Introduction
Gastric cancer (GC) is the second most common cause
of cancer-related death in the world, with the estimated 5-year
survival rate ranges from 4 to 20% (1). High mortality rate in GC is mostly due
to its detection at advanced stage (IIIA-IV), which based on
endoscopic examination followed by the histological analysis of
gastric biopsy, which is an invasive technique not applicable for
the screening of asymptomatic population (2,3). Hence,
development of noninvasive or minimally invasive tests for GC, body
fluids such as plasma, serum or urine is really necessary. For the
development of serological tests for early cancer detection,
Autoantibodies against tumor-associated antigens (TAAs) are very
attractive biomarkers for the development of noninvasive
serological tests for the early detection of cancer because of
their specificity and stability in the sera (4). By using the classical serological
identification of antigens by recombinant expression cloning
(SEREX) and proteomics techniques, a variety of TAAs of GC were
identificated, while the antibody repertoire in GC has not been
comprehensively characterized and the diagnostics significance of
the autoantibodies has not been evaluated so far (4).
In recent years, major efforts have been made to
develop sophisticated experimental and bioinformatic workflows for
sequencing adaptive immune repertoires (5). B cells are selectively activated by the
specific recognition of an antigen via the variable region of
surface B cell receptor (BCR). The BCRs undergo sequential
mechanisms to maximize diversity, mainly including rearrangement of
various V, D and J gene segments, insertion and deletion of
nucleotide at V-D or D-J joint and somatic hyper-mutation of the V
region to generate BCRs with high-affinity antigen binding sites.
The hypervariable regions in BCRs are also called CDR. Within the
variable domain, CDR1 and CDR2 are found in the V region of a
polypeptide chain, and CDR3 includes some of V, all of D (heavy
chains only) and J regions (6) We and
other researchers focused on CDR3 because of its contribution to
the generation of antibody diversity. The diversity of distinct
BCRs were estimated about 3×109 in peripheral blood for
healthy people, making the repertoire particularly difficult to
analyze (6). Recently,
high-throughput sequencing (HTS) technologies have transformed our
ability to examine antigen receptor repertoires at single
nucleotide resolution, which makes possible the study of BCR
repertoire diversity and selection mechanisms at a greater depth
than in the past (7–9).
In the present study, we studied the composition and
variation of the BCR CDR3s in peripheral blood, cancer tissues, and
peri-cancer tissues included from GC individuals by multiplex
(polymerase chain reaction) PCR and HTS. Millions of BCR reads will
be obtained for each sample, such large-scale sequencing of the BCR
repertoire in peripheral blood, cancer tissues, and peri-cancer
tissues from GC individuals may reform our perception of the immune
system. Moreover, a deep understanding of CDR3s from GC may provide
more information for the development of serological tests for early
cancer detection and promising treatment target.
Materials and methods
Clinical samples
5 ml peripheral blood samples, cancer and
peri-cancer tissue from 4 males' and 3 females' GC were collected
at Shenzhen People's Hospital (Shenzhen, China). GC patients
enrolled in the group were required to comply with the pathologic
diagnosis, and the patients had a mean age of 62.4 years, ranging
from 26 to 85 years. All patients gave written informed consent and
the present study was approved by the Medical Ethics Committee of
Shenzhen People's Hospital.
DNA extraction and mix
5 ml peripheral blood samples, 10 mg cancer and 10
mg peri-cancer tissue were obtained from each patient and DNA was
extracted by standard methods. Briefly, dewaxing was done by xylene
and followed by overnight proteinase K digestion for tissues.
QIAamp DNA Mini kit (Qiagen GmbH, Hilden, Germany) was further used
for DNA extraction following the manufacturer's instructions. DNA
quality was evaluated by loading on a 0.8% agarose gel
electrophoresis and DNA concentration was quantified by Qubit
fluorometer. DNA from 7 patients' peripheral blood samples were
mixed together by 1:1:1:1:1:1:1 according to Qubit value, renamed
one blood sample. Meanwhile, DNA from 7 patients' cancer tissues
and peri-cancer tissues were mixed by the same way.
Multiplex-PCR amplification of the IGH
CDR3 region
The utilized 12 forward primers and 4 reverse
primers were used for multiplex PCR to amplify rearranged IGH CDR3
region (10). The PCR condition was
set as 95°C for 15 min, followed by 25 cycles of 94°C for 15 sec,
60°C for 3 min, with a final extension for 10 min at 72°C. And the
PCR products were purified by AMPure XP beads to remove primer
sequences (Beckman Coulter, Inc., Brea, CA, USA). A second round
PCR was performed to add a sequencing index to each sample. The PCR
condition was set as 98°C for 1 min, followed by 25 cycles of 98°C
for 20 sec, 65°C for 30 sec and 72°C for 30 sec, with a final
extension for 5 min at 72°C. The library was separated on agarose
gel, and the target region was isolated and cleaned by QIAquick Gel
Extraction kits (Qiagen GmbH).
HTS and data analysis
The library was quantitated by Agilent 2100
bioanalyzer instrument (Agilent DNA 1000 Reagents; Agilent
Technologies, Inc., Santa Clara, CA, USA) and real-time
quantitative PCR (TaqMan Probe), sequenced by Illumina Miseq.
