Introduction
The ABO blood group polymorphism has been associated
with different diseases, cancer included (1). The cancer developments are variegated
processes associated with aging. Protection from cancer and
atherosclerosis is the main longevity reason. Long-survivors are an
important group for the evaluation of genetic markers in cancer
pathogenesis (2).
The ‘histo-blood system’ was first proposed for the
ABO system characterized by the presence or absence of the
carbohydrate antigens A and B on the erythrocyte membrane and blood
plasma regular antibodies (anti-A, anti-B) (3). The A and B alleles encode a specific
glycosyl-transferring enzyme. The blood groups are characterized by
the presence of carbohydrate sugars on the red blood cell surface:
N-acetylgalactosamine for the A antigen and D-galactose for the B
antigen, both for the blood type AB and neither for the phenotype
O. The A, B and AB-related carbohydrate sugars are situated on the
H antigen, and the unmodified H antigen defines the blood group O
(4). The A and B blood groups differ
mainly by four amino acid substitutions (R176G, G235S, L266M, and
G268A) from four common variants (rs7853989:C>G,
rs8176743:G>A, rs8176746:C>A, and rs8176747:G>C), while
the blood group O is characterized by a single nucleotide deletion
(rs8176719(−;G)) which results in early protein termination
(5,6).
Population studies have demonstrated that the ABO
group phenotypes frequencies vary widely from one ethnicity to
another: The O blood group is common in South American and Africans
natives, A and B being common in Asian and North European
countries, respectively, while AB is more frequent in Korea, China
and Japan (7).
The ABO (ABO, 9q34.1) genes control the
expression of part of the carbohydrates by the epithelial cells in
the respiratory, genitourinary and gastrointestinal systems; the
carbohydrate variability acts as a potential receptor for
non-pathogenic and pathogenic microorganisms influencing immune
responses (8,9).
The first report on the relation between the ABO
blood group and cancer was published by England and Scotland
researchers. A study of the frequency of the ABO blood groups in
patients with stomack cancer indicated the A blood group to
increase the risk, while the O blood type was protective (10). Thereafter, the correlation between the
ABO blood type and other malignancies, such as gastric (11) and pancreatic (12–15), has
been continuously reported. A total of 1.6 million healthy blood
donors were followed in Denmark and Sweden: The A, B and AB blood
groups were associated either with the increased or decreased risk
of cancer at 13 anatomical sites as compared with the O blood group
(16). Multiple mechanisms have been
indicated to explain the blood type role in cancer progression,
including altered immune response, inflammation and cellular
adhesion (6,17–19).
The role of the specific genetic differences
contributing to life expectancy is hardly known. Several genome
sequencing and GWAS studies compared the total number of disease
variants in centenarians and controls, indicating that there are
some evidences that centenarians harbor the anti-aging
polymorphisms which protect them from diseases, although
long-survivors may show numerous disease variants at a rate similar
to normal people, but they are protected from their effects
(20,21). The SNP (rs8176719) defining the most
common allele responsible for the O blood group is related with
longevity; the centenarians are more likely than controls to have
the O blood group. In the GWAS analysis, this SNP has shown that
the O blood group is associated with numerous protective effects
(22). Genetic association studies
identified that the polymorphism located near APOE was found to be
consistently associated with extreme longevity (23). The longevity genetic markers in the
Lithuanian population were found to be the low levels of
apolipoprotein B (apoB) and the apoE and the low apoB/apoA-1 ratio
(24,25). The Gc1 allele of the vitamin D-binding
protein demonstrated a marked decrease in the oldest-old cohort,
and the Gc2 allele frequency is expressed in the opposite way in
the Lithuanian population (26).
Long-survivors as the cohort are important for
evaluating the role of genetic determinants in cancerogenesis and
the related mortality. The further change in mortality patterns
will increase success in reducing the number of cancer-related
mortalities (27). In our study, we
evaluated the ABO genetic variation in prostate, renal cell and
bladder cancer patients, because the frequency of kidney and
prostate cancer and mortality from these cancer types in the
Lithuanian population is the higest in Europe (28,29). The
second task was to determine if the ABO blood type could be one of
the multifactorial determinants in relation with the longevity as
the resistance to cancer. The cluster analysis of the ABO blood
group system in the Lithuanian population was selected to compare
the structure of the analyzed data, their mathematical similarity
and to form groups (clusters) in the population of Lithuania
comparing the groups of prostate, kidney, bladder cancer patients
with the healthy blood donors, long-survivors. The cluster method
helps to estimate differences (similarities) and genetic distances
among the groups of study subjects by showing the cluster hierarchy
and their interconnectedness regarding the ABO group frequency. In
the literature review, we included the ABO relationship with
cancer, whereas resistance to cancerogenesis is an additional
important determinant for longevity.
Materials and methods
The patient groups studied
The ABO blood group system has been identified in
the following individual groups of the Lithuanian population:
Control group (blood donors), long-lived individuals, individuals
suffering from the prostate, bladder, kidney cancer. The study
groups contained only the Caucasian population. The ethical
approval of the study was obtained from Kaunas Regional Biomedical
Research Ethics Committee (Kaunas, Lithuania; protocol no. BE-2-10;
2017); written informed consent was obtained from the patients. The
blood donors group consisted of 994 individuals (aged 18 to 64
years) randomly selected from the National Blood Center.
Individuals suffering from the urogenital system cancer were
treated from 2005 to 2015 in the Department of Oncourology of the
National Cancer Institute. Information about patients was collected
from the information system database of the Department of
Oncourology. As a control we also used data on the ABO blood group
distribution from the oldest-old cohort individuals. As a function
of age, the SNP allele frequency may not change in a monotonic
fashion (30); alleles for some SNPs
show a pattern such that 80 years old individuals have lower levels
of alleles frequency, but young and very old people have similarly
high allele frequencies (22). From
twin studies, the survival heritability to the late eighties is
estimated to be approximately 25–30% (31). In this case, our choice of the
oldest-old cohort was accepted to be ≥80 years as long-survivors
are an important group for the evaluation of genetic markers in the
tumorigenesis.
Data of the following groups of cancer patients are
analyzed: Prostate cancer (n=2,200, men aged 40 to 93 years);
bladder cancer (n=1,530): Men (n=1,201, aged 21 to 98 years), women
(n=329, aged 27 to 93 years); renal cell cancer (n=2,650): Men
(n=1,553, aged 19 to 89 years), women (n=1,097, aged 19 to 90
years). The data criteria used for the selection of patients were
as follows: Gender, blood group, age, date of the first diagnosis,
disease codes (according to the International Classification of
Diseases (ICD-10)). The group of oldest-old individuals consisted
of 166 persons: Men aged 82 to 102 years (n=54) and women
aged 81 to 103 years (n=112). For 48 long-lived individuals,
the ABO blood group system phenotype was studied in patients
undergoing cataract surgery (this subgroup was collected in the Eye
Clinic of Kaunas Clinics of the Lithuanian University of Health
Sciences Hospital), and others were collected as out-patients; the
oldest-old individuals’ selection criteria were age ≥80 years and
history of the disease with no data on cancer.
There were no significant differences in ABO blood
types between females and males in the blood donors and oldest-old
control groups, so the pooled group was used.
The phenotypes of ABO blood groups were identified
using the seraclones anti-A, B (Bio-Rad Medical Diagnostics GmbH,
Dreieich, Germany) antibodies.
Statistical analysis
Statistical analysis was performed using the SPSS
21.0 (IBM Corp., Armonk, NY, USA) and R project 3.4.0 (cran.r-project.org) software package. Categorical data
are expressed as frequencies and percentages. The Chi-square test
was used to compare the ABO blood group phenotype frequencies among
the studied groups. The normal approximation method was used to
calculate the odds ratio (OR) with the 95% confidence interval (CI)
to measure the association of ABO blood group phenotypes with
prostate, renal cell and bladder cancer in comparison with blood
donors and long-survived in the population. In the study, the OR
was used to compare the relative odds of the cancer patients having
a different ABO blood group. The risk of disease as the OR was
calculated as follows: OR=[(n of patients with blood group X
× n of control with not blood group X)/(n of patients
with not blood group X × n of patients with blood group
X)].
Also, the OR has been calculated with the aim to
find out how the ABO blood groups are associated with the
likelihood of reaching the oldest-old age (≥80 years) in the cancer
patients groups. This was calculated as follows: OR=[(n of
oldest-olds with blood group X × n of control with not blood
group X)/(n of oldest-olds with not blood group X × n
of control with blood group X)].
P<0.05 was considered to indicate a statistically
significant difference.
Hierarchical clustering was used to evaluate the
structure of the ABO blood group system data among the analyzed
groups, their mathematical similarities and to form groups
(clusters). In order to decide where a cluster should be split and
to measure dissimilarity among the study groups the Chord metric
was used. Cluster analysis was accomplished using the PAST
3.15 software package (folk.uio.no/ohammer/past/). The
literature retrieval was accessed through PubMed (1986–2017) using
the terms of ABO, cancer, longevity, including the appropriate
Boolean operators AND and OR.
