Introduction
Hepatic lesions of hepatocellular carcinoma (HCC) in
the late stage can cause extensive hepatic failure and the
compression of bile duct tumor, and jaundice occurs in about 10 to
40% of the patients. This change is often referred to as jaundice
or cholestatic hepatocytes. Neoadjuvant chemotherapy not only
narrows the scope of tumor surgeries, but also can significantly
improve the survival rate of patients (1–3). Unlike
other cancer patients, the central nervous system and peripheral
nervous system of patients are damaged after neoadjuvant
chemotherapy (4–6). At present, neoadjuvant chemotherapy has
been clinically promoted, and one of the key issues of
postoperative survival rate of patients is the depth of anesthesia,
so exploring the impact of chemotherapy on patients is an important
factor for safe anesthesia in patients. The target effect-site
concentration EC50 of patients receiving neoadjuvant
chemotherapy before operation is reduced compared with that of
patients receiving no chemotherapy (7,8).
Sevoflurane and desflurane are relatively new types of inhaled
anesthetics that have been widely used clinically (9,10).
Desflurane has been widely used for the maintenance
of general anesthesia for ambulatory surgery in adults and a type
of inhalational agent with the least blood gas solubility
coefficient and fastest recovery. However, desflurane has not been
widely used in the pediatric population because of its two
disadvantages: Its pungent smell and irritant nature, which makes
it unsuitable for its use for induction of general anesthesia; and
it association with inducing airway complications, such as a
laryngospasm, breath holding, and cough (11). Because of the low blood-gas and
blood-tissue solubility, sevoflurane has increasingly become more
popular, and may provide rapid recovery after general anesthesia
(12). Notably, sevoflurane is well
known to cause emergence agitation (EA). A recent Cochrane review
revealed that compared to sevoflurane, desflurane has a relative
risk of EA of 1.46 with 95% confidence interval of 0.92–2.31
(13).
Due to changes in neuropathology caused by
chemotherapy drugs, whether the neoadjuvant chemotherapy for
patients will affect the minimum alveolar concentration (MAC)
values of sevoflurane and desflurane during anesthesia needs to be
further studied. Therefore, in this study, the effects of
neoadjuvant chemotherapy drugs on sevoflurane and desflurane were
investigated by the further study on the MAC values of sevoflurane
and desflurane used to anesthetize HCC patients complicated with
jaundice after neoadjuvant chemotherapy, to develop a much safer
and more effective surgical program for HCC patients complicated
with jaundice after neoadjuvant chemotherapy and provide a
theoretical basis.
Materials and methods
General data
80 HCC patients complicated with jaundice were
selected, in which 40 patients received neoadjuvant chemotherapy.
The present study was approved by the ethics committee of Xiangyang
No. 1 People's Hospital, Hubei University of Medicine and informed
consents were signed by the patients and/or guardians. The
chemotherapy regimen was oxaliplatin combined with tegafur: 150
mg/m2 oxaliplatin was intravenously instilled for 3 h on
the 1st day; patients orally took tegafur after a meal for
consecutive 14 days, and the initial dose was adjusted according to
the body surface area of patients (the dose was adjusted to 40
mg/m2 for patients with less than 1.25 m2
body surface area; the dose was adjusted to 50 mg/m2 for
patients with 1.25~1.5 m2 body surface area; the dose
was adjusted to 60 mg/m2 for patients with more than 1.5
m2 body surface area). 21 days formed 1 cycle. The
sevoflurane and desflurane treatment time for each patient was
once. The concentration was from 0 to 2 times the concentration of
the basis of MAC. The concentration was adjusted continuously by a
special device.
Inclusion criteria: Patients whose American Society
of Anesthesiologists (ASA) Score reached Grade I–II; HCC patients
complicated with jaundice; patients with basically normal results
in preoperative routine examinations; patients whose body mass
index (BMI) was 22–23; patients aged 30–62 years old.
Exclusion criteria: Patients with a history of
severe cardiovascular system or respiratory system disease, or
renal dysfunction; patients with hepatic encephalopathy; patients
with a history of mental or neurological disease; patients with a
long history of taking psychiatric drugs or alcohol dependence;
patients whose heart rate (HR) were less than 50 bpm before skin
incision; patients whose mean arterial blood pressure (MAP) was
less than 50mmHg; patients receiving drug intervention; patients
whose intubation was not successfully conducted for the first
time.
Grouping of subjects: A total of 40 patients
receiving chemotherapy were randomly divided into the desflurane
group (Group D, n=20) and the sevoflurane group (Group S, n=20).
Patients in all the chemotherapy groups were treated with
chemotherapy for 2 cycles (14 days for 1 chemotherapy cycle), and
received surgical treatment 3 weeks after chemotherapy. A total of
40 patients receiving no neoadjuvant chemotherapy were selected as
the control group, and were randomly divided into the desflurane
group (Group C1, n=20) and the sevoflurane group (Group C2,
n=20).
