Introduction
Choroidal melanoma is a rare neoplasm, although it
is the most common primary intraocular malignant tumor in adults.
The malignancy predominantly appears during the 6th decade of life.
The malignancy arises at the uveal tract melanocytes and the
choroid is most commonly involved. Clinical presentation is
non-specific, depends on the location and size of the choroidal
melanoma and includes blurring of vision, painless and progressive
visual field loss, floaters and photopsia (1). Quite often the tumor is visible during
fundoscopy, however, the most valuable diagnostic test is combined
A- and B-mode ultrasonography (2).
Prognosis depends on several factors including, age of the patient,
tumor size, histological characteristics and the presence of
metastases. Nonetheless, even with early diagnosis and appropriate
treatment by using radiation or enucleation, an estimated 40–50% of
all the patients eventually succumb to distal metastases (3,4). In the
present study, we report a case of choroidal melanoma, in a
66-year-old male. The aim of the current study is to demonstrate
the importance of identifying a choroidal mass timely, in order to
avoid possible metastases and to present the favorable aesthetic
post-operative result.
Case report
In February 2017, a 66-year-old male presented to
our clinic with chronic headache, photopsia and progressive vision
loss of 11 months duration. In his history, the patient reported
chronic obstructive pulmonary disease (COPD), five STEMIs (last
one, one year prior) for which the patient was under antiplatelet
therapy and cardiac arrhythmia for which he was administered
amiodarone treatment. Complete ocular examination was performed:
7/10 cc right eye (RE) and 2/10 cc left eye (LE). Anterior segment
was within normal in both eyes. Intraocular pressure was 15 mmHg in
both eyes. After mydriasis, fundoscopy of the left eye revealed a
retinal detachment and underneath a solid dark gray mass in the
nasal choroid. Fundoscopy of the right eye was unremarkable. B-scan
ultrasound of the left eye was performed to assess the extent of
the mass. A clinical diagnosis of choroidal melanoma was made based
on our findings, and enucleation of the left eye was determined as
the line of treatment.
Regarding surgery, conjunctival peritomy, isolation