Results
The ABO blood group frequency and OR
in cancer patient groups
The frequency of ABO blood groups in the population
(blood donors control group) was as follows: O-38.6%; A-37.5%,
B-16.2%, AB-7.6% (Table I).
 | Table I.Frequency of the ABO blood groups and
OR with 95% CI in the study cancer and blood donor groups. |
Table I.
Frequency of the ABO blood groups and
OR with 95% CI in the study cancer and blood donor groups.
|
|
| Blood groups, % (n)
[OR (95% CI)] |
|---|
|
|
|
|
|---|
| Study groups | n | O | A | B | AB |
|---|
| Blood donors | 994 | 38.6 (384) | 37.5 (373) | 16.2 (161) | 7.6 (76) |
| Prostate
cancer | 2,200 | 36.0 (792) [0.89
(0.77–1.04)] | 37.3 (821) [0.99
(0.85–1.16)] | 19.2 (422) [1.23
(1.01–1.50)]a | 7.5 (165) [0.98
(0.74–1.30)] |
| Kidney cancer |
|
|
|
|
|
| Pooled
group | 2,650 | 37.4 (990) | 38.2 (1012) | 17.2 (457) | 7.2 (191) |
|
|
| [0.95
(0.82–1.01)] | [1.03
(0.89–1.20)] | [1.08
(0.89–1.31)] | [0.94
(0.71–1.24)] |
|
Men | 1,553 | 37.7 (586) | 39.3 (610) | 16.2 (251) | 6.8 (106) |
|
|
| [0.96
(0.82–1.13)] | [1.08
(0.91–1.27)] | [1.00
(0.80–1.24)] | [0.89
(0.65–1.20)] |
|
Women | 1,097 | 36.8 (404) | 36.7 (402) | 18.8 (206) | 7.7 (85) |
|
|
| [0.93
(0.78–1.11)] | [0.96
(0.81–1.15)] | [1.20
(0.95–1.50)] | [1.02
(0.74–1.40)] |
| Bladder cancer |
|
|
|
|
|
| Pooled
group | 1,530 | 36.5 (559) | 37.6 (575) | 19.5 (299) | 6.4 (97) |
|
|
| [0.92
(0.78–1.08)] | [1.00
(0.85–1.18)] | [1.26
(1.02–1.55)]a | [0.82
(0.60–1.12)] |
|
Men | 1,201 | 37.7 (453) | 36.5 (438) | 19.7 (237) | 6.1 (73) |
|
|
| [0.96
(0.81–1.14)] | [0.96
(0.80–1.14)] | [1.27
(1.02–1.59)]a | [0.78
(0.56–1.09)] |
|
Women | 329 | 32.2 (106) | 41.6 (137) | 18.9 (62) | 7.3 (24) |
|
|
| [0.76
(0.58–0.98)]a | [1.18
(0.92–1.53)] | [1.20
(0.87–1.66)] | [0.95
(0.59–1.53)] |
The comparison of blood type frequency between
prostate cancer and blood donors has shown that the blood group B
was significantly more frequent in the prostate cancer group
(P<0.05), and the blood type B was found to be associated with a
significantly increased risk of prostate cancer (OR-1.23,
P<0.05; Table I). A comparison of
the frequency of ABO blood groups between blood donors and the
kidney cancer group revealed no significant difference (P>0.05;
Table I). Also, no OR significance of
any ABO blood group type in the studied kidney cancer groups in
association with the blood donor population was found
(P>0.05).
The blood group B was found to be significantly more
frequent in the pooled group suffering from bladder cancer as
compared with the frequency in the control group of blood donors
(P<0.04). The blood group O frequency was lower in the bladder
cancer women group (P<0.05), and the blood type B was more
frequent in the bladder cancer men group (P<0.04) as compared
with the blood donor group (Table I).
The blood group O was found to be significantly decreasing the risk
of bladder cancer for females (OR-0.76, P<0.05) as tested in
association with the blood donors. A statistically significant risk
association of the blood type B in the pooled group of the bladder
cancer (OR-1.26, P<0.04) was found also; the male bladder cancer
risk was found to be related with the blood type B (OR-1.27,
P<0.04), and no risk related with the blood type B was
determined for the female bladder cancer (P>0.05; Table I) as tested in association with the
control group of blood donors.
The ABO blood group frequency and OR
in the cancer group as compared with the oldest-old group
A comparison of the oldest-old and blood donor
groups has revealed that the blood group A is significantly more
frequent in the oldest-old group (P<0.05). In the control group
of blood donors, the blood type B was more frequent than in the
oldest-old group (P<0.05).
A statistically significant difference in the
frequency of blood groups was found by comparing the prostate
cancer and the oldest-old groups: The blood type A frequency was
significantly lower in the prostate cancer as compared with
oldest-old group (37.3 and 45.8%, respectively, P<0.04). The
blood group B was more frequent in the prostate cancer group than
in the oldest-old group (19.2 and 10.2%, P<0.006). The study of
OR between the prostate cancer and the oldest-old groups has shown
that the blood type B significantly reduces the oldest-old age
likelihood in prostate cancer patients (OR-0.48, P<0.006), and
the A blood group has shown an opposite association (OR-1.42,
P<0.04; Table II).
 | Table II.Frequency of the ABO blood groups in
the oldest-old group and the OR with 95% CI in the studied cancer
groups in association with the oldest-old group. |
Table II.
Frequency of the ABO blood groups in
the oldest-old group and the OR with 95% CI in the studied cancer
groups in association with the oldest-old group.
|
|
| Blood group, OR
(95% CI) |
|---|
|
|
|
|
|---|
| Study groups | n | O | A | B | AB |
|---|
| Oldest-olds, %
(n) | 166 | 39.2 (65) | 45.8 (76) | 10.2 (17) | 4.8 (8) |
| Prostate
cancer | 2,200 | 1.14
(0.83–1.58) | 1.42
(1.03–1.95)a | 0.48
(0.29–0.80)b | 0.645
(0.30–1.29) |
| Kidney cancer |
|
|
|
|
|
| Pooled
group | 2,650 | 1.08
(0.78–1.49) | 1.37
(1.0–1.87) | 0.55
(0.33–0.91)a | 0.65
(0.32–1.35) |
| Bladder cancer |
|
|
|
|
|
| Pooled
group | 1,530 | 1.12
(0.81–1.55) | 1.40
(1.02–1.94)a | 0.47
(0.28–0.79)b | 0.75
(0.36–1.57) |
No significant difference in the blood group A
frequency was found when comparing the pooled and the men kidney
cancer patient groups with the oldest-old group (P>0.05). A
comparison of the women group suffering from kidney cancer with
oldest-old individuals showed that the blood group A frequency was
lower in the women kidney cancer group (P<0.03); also, the blood
type B was more frequent in the pooled (P<0.03), women
(P<0.007) and men (P<0.05) kidney cancer groups as compared
with its frequency in the oldest-olds. The study of OR between the
kidney cancer and oldest-old groups has shown that the blood type B
significantly reduces the oldest-old age likelihood in all studied
kidney cancer groups: Pooled (OR-0.55, P<0.03), men (OR-0.60,
P<0.05) and women (OR-0.49, P<0.009), and the A blood group
has shown a significant opposite association only for the kidney
cancer women group (OR-1.46, P<0.03). The blood type A frequency
was found to be significantly lower in pooled individuals suffering
from bladder cancer (P<0.05) as compared with the oldest-old
group; also, in oldest-olds the blood type A was more frequent than
in the group of men with bladder cancer (P<0.05). The blood
group B frequency in the bladder cancer pooled group was
significantly higher than in the oldest-old group (P<0.004). A
comparison of the blood B group frequency in the oldest-old group
with the men and women bladder cancer groups revealed a
statistically higher frequency of the B blood type in the men
(P<0.003) and women (P<0.02) bladder cancer groups. The study
of OR between the bladder cancer and oldest-old groups has shown
that the blood type B significantly reduces the oldest-old age
likelihood in bladder cancer patients for the pooled (OR-0.47,
P<0.005), men (OR-0.46, P<0.004) and women (OR-0.49,
P<0.02) groups, whereas the A blood group has shown a
significant opposite association only for the pooled (OR-1.40,
P<0.04) and men (OR-1.47, P<0.03) bladder cancer groups
(Table II).