Briefly, the adaptor reads and the low quality reads were filtered
from raw data, and the clean data was used in further alignment.
Subsequently, the clean data was aligned to human IGH database and
analyzed by online IMGT/HighV-QUSET tool. The data were including
V, D, J assignment, CDR3 length distribution, clustering and other
analyses (11). Statistical
significance was determined using Student's t-test and P<0.05
was considered to indicate a statistically significant
difference.
Results
Summary of sequencing
We obtained 519,954 reads number of blood sample,
544,159 reads number of cancer sample and 503,171 reads number of
peri-cancer sample from 7 GC patients. After filtering, including
the removal of contamination, adaptor sequences and low-quality
reads, the data were aligned to human IGH database. On average, the
mapping IGH sequences reads number was 519,197 per sample. The mean
unknown sequences number (reads=3,031), productive sequences number
(reads=427,748), Non_productive sequences number (reads=91,449),
In_frame sequences number (458,728), and Out-of_frame sequences
number (reads=60,299) per sample were listed on Table I. The CDR3 sequences are identified by
the conserved motif. The abundance of each CDR3 clone and the
number of distinct CDR3 clone species are calculated. On average,
there were 403,959 total CDR3 sequences, 72,367 unique cdr3 nt
sequences number, 61,709 Unique cdr3 aa sequences number per sample
(Table I).
 | Table I.Human immunoglobulin heavy chain
sequence statistics. |
Table I.
Human immunoglobulin heavy chain
sequence statistics.
| Data analysis | Blood | Cancer | Peri-cancer |
|---|
| Total reads
number | 519954 | 544159 | 503171 |
| Immune sequences
number | 515136 | 541462 | 500994 |
| Unknown sequences
number | 4818 | 2697 | 2177 |
| Productive sequences
number | 407885 | 461434 | 413926 |
| Non_productive
sequences number | 107251 | 80028 | 87068 |
| In-frame sequences
number | 438135 | 494654 | 443395 |
| Out-of_frame
sequences number | 76828 | 46654 | 57415 |
| Total CDR3 sequences
number | 382253 | 437438 | 392186 |
| Unique CDR3 nt
sequences number | 57658 | 76166 | 83278 |
| Unique CDR3 aa
sequences number | 48547 | 64121 | 72460 |
Distribution characteristics of CDR3
length
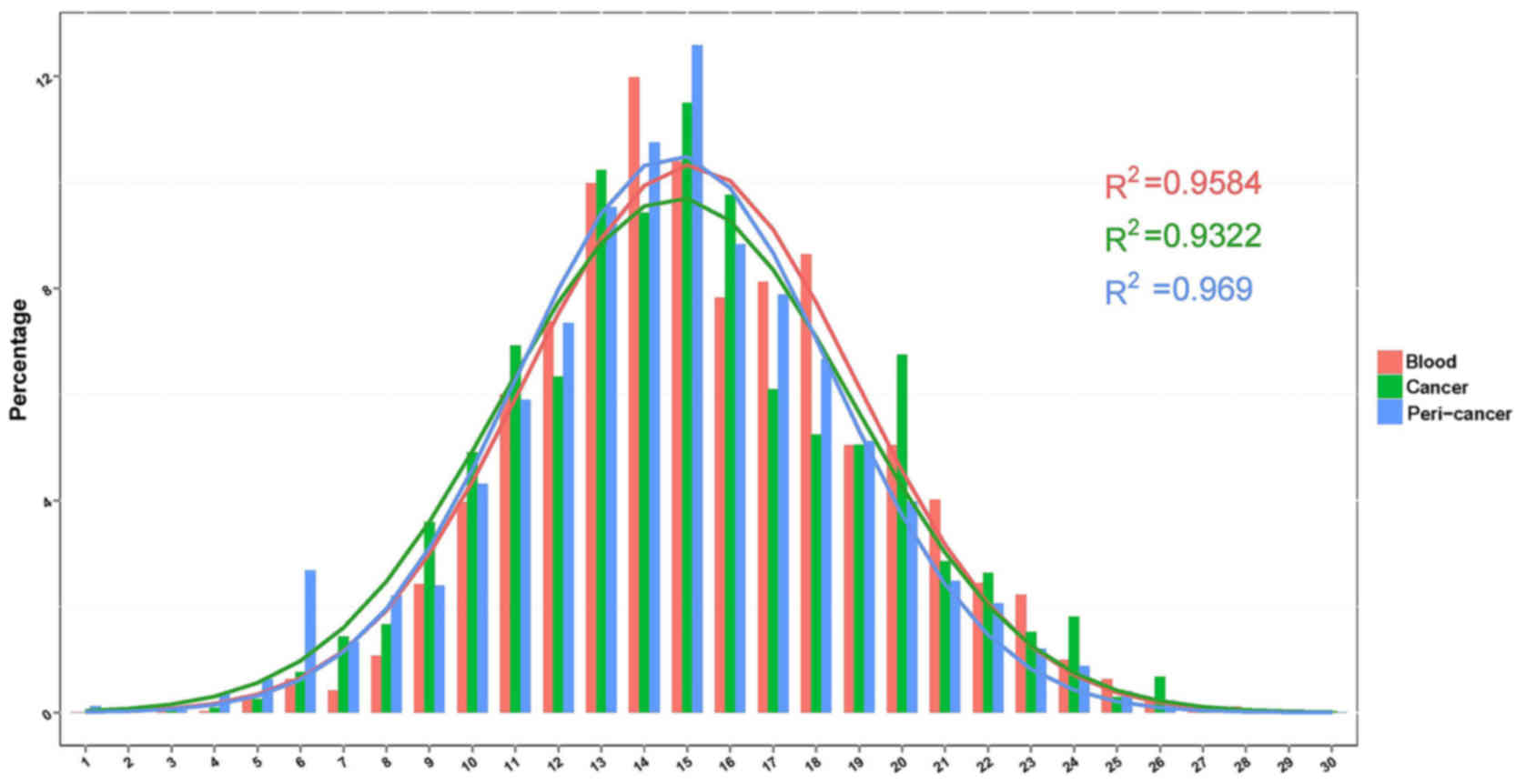