Study methods
Anesthesia methods
All patients did not undergo preoperative
medication. Patients were infused with sodium chloride by injection
via the peripheral venous route at a rate of 10 ml kg−1
h−1. Electrocardiography, pulse, oxygen saturation and
bispectral index (BIS) were monitored, and radial artery
catheterization was performed under local anesthesia as an invasive
method to measure arterial blood pressure. Anesthesia was induced
by target-controlled infusion of propofol (Approval no.: National
Medicine Permission no. H20123318; manufacturing enterprise: Xi'an
Libang Pharmaceutical Co., Ltd.; target plasma concentration: 2
µg/ml) and remifentanil [Approval no.: National Medicine Permission
no. H20123422; manufacturing enterprise: China National
Pharmaceutical Industrial Corporation Ltd., (Langfang Branch);
target plasma concentration: 4 ng/ml], and changes in consciousness
of patients were carefully observed. Endotracheal intubation was
carried out for mechanical ventilation. After that, patients began
to inhale sevoflurane (Approval no.: National Medicine Permission
no. H20070172; manufacturing enterprise: Shanghai Hengrui
Pharmaceutical Co., Ltd.) and desflurane to maintain anesthesia,
respectively, and the infusion of propofol and remifentanil was
stopped. The volatilization pot was adjusted according to the end
tidal concentrations of sevoflurane and desflurane so that the end
tidal concentrations of them reached the target values and lasted
for 15 min or longer. HR, MAP and BIS before anesthesia induction
(T0), at 2 min before skin incision (T1), at 1 min before skin
incision (T2), immediately before skin incision (T3), immediately
after skin incision (T4), at 1 min after skin incision (T5) and at
2 min after skin incision were recorded, after which 0.15 mg
kg−1 cisatracurium (Approval no.: National Medicine
Permission no. H20090202; manufacturing enterprise: Zhejiang Xianju
Pharmaceutical Co., Ltd.) was used according to the operation
condition.
Test methods
The mean values of HR, MAP and BIS measured at from
T0 to T6 were calculated, and changes in HR, MAP and BIS at each
time point were compared, which was recorded by Multifunctional
monitor. If the amplitude of changes in the mean values of HR, MAP
and BIS was greater than or equal to 15%, it was considered to be
positive; if the amplitude was less than 15%, it was considered to
be negative. According to the principle of sequential allocation
method, the test would be terminated when more than or equal to 6
waveforms alternating from a positive direction to a negative
direction appeared.
The mean values of the end tidal concentrations of
sevoflurane and desflurane were selected at each pair of crossings,
which were the concentrations when half of the patients had no
response to plantar acupuncture. According to the principle of
sequential allocation method, MAC values of sevoflurane and
desflurane and 95% confidence interval (CI) were calculated.
If the MAP value was less than 50 mmHg before the
skin incision, 6 mg ephedrine (Approval no.: National Medicine
Permission no. H42021159; manufacturing enterprise: Hubei Kelun
Pharmaceutical Co., Ltd.) was intravenously injected, and if the HR
was less than 50 beats/min, 0.5 mg atropine (Approval no.: National
Medicine Approval no. H42021159; manufacturing enterprise: Hubei
Kelun Pharmaceutical Co., Ltd.) was intravenously injected.
Statistical treatments
Statistical analysis of the data was carried out
using SPSS v22.0 software (IBM Corp., Armonk, NY, USA). Measurement
data were expressed as mean ± standard deviation (x±s), and the
one-way analysis of variance was used to reveal the age, height,
weight, preoperative fasting time and preoperative infused fluid
volume, and to detect the intergroup differences in HR and MAP
values at each time point among various groups. The repeated
measures analysis of variance was used to analyze the intragroup
differences in HR and MAP values in each group. The pairwise
comparisons were detected using Student-Newman-Keuls-q (SNK-q)
test. The logistic regression analysis, Dixon method and mean
method were used to determine MAC values of sevoflurane and
desflurane, respectively. The changes in MAC values of sevoflurane
and desflurane were detected via linear regression analysis and
correlation analysis so as to determine the sex distribution ratio.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparisons of general data of four
groups of patients
80 patients completed the test, and intraoperative
and postoperative accidents did not occur in the follow-ups. The
general conditions of four groups of patients were generally
similar (P>0.05), and there were no significant differences in
all indicators during the operation (Table I).
 | Table I.Comparisons of baseline data of four
groups of patients. |
Table I.