The cluster data analysis of the study
groups
The clustering analysis of the pooled male and
female groups has shown that there are three main clusters. The
first cluster is composed of two smaller clusters: The prostate and
bladder cancer groups was the first one, and the kidney cancer and
blood donors comprised the second cluster. A separate main cluster
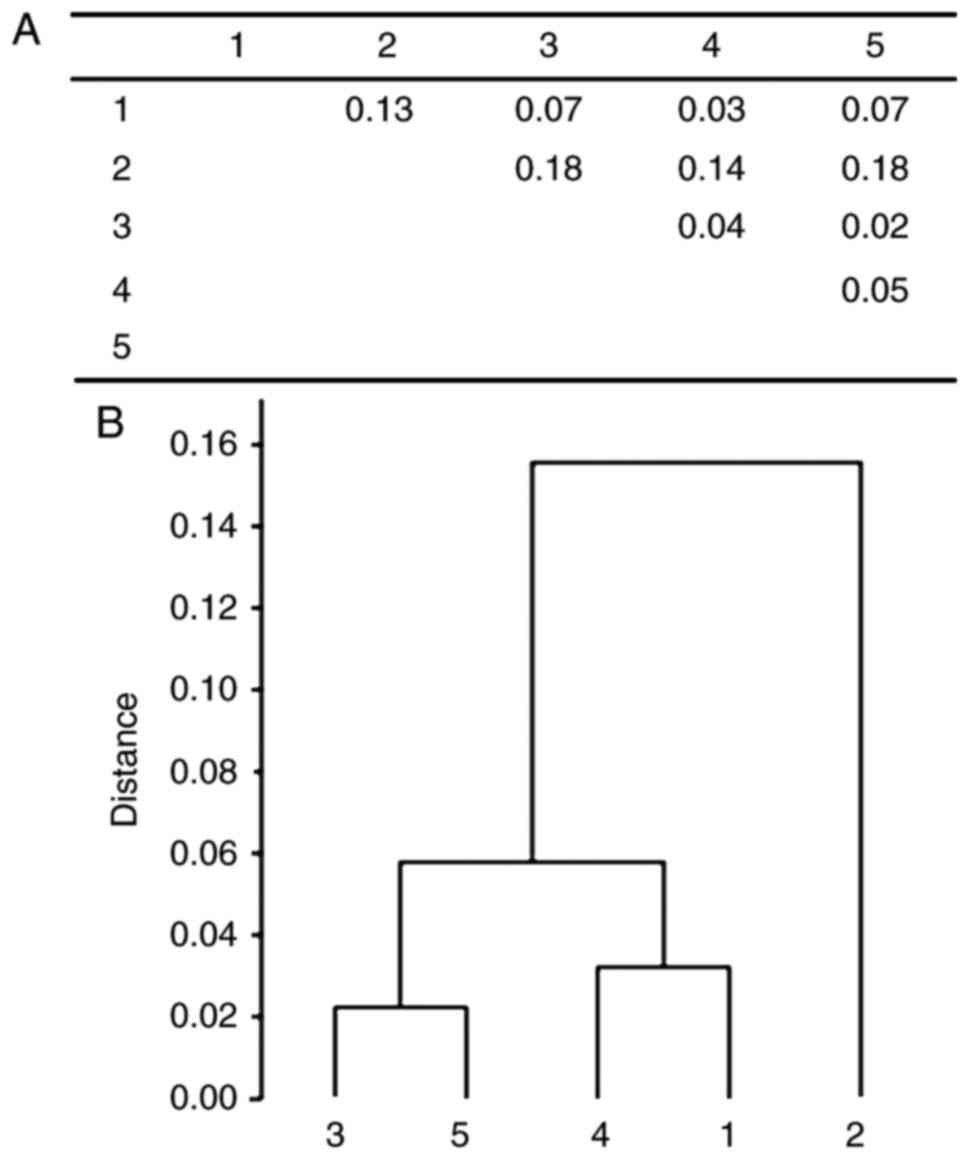
was the oldest-old group (Fig.
1).
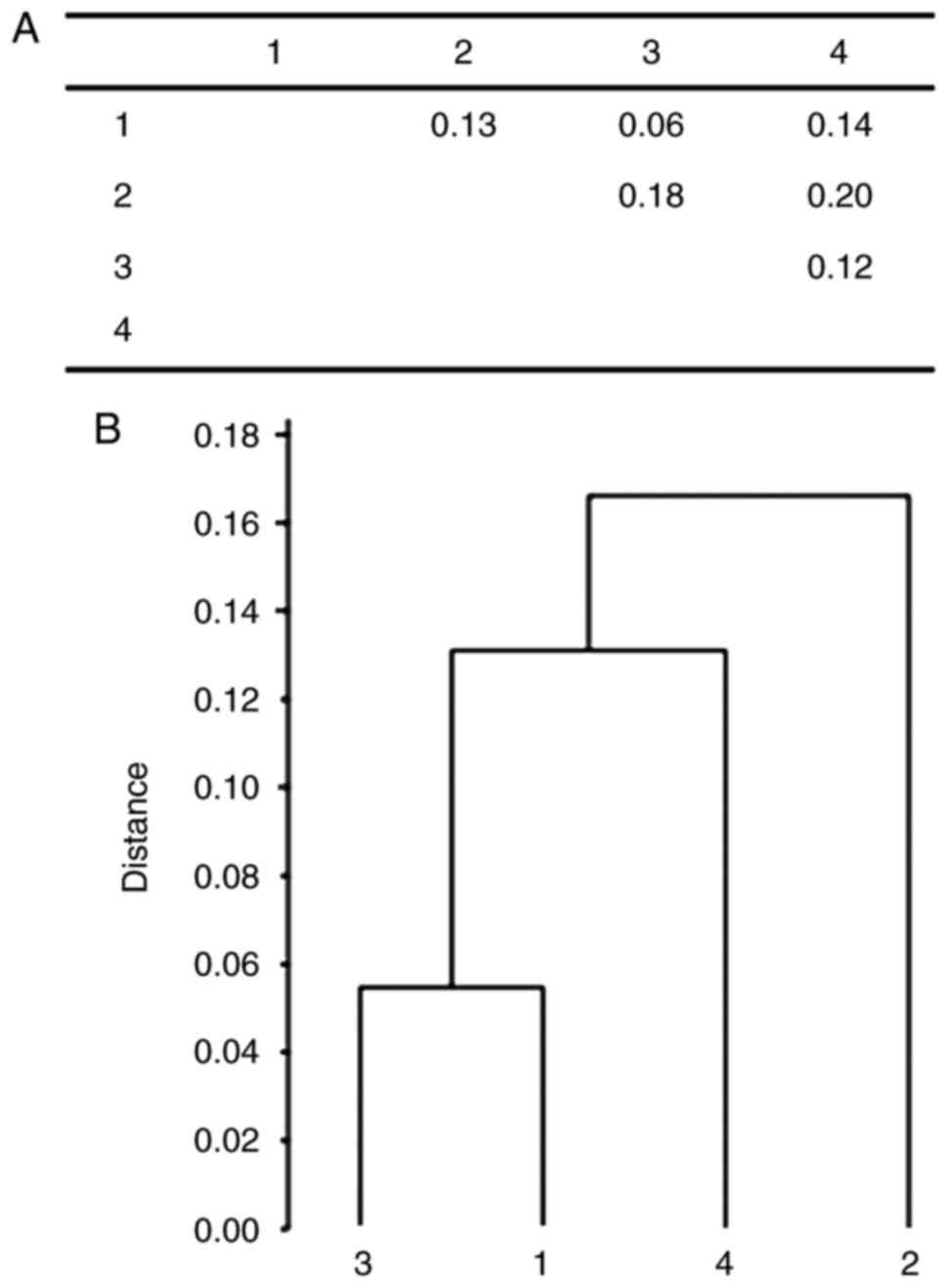
Fig. 2 represents the
structural analysis of the cancer female, control and oldest-old
groups where females suffering from kidney cancer formed the first
cluster with blood donors, and the second and third main clusters
consisted of bladder cancer and oldest-old individuals.