The length of the BCR CDR3 loop is an important
determinant of B cell repertoire diversity. In our study, we first
assessed length distribution of BCR CDR3 sequences (aa) in the
blood, cancer and peri-cancer group of GC (Fig. 1). Each group displayed good Gaussian
distribution of CDR3 length. Then we fitted Gaussian distribution
curve for each sample by excel. And the goodness of fit was
quantified by R2, which ranges from 0 to 1 (that is, from worst
fitness to best fitness). R2 were calculated for each sample, and
for the R2 value peri-cancer (R2=0.969) >blood (R2=0.9584)
>cancer (R2=0.9322).
Highly expanded clones (HEC)
The expression level of each clone was calculated
according to the identity of each sequence after alignment. And the
degree of expansion of each sample clone was based on the unique
CDR3 sequence frequency. Here, we defined that BCR clones with
frequency above 0.1% of total reads in a sample were HECs. In the
blood sample, we observed 180 clones were HECs, with HEC ratio
29.80% (Table II), clone aa sequence
‘ARDLSSWYRDMDV’ was the only sequence above 0.5% (0.52%). While in
the cancer sample, 82 clones were defined HECs, with HEC ratio
17.24% (Table III), there were 8 aa
sequences with ratio above 0.5% among them listed in Table III. And for peri-cancer sample, we
observed 31 clones were HECs (Table
IV), with HEC ratio 7.07%, and among them 4 aa sequences were
HEC above 0.5%. Next, we investigated whether HECs overlap among
blood, cancer and peri-cancer sample. Only two HEC sequences were
found in both cancer and peri-cancer sample, with sequence
‘ASEIHLGADRLGMGV’ 0.11% in cancer vs. 0.14% in peri-cancer,
‘ARDIGSSSWYEHLEQ’ 0.11% in cancer vs. 0.39% in peri-cancer. While
sequence ‘ASEIHLGADRLGMGV’ and sequence ‘ARDIGSSSWYEHLEQ’ were
found 0.0007 and 0.001% in blood sample.
 | Table II.Blood sample's HEC aa sequences for
ratios ≥0.1%. |
Table II.
Blood sample's HEC aa sequences for
ratios ≥0.1%.
| HEC aa sequence | HEC ratio (%) |
|---|
| ARDLSSWYRDMDV | 0.52 |
| ARDQRYYYYMDV | 0.48 |
| ARDRGYWYFDL | 0.45 |
| ARLLTMIHYYMDV | 0.38 |
| ARTTDSGQHWYFDL | 0.37 |
| ARDVIAIYYYYMDV | 0.36 |
| ARGYNPDYGMDV | 0.35 |
| ARLHRSWNEVYYGMDV | 0.33 |
| ARFGTRSNFQH | 0.31 |
| ARVGCGGGRCSLGMHV | 0.31 |
| ARGMYGDYVYYGMDV | 0.30 |
| ARDRAAPYYYYYMDV | 0.28 |
| ARQQLNLHMDV | 0.28 |
| AREDYSYYYYMDV | 0.27 |
