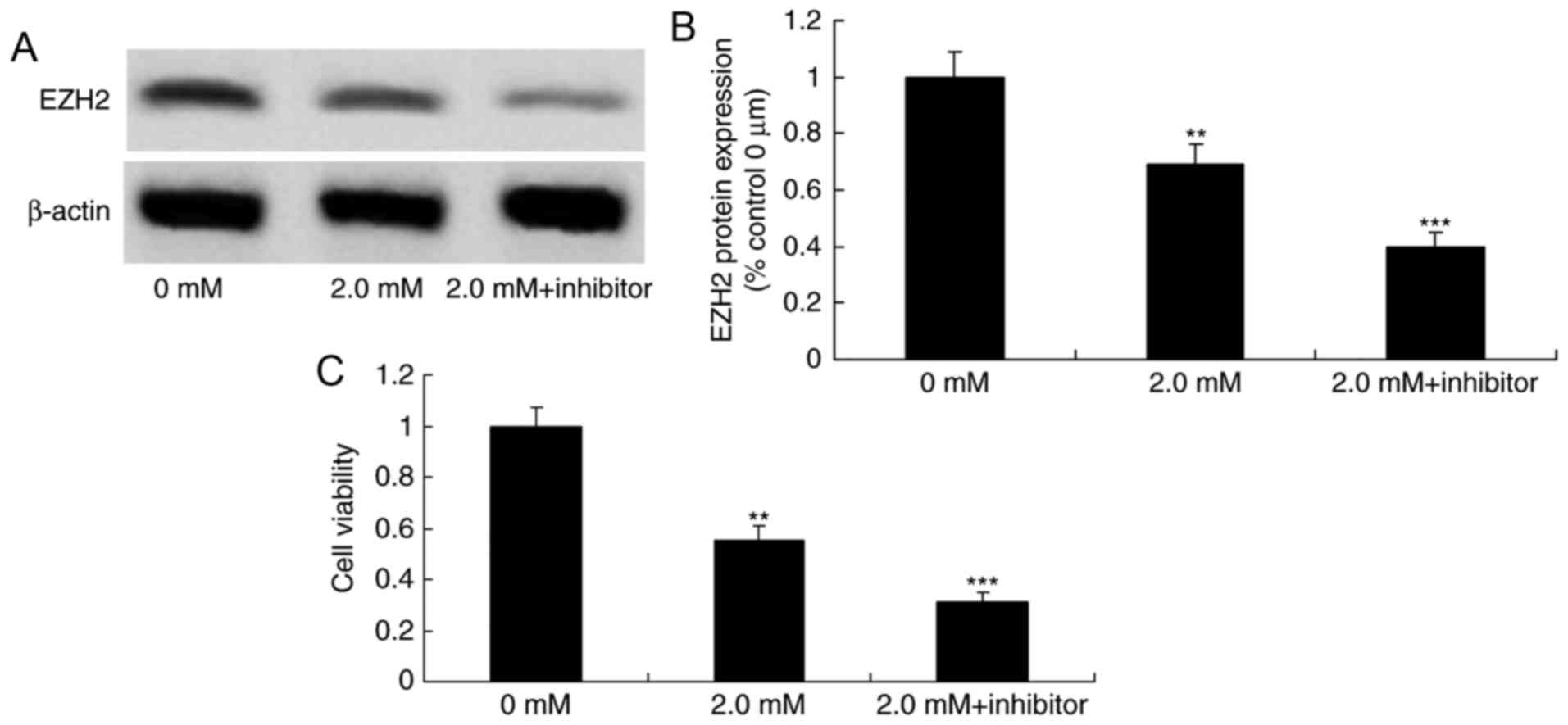
Downregulation of the expression of
EZH2 enhances the anticancer effect of methyl jasmonate on human
colorectal cancer cells
To elucidate the mechanism by which apoptosis was
induced when cells were treated with methyl jasmonate, the
viability of methyl jasmonate-treated T24 cells following the
downregulation of the expression of EZH2 was examined. GSK343, an
EZH2 inhibitor, significantly suppressed EZH2 protein expression
levels in T24 cells compared with cells treated with methyl
jasmonate alone (P<0.01) and significantly inhibited the cell
viability of T24 cells treated with 2.0 mM methyl jasmonate,
compared with the group treated with methyl jasmonate alone
(P<0.01; Fig. 8).