of the four rectus muscles, isolation and displacement of the
oblique muscles and finally blunt approach to optic nerve was
carried out with curved blunt end scissors in order to cut it as
far as possible from the sclera. The Mesh-Wrapped Bioceramic
Implant, a 22 mm diameter sphere covered with mesh (FCI
ophthalmics), was implanted in the orbit to restore the volume and
the recti muscles were attached in the appropriate position and
secured at the mesh with 6/0 vicryl suture (Fig. 1). Tennon's capsule and conjunctiva
were closed separately. A conformer was placed to cover the ocular
surface and keep the fornixes in shape. Postoperative care was
given systematically and topically.
The enucleated eye was subjected to
histopathological examination. Histopathology report describes a
choroidal melanoma 2 cm in diameter and 0.8 cm in elevation,
occupying almost half of the globe, projecting in the vitreous
cavity, detaching the retina and in contact with the ciliary body.
Microscopically, the neoplasm comprised mostly of epithelioid cells
and fewer Type B spindle cells, with intense pigmentation, no
lymphocytic infiltration and no apparent necrosis, while it
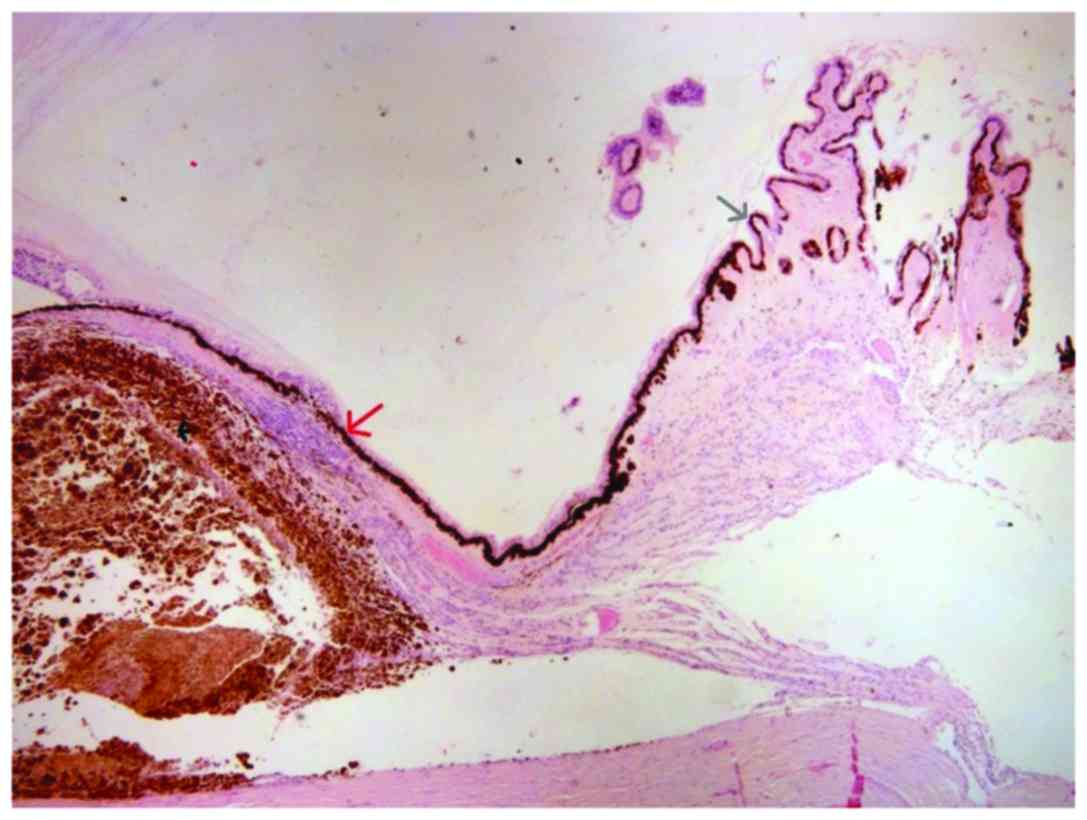
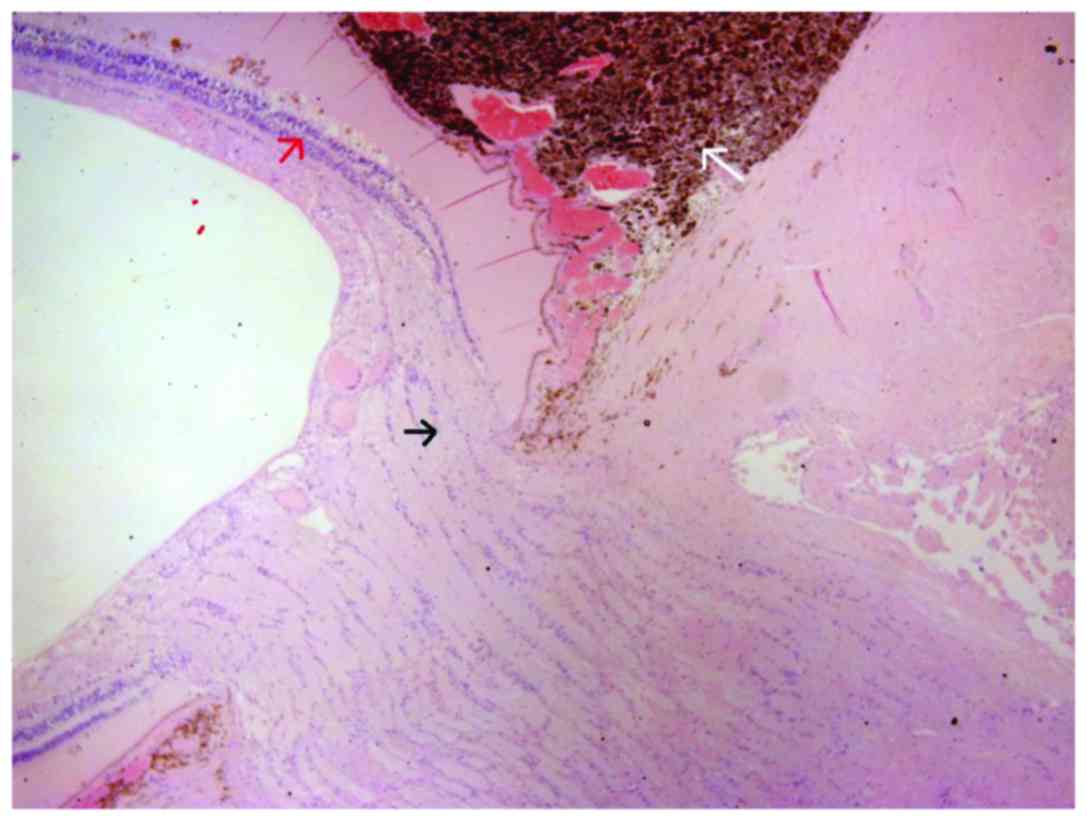
infiltrated the radial portion of the ciliary body (Fig. 2), the sclera and extended near the
optic nerve without infiltrating it (Fig.
3). AJCC staging for the melanoma was T4b. After two months a
smooth ocular surface was preset and a custom-made artificial eye
was provided with excellent aesthetic result. A follow-up of 13
months shows unremarkable ocular examination; however, on the 14th
month after initial diagnosis, oncology screening revealed several
metastases in the liver.
The Ethics Committee for Human Research of the Human
Research of the Konstantopouleio-Patission General Hospital
approved the study.
Discussion
Uveal melanoma is an uncommon entity. The estimated
incidence of primary choroidal melanomas 6–7.5 cases per 1 million
population with Caucasians most frequently affected. In most cases,
melanomas arise at about 55 years; however they can occur at any
age. These tumors are found slightly more frequently in men.
Nonetheless, choroidal melanoma is the most common primary
intra-ocular malignant tumor (1).