| ARSSYYYGMDV | 0.27 |
| ARIPWDYDWYFDL | 0.27 |
| ARRRSIAAGPLDV | 0.27 |
| ARRDTLGV | 0.25 |
| ARAPYVPMDV | 0.25 |
| ARDSLHSWYDDFQN | 0.25 |
| ARDPLYYGMDV | 0.25 |
| ARSSIGYYYMDV | 0.25 |
|
ARHWSDQHLHYYYGMDV | 0.25 |
| ARGRGYVTYGMDV | 0.25 |
| ATGRYYYYGMDV | 0.24 |
| ARLPWLGGMDV | 0.24 |
|
ARLPLAYSNYYYYYGMDV | 0.23 |
| ARDSGGYYGMDV | 0.23 |
| ARDGGDYYYYYMDV | 0.22 |
| AMGGGYYYYYGMDV | 0.22 |
|
ARHPRPVTDYYYYYYMDV | 0.21 |
|
ARDTYGDYASYYYYGMDV | 0.21 |
|
ARFWTNSSSWYYYGMDV | 0.21 |
| ARDWSYGLDV | 0.21 |
| ARDHWNYDGGYMDV | 0.21 |
|
ARTSSGWYLGSYYYGMDV | 0.21 |
| ARDNDYSDYYGMDV | 0.20 |
| ARDGAAGTQYGMDV | 0.20 |
|
ARDTAMVGRGRGTYGMDV | 0.20 |
| ARDPGSSNWYFDL | 0.20 |
| ATGRRDYYYYGMDV | 0.20 |
| ARVAYCGGDCYRNLDV | 0.19 |
| ARQGFDYYYMDV | 0.19 |
| ARSLSSTYYYYYMDV | 0.18 |
| ARAVVPAAIDGWYFDL | 0.18 |
| AKCGPRGARGTMDV | 0.18 |
| AKAGRAAAGTGYFQH | 0.18 |
| ARSQMATIDYYYGMDV | 0.18 |
| ARDFGDYMDV | 0.18 |
| ARGGWSWYFDL | 0.18 |
|
ARSPGKHIVVVTVVFDL | 0.18 |
| ARDEATGVAGGMDV | 0.18 |
| ARLSPTQYYYYGMDV | 0.17 |
| ARDGIAGLDY | 0.17 |
| ARDMELGFDYYMDV | 0.17 |
|
ARVYSGYTITRWYYMDV | 0.17 |
| AKQRHTRMNYMDV | 0.17 |
| ARFKNYYYYGMDV | 0.17 |
|
ARGWNTDSYYFYMDV | 0.17 |
|
ARVGHMVRIYYYYGMDV | 0.17 |
|
AREFPAVTTPGGMDV | 0.17 |
| ARDAEGMDV | 0.17 |
|
ARDRTRLLIDYYYYYMDV | 0.17 |
|
ARDTGASHFYYYYGMDV | 0.16 |
| ARDTRYDYYYYMDV | 0.16 |
|
ARTDGLGTYYYGMDV | 0.16 |
| GRVDTLIMYGMDV | 0.16 |
| AREDEYYGMDV | 0.16 |
| ARDAF | 0.16 |
|
ARVTVAAADREGMDV | 0.15 |
|
AKADFRSAGDYYYYMDV | 0.15 |
|
ARVRFDGSGSRHYYYYGMDV | 0.15 |
| ATYTSSWYFYGMDV | 0.15 |
| ARDSPWNYYYGMDV | 0.15 |
|
ARHTKVYGDYDDSYYYYYMDV | 0.15 |
|
ARDDEAYRRSSYYYYGMDV | 0.15 |
|
ARGQRTYGRGYYYYGMDV | 0.15 |
| ARGGSSSWYMDV | 0.15 |
|
ARHPYGYNWNELGTEPNYYYMDV | 0.15 |
| ARDRGSGYFDL | 0.15 |
|
ARDQASIAAQNYGMDV | 0.15 |
|
ARLSQGYGDRDDIFQFYWYFDL | 0.15 |
|
AKDGMSYSSSWHYWYFDL | 0.15 |
| TRDAEGMDV | 0.15 |
|
ARDRGIAAAGTPYYYYGMDV | 0.15 |
|
ARDGTHCSGSRCYGYFDL | 0.15 |
|
AKAGPRWYYYYYMDV | 0.15 |
|
AREGLSGSYYYYYGMDV | 0.15 |
| AREISYYYYMDV | 0.15 |
|
AKHGGDIAGLRYFHY | 0.15 |
|
ARHSMTTSYYYGMDV | 0.14 |
|
ARDRTAVVVAASLLYGMDV | 0.14 |
|
ARGEWEPPIGYYYYGMDV | 0.14 |
| ARDIAARPEQSVQH | 0.14 |
|
ARDFEQQLLYYYYYMDV | 0.14 |
|
ARIAAAGHYYYYGMDV | 0.14 |
|
AKSATGPRPYWYFDL | 0.14 |
| ARPPEGSYYAFDI | 0.14 |
|
ARGGAANDYYYYYMDV | 0.14 |
|
ARDGQTTWLYYYYMDV | 0.14 |
|
ARDKVPAANYYYGMDV | 0.14 |