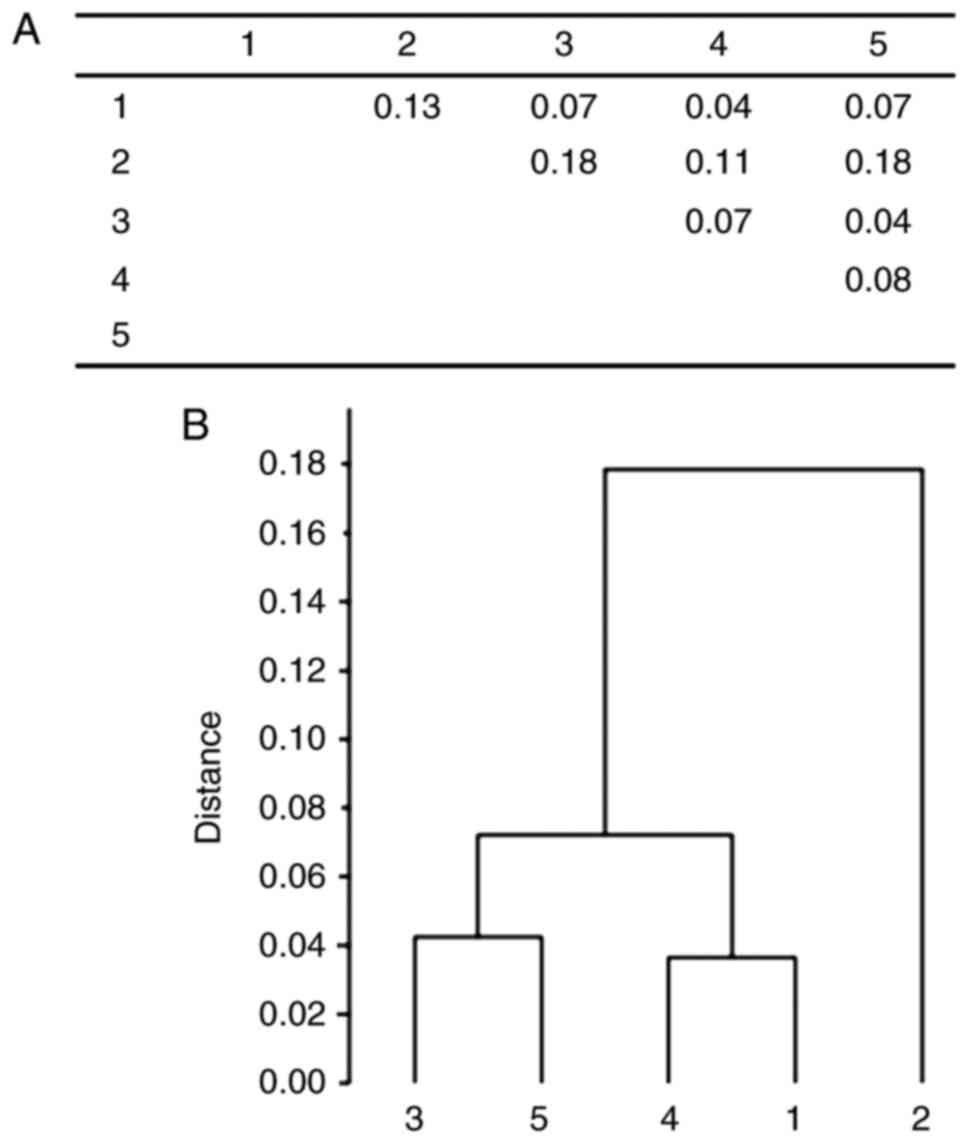
A clustering analysis of cancer male individuals and
control groups has shown three main clusters: The first comprised
the prostate and bladder groups, the second consisted of kidney
cancer and blood donor groups, and the third main cluster contained
the oldest-old individuals (Fig.
3).
Clusters of the pooled groups showed a close
similarity with clusters formed by gender of the male and female
groups (Figs. 1–3).
Discussion
The ABO blood phenotype frequencies vary in
different ethnic/racial groups; they vary globally, where the type
O frequency is most common and approaches 100% among the indigenous
populations of South and Central America; the phenotype A is more
common in Eastern and Central Europe, the phenotype B being more
common in China, India and the AB type is more frequent in Korea,
China and Japan (32). In the US
Caucasians, the frequency of blood groups O, A, B and AB is 45, 40,
11 and 4%, respectively, in Hispanics the distribution being 57,
31, 10 and 3%; and in Blacks 50, 26, 20 and 4% (33); in the Lithuanian population, the blood
type frequencies (in the blood donors cohort) are as follows:
O-38.6%; A-37.5%, B-16.2%, AB-7.6%. The geographical and ethnic
differences in the distribution of ABO blood groups may influence
associations between the ABO blood group phenotypes and the risk of
diseases.
The clustering analysis of our studied groups has
shown that there are separate main clusters of oldest-old
individuals in the analysis of pooled as well as of separate male
and female cancer (prostate, bladder, kidney) in comparison with
control blood donors and oldest-old groups.
The study data on the ABO blood type frequency and
OR in prostate cancer patients (all cases pooled independently of
the Gleason score and stage) showed a significantly higher blood
group B frequency as compared with the blood donors in the
Lithuanian population. Other researchers using three single
nucleotide polymorphisms (rs8176746, rs505922, and rs8176704) to
determine the ABO genotype in 2,774 aggressive prostate cancer
cases (the Gleason score ≥8 or locally advanced/metastatic disease)
found no association between the ABO blood group phenotype and
aggressive prostate cancer (34). The
lack of association between the ABO blood type and the prostate
cancer risk or survival was reported (35). Notably, blood type O prostate cancer
patients were at a significantly lower risk of biochemical
recurrence as compared with the blood type A patients. The analysis
of radical prostatectomy patients and their 5-year biochemical
recurrence-free rate showed the blood type O to be significantly
associated with a decreased risk of biochemical recurrence as
compared with blood type A patients (36). A significant survival has been
reported for prostate cancer blood types B and O patients treated
with the therapeutic cancer vaccine PROSTVAC-VF, and the type B and
O PROSTVAC-VF patients demonstrated markedly improved clinical
outcomes as compared with the blood types A and AB patients,
including a longer median survival and the improved overall
survival. This indicates that the ABO blood group may provide a
screen to pre-select patients for PROSTVAC-VF therapy (37).
The loss of the blood group A antigen expression in
prostate cancer tissue and the retention of the H antigen (38) as well as the A antigen expression
decrease with tumor progression have been reported (39). The lost A antigen expression on cancer
cells might be related with the increased risk of biochemical
recurrence of prostate cancer in patients with A blood group
(36). An alternative explanation of
this phenomenon could be the cross-reaction of anti-A antibodies
with the tumor-associated Tn antigen
(alpha-N-acetylgalactosamine-O-serine/threonine), the
latter being expressed in the prostate cancer tissue (40). The Tn antigen is a marker for the
design of anti-tumor vaccine studies (40,41). The
anti-A antibodies can cross-react with the Tn antigen (42); this cross-reaction might be related
with the decreased risk of biochemical recurrence in the blood type
O patients (37). The risk of venous
thromboembolic events which are the most common complication and
the cause of death within 30 days after radical prostatectomy is
greater in patients with a non-O blood phenotype than in those with
the O blood group (43).
Our study of the OR and of the frequency of ABO
blood groups shows the bladder cancer risk (in pooled males and
females and men bladder cancer groups) to be significantly related
with the blood group B, but no such association was determined in
Lithuanian cancer female patients; also, the blood group O was
found to be significantly decreasing the risk of bladder cancer for
females as tested in association with the blood donors.
The literature data on the ABO blood group
prognostic value in bladder cancer patients are conflicting.
Bladder cancer patients with the O blood type seemed to have a
worse clinical outcome than those with non-O blood types. A
tendency for high-grade and large-sized tumours was observed in the
blood groups O and B patients (44).
A significantly worse 5-year recurrence-free and cancer-specific
survival was associated with a non-O blood type, but no significant
difference in the overall survival was found. Non-O blood types
were associated with the highest cancer-specific mortality,
especially in patients with the blood type A (45). In patients with nonmuscle invasive
bladder urothelial carcinoma with blood type O, the recurrence and
progression rates were significantly worse than those with blood
types A or B (46). In contrast to
findings among Europeans and Americans, in the Japan population
there were no significant differences among blood groups for the
stage, histological grade or survival rate of bladder cancer
patients (47). In urothelial bladder
carcinoma patients after radical cystectomy, the blood type B was
associated with a greater likelihood of lymphovascular invasion and
positive soft tissue margins, and blood type B patients showed a
significantly higher urothelial bladder carcinoma-related mortality
than those with the blood types O and AB (48). By other researchers, the ABO blood
group was not associated with the prognosis of bladder cancer
patients who underwent radical cystectomy (49).
The cancer originating in epithelial cells and the
changes in blood group antigens in the cancer tissue constitute an
important aspect in tumor immunology. The expression of soluble A
and B antigens was decreased in patients with bladder cancer in
comparison with the normal subjects (50). The reduction of A antigen expression
in the bladder cancer tissue was found, while the expression of the
A antigen was maintained in the normal or concomitant dysplasia
urothelium, indicating that the ABO gene promoter hypermethylation
and/or the gene loss is a specific marker of the bladder cancer
(51). The others reported the ABO
blood group antigen expression to be associated with a higher
bladder tumor grade, but in adjuvant chemotherapy receiving
patients the AB phenotype antigen expression was associated with a
reduced bladder overall and cancer-specific survival (52).
A comparison of the frequency of ABO blood groups
between blood donors and the kidney cancer group revealed no
significant difference, and no OR significance of any ABO blood
group type in the studied kidney cancer (polled, male and female)
groups in association with the blood donors was found in our study.
By others, the ABO type frequency differences according to the
renal cell carcinoma (RCC) histological subtype (clear cell type
and non-clear cell type) reached no statistical significance
(32) or the kidney cancer risk was
inversely associated with blood type AB compared with the type O
(53). The follow-up cohort study has
revealed the RCC incidence to be higher in non-O blood type
subjects than in blood type O women (54).