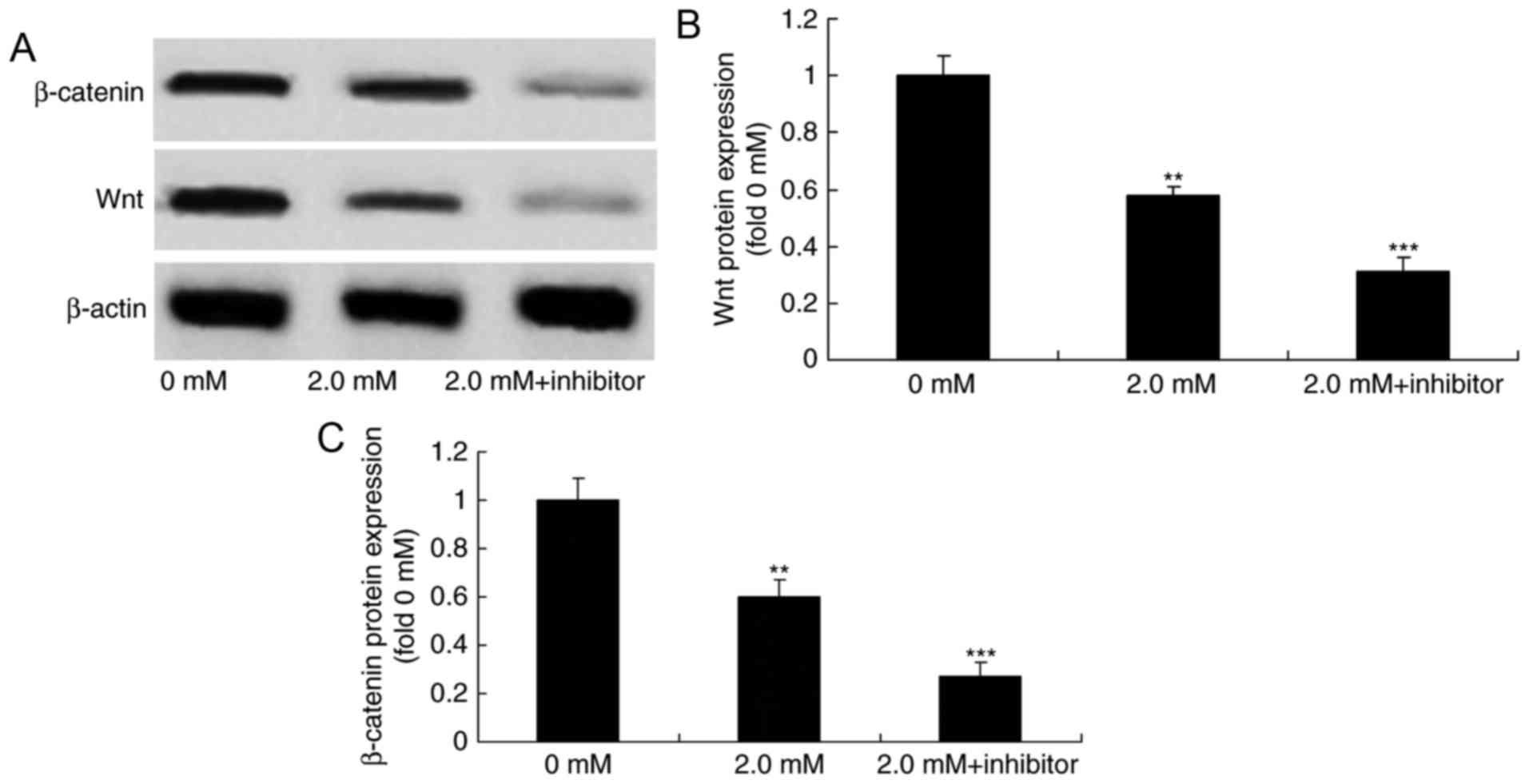
Downregulation of the expression of
EZH2 enhances the anticancer effect of methyl jasmonate on human
colorectal cancer cells through the suppression of Wnt/β-catenin
pathway
Next, the effect of the downregulation of the
expression of EZH2 on Wnt and β-catenin expression was examined
using western blot analysis. Downregulation of the expression of
EZH2 significantly suppressed Wnt and β-catenin protein expression
in T24 cells treated with 2.0 mM methyl jasmonate, compared with
the group treated with methyl jasmonate alone (P<0.01; Fig. 9).