Comparisons of baseline data of four
groups of patients.
|
|
| Sex |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Group | n | Male | Female | Age (years) | Weight (kg) | Height (cm) | BMI
(kg/m2) |
|---|
| Group D | 20 | 12 | 8 | 50.21±8.17 | 64.45±11.78 | 165.52±6.93 | 22.45±2.31 |
| Group S | 20 | 10 | 10 | 52.32±7.55 | 66.12±13.01 | 168.37±6.29 | 22.39±2.45 |
| Group C1 | 20 | 6 | 14 | 51.75±7.33 | 63.33±14.08 | 167.06±6.42 | 22.57±2.60 |
| Group C2 | 20 | 12 | 8 | 52.51±7.11 | 63.22±14.14 | 166.72±6.31 | 22.49±2.98 |
| χ2 or
t-value |
| 0.480 | 6.457 | 4.251 | 5.085 | 2.059 |
| P-value |
| 0.187 | 0.207 | 0.235 | 0.591 | 0.436 |
Changes in MAP values of four groups
of patients at different time points before anesthesia induction
and before and after skin incision
There was no significant difference in the
comparison of MAP value at T0 among four groups of patients
(P>0.05). At each time point from T1 to T6, the MAP values of
Group D were significantly lower than those of Group C1, and the
differences were statistically different (P<0.05). The MAP
values of Group S were significantly lower than those of Group C2,
and the differences were statistically different (P<0.05). The
differences in MAP values among the four groups were statistically
significant (P<0.05; Table
II).
 | Table II.Changes in MAP of four groups of
patients at different time points before anesthesia induction and
before and after skin incision. |
Table II.
Changes in MAP of four groups of
patients at different time points before anesthesia induction and
before and after skin incision.
| Group | n | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|
| Group D | 20 | 90.25±5.13 |
60.88±5.22a |
62.50±5.29a |
63.37±5.61a |
64.59±5.87a |
66.32±6.03a |
67.06±6.33a |
| Group S | 20 | 91.33±5.59 |
62.02±5.31b |
63.15±5.12b |
64.37±5.50b |
65.45±5.99b |
67.09±5.25b |
68.18±5.27b |
| Group C1 | 20 | 88.71±5.01 | 66.21±6.01 | 67.30±6.26 | 67.54±6.54 | 70.07±6.72 | 73.92±6.88 | 76.39±6.99 |
| Group C2 | 20 | 88.92±5.34 | 67.56±6.21 | 69.59±6.77 | 70.32±6.79 | 72.55±6.98 | 76.90±7.21 | 77.99±7.45 |
| F-value |
| 1.083 | 6.371 | 6.593 | 5.427 | 6.486 | 8.443 | 14.477 |
| P-value |
| 0.362 | 0.0007 | 0.0005 | 0.0011 | 0.0006 | 0.0004 | 0.0000 |
Changes in HR values of four groups of
patients at different time points before anesthesia induction and
before and after skin incision
There was no significant difference in the
comparison of HR value at T0 among four groups of patients
(P>0.05). The HR values of Group D were significantly lower than
those of Group C1, and the differences were statistically
significant (P<0.05). The MAP values of Group S were
significantly lower than those of Group C2, and the differences
were statistically different (P<0.05). The differences in MAP
values among the four groups were statistically significant
(P<0.05; Table III).
 | Table III.Changes in HR of four groups of
patients at different time points before anesthesia induction and
before and after skin incision. |
Table III.
Changes in HR of four groups of
patients at different time points before anesthesia induction and
before and after skin incision.
| Group | n | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|
| Group D | 20 | 87.52±5.96 |
73.07±6.88a |
70.22±6.53a |
68.19±6.73a |
75.72±6.90a |
78.14±7.52a |
78.99±7.83a |
| Group S | 20 | 89.59±6.10 |
74.01±6.21b |
71.05±6.31b |
69.97±6.81b |
74.99±6.93b |
79.83±6.99b |
80.07±7.92b |
| Group C1 | 20 | 85.23±5.66 | 78.15±7.03 | 75.32±7.01 | 73.81±6.82 | 78.15±7.03 | 81.33±7.95 | 88.45±8.52 |
| Group C2 | 20 | 85.91±5.83 | 79.31±7.42 | 75.07±6.98 | 72.91±6.52 | 80.56±7.69 | 85.68±7.92 | 90.39±9.21 |
| F-value |
| 2.622 | 3.927 | 3.128 | 2.978 | 3.342 | 4.127 | 4.595 |
| P-value |
| 0.0567 | 0.0116 | 0.0305 | 0.0365 | 0.0213 | 0.0186 | 0.0133 |
Changes in BIS values of four groups
of patients at different time points before anesthesia induction
and before and after skin incision
There was no significant difference in the
comparison of BIS value at T0 among four groups of patients
(P>0.05). The BIS values of Group D were significantly lower
than those of Group C1, and the differences were statistically
significant (P<0.05). The BIS values of Group S were
significantly lower than those of Group C2, and the differences
were statistically different (P<0.05). The differences in MAP
values among the four groups were statistically significant
(P<0.05; Table IV).
 | Table IV.Changes in bispectral index values of
four groups of patients at different time points before anesthesia
induction and before and after skin incision. |
Table IV.