There are several conflicting reports on the ABO
blood type relationship with the survival of RCC patients. The ABO
blood type was reported to be significantly associated with the
progression-free survival and cancer-specific survival in patients
with RCC following partial or radical nephrectomy: A non-O blood
type (A, B or AB) is an independent prognostic factor for a worse
progression-free survival outcome, and the blood type A is an
independent factor associated with a worse cancer-specific survival
(32). In patients undergoing surgery
for loco-regional RCC, the O blood group was found to be
independently associated with the better overall survival, and the
non-O blood type was identified as an independent predictor of
mortality (55). In other studies,
the O blood group was associated with the absence or a lower level
of lymph node metastases, and this finding did not generate a
favourable impact on the RCC prognosis (56,57).
Others have reported the ABO blood groups to be not associated with
survival outcomes and not a prognostic factor in renal cancer
patients after surgery (57). The
risk of bilateral RCC was found to be significantly increased in
relationship with the O blood type (56).
ABO antigens may be related with systemic
inflammation, and chronic inflammation is associated with RCC
(58). The C-reactive protein level
was related to the ABO blood type: RCC patients with the blood type
O had a significantly lower C-reactive protein level than those
with a non-O blood type (32). There
is a relationship between the ABO gene and the the tumor necrosis
factor alpha (59); the ABO gene
locus is linked with TNF-α serum levels (60), so blood group antigens may contribute
by modifying the immune surveillance (17). Non-O blood group patients have higher
levels of the von Willebrand (vWF) and VIII factors (61). Thus, non-O blood group patients have a
greater tendency of hypervascularity and hypercoagulability which
are typical RCC characteristics. The inquired A and/or B antigen is
lost in the RCC tissue; these structural changes in the ABH
antigen, occuring in the RCC, may enhance the progression of the
disease or survival (62–64).
The blood group phenotype O is characterized by the
presence of the H antigen and the anti-A and anti-B antibodies in
the serum (natural isoagglutinins, alloantibodies) which could act
as a cancer defense factor (6,64); these
isoagglutinins can react to the Tn and T pancarcinoma antigens
(65); the A and B antigens were
suggested to be structurally related to Tn and T and could make
cancer cells immunologically less recognizable in the blood groups
A and B individuals (66).
The hypothesis encompasses the enzymatic activity
dysregulation of the ABH glycosyltransferases involved in the
processes of cellular adhesion, membrane signaling and immune
response (17,67,68). The
A/B glycosyltransferases modulate the circulating plasma levels of
vWF (69,70); the vWF has been recently found to be
an important modulator of angiogenesis and apoptosis which are
tumorigenic processes (71). The A, B
or H antigens are involved in controlling cell proliferation,
adhesion and motility as these antigens are present on receptors
for the epidermal growth factor, cadherins, CD44 and integrins
(17,72–74). The
ABO blood group carbohydrates on the surface of metastatic cancer
cells are functioning as cell adhesion molecules (75). There are reports on the association
between circulating levels of tumor necrosis factor-alpha and the
ABO gene locus polymorphisms (60), the latter being associated with the
soluble intercellular adhesion molecule (ICAM-1) (76–78),
E-selectin (79,80) and P-selectin (78). All these molecules are immune cell
recruitment mediators in chronic inflammation which may have a
directly linking ABO blood group and tumor initiation and spread
(81,82). Compared with the blood group O, the
expression of ICAM-1, which inhibits lymphocyte attachment to
endothelial cells, is significantly reduced in patients with a
non-O blood group (blood group A in particular) (6,83). The
decreased levels of the soluble ICAM in non-O blood group patients
may be related to tumor spread (84).
This suggestion has been supported with a number of studies showing
a decreased survival in non-O blood group and a favorable prognosis
in cancer patients with the O blood group (85,86). With
the A1 allele, the expression of P-selectin and ICAM-1 is
associated (78). During
inflammation, E-selectin increases leukocyte accumulation, and
P-selectin enables leukocytes to interact with endothelial cells or
platelets. Inflammatory cytokines rapidly upregulate ICAM-1 and can
inhibit lymphocyte adhesion to endothelial cells (83). Compared with non-O blood groups,
monocytes of O blood group individuals produced higher levels of
interleukin-6, nitric oxide and tumor necrosis factor-alpha
(87).
The ABH antigens are expressed on the surfaces of
several normal and tumour tissues, including the kidneys and renal
cell carcinoma lines (17,88), bladder (50–52) and
prostate tissue cells (36,38,39).
Compared to normal cells, aberrant ABH antigens have been found on
tumor tissue cells, such as A or B epitope loss and H antigen
accumulation, or an incompatible expression of tumor A antigens is
notable in blood type O subjects (17). The loss or diminished expression of
ABH antigens has been reported in both solid and hematological
malignancies. There have been occasional case reports of ABO blood
group antigen change in malignant conditions, and the antigens were
re-expressed when the patients attained remission. There are two
possible mechanisms for the weakening of ABO antigens expression
during malignancy: 1) Inactivation of A and B transferases (there
is a decreased expression of the A and B antigens with a concurrent
increase in the H antigen), 2) the inactivation of H transferase,
related to a decreased H substance, and a resulting decrease in A
and/or B substance (89). An altered
expression of ABO antigens may contribute to a malignant phenotype
via increased cell motility or enhanced evasion of apoptosis
(18). The loss of A or B
glycosyltransferase enhances malignancy in human carcinoma cell
lines (90) and is associated with a
less favorable prognosis (91). In
the non-A individuals, tumor cells can express the A antigen, while
glycosylation can lead to conformational changes in proteins such
as the epidermal growth factor receptor, or alter the immune
recognition of natural killer cells which are the conditions
favoring tumorigenesis (92). The
underlying mechanisms by which the ABO blood group may interplay
with tumorigenesis need further research (81). A deletion of A or B antigens in non-O
blood group patients leads to the upregulation of the H precursor
and Lewis factor, both of them stimulating neoangiogenesis
(93).
In our study, a comparison of the oldest-old and
blood donor groups has revealed the blood group A to be
significantly more frequent, and the blood type B was significantly
rarer in the oldest-old group; the blood type A frequency was
significantly lower and the blood group B was significantly more
frequent in the prostate cancer group as compared with the
oldest-old group, and the study of OR has shown that the blood type
B significantly reduces the oldest-old age likelihood in prostate
and bladder cancer patients, while the A blood type has shown a
significant opposite association in prostate cancer patients.
Our finding that the blood group A was significantly
overrepresented in the oldest-old cohort deserves special
attention. When comparing cancer patient groups with oldest-olds,
the blood group A was found to be significantly overrepresented
among urogenital cancer patients. There are two blood group A
subtypes, namely A1 and A2, and the A1 allele increases the ABO
protein level with the highest enzymatic activity (94). The increased susceptibility of
pancreatic cancer has been reported to be possibly connected with
the blood group A which is likely to be related with the
A1 subtype (15). The
possible associations with the blood type A have been found in
ovarian cancer: Cases with minor alleles of rs1053878 had a 50%
lower risk of death and a better survival related with the blood
type A and imply that this relationship may be caused by the A2
allele (95). The meta-analysis of
eight studies indicated that women with genetic blood type A
variants had a 9% greater ovarian cancer risk than did blood group
O ones, and the increased risk was only evident for A1 genotype
cases (89).
Furthermore, thromboembolism is the additional way
by which the ABO blood type may influence the survival of cancer
patients. Individuals with the blood type O are at a lower venous
thromboembolism risk and have lower vWF levels than the ones with
non-O blood groups, and the A2 blood phenotype was found to be
independently related with a lower thromboembolism risk and to have
the lowest vWF levels among all other ABO blood phenotypes
(96,97). The better survival in relationship
with the lower risk of tromboembolism among cancer patients with
blood type A could be due to the A2 allele. The limitation of our
and many other studies of the role of ABO blood groups in longevity
is that they do not distinguish between the A1 and A2 subgroups
evaluating the phenotype rather than the ABO genotype, whereas this
might be of biological significance as the A1 and A2
glycosyltransferases display different catalytic activities, the A2
isoform having a higher Km and the estimated enzymatic
activity 30–50 times lower than A1 (17). The differences in the A1 and A2
allelic proportions across different populations may produce some
inconsistencies across the studies. A significant increase of the A
blood phenotype was observed in the population of healthy elderly
males aged over 64 years in the UK (98).
Our OR analysis of the association among cancer and
oldest-old groups shows that the blood type B significantly reduces
the old-oldest age likelihood in all studied kidney cancer (pooled,
men, and women), bladder cancer patients for the pooled and
separate men and women groups; notably, the A blood group has shown
a significant opposite association for the women kidney cancer, in
pooled and men bladder cancer groups in the Lithuanian population.
We and others have found a significant association between the
blood group B and the higher prevalence of tumors, but there are no
clear biological mechanisms to correlate the association of the
blood type B with different tumor development (13). The ABO blood type may influence cancer
patients’ survival by mechanisms related to coagulation. The ABO
gene is responsible for the post-translational glycosylation of the
procoagulant molecule vWF (99).