Discussion
In recent years in China, colorectal cancer rates
have been gradually increasing, with this increase primarily
occurring in large and medium-sized cities (17). According to incomplete statistics, in
big cities such as Beijing and Shanghai, the incidence rate by year
of colorectal cancer has reached or exceeded the average level in
developed Western countries (18).
The morbidity rate of colorectal cancer is gradually rising and its
malignant biological behavior is closely associated with a small
group of tumor stem cells (19).
Early-stage diagnosis of colorectal cancer can be missed and in
later stages, distant metastasis may occur, together resulting in a
poor patient prognosis (20).
Therefore, investigations into the molecular mechanism of the
occurrence and development of colorectal cancer can aid the
prophylaxis and treatment of colorectal tumor types. In the present
study, methyl jasmonate significantly suppressed cell growth,
inducing cytotoxicity, apoptosis and caspase-3 activity in T24
cells. Zheng et al (13)
previously reported that methyl jasmonate abolished the migration,
invasion and angiogenesis of gastric cancer cells.
To date, the most insurmountable problems in cancer
treatment are the invasion and metastasis of tumors, which results
in the mortality of the majority of patients with cancer and is
primarily promoted by the migration of tumor cells (21). EZH2 is a transcription inhibition
factor that inhibits the transcription of multiple cancer
suppressor genes and gives rise to the enhancement of invasion and
metastasis; it breaks the balance between the promotion and the
inhibition of associated genes by migration, which results in the
invasion and metastasis of tumors (22). Abnormally high expression of EZH2 may
promote cell proliferation: EZH2 gene expression is markedly
increased in multiple tumor types including in the prostate,
breast, kidney and lung (8).
Therefore, it can be concluded that EZH2 is an oncogene. The
present study revealed that methyl jasmonate significantly
downregulated EZH2 protein expression in T24 cells in a
dose-dependent manner. Wang et al (23) previously reported that methyl
jasmonate sensitizes gambogic acid-induced apoptosis of human
bladder cancer cells through the downregulation of EZH2 expression
by microRNA-101.
The Wnt/β-catenin signaling pathway is highly
evolutionarily conserved and controls numerous different events,
including human embryonic development, cell fate, tissue and organ
morphogenesis, in addition to tumorigenesis (24). Wnt/β-catenin signaling is involved in
the development of the central nervous system, reproductive tract,
breast, kidney, limbs, placenta, hair and bone (25). The expression imbalance of
Wnt/β-catenin pathway constituents may result in embryonic death or
abnormal embryonic development (25).
There is a close association between the Wnt/β-catenin signaling
pathway and tumor development (26).
The abnormal activation of the Wnt/β-catenin signaling pathway has
been identified in cancer of the breast, liver, stomach, thyroid,
lung, prostrate, skin and other malignant tumor types (27). In the present study, it was revealed
that the anticancer effect of methyl jasmonate downregulates
Wnt/β-catenin expression in human colorectal cancer cells. Raviv
et al (28) previously
reported that methyl jasmonate downregulated survivin expression
and sensitized colon carcinoma cells through the β-catenin
pathway.
The present study, to the best of our knowledge for
the first time, revealed that the anticancer effect of methyl
jasmonate induced apoptosis in human colorectal cancer cells,
mediated through the EZH2/Wnt/β-catenin pathway. The findings in
the present study suggest that methyl jasmonate may be a potential
novel drug for the clinical treatment of human colorectal
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TTL designed the experiment, analyzed the data and
wrote the manuscript. YW, LF, CGC, YKW and TTL performed the
experiments.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kwakman R, Schrama AM, van Olmen JP, Otten
RH, de Lange-de Klerk ES, de Cuba EM, Kazemier G and Te Velde EA:
Clinicopathological parameters in patient selection for
cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
for colorectal cancer metastases: A meta-analysis. Ann Surg.