| ARDLITGTTGMDV | 0.14 |
| APKPGRRLVDV | 0.14 |
| ARDLRGGSSYGMDV | 0.14 |
| ARHDDTSGQDLHEH | 0.13 |
|
ARDTLSGGYYYYYYMDV | 0.13 |
| ARDFPGGMDV | 0.13 |
| ARDVDP | 0.13 |
|
ARDRPYSSGWSWGYGMDV | 0.13 |
|
AKSAYCTPKCNALDV | 0.13 |
|
AKVVVFPVVVPAASDYMDV | 0.13 |
|
ARQHRRVRGVMNYYGMDV | 0.13 |
|
ARDRRGGDYYYYYGMDV | 0.13 |
|
AKDSWYSRPYYGMDV | 0.13 |
|
ARHGWFGELGVYYYYGMDV | 0.13 |
| AKVYRDYYYYGMDV | 0.13 |
|
ARRRYSGYRDDAFDI | 0.12 |
|
ARDLRSGEMATPNYYYYGMDV | 0.12 |
|
ARGGVDTAMGYYYYYMDV | 0.12 |
|
ARVRGSSSWYRPRGMDV | 0.12 |
|
ARVGVPIADEDYYYYMDV | 0.12 |
|
ARDGRDSSGWYSYWYFDL | 0.12 |
| ARHLTGELFGMDV | 0.12 |
|
ASVAATTNYYYVLDV | 0.12 |
| ARGNWGTSWYFDL | 0.12 |
|
ARHKLRDGSGSYFLYMDV | 0.12 |
|
ARDLAVLGGSGLGGL | 0.12 |
|
ARDRGPYYYYYGMDV | 0.12 |
|
ARDPGGGTTGMTYYYYGMDV | 0.12 |
|
ARVVLPFGELEAMDV | 0.12 |
|
ARGVTIFGVVTYYYYMDV | 0.12 |
|
ARFRVSYGYVDYYYYGMDV | 0.12 |
| ASAYGDYGAAFDI | 0.12 |
| AKDEKGVIYYGMDV | 0.12 |
|
AREGLRNYYYYYMDV | 0.12 |
| ARGKLLSPMDV | 0.12 |
|
ARDLGTTVTTERSYYYGMDV | 0.12 |
|
ARDIRREWEPSFYYGMDV | 0.12 |
|
ARDFGHIYDEYDFDF | 0.12 |
|
ATIRCSGGSCPYYYYGMDV | 0.12 |
| ARSGYYDRYYYMDV | 0.12 |
|
ARDFTDQNTVYYGMDV | 0.12 |
|
ARGGPYYYYYYGMDV | 0.12 |
|
AREYSSNHYYYYMDV | 0.11 |
|
ARGRVPAAKGYYYYYMDV | 0.11 |
| ARDRVADDAFDI | 0.11 |
|
ARDLGGYFLYRYYGMDV | 0.11 |
|
ARDRNYYGSGSYYSTYMDV | 0.11 |
| AKDDGPYYYGMDV | 0.11 |
|
AKDPHGSSWYYYYYYYYMDV | 0.11 |
|
ARHSYDILTGYYAYYYGMDV | 0.11 |
| AKGPRSGIRFMDV | 0.11 |
|
ARQDYYDSSGYYYDYYYYGMDV | 0.11 |
| ARRVSSSWYGWSDY | 0.11 |
| ARAHGPSSWGGMDV | 0.11 |
| ASPNSSSTAYTFDY | 0.11 |
|
AREAMVRGALYYYYYGMDV | 0.11 |
|
ARECFSSWYCYYYYGMDV | 0.11 |
| AKDSIAVAGDGMDV | 0.11 |
|
ARHVGHYYGSGSYYNTYMDV | 0.11 |
|
ARALRRYVVVPAAYYYYYMDV | 0.11 |
|
ATASGRYFDYYYYMDV | 0.11 |
|
ARPGHVVPAAPAAFDI | 0.11 |
|
ARHGLRGCSDRCYTSFYYNGMDD | 0.10 |
| ARMSMTTVPK | 0.10 |
| ARGHMTPDYMDV | 0.10 |
| ARESDYYYGMDV | 0.10 |
|
AGPDTAMVSRDYYYYGMDV | 0.10 |
|
ARHEAGDLGYDAFDI | 0.10 |
|
ARDQGLVVVIKDYYYGMDV | 0.10 |
| ARDKEDPWNALDL | 0.10 |
| ALEEVGY | 0.10 |
| AKVRRAQGYYAMDV | 0.10 |
|
ARDRGGIQLWRTRYRYYYYGMDV | 0.10 |
|
ARVPTPFDYYYYMDV | 0.10 |
|
ARGRQVDSSGWYDYYYYGMDV | 0.10 |
| ARDHYSSDAFDI | 0.10 |
|
ATLYYDFFVRPGGMDV | 0.10 |
| GRDCVRAGDYGVDV | 0.10 |
| AREPLYYDYYMDV | 0.10 |
 | Table III.Cancer sample's HEC aa sequences for
ratio >0.1%. |
Table III.
Cancer sample's HEC aa sequences for
ratio >0.1%.