Thromboembolism has an impact on the poor cancer patients’
survival, and patients with the blood type O have lower rates of
thromboembolism because of the lower levels of vWF and the
increased risk for those with blood type B (100,101).
Another study shows a negative relationship between
the B blood type and life expectancy in a large cohort (n=28,129);
its authors indicate that this evidence may be attributable to the
association between the B blood group and some aging-associated
conditions raising a new link between the ABO blood type and cancer
(102). Others have found the
percentage of patients with the blood group B and their survival to
decline with age, and the blood group B may be a marker for earlier
death (103). On the contrary,
compared to the controls, the B group more frequently was observed
in Japanese centenarians, suggesting that the group B might be
associated with longevity; the group B individuals were more likely
to survive age-related diseases, since 33% of the centenarians were
free of such diseases, and this did not correlate with the group B.
These conflicting results regarding the blood type B demonstrate
that demographic evidences suggest longevity to be possibly caused
by different combinations of genes in relation with the different
environmental and geographical quantitative and qualitative
differences in which the population-specific genetic factors play a
role in the longevity (104).
The ABO blood group system is a critical player in
the genomic medicine. The structural analysis of our study and
other researchers’ data indicate the blood type B to be related
with the risk of prostate and bladder cancer and could be evaluated
as a congenital determinant in the negative relationship with
longevity, and that blood types O and A could be the positive
factors increasing the oldest-old age likelihood. Moreover, the
further studies of the ABO blood groups evaluating the
A1 or A2 in relationship with the ABH
enzymatic activities, their role in cellular adhesion, immune
response in the pathogenesis of cancer could be also important for
longevity elucidation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DoS, MJ, VL and DŽ were involved in the research
area and study design. DoS, AU, DŽ and AS collected patients’ data.
MJ, AU, DŽ, DoS, DaS, RŠ, AS, KS and VL performed the data
analysis. Statistical analysis was conducted by MJ and DoS. All
authors contributed important intellectual content during
manuscript drafting and revisions, and all accept accountability
for the overall work by ensuring that questions pertaining to the
accuracy or integrity of any portion of the work are appropriately
investigated and resolved. All authors approved the final
manuscript version.
Ethics approval and consent to
participate
The present study was approved by the Kaunas
Regional Biomedical Research Ethics Committee (Kaunas, Lithuania).
Written informed consent was obtained from the patients.
Consent for publication
Written informed consent was obtained from the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rummel SK and Ellsworth RE: The role of
the histoblood ABO group in cancer. Future Sci OA. 2:FSO1072016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stakisaitis D, Lesauskaitė V, Girdauskaitė
M, Janulionis E, Ulys A and Benetis R: Investigation of vitamin
D-binding protein polymorphism impact on coronary artery disease
and relationship with longevity: Own data and a review. Int J
Endocrinol. 2016:83473792016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clausen H and Hakomori SI: ABH and related
histo-blood group antigens; immunochemical differences in carrier
isotypes and their distribution. Vox Sang. 56:1–20. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dean L: ABO Blood Group. In: Medical
genetics summaries [internet]. Pratt V, McLeod H, Dean L, Malheiro
A and Rubinstein W: National Center for Biotechnology Information.
Bethesda, MD; 2012, https://www.ncbi.nlm.nih.gov/books/NBK100894/Updated
2015.
|
|
5
|
Hamosh A, Scott AF, Amberger J, Bocchini
C, Valle D and McKusick VA: Online Mendelian Inheritance in Man
(OMIM), a knowledgebase of human genes and genetic disorders.
Nucleic Acids Res. 30:52–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto F, Cid E, Yamamoto M and Blancher
A: ABO research in the modern era of genomics. Transfus Med Rev.
26:103–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mourant AE: Blood relations: Blood groups
and anthropology. Oxford University Press; Oxford; pp. 13–20.
1983
|
|
8
|
Maynard CL, Elson CO, Hatton RD and Weaver
CT: Reciprocal interactions of the intestinal microbiota and immune
system. Nature. 489:231–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Mattos LC: Structural diversity and
biological importance of ABO, H, Lewis and secretor histo-blood
group carbohydrates. Rev Bras Hematol Hemoter. 38:331–340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aird I, Bentall HH and Roberts JA: A
relationship between cancer of stomach and the ABO blood groups. Br
Med J. 1:799–801. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu YQ, Jiang TW, Cui YH, Zhao YL and Qiu
LQ: Prognostic value of ABO blood group in patients with gastric
cancer. J Surg Res. 201:188–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amundadottir L, Kraft P,
Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA,
Bueno-de-Mesquita HB, Gross M, Helzlsouer K and Jacobs EJ: et
alGenome-wide association study identifies variants in the ABO
locus associated with susceptibility to pancreatic cancer. Nat
Genet. 41:986–990. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben Q, Liu J, Wang W, Guo F, Yao W, Zhong
J and Yuan Y: Association between ABO blood types and sporadic
pancreatic neuroendocrine tumors in the Chinese Han population.
Oncotarget. 8:54799–54808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Engin H, Bilir C, Üstün H and Gökmen A:
ABO blood group and risk of pancreatic cancer in a Turkish
population in Western Black Sea region. Asian Pac J Cancer Prev.
13:131–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Jellas K, Hoem D, Hagen KG, Kalvenes
MB, Aziz S, Steine SJ, Immervoll H, Johansson S and Molven A:
Associations between ABO blood groups and pancreatic ductal
adenocarcinoma: Influence on resection status and survival. Cancer
Med. 6:1531–1540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vasan SK, Hwang J, Rostgaard K, Nyrén O,
Ullum H, Pedersen OBV, Erikstrup C, Melbye M, Hjalgrim H, Pawitan Y
and Edgren G: ABO blood group and risk of cancer: A register-based
cohort study of 1.6 million blood donors. Cancer Epidemiol.
44:40–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hakomori S: Antigen structure and genetic
basis of histo-blood groups A, B and O: Their changes associated
with human cancer. Biochim Biophys Acta. 1473:247–266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Pendu J, Marionneau S, Cailleau-Thomas
A, Rocher J, Le Moullac-Vaidye B and Clément M: ABH and Lewis
histo-blood group antigens in cancer. APMIS. 109:9–31. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franchini M, Liumbruno GM and Lippi G: The
prognostic value of ABO blood group in cancer patients. Blood
Transfus. 14:434–440. 2016.PubMed/NCBI
|
|
20
|
Freudenberg-Hua Y, Freudenberg J, Vacic V,
Abhyankar A, Emde AK, Ben-Avraham D, Barzilai N, Oschwald D,
Christen E and Koppel J: et alDisease variants in genomes of
44 centenarians. Mol Genet Genomic Med. 2:438–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sebastiani P, Riva A, Montano M, Pham P,
Torkamani A, Scherba E, Benson G, Milton JN, Baldwin CT and
Andersen S: et alWhole genome sequences of a male and female
supercentenarian, ages greater than 114 years. Front Genet.
2:902012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fortney K, Dobriban E, Garagnani P,
Pirazzini C, Monti D, Mari D, Atzmon G, Barzilai N, Franceschi C,
Owen AB and Kim SK: Genome-wide scan informed by age-related
disease identifies loci for exceptional human longevity. PLoS
Genet. 11:e10057282015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Christensen K, Johnson TE and Vaupel JW:
The quest for genetic determinants of human longevity: Challenges
and insights. Nat Rev Genet. 7:436–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spitsyn VA and Stakishaĭtis DV:
Interrelation of genetic dimorphism of ear wax and the level of
apolipoproteins with atherogenesis and longevity in the Lithuanian
population. Genetika. 29:334–341. 1993.(In Russian). PubMed/NCBI
|
|
25
|
Stakishaĭtis DV, Ianchauskene SM,
Ivashkiavichene LI and Priaĭksha RA: Atherogenesis and serum levels
of apolipoprotein E. Kardiologija. 32:14–16. 1992.
|
|
26
|
Stakisaitis D, Maksvytis A, Benetis R and
Viikmaa M: Coronary atherosclerosis and blood groups of ABO system
in women (own data and review). Medicina (Kaunas). 38(Suppl 2):
S230–S235. 2002.
|
|
27
|
Stini WA: Sex differences in bone loss-an
evolutionary perspective on a clinical problem. Coll Antropol.
27:23–46. 2003.PubMed/NCBI
|
|
28
|
Znaor A, Laversanne M and Bray F: Less
overdiagnosis of kidney cancer? an age-period-cohort analysis of
incidence trends in 16 populations worldwide. Int J Cancer.
141:925–932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gondos A, Krilaviciute A, Smailyte G, Ulys
A and Brenner H: Cancer surveillance using registry data: Results
and recommendations for the Lithuanian national prostate cancer
early detection programme. Eur J Cancer. 51:1630–1637. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huffman DM, Deelen J, Ye K, Bergman A,
Slagboom EP, Barzilai N and Atzmon G: Distinguishing between
longevity and buffered-deleterious genotypes for exceptional human
longevity: The case of the MTP gene. J Gerontol A Biol Sci Med Sci.