263:1102–1111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krebs B, Horvat M, Golle A, Krznaric Z,
Papeš D, Augustin G, Arslani N and Potrč S: A randomized clinical
trial of synbiotic treatment before colorectal cancer surgery. Am
Surg. 79:E340–E342. 2013.PubMed/NCBI
|
|
3
|
Scorsetti M, Comito T, Tozzi A, Navarria
P, Fogliata A, Clerici E, Mancosu P, Reggiori G, Rimassa L,
Torzilli G, et al: Final results of a phase II trial for
stereotactic body radiation therapy for patients with inoperable
liver metastases from colorectal cancer. J Cancer Res Clin Oncol.
141:543–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng G, Wang H, Zhang X, Yang Y, Wang L,
Du L, Li W, Li J, Qu A, Liu Y and Wang C: Identification and
validation of reference genes for qPCR detection of serum microRNAs
in colorectal adenocarcinoma patients. PLoS One. 8:e830252013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aranda E, Aparicio J, Alonso V,
Garcia-Albeniz X, Garcia-Alfonso P, Salazar R, Valladares M, Vera
R, Vieitez JM and Garcia-Carbonero R: SEOM clinical guidelines for
diagnosis and treatment of metastatic colorectal cancer 2015. Clin
Transl Oncol. 17:972–981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei FZ, Cao Z, Wang X, Wang H, Cai MY, Li
T, Hattori N, Wang D, Du Y, Song B, et al: Epigenetic regulation of
autophagy by the methyltransferase EZH2 through an MTOR-dependent
pathway. Autophagy. 11:2309–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Wang F, Zong G, Liu R, Zhang Y,
Luan Y, Xu L and Xuan J: Prognostic significance of EZH2 expression
in patients with digestive cancers: A meta-analysis. Int J Clin Exp
Med. 8:16043–16049. 2015.PubMed/NCBI
|
|
8
|
Liu YL, Gao X, Jiang Y, Zhang G, Sun ZC,
Cui BB and Yang YM: Expression and clinicopathological significance
of EED, SUZ12 and EZH2 mRNA in colorectal cancer. J Cancer Res Clin
Oncol. 141:661–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scholer-Dahirel A and McLaughlin ME:
Determinants of Wnt/β-catenin pathway dependency in colorectal
cancer. Cell Cycle. 11:9–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Q, Shen S, Liao M, Lian P and Wang X:
NGX6 inhibits cell invasion and adhesion through suppression of
Wnt/beta-catenin signal pathway in colon cancer. Acta Biochim
Biophys Sin (Shanghai). 42:450–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao C, Chen G, Kuan SF, Zhang DH,
Schlaepfer DD and Hu J: FAK/PYK2 promotes the Wnt/β-catenin pathway
and intestinal tumorigenesis by phosphorylating GSK3β. Elife.
4:2015. View Article : Google Scholar
|
|
12
|
Yu Q, Shang LU, Yu H, Yang Z and Xu D:
Silencing of FRAT1 by siRNA inhibits the proliferation of SGC7901
human gastric adenocarcinoma cells. Biomed Rep. 4:223–226. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng L, Li D, Xiang X, Tong L, Qi M, Pu
J, Huang K and Tong Q: Methyl jasmonate abolishes the migration,
invasion and angiogenesis of gastric cancer cells through
down-regulation of matrix metalloproteinase 14. BMC Cancer.
13:742013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milrot E, Jackman A, Kniazhanski T, Gonen
P, Flescher E and Sherman L: Methyl jasmonate reduces the survival
of cervical cancer cells and downregulates HPV E6 and E7, and
survivin. Cancer Lett. 319:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen S and Flescher E: Methyl jasmonate:
A plant stress hormone as an anti-cancer drug. Phytochemistry.