Changes in bispectral index values of
four groups of patients at different time points before anesthesia
induction and before and after skin incision.
| Group | n | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|
| Group D | 20 | 93.23±6.90 |
81.21±6.01a |
67.30±6.26a |
61.54±6.54a |
54.07±6.72a |
48.92±6.88a |
46.39±6.99a |
| Group S | 20 | 91.56±6.21 |
80.56±6.21b |
66.59±6.77b |
60.32±6.79b |
52.55±6.98b |
46.90±6.21b |
47.99±7.45b |
| Group C1 | 20 | 93.52±6.77 | 85.08±5.22 | 72.50±5.29 | 66.37±5.61 | 58.59±5.87 | 53.32±6.03 | 57.06±6.33 |
| Group C2 | 20 | 93.84±6.82 | 87.34±5.31 | 73.15±5.12 | 67.37±5.50 | 57.45±5.99 | 55.09±5.25 | 58.18±5.27 |
| F-value |
| 0.46 | 3.927 | 3.247 | 4.957 | 3.683 | 4.325 | 4.568 |
| P-value |
| 0.709 | 0.0129 | 0.0322 | 0.0065 | 0.0232 | 0.0166 | 0.0147 |
Determination of MAC values of
sevoflurane and desflurane by mean method
The MAC values of sevoflurane and desflurane were
calculated using the mean method. The MACMean value was
2.17±0.13% (95% CI: 2.00–2.31%) in Group D, 2.09±0.17% (95% CI:
2.01–2.32%) in Group S, 3.13±0.11% (95% CI: 3.01–3.35%) in Group C1
and 3.15±0.12% (95% CI: 3.05–3.57%) in Group C2. Comparisons of
MACMean values of sevoflurane and desflurane among four
groups of patients showed that MACMean values of Group D
were significantly lower than those of Group C1, and the
differences were statistically significant (P<0.05);
MACMean values of Group S were significantly lower than
those of Group C2, and the differences were statistically
significant (P<0.05). The differences in MACMean
values among four groups were statistically significant (P<0.05;
Table V).
 | Table V.Determination of MAC values of
sevoflurane and desflurane by mean method. |
Table V.
Determination of MAC values of
sevoflurane and desflurane by mean method.
| Group | n | MACMean
(%) |
|---|
| Group D | 20 |
2.17±0.13a |
| Group S | 20 |
2.09±0.17b |
| Group C1 | 20 | 3.13±0.11 |
| Group C2 | 20 | 3.15±0.12 |
| F-value | 377.547 |
| P-value | 0.000 |
Determination of MAC values of
sevoflurane and desflurane by Dixon method
The MAC values of sevoflurane and desflurane were
calculated using the Dixon method. The MACDixon value
was 2.18% (95% CI: 2.11–2.42%) in Group D, 2.03% (95% CI:
2.01–2.53%) in Group S, 3.08% (95% CI: 3.04–3.52%) in Group C1 and
3.109% (95% CI: 3.04–3.59%) in Group C2. Comparisons of
MACDixon values of sevoflurane and desflurane among four
groups of patients showed that MACDixon values of Group
D were significantly lower than those of Group C1, and the
differences were statistically significant (P<0.05);
MACDixon values of Group S were significantly lower than
those of Group C2, and the differences were statistically
significant (P<0.05). The differences in MACDixon
values among four groups were statistically significant (P<0.05;
Table VI).
 | Table VI.Determination of MAC values of
sevoflurane and desflurane by the Dixon method (n=20). |
Table VI.
Determination of MAC values of
sevoflurane and desflurane by the Dixon method (n=20).
| Group | n | MACDixon
(%) |
|---|
| Group D | 20 | 2.18 |
| Group S | 20 | 2.03 |
| Group C1 | 20 | 3.08 |
| Group C2 | 20 | 3.09 |
| F-value | 118.322 |
| P-value | 0.000 |
Logistic regression analyses of MAC
values of sevoflurane and desflurane
Logistic regression analyses showed that the MAC
values of sevoflurane and desflurane were closely related to
whether patients received neoadjuvant chemotherapy, and
MACLog values of sevoflurane and desflurane were
decreased in patients receiving neoadjuvant chemotherapy. The
MACLog value was 2.19% (95% CI: 2.10–2.51%) in Group D,
2.05% (95% CI: 2.00–2.47%) in Group S, 3.08% (95% CI: 3.03–3.55%)
in group C1 and 3.09% (95% CI: 3.04–3.59%) in Group C2. The
differences in MACLog values among four groups of
patients were significant (P<0.05), and the comparisons between
Group D and Group C1 and between Group S and Group C2 showed
P<0.05 (Table VII).
 | Table VII.Determination of MAC values of
sevoflurane and desflurane by logistic regression analyses. |
Table VII.