| HEC aa
sequence | HEC ratio (%) |
|---|
|
ARWRAAAGTRRNYSYHYMDV | 1.23 |
|
AKDFGAGLGGYYMDV | 1.01 |
| ARLQGGAVFQH | 0.79 |
|
ARGSMSIRAGWYFDL | 0.75 |
|
AADRGDYGDYEDYYYYIDV | 0.64 |
|
AREVHQRQQSEDAFDV | 0.57 |
|
ARDLCSDGVCDWYYFMDV | 0.52 |
|
ARDGRTWHYESRGFHGWFDA | 0.51 |
|
AKEGIPAAGMSEGYYYYMDV | 0.38 |
|
ARELRGGSWAGGMDV | 0.29 |
| ARTRITILGDMDV | 0.27 |
| ARDLRGSDDY | 0.27 |
| TIGHYST | 0.26 |
| ARTRITIFGDMDV | 0.23 |
| AGSSGFDPFDC | 0.23 |
| AQEIRPNDC | 0.22 |
| ATDAVNNWNSHY | 0.21 |
| EAGGAGFDC | 0.21 |
|
ARDASSSGLRYYGMDV | 0.21 |
| ARRNKGSLGWDFDY | 0.21 |
|
AKGGNTGGTNQFLSYYYHYMDV | 0.20 |
| ARDRAGDYAADD | 0.20 |
| ARQRGYYYNMDV | 0.20 |
|
AADGFQLEDFRYGMDV | 0.20 |
| ARTQWEYWYFDL | 0.18 |
|
ARLWTVTPTDYGMDV | 0.18 |
| TIGHYSS | 0.18 |
| ARTVTWGYMDV | 0.17 |
|
AKDDTTYCGGDCYFDL | 0.17 |
|
AKGGHMGGPNQFLSYYYHYMDV | 0.17 |
|
TRGGSRGDSISWYTGMHSYYGMDV | 0.17 |
| ARVLHGGGGHFHH | 0.17 |
|
ARSPYDSSGSKYYGMDV | 0.16 |
|
TRDERGRAAQTNYYYYYMDV | 0.16 |
| ARGRQDYFDF | 0.15 |
|
AKDARYCTPTSCNTPLSYSSFYYMDA | 0.15 |
| ATEDSNGGSYRRH | 0.15 |
| ARTSGSSGDAFDI | 0.15 |
|
AKDRGGPNIPDWYFDL | 0.15 |
| ARPSGAVTTMRMDV | 0.14 |
| ARVLHGGGGNFHH | 0.14 |
| ATDSLDV | 0.14 |
|
ARGGSRGDSTSWYTGMHSYYGMDV | 0.14 |
|
AKDDTTYCGGDCYFDI | 0.14 |
|
ATLDTHPAHYYYGMDV | 0.13 |
|
AKGGEGRTTYWYLDL | 0.13 |
| ARGRQDYFDY | 0.13 |
| ARFHLKDGTSSEY | 0.13 |
|
ARLSGSYYYYYYMDV | 0.13 |
| AKDQQWLIRLVHDY | 0.12 |
|
ARDPYYYGSGSRYYYYMDV | 0.12 |
|
ARGPGAAAGTYYYYSMDV | 0.12 |
| ARDYGEN | 0.12 |
|
ARGPRQIGARSSSGLEDWHFEL | 0.12 |
|
ARDISPAYRGNHAFDI | 0.12 |
|
AKARTPGTPYYYHMDF | 0.12 |
| ARSSGRAAGVDS | 0.11 |
| ARETQGFDP | 0.11 |
| VRGTITFDY | 0.11 |
|
AKTYGDYGGETAFDM | 0.11 |
|
ASEVHLQSDRLGMDV | 0.11 |
|
ARGGLPSSYYYYMDV | 0.11 |
| ARTSQLGRYLDL | 0.11 |
|
ASEIHLGADRLGMGV | 0.11 |
|
ARESGGYRYGHIDHYYNAMDV | 0.11 |
|
ARLDCSSTSCYPDLGLGYFDL | 0.11 |
|
ARDIGSSSWYEHLEQ | 0.11 |
|
AKLNSALGVTGRRGRPVYFED | 0.11 |
| ARSRDRYYSYYMDV | 0.11 |
| ARTTYSDV | 0.11 |
| ARRRYGEGFQH | 0.11 |
|
ASGTASWYDYYYGMDV | 0.11 |
| ARDPGGYSFDH | 0.11 |
|
VRGEDDDGDYVDYYYGMDV | 0.11 |
| ARIRPVYTIDF | 0.11 |
|
AKGGEGRTTYWYFDL | 0.11 |
| VTQGDGSLDF | 0.10 |
| SRARITIFGDMDV | 0.10 |
| ARETEGFDP | 0.10 |
|
ARGRGNDYGDYSYYYYMDV | 0.10 |
| GHIGTFDL | 0.10 |
|
ARGGSRGDSTSWYTGMHVYYGLDV | 0.10 |
 | Table IV.Peri-cancer sample's HEC aa sequences
for ratio >0.1%. |
Table IV.
Peri-cancer sample's HEC aa sequences
for ratio >0.1%.