67:1153–1160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
vB Hjelmborg J, Iachine I, Skytthe A,
Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL and
Christensen K: Genetic influence on human lifespan and longevity.
Hum Genet. 119:312–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bodmer W: Genetic characterization of
human populations: From ABO to a genetic map of the British people.
Genetics. 199:267–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garratty G, Glynn SA and McEntire R;
Retrovirus Epidemiology Donor Study: ABO and Rh(D) phenotype
frequencies of different racial/ethnic groups in the United States.
Transfusion. 44:703–706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Markt SC, Shui IM, Unger RH, Urun Y, Berg
CD, Black A, Brennan P, Bueno-de-Mesquita HB, Gapstur SM and
Giovannucci E: et alABO blood group alleles and prostate
cancer risk: Results from the breast and prostate cancer cohort
consortium (BPC3). Prostate. 75:1677–1681. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kvist E, Krogh J and Hjortberg P:
Prognostic variables in patients with prostate cancer: Influence of
blood group ABO (H), the Rhesus system, age, differentiation,
tumour stage and metastases. Int Urol Nephrol. 24:417–423. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohno Y, Ohori M, Nakashima J, Okubo H,
Satake N, Takizawa I, Hashimoto T, Hamada R, Nakagami Y, Yoshioka K
and Tachibana M: Associations between ABO blood groups and
biochemical recurrence after radical prostatectomy. Int J Clin Exp
Med. 8:2642–2648. 2015.PubMed/NCBI
|
|
37
|
Muthana SM, Gulley JL, Hodge JW, Schlom J
and Gildersleeve JC: ABO blood type correlates with survival on
prostate cancer vaccine therapy. Oncotarget. 6:32244–32256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abel PD, Marsh C, Henderson D, Leathem A,
Powell PH and Williams G: Detection of blood group antigens in
frozen sections of prostatic epithelium. Br J Urol. 59:430–435.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chastonay P, Hurlimann J and Gardiol D:
Biological tissue markers in benign and malignant disease of the
human prostate. Virchows Arch A Pathol Anat Histopathol.
410:221–229. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Q, Anver MR, Butcher DO and
Gildersleeve JC: Resolving conflicting data on expression of the Tn
antigen and implications for clinical trials with cancer vaccines.
Mol Cancer Ther. 8:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slovin SF, Ragupathi G, Musselli C,
Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky
S, Livingston PO and Scher HI: Fully synthetic carbohydrate-based
vaccines in biochemically relapsed prostate cancer: Clinical trial
results with alpha-N-acetylgalactosamine-O-serine/threonine
conjugate vaccine. J Clin Oncol. 21:4292–4298. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hirohashi S, Clausen H, Yamada T,
Shimosato Y and Hakomori S: Blood group A cross-reacting epitope
defined by monoclonal antibodies NCC-LU-35 and −81 expressed in
cancer of blood group O or B individuals: Its identification as Tn
antigen. Proc Natl Acad Sci USA. 82:7039–7043. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Clyne M: Prostate cancer: Non-O blood type
is VTE risk factor after radical prostatectomy. Nat Rev Urol.
10:6802013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Raitanen MP and Tammela TL: Relationship
between blood groups and tumour grade, number, size, stage,
recurrence and survival in patients with transitional cell
carcinoma of the bladder. Scand J Urol Nephrol. 27:343–347. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang SS: Re: The association of ABO blood
type with disease recurrence and mortality among patients with
urothelial carcinoma of the bladder undergoing radical cystectomy.
J Urol. 196:3522016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Klatte T, Xylinas E, Rieken M, Kluth LA,
Rouprêt M, Pycha A, Fajkovic H, Seitz C, Karakiewicz PI and Lotan
Y: et alImpact of ABO blood type on outcomes in patients
with primary nonmuscle invasive bladder cancer. J Urol.
191:1238–1243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamada T, Fukui I, Yokokawa M and Oshima
H: A study of prognosis and clinicopathology of bladder cancer to
blood group type of host patients in Japan. Scand J Urol Nephrol.
27:199–203. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Klatte T, Xylinas E, Rieken M, Rouprêt M,
Fajkovic H, Seitz C, Karakiewicz PI, Lotan Y, Babjuk M, de Martino
M and Shariat SF: Effect of ABO blood type on mortality in patients
with urothelial carcinoma of the bladder treated with radical
cystectomy. Urol Oncol. 32:625–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Süer E, Özcan C, Gökçe I, Gülpınar Ö,
Göğüş C, Türkölmez K, Baltac S and Bedük Y: Do blood groups have
effect on prognosis of patients undergoing radical cystectomy? Int
Urol Nephrol. 46:1521–1526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Foresto P, Bioindi C, Racca L, Brufman A,
Yaber F, Solis E, Provenzal O and Valverde J: Abnormal
glycosylation of soluble ABH antigens in tumors of the urinary
tract. Arch Esp Urol. 53:196–199. 2000.(In Spanish). PubMed/NCBI
|
|
51
|
Chihara Y, Sugano K, Kobayashi A, Kanai Y,
Yamamoto H, Nakazono M, Fujimoto H, Kakizoe T, Fujimoto K,
Hirohashi S and Hirao Y: Loss of blood group A antigen expression
in bladder cancer caused by allelic loss and/or methylation of the
ABO gene. Lab Invest. 85:895–907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Engel O, Soave A, Peine S, Kluth LA,
Schmid M, Shariat SF, Dahlem R, Fisch M and Rink M: The impact of
the AB0 and the Rhesus blood group system on outcomes in bladder
cancer patients treated with radical cystectomy. World J Urol.
33:1769–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun W, Wen CP, Lin J, Wen C, Pu X, Huang
M, Tsai MK, Tsao CK, Wu X and Chow WH: ABO blood types and cancer
risk-a cohort study of 339,432 subjects in Taiwan. Cancer
Epidemiol. 39:150–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Joh HK, Cho E and Choueiri TK: ABO blood
group and risk of renal cell cancer. Cancer Epidemiol. 36:528–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kaffenberger SD, Morgan TM, Stratton KL,
Boachie AM, Barocas DA, Chang SS, Cookson MS, Herrell SD, Smith JA
and Clark PE: ABO blood group is a predictor of survival in
patients undergoing surgery for renal cell carcinoma. BJU Int.
110:E641–E646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
de Martino M, Waldert M, Haitel A, Schatzl
G, Shariat SF and Klatte T: Evaluation of ABO blood group as a
prognostic marker in renal cell carcinoma (RCC). BJU Int.
113:E62–E66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee C, You D, Sohn M, Jeong IG, Song C,
Kwon T, Hong B, Hong JH, Ahn H and Kim CS: Prognostic value of ABO
blood group in patients with renal cell carcinoma:
Single-institution results from a large cohort. J Cancer Res Clin
Oncol. 141:1441–1447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Soyupek S, Tulunay O, Armağan A, Hoscan B
and Perk H: Clinical importance of intratumoral and normal renal
parenchymal inflammatory cell infiltration in renal cell carcinoma.
Scand J Urol Nephrol. 41:387–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harrison ML, Obermueller E, Maisey NR,
Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C and Timotheadou E:
et alTumor necrosis factor alpha as a new target for renal
cell carcinoma: Two sequential phase II trials of infliximab at
standard and high dose. J Clin Oncol. 25:4542–4549. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Melzer D, Perry JR, Hernandez D, Corsi AM,
Stevens K, Rafferty I, Lauretani F, Murray A, Gibbs JR and Paolisso
G: et alA genome-wide association study identifies protein
quantitative trait loci (pQTLs). PLoS Genet. 4:e10000722008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tirado I, Mateo J, Soria JM, Oliver A,
Martínez-Sánchez E, Vallvé C, Borrell M, Urrutia T and Fontcuberta
J: The ABO blood group genotype and factor VIII levels as
independent risk factors for venous thromboembolism. Thromb
Haemost. 93:468–474. 2005.PubMed/NCBI
|
|
62
|
Iodice S, Maisonneuve P, Botteri E, Sandri
MT and Lowenfels AB: ABO blood group and cancer. Eur J Cancer.
46:3345–3350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cordon-Cardo C, Reuter VE, Finstad CL,
Sheinfeld J, Lloyd KO, Fair WR and Melamed MR: Blood group-related
antigens in human kidney: Modulation of Lewis determinants in renal
cell carcinoma. Cancer Res. 49:212–218. 1989.PubMed/NCBI
|
|
64
|
Reid ME and Mohandas N: Red blood cell
blood group antigens: Structure and function. Semin Hematol.