Determination of MAC values of
sevoflurane and desflurane by logistic regression analyses.
| Group | n | MACLog
(%) |
|---|
| Group D | 20 | 2.19 |
| Group S | 20 | 2.05 |
| Group C1 | 20 | 3.08 |
| Group C2 | 20 | 3.09 |
| F-value |
| 19.564 |
| P-value |
| 0.003 |
Variation trend of sevoflurane and
desflurane concentrations to stimulus responses of skin
incision
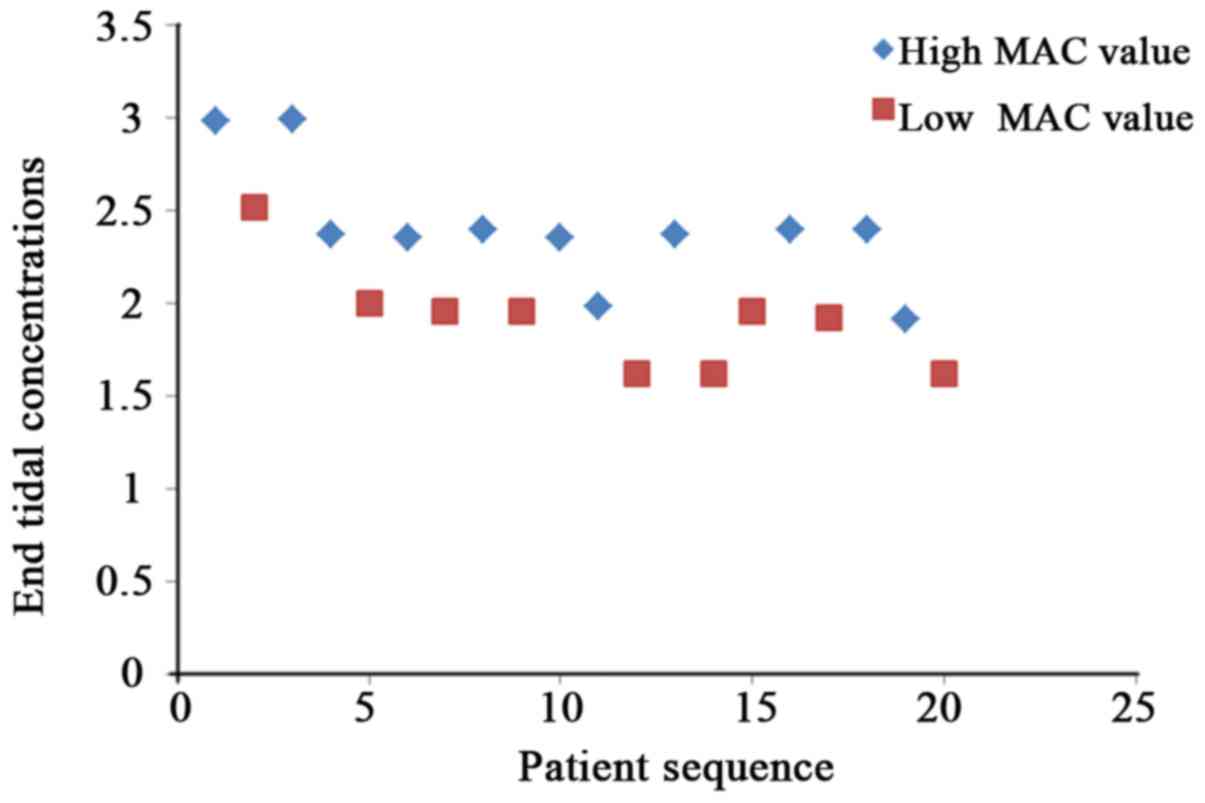
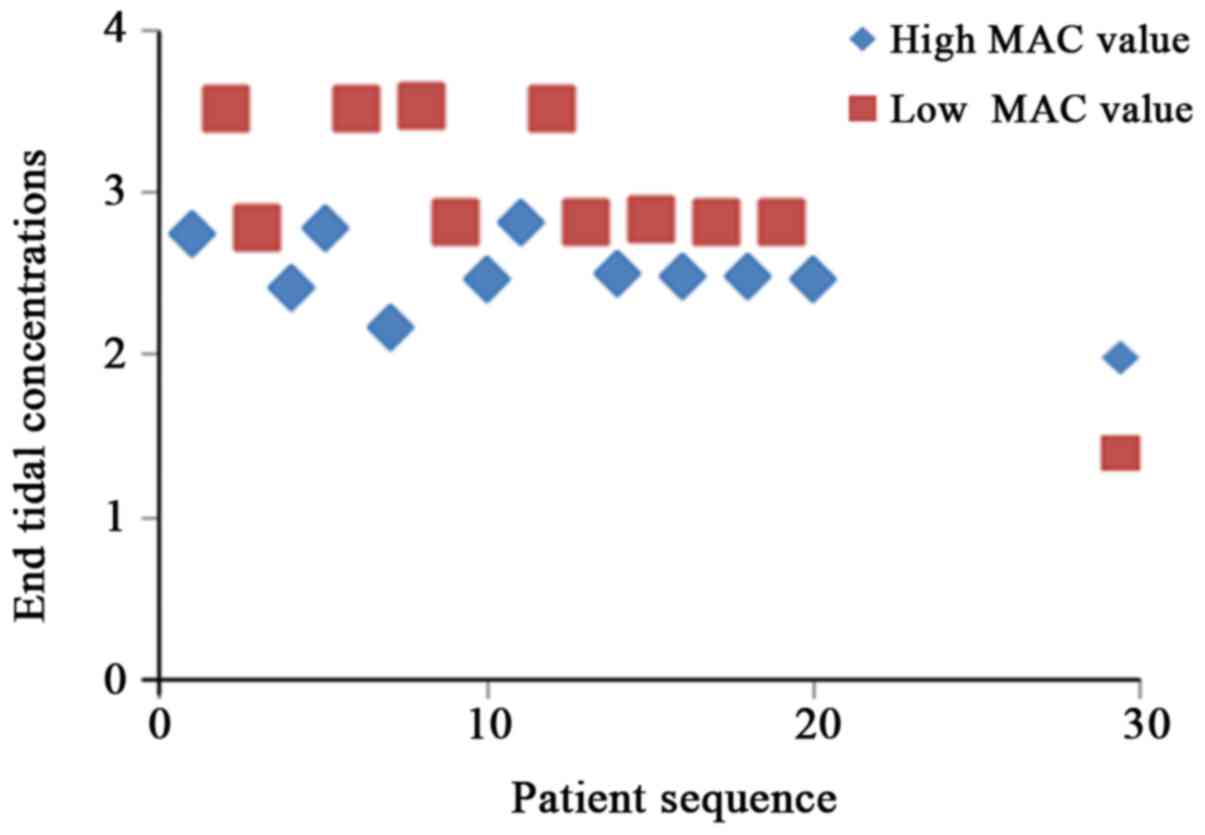
According to the principle of sequential allocation
method, the variation trend graphs of different end tidal
concentrations of sevoflurane and desflurane to stimulus responses
in four groups of patients were obtained (Figs. 1–4).
Discussion
HCC complicated with jaundice rarely occurs, and
lesions often occur in the late tumor phase with a relatively lower
surgical resection rate and a relatively higher incidence rate of
complications. Neoadjuvant chemotherapy is a systemic chemotherapy
method prior to operation or radiotherapy based on the local
treatment of malignant tumors (14,15). At
present, the study results of many researchers have gradually
confirmed the effect of neoadjuvant chemotherapy in the treatment
of malignant tumors, which is significant in patients with
malignant tumors, so in practical clinical applications,
neoadjuvant chemotherapy has been gradually applied in patients
with malignant tumors prior to operation or radiotherapy. However,
there is a need for further studies on the relationship between
chemotherapy drugs and anesthetics, so in this study, a reasonable
treatment regimen was selected before operation or radiotherapy.
Besides, HCC patients complicated with jaundice with same treatment
courses inhaled sevoflurane and desflurane for anesthesia together
with patients receiving no neoadjuvant chemotherapy. MAC values
were detected so as to explore whether neoadjuvant chemotherapy
could improve the sensitivity of HCC patients complicated with
jaundice to sevoflurane and desflurane.
There were no significant differences in the age,
sex, height, BMI and other conditions among the three groups of
patients. The surgical treatment regimen was to perform
preoperative chemotherapy for patients in the neoadjuvant
chemotherapy group. The specific action dose of oxaliplatin was 150
mg/qd and that of tegafur was 60 mg/bid. After the chemotherapy,
the computed tomography (CT) was used to assess the tumor changes,
and patients were further examined. Operation patients underwent
the surgical treatment at the end of 3-week 2 cycles of
chemotherapy, thus ensuring the homogeneity within the chemotherapy
group.
The test results also revealed that compared with
those of patients in Group C, MAC values of patients in Group S and
Group D were reduced, confirming that neoadjuvant chemotherapy
could reduce MAC values of HCC patients complicated with jaundice.