| HEC aa
sequence | HEC ratio (%) |
|---|
|
VRHSSGDYRNWYFDL | 1.16 |
| ARAGYTYGEDMDV | 0.72 |
| AREMDV | 0.59 |
| CAYHDY | 0.52 |
|
ARDIGSSSWYEHLEQ | 0.39 |
| ASQRAIFRPMDV | 0.30 |
| AALLQHNGRGTFDF | 0.26 |
| AKDDEYHDSFGLDV | 0.21 |
|
ARHEVASHDSYYMDV | 0.19 |
| ARLPEGLDWHLDL | 0.17 |
|
ARGRRPDHTYFYMDV | 0.16 |
| GVYV | 0.16 |
| ARGRTPGRMDV | 0.15 |
| AVAVHGTYFWYFDV | 0.15 |
| ATSDNYYMDV | 0.14 |
|
ASEIHLGADRLGMGV | 0.14 |
| ARVPWGWFFDY | 0.12 |
| AREGI | 0.12 |
| A | 0.12 |
|
AKDVGTYYIYNYMDV | 0.12 |
|
ARSSDRAEFGGNYYYSMDV | 0.11 |
| ARPGQQRGGWYFDL | 0.11 |
|
VRRGFWSEAAIGKDGNYYYMDV | 0.11 |
| ARSATTAANWYFNL | 0.11 |
| AASSDY | 0.11 |
|
AKDRIRWSLYDFCSGFDV | 0.10 |
| AKAGGAFLYMDV | 0.10 |
|
ARGRRGYSGYENRPLLDQ | 0.10 |
|
ATLQQGHSRGSLTNPSFDYYTMDV | 0.10 |
|
ARHRRYYGSGYYMDV | 0.10 |
| ARDGMRTGNMDV | 0.10 |
Cancer special CDR3nt and aa
We further to investigate the cancer special CDR3s
with blood sample and peri-cancer sample no expression, which may
be the perfect B cell treatment target. And the top 20 cancer
special CDR3nt and aa were listed in Table V, with the sequence ratio from 0.04 to
0.11%. And the identified cancer special CDR3s need further
research.
 | Table V.Top 20 cancer special CDR3 aa and nt
sequence. |
Table V.
Top 20 cancer special CDR3 aa and nt
sequence.
| aa sequence | nt sequence | Sequence ratio
(%) |
|---|
|
GCGAGAGATCCCGGTGGATACTCCTTTGACCAC | ARDPGGYSFDH | 0.11 |
|
GCGAGACAGGGTGTTGAAGCAGCAGCTGATTCCTACTACTACTACGGTATGGACGTC |
ARQGVEAAADSYYYYGMDV | 0.08 |
|
GCGAGAGTATTACATGGCGGTGGCGGGATCCTCCATCAC | ARVLHGGGGILHH | 0.07 |
|
GCGAGAGATGGTTCGGGGAGCCCCATGGACGTC | ARDGSGSPMDV | 0.07 |
|
GCGAGAGATCCTTATTACTATGGTTCGGGAAGTTCTTCGTACTACTACTTTGACTCC |
ARDPYYYGSGSSSYYYFDS | 0.07 |
|
GCGAGAACGGCAATTGCTACGAGGGGCTACTACTACATGGACGTC |
ARTAIATRGYYYMDV | 0.07 |
|
GCAAGAGGTACTATGGTTCGGGGAGTCCTCAGAGGCTACATGGACGTC |
ARGTMVRGVLRGYMDV | 0.06 |
|
GCGAGAGATTTGACTGCAGCAGCTGGCACCCCTTTCTACTACTACAAAGGTTTGGACGTC |
ARDLTAAAGTPFYYYKGLDV | 0.06 |
|
GCGAAATGGGAGGGCACTTACCCTAAGTATTACATGGACATC | AKWEGTYPKYYMDI | 0.06 |
|
GCGAGAGCTCGTGCTTTTGATATC | ARARAFDI | 0.06 |
|
GCGAGAGGGGGTTTGGTAGCTGGAGCTATGGACGTC | ARGGLVAGAMDV | 0.06 |
|
GCGAGAGATCCTTCTTACGGTATGGACGTC | ARDPSYGMDV | 0.05 |
|
GCGAGAGGTTCGGGGAGTTCTTACTACTACTACTACGGTATGGACGTC |
ARGSGSSYYYYYGMDV | 0.05 |
|
GCGAGAGTATTACATGGCGGTGGCGGGATCTTCCACCAC | ARVLHGGGGIFHH | 0.05 |
|
GCGAGACTTGCCCGTGACCCTGCTATGGAGACTTTAGCCGGCTGGTACTTCGATCTC |
ARLARDPAMETLAGWYFDL | 0.05 |
|
GCGAGACCGCGGTATAACTGGAACTACATGGGGGACTACTACTACGGTATGGACGTC |
ARPRYNWNYMGDYYYGMDV | 0.05 |
|
GCGAGAACAGGCAAAGAAATGATGGACTACATGGTCGTC | ARTGKEMMDYMVV | 0.05 |
|
GTGAGAGGGGAGGGCGGCTACTACTACGGTATGGAAGTC | VRGEGGYYYGMEV | 0.05 |
|
GCGAGATCGTCTGGAAGGGCAGCGGGCGTTGATTCC | ARSSGRAAGVDS | 0.05 |
|
GCGAAATATAGTAGTTCGTCTAAGTACTATTATTATATGGACGTC |
AKYSSSSKYYYYMDV | 0.04 |
Discussion
GC is biologically and genetically heterogeneous
with a poorly understood carcinogenesis at the molecular level.
Despite there were many prognostic, predictive, and therapeutic
biomarkers: HER2, E-cadherin, fibroblast growth factor receptor,
mammalian target of rapamycin, and hepatocyte growth factor
receptor as well as sections on microRNAs, long noncoding RNAs,
matrix metalloproteinases, PD-L1, Baniak et al investigated
to date (12), GC continues to be
detected at an advanced stage with resultant poor clinical outcomes
(13,14).