70:1600–1609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cesari IM, Carvalho E, Figueiredo
Rodrigues M, Mendonça Bdos S, Amôedo ND and Rumjanek FD: Methyl
jasmonate: Putative mechanisms of action on cancer cells cycle,
metabolism, and apoptosis. Int J Cell Biol. 2014:5720972014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sofocleous CT, Garcia AR, Pandit-Taskar N,
Do KG, Brody LA, Petre EN, Capanu M, Longing AP, Chou JF,
Carrasquillo JA and Kemeny NE: Phase I trial of selective internal
radiation therapy for chemorefractory colorectal cancer liver
metastases progressing after hepatic arterial pump and systemic
chemotherapy. Clin Colorectal Cancer. 13:27–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allegra CJ, Yothers G, O'Connell MJ, Beart
RW, Wozniak TF, Pitot HC, Shields AF, Landry JC, Ryan DP, Arora A,
et al: Neoadjuvant 5-FU or capecitabine plus radiation with or
without oxaliplatin in rectal cancer patients: A phase III
randomized clinical trial. J Natl Cancer Inst. 107(pii):
djv2482015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi T, Masaki T, Kogawa K, Matsuoka
H and Sugiyama M: Efficacy of gum chewing on bowel movement after
open colectomy for left-sided colorectal cancer: A randomized
clinical trial. Dis Colon Rectum. 58:1058–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du M, Liu S, Gu D, Wang Q, Zhu L, Kang M,
Shi D, Chu H, Tong N, Chen J, et al: Clinical potential role of
circulating microRNAs in early diagnosis of colorectal cancer
patients. Carcinogenesis. 35:2723–2730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao F, Chang VT, Cleeland C, Cleary JF,
Mitchell EP, Wagner LI and Fisch MJ: Determinants of pain severity
changes in ambulatory patients with cancer: An analysis from
Eastern Cooperative Oncology Group trial E2Z02. J Clin Oncol.
32:312–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song-Bing H, Hao Z, Jian Z, Guo-Qiang Z,
Tuo H, Dai-Wei W, Wen G, Lin G, Yi Z, Xiao-Feng X, et al:
Inhibition of EZH2 expression is associated with the proliferation,
apoptosis and migration of SW620 colorectal cancer cells in vitro.
Exp Biol Med (Maywood). 240:546–555. 2015.PubMed/NCBI
|
|
23
|
Wang Y, Xiang W, Wang M, Huang T, Xiao X,
Wang L, Tao D, Dong L, Zeng F and Jiang G: Methyl jasmonate
sensitizes human bladder cancer cells to gambogic acid-induced
apoptosis through down-regulation of EZH2 expression by miR-101. Br
J Pharmacol. 171:618–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Wang X, Jia T, Wei W, Chua MS and So
S: Tankyrase inhibitors attenuate WNT/β-catenin signaling and
inhibit growth of hepatocellular carcinoma cells. Oncotarget.
6:25390–25401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sebio A, Kahn M and Lenz HJ: The potential
of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther
Targets. 18:611–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Serafino A, Moroni N, Zonfrillo M,
Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A,
Rasi G and Pierimarchi P: WNT-pathway components as predictive
markers useful for diagnosis, prevention and therapy in
inflammatory bowel disease and sporadic colorectal cancer.
Oncotarget. 5:978–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan BL, Norhaizan ME, Huynh K, Heshu SR,
Yeap SK, Hazilawati H and Roselina K: Water extract of brewers'
rice induces apoptosis in human colorectal cancer cells via
activation of caspase-3 and caspase-8 and downregulates the
Wnt/β-catenin downstream signaling pathway in brewers' rice-treated
rats with azoxymethane-induced colon carcinogenesis. BMC Complement
Altern Med. 15:2052015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raviv Z, Zilberberg A, Cohen S,
Reischer-Pelech D, Horrix C, Berger MR, Rosin-Arbesfeld R and
Flescher E: Methyl jasmonate down-regulates survivin expression and
sensitizes colon carcinoma cells towards TRAIL-induced
cytotoxicity. Br J Pharmacol. 164:1433–1444. 2011. View Article : Google Scholar : PubMed/NCBI
|