Chemotherapy leads to the emergence of many adverse reactions in
patients, and as the basic principle of selecting neoadjuvant
chemotherapy drugs and regimens is the high efficiency and low
toxicity, the regimen selected for the neoadjuvant chemotherapy
group was oxaliplatin combined with tegafur (16,17). Most
of inhaled anesthetics are discharged from the body through the
lung, and a small part of them can be discharged from the human
body through the skin and urine, but they are not discharged
through the liver, so despite of the impact of chemotherapy drugs,
the liver function of patients receiving neoadjuvant chemotherapy
may be damaged to a certain degree. However, in the body's
metabolic process, significant changes will not occur in
sevoflurane and desflurane, so that the potency of sevoflurane and
desflurane will not be affected. The reason for the decrease in MAC
values of halothane and desflurane is likely to the changes in the
efficacy of inhaled anesthetics. As the inhalation of anesthetics
calms patients down and hypnotize them, it hinders the normal brain
function of patients (18–20). Therefore, changes in the brain
functional status of patients after neoadjuvant chemotherapy result
in decreased MAC values of sevoflurane and desflurane titers
(21–23). The MAC value of sevoflurane of
patients receiving no chemotherapy detected in the test was 3.08%,
which was consistent with that in other studies (24,25).
In summary, preoperative neoadjuvant chemotherapy
can reduce MAC values of sevoflurane and desflurane of HCC patients
complicated with jaundice. Therefore, from the perspective of
improving the safety of anesthesia for patients, the dose of
anesthetics needs to be appropriately reduced for patients
receiving neoadjuvant chemotherapy. Neoadjuvant chemotherapy can
increase the sensitivity of patients to sevoflurane and desflurane,
but the specific mechanism of action still needs to be further
studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ drafted this manuscript. LZ and XM were
responsible for the conception and design of the study. MZ
collected the patient data and revised the manuscript critically
for important intellectual content. MZ and YD analyzed and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xiangyang No. 1 People's Hospital, Hubei University of Medicine.
Signed written informed consents were obtained from the patients
and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ronellenfitsch U, Schwarzbach M, Hofheinz
R, Kienle P, Nowak K, Kieser M, Slanger TE, Burmeister B, Kelsen D,
Niedzwiecki D, et al: Predictors of overall and recurrence-free
survival after neoadjuvant chemotherapy for gastroesophageal
adenocarcinoma: Pooled analysis of individual patient data (IPD)
from randomized controlled trials (RCTs). Eur J Surg Oncol.
43:1550–1558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kümmel S, Paepke S, Huober J, Schem C,
Untch M, Blohmer JU, Eiermann W, Gerber B, Hanusch C, Hilfrich J,
et al: Randomised, open-label, phase II study comparing the
efficacy and the safety of cabazitaxel versus weekly paclitaxel
given as neoadjuvant treatment in patients with operable
triple-negative or luminal B/HER2-negative breast cancer
(GENEVIEVE). Eur J Cancer. 84:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng X, Chen A, Zhang Y, Wang J, Shao L
and Wei L: Central nervous system toxicity of metallic
nanoparticles. Int J Nanomedicine. 10:4321–4340. 2015.PubMed/NCBI
|
|
4
|
Winocur G, Henkelman M, Wojtowicz JM,
Zhang H, Binns MA and Tannock IF: The effects of chemotherapy on
cognitive function in a mouse model: A prospective study. Clin
Cancer Res. 18:3112–3121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sipocz I, Pinter T, Skaliczky Z and
Kullmann T: Effective systemic palliative chemotherapy for
intracranial metastases of breast cancer. Orv Hetil. 157:1809–1813.
2016.(In Hungarian). PubMed/NCBI
|
|
6
|
Nudelman KN, McDonald BC, Wang Y, Smith
DJ, West JD, O'Neill DP, Zanville NR, Champion VL, Schneider BP and
Saykin AJ: Cerebral perfusion and gray matter changes associated
with chemotherapy-induced peripheral neuropathy. J Clin Oncol.
34:677–683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He ZJ, Hu YH and Fan ZY: Median effective
effect-site concentration of intravenous anesthetics for loss of
consciousness in neoadjuvant chemotherapy patients. Chin Med J
(Engl). 124:504–508. 2011.PubMed/NCBI
|
|
8
|
Trenerry C, Peters MDJ, Corsini N,
Damarell RA, Wilson C and Flight I: Patient-reported outcomes
following neoadjuvant chemotherapy or chemoradiotherapy treatment
for esophageal cancer: A scoping review protocol. JBI Database
System Rev Implement Rep. 15:1499–1507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weng Y, Yang L, Corringer PJ and Sonner
JM: Anesthetic sensitivity of the Gloeobacter violaceus
proton-gated ion channel. Anesth Analg. 110:59–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Q, Anderson WD, Jones ST, Souza CS,
Hosoume JM, Treptow W and Covarrubias M: Positive allosteric
modulation of Kv channels by sevoflurane: Insights into the
structural basis of inhaled anesthetic action. PLoS One.