Cancer immunoediting, which is the capacity of
immunity to control and shape cancer, that is, the result of three
processes: Elimination, equilibrium, and escape (15,16). While
the influence of gastrointestinal tract tumor differentiation on
the diversity of T-cell repertoire was investigated by Luo et
al, and they found TCR repertoire diversity have a significant
correlation with the degree of tumor differentiation (17). In this study, we used a novel HTS
protocal to investigate the BCR CDR3s in blood, cancer tissues and
peri-cancer tissues of GC individuals. On average, we obtained
403,959 total CDR3 sequences, 72,367 unique cdr3nt sequences
number, 61,709 Unique cdr3 aa sequences number per sample. Among
them, we defined that BCR clones with frequency above 0.1% of total
reads in a sample were HECs. The overlap HECs and cancer special
HECs were counted and listed in the data. Furthermore, although
this study provided some insight into the pathological processes
associated with the cancer-specific BCR CDR3s, the function role of
these sequences in the development and/or progression of cancer and
antitumor immune response is still elusive, and it would be of
great interest to explore their predictive and prognostic
significance.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds received
from Science and Technology Plan of Shenzhen, Guangdong (grant nos.
JCYJ20160422150329190 and JCYJ20140416122811914) and China
Postdoctoral Science Foundation Grant (grant no. 2017M610575).
Availability of data and materials
The aa sequence raw data is available from the
National Center for Biotechnology Information database at
ncbi.nlm.nih.gov/bioproject/438006.
Authors' contributions
SL and YZ conceived and designed the study. LWL,
SKD, XCL and KLZ performed the experiments. SL wrote the paper. KP
and YD interpreted the data and gave final approval of the version
to be published. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent and
the study was approved by the Medical Ethics Committee of Shenzhen
People's Hospital.
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park JM and Kim YH: Current approaches to
gastric cancer in Korea. Gastrointest Cancer Res. 2:137–144.
2008.PubMed/NCBI
|
|
2
|
Rosati G, Ferrara D and Manzione L: New
perspectives in the treatment of advanced or metastatic gastric
cancer. World J Gastroenterol. 15:2689–2692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi IS and Tsungteh TT: Epigenetic
alterations in gastric carcinogenesis. Cell Res. 15:247–254. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zayakin P, Ancāns G, Siliņa K, Meistere I,
Kalniņa Z, Andrejeva D, Endzeliņš E, Ivanova L, Pismennaja A,
Ruskule A, et al: Tumor-associated autoantibody signature for the
early detection of gastric cancer. Int J Cancer. 132:137–147. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedensohn S, Khan TA and Reddy ST:
Advanced methodologies in high-throughput sequencing of immune
repertoires. Trends Biotechnol. 35:203–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung D, Giallourakis C, Mostoslavsky R and
Alt FW: Mechanism and control of V (D)J recombination at the
immunoglobulin heavy chain locus. Annu Rev Immunol. 24:541–570.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calis JJA and Rosenberg BR: Characterizing
immune repertoires by high throughput sequencing: Strategies and
applications. Trends Immunol. 35:581–590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mori A, Deola S, Xumerle L, Mijatovic V,
Malerba G and Monsurro V: Next generation sequencing: New tools in
immunology and hematology. Blood Res. 48:242–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weinstein JA, Jiang N, White RA III,
Fisher DS and Quake SR: High-throughput sequencing of the zebrafish
antibody repertoire. Science. 324:807–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Hou XL, Sui WG, Lu QJ, Hu YL and
Dai Y: Direct measurement of B-cell receptor repertoire's
composition and variation in systemic lupus erythematosus. Genes
Immun. 18:22–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobi AM and Diamond B: Balancing
diversity and tolerance: Lessons from patients with systemic lupus
erythematosus. J Exp Med. 202:341–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baniak N, Senger J, Ahmed S, Kanthan SC
and Kanthan R: Gastric biomarkers: A global review. World J Surg
Oncol. 14:2122016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kodera Y: The current state of stomach
cancer surgery in the world. Jpn J Clin Oncol. 30–Aug;2016.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lazăr DC, Tăban S, Cornianu M, Faur A and
Goldis A: New advances in targeted gastric cancer treatment. World
J Gastroenterol. 22:6776–6799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koebel CM, Vermi W, Swann JB, Zerafa N,
Rodig SJ, Old LJ, Smyth MJ and Schreiber RD: Adaptive immunity
maintains occult cancer in an equilibrium state. Nature.
450:903–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee K, Hwang H and Nam KT: Immune response
and the tumor microenvironment: How they communicate to regulate
gastric cancer. Gut Liver. 8:131–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo W, Liao WJ, Huang YT, Shi M, Zhang Y,
Wen Q, Zhou MQ and Ma L: Cancer of the gastrointestinal tract
results in a restricted T-cell repertoire dependent upon tumor
differentiation. Cell Immunol. 270:47–52. 2011. View Article : Google Scholar : PubMed/NCBI
|