41:93–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hofmann BT, Stehr A, Dohrmann T, Güngör C,
Herich L, Hiller J, Harder S, Ewald F, Gebauer F and Tachezy M:
et alABO blood group IgM isoagglutinins interact with
tumor-associated O-glycan structures in pancreatic cancer. Clin
Cancer Res. 20:6117–6126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ju T, Otto VI and Cummings RD: The Tn
antigen-structural simplicity and biological complexity. Angew Chem
Int Ed Engl. 50:1770–1791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang S, Zhang HS, Cordon-Cardo C, Reuter
VE, Singhal AK, Lloyd KO and Livingston PO: Selection of tumor
antigens as targets for immune attack using immunohistochemistry:
II. Blood group-related antigens. Int J Cancer. 73:50–56. 1997.
|
|
68
|
Hakomori S: Tumor-associated carbohydrate
antigens defining tumor malignancy: Basis for development of
anti-cancer vaccines. Adv Exp Med Biol. 491:369–402. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Williams SR, Hsu FC, Keene KL, Chen WM,
Dzhivhuho G, Rowles JL III, Southerland AM, Furie KL, Rich SS and
Worrall BB: et alGenetic drivers of von Willebrand factor
levels in an ischemic stroke population and association with risk
for recurrent stroke. Stroke. 48:1444–1450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Franchini M, Crestani S, Frattini F, Sissa
C and Bonfanti C: ABO blood group and von Willebrand factor:
Biological implications. Clin Chem Lab Med. 52:1273–1276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Franchini M, Frattini F, Crestani S,
Bonfanti C and Lippiet G: Von Willebrand factor and cancer: A
renewed interest. Thromb Res. 131:290–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Furnari FB, Cloughesy TF, Cavenee WK and
Mischel PS: Heterogeneity of epidermal growth factor receptor
signalling networks in glioblastoma. Nat Rev Cancer. 15:302–310.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Breier G, Grosser M and Rezaei M:
Endothelial cadherins in cancer. Cell Tissue Res. 355:523–527.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Prochazka L, Tesarik R and Turanek J:
Regulation of alternative splicing of CD44 in cancer. Cell Signal.
26:2234–2239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Akhtar K, Mehdi G, Sherwani R and Sofi L:
Relationship between various cancers and ABO blood groups-a
Northern India experience. Internet J Pathol. 13:12010.
|
|
76
|
Paré G, Chasman DI, Kellogg M, Zee RY,
Rifai N, Badola S, Miletich JP and Ridkeret PM: Novel association
of ABO histo-blood group antigen with soluble ICAM-1: Results of a
genome-wide association study of 6,578 women. PLoS Genet.
4:e10001182008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang W, Xu Q, Zhuang Y and Chen Y: Novel
association of soluble intercellular adhesion molecule 1 and
soluble P-selectin with the ABO blood group in a Chinese
population. Exp Ther Med. 12:909–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Barbalic M, Dupuis J, Dehghan A, Bis JC,
Hoogeveen RC, Schnabel RB, Nambi V, Bretler M, Smith NL and Peters
A: et alLarge-scale genomic studies reveal central role of
ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 19:1863–1872.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Paterson AD, Lopes-Virella MF, Waggott D,
Boright AP, Hosseini SM, Carter RE, Shen E, Mirea L, Bharaj B and
Sun L: et alGenome-wide association identifies the ABO blood
group as a major locus associated with serum levels of soluble
E-selectin. Arterioscler Thromb Vasc Biol. 29:1958–1967. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Qi L, Cornelis MC, Kraft P, Jensen M, van
Dam RM, Sun Q, Girman CJ, Laurie CC, Mirel DB and Hunter DJ: et
alGenetic variants in ABO blood group region, plasma soluble
E-selectin levels and risk of type 2 diabetes. Hum Mol Genet.
19:1856–1862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Franchini M, Favaloro EJ, Targher G and
Lippi G: ABO blood group, hypercoagulability, and cardiovascular
and cancer risk. Crit Rev Clin Lab Sci. 49:137–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rieckmann P, Michel U, Albrecht M, Brück
W, Wöckel L and Felgenhauer K: Soluble forms of intercellular
adhesion molecule-1 (ICAM-1) block lymphocyte attachment to
cerebral endothelial cells. J Neuroimmunol. 60:9–15. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kobayashi H, Boelte KC and Lin PC:
Endothelial cell adhesion molecules and cancer progression. Curr
Med Chem. 14:377–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rahbari NN, Bork U, Hinz U, Leo A,
Kirchberg J, Koch M, Büchler MW and Weitz J: AB0 blood group and
prognosis in patients with pancreatic cancer. BMC Cancer.
12:3192012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ko K, Park YH, Jeong CW, Ku JH, Kim HH and
Kwak C: Prognostic significance of blood type A in patients with
renal cell carcinoma. Urol J. 13:2765–2772. 2016.PubMed/NCBI
|
|
87
|
Alkout AM, Blackwell CC and Weir DM:
Increased inflammatory responses of persons of blood group O to
Helicobacter pylori. J Infect Dis. 181:1364–1369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Breimer ME, Mölne J, Nordén G, Rydberg L,
Thiel G and Svalander CT: Blood group A and B antigen expression in
human kidneys correlated to A1/A2/B, Lewis, and secretor status.
Transplantation. 82:479–485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nambiar RK, Narayanan G, Prakash NP and
Vijayalakshmi K: Blood group change in acute myeloid leukemia. Proc
(Bayl Univ Med Cent). 30:74–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ichikawa D, Handa K and Hakomori S:
Histo-blood group A/B antigen deletion/reduction vs. continuous
expression in human tumor cells as correlated with their
malignancy. Int J Cancer. 76:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Moldvay J, Scheid P, Wild P, Nabil K, Siat
J, Borrelly J, Marie B, Farré G, Labib T and Pottier G: et
alPredictive survival markers in patients with surgically
resected non-small cell lung carcinoma. Clin Cancer Res.
6:1125–1134. 2000.PubMed/NCBI
|
|
92
|
Greenwell P: Blood group antigens:
Molecules seeking a function? Glycoconj J. 14:159–173. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Halloran MM, Carley WW, Polverini PJ,
Haskell CJ, Phan S, Anderson BJ, Woods JM, Campbell PL, Volin MV,
Bäcker AE and Koch AE: Ley/H: An endothelial-selective,
cytokine-inducible, angiogenic mediator. J Immunol. 164:4868–4877.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yamamoto F, McNeill PD and Hakomori S:
Human histo-blood group A2 transferase coded by A2 allele, one of
the A subtypes, is characterized by a single base deletion in the
coding sequence, which results in an additional domain at the
carboxyl terminal. Biochem Biophys Res Commun. 187:366–374. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cozzi GD, Levinson RT, Toole H, Snyder MR,
Deng A, Crispens MA, Khabele D and Beeghly-Fadiel A: Blood type,
ABO genetic variants, and ovarian cancer survival. PLoS One.
12:e01751192017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Trégouët DA, Heath S, Saut N,
Biron-Andreani C, Schved JF, Pernod G, Galan P, Drouet L, Zelenika
D and Juhan-Vague I: et alCommon susceptibility alleles are
unlikely to contribute as strongly as the FV and ABO loci to VTE
risk: Results from a GWAS approach. Blood. 113:5298–5303. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ozel AB, McGee B, Siemieniak D, Jacobi PM,
Haberichter SL, Brody LC, Mills JL, Molloy AM, Ginsburg D, Li JZ
and Desch KC: Genome-wide studies of von Willebrand factor
propeptide identify loci contributing to variation in propeptide
levels and von Willebrand factor clearance. J Thromb Haemost.
14:1888–1898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Murray S: ABO groups and Rh genotypes in
the elderly. Br Med J. 2:1472–1474. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Matsui T, Titani K and Mizuochi T:
Structures of the asparagine-linked oligosaccharide chains of human
von Willebrand factor. Occurrence of blood group A, B, and H(O)
structures. J Biol Chem. 267:8723–8731. 1992.PubMed/NCBI
|
|
100
|
Ohira T, Cushman M, Tsai MY, Zhang Y,
Heckbert SR, Zakai NA, Rosamond WD and Folsom AR: ABO blood group,
other risk factors and incidence of venous thromboembolism: The
Longitudinal Investigation of Thromboembolism Etiology (LITE). J
Thromb Haemos. 5:1455–1461. 2007. View Article : Google Scholar
|
|
101
|
Zhou S and Welsby I: Is ABO blood group
truly a risk factor for thrombosis and adverse outcomes? World J
Cardiol. 6:985–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mengoli C, Bonfanti C, Rossi C and
Franchini M: Blood group distribution and life-expectancy: A
single-centre experience. Blood Transfus. 13:313–317.
2015.PubMed/NCBI
|
|
103
|
Brecher ME and Hay SN: ABO blood type and
longevity. Am J Clin Pathol. 135:96–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shimizu K, Hirose N, Ebihara Y, Arai Y,
Hamamatsu M, Nakazawa S, Masui Y, Inagaki H, Gondo Y and Fujimori
J: et alBlood type B might imply longevity. Exp Gerontol.
39:1563–1565. 2004. View Article : Google Scholar : PubMed/NCBI
|