10:e01433632015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klock PA Jr, Czeslick EG, Klafta JM,
Ovassapian A and Moss J: The effect of sevoflurane and desflurane
on upper airway reactivity. Anesthesiology. 94:963–967. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotwani MB and Malde AD: Comparison of
maintenance, emergence and recovery characteristics of sevoflurane
and desflurane in pediatric ambulatory surgery. J Anaesthesiol Clin
Pharmacol. 33:503–508. 2017.PubMed/NCBI
|
|
13
|
Costi D, Cyna AM, Ahmed S, Stephens K,
Strickland P, Ellwood J, Larsson JN, Chooi C, Burgoyne LL and
Middleton P: Effects of sevoflurane versus other general
anaesthesia on emergence agitation in children. Cochrane Database
Syst Rev. 12:CD0070842014.
|
|
14
|
Chen VE, Gillespie EF, Zakeri K, Murphy
JD, Yashar CM, Lu S and Einck JP: Pathologic response after
neoadjuvant chemotherapy predicts locoregional control in patients
with triple negative breast cancer. Adv Radiat Oncol. 2:105–109.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirkin KA, Luke FE, Gangi A, Pimiento JM,
Jeong D, Hollenbeak CS and Wong J: Sarcopenia related to
neoadjuvant chemotherapy and perioperative outcomes in resected
gastric cancer: A multi-institutional analysis. J Gastrointest
Oncol. 8:589–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hentic O, Safi D, Jerome C, Magaly Z,
Vinciane R, Sebastien G, Frederique M, Anne C, Philippe L, Philippe
R, et al: Neoadjuvant gemcitabine-oxaliplatin (GemOx) combination
followed by chemoradiotherapy (CRT) in borderline pancreatic
adenocarcinoma (BPC): A promising management. Pancreatology. 15
Suppl:S1122015. View Article : Google Scholar
|
|
17
|
Seigers R, Loos M, Van Tellingen O,
Boogerd W, Smit AB and Schagen SB: Cognitive impact of cytotoxic
agents in mice. Psychopharmacology (Berl). 232:17–37. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou C, Liang P, Liu J, Ke B, Wang X, Li
F, Li T, Bayliss DA and Chen X: HCN1 channels contribute to the
effects of amnesia and hypnosis but not immobility of volatile
anesthetics. Anesth Analg. 121:661–666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Liu J, Wang Y, Bai X, Chen Y, Qian
J, Zhu H, Liu F, Qiu X, Sun S, et al:
Methotrexate-cytarabine-dexamethasone combination chemotherapy with
or without rituximab in patients with primary central nervous
system lymphoma. Oncotarget. 8:49156–49164. 2017.PubMed/NCBI
|
|
20
|
Lutterbeck CA, Kern DI, Machado ÊL and
Kümmerer K: Evaluation of the toxic effects of four anti-cancer
drugs in plant bioassays and its potency for screening in the
context of waste water reuse for irrigation. Chemosphere.
135:403–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Dingley J, Elstad M, Scull-Brown E,
Steen PA and Thoresen M: Minimum alveolar concentration (MAC) for
sevoflurane and xenon at normothermia and hypothermia in newborn
pigs. Acta Anaesthesiol Scand. 57:646–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mishra RK, Mahajan C, Prabhakar H, Kapoor
I and Bithal PK: Effect of nitrous oxide on bispectral index values
at equi-minimum alveolar concentrations of sevoflurane and
desflurane. Indian J Anaesth. 61:482–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du W, Li C, Wang H, Zhao A, Shen J, Yong F
and Jia H: Effect of neoadjuvant chemotherapy on sevoflurane
MAC-BAR value of patients undergoing radical stomach carcinoma
surgery. Int J Clin Exp Med. 8:5649–5657. 2015.PubMed/NCBI
|
|
24
|
Voulgaris DA, Egger CM, Seddighi MR,
Rohrbach BW, Love LC and Doherty TJ: The effect of nitrous oxide on
the minimum alveolar concentration (MAC) and MAC derivatives of
isoflurane in dogs. Can J Vet Res. 77:131–135. 2013.PubMed/NCBI
|
|
25
|
Suarez MA, Seddighi R, Egger CM, Rohrbach
BW, Cox SK, KuKanich BK and Doherty TJ: Effect of fentanyl and
lidocaine on the end-tidal sevoflurane concentration preventing
motor movement in dogs. Am J Vet Res. 78:12–16. 2017. View Article : Google Scholar : PubMed/NCBI
|