This malignancy arises from melanocytes in the
choroid, ciliary body, or iris. The most common site involved is
the choroid posterior to the equator, with approximately 85% of
cases localized to this area (5). The
pathophysiology of uveal melanoma, which includes choroidal and
ciliary body tumors, remains enigmatic. Apart from the progress
thus far regarding the establishment of some prognostic tools, the
molecular prognostic information remains obscure. There is a
well-known association of monosomy 3 and the development of
aggressive uveal melanoma. More recently, specific abnormalities in
loci associated with high-risk melanoma have been identified
including 3p and 1p losses and 8q gain; however no common molecular
pathway has yet been found (6).
Genetics studies have emphasized the role of
specific mutations of GNAQ and GNA11 (members of
large G proteins) as well as CDKN2A, BRCA2, p14/ARF and
BAP1 genes in the development of choroidal melanoma
(7–10). A previous study, which focused on
genetic factors associated with pigmentation traits, demonstrated
the importance of rs12913832, rs1129038 and rs916977 polymorphisms
of HERC2/OCA2 genomic region as susceptibility factors of
uveal melanoma (11).
Next generation sequence performed by using uveal
melanoma tumors indicated the significance of mutations of
EIF1AX and SF3B1 genes as predisposition factors
(12,13). Furthermore, an RNA-based assay for
choroidal melanoma prognosis that examines the expression profile
of 15 genes has been developed aiming to generate prognostic
subgroups for metastasis risk (14).
Of note, comparative genomic hybridization failed to detect any
defects or deletions when DNA from tumors was analyzed (15).
Suspected risk factors include iris color, skin
color, hormonal influence and acute or intense exposure to
ultraviolet light; however, host factors remain the prominent known
risk factor with ancestry playing the strongest role (16,17). The
risk of choroidal and ciliary body melanomas in patients with nevi
of the uveal tract is low, according to the available literature
(18).
The clinical presentation of choroidal melanomas
tends to vary as findings can be vague or non-specific and
associated with the location of the tumor. Patients usually present
with blurring of vision, painless and progressive visual field
loss, floaters and photopsia. Ocular pain can present as the tumor
presses on the ciliary nerves or due to acute angle closure
glaucoma. However, frequently the patient remains asymptomatic
until the mass has grown sufficiently to produce such symptoms.
Notably, with the choroid layer being devoid of lymphatics,
choroidal melanomas mostly metastasize via haemotogenous route
mainly to the liver (19,20).
The classic appearance of the neoplasm on dilated
fundoscopy is a pigmented, dome or mushroom shaped tumor
(indicating extension through Bruch's membrane) with surface
vasculature and orange lipofuscin pigmentation, with an associated
exudative retinal detachment (usually with melanomas greater than 4
mm in thickness). Notably, choroidal melanomas are usually
pigmented, but their pigmentation can be vary and even be
amelanotic. Findings that are not typical of choroidal melanomas
and may indicate an alternative condition are drusen overlying the
lesion, choroidal neovascularization over the surface of the tumor
and bilateral observation of the lesion (21).
Combined A- and B-mode ultrasonography represent the
most valuable diagnostic test. On A-scan ultrasonography, choroidal
melanoma shows medium to low internal echoes with smooth
attenuation and usually visible vascular pulsations. On B-scan
ultrasonography, an acoustically silent zone within the melanoma,
choroidal excavation and shadowing in the orbit are classically
observed. For tumors greater than 3 mm in thickness, a combination
of A and B-scan ultrasonography has 95% accuracy in choroidal
melanomas diagnosis (2).
Fluorescein angiography is helpful in identifying
features suspicious for melanoma, including areas of fluorescein
dye leakage and intrinsic tumor circulation (double circulation)
located in and around the lesion (3).
The overall prognosis of this rare entity is based
on several factors: The patient's age, tumor size, histological
characteristics and the presence of metastasis (3). However, even with early diagnosis and
appropriate treatment by using radiation or enucleation and
follow-up, it is estimated that 40–50% of all patients will
eventually die due to distal metastases (4).
Management depends on tumor size and targets maximum
vision sparing, quality of life and emotional result, considering
that enucleation is a form of amputation for the patient. For this
reason, important and of great consideration is the postoperative
restoration of the appearance of the enucleated orbit. The use of
new materials of intraorbital implants to restore orbit volume,
that allow suturing of the muscles on them while minimizing
complications of extrusion and inflammation of the implant,
provides implant motility and a good infrastructure for fitting
custom made artificial eyes with excellent aesthetic result,
eliminating one of the emotional stresses of these patients.
Metastases and general health status should be
considered in the treatment decision. Enucleation tends to be the
method usually preferred for medium and large ocular melanomas,
considered primarily in cases of diffuse melanomas and in the
presence of extraocular extension. Other published techniques
described, are plaque brachytherapy with iodine-125, gold-198 or
palladium-103, external beam irradiation with charged particles,
protons, or helium nuclei and Gamma knife surgery. Eyewall
resection or sclerouvectomy is an alternative option proposed in
the literature (22).
In conclusion, although uveal melanomas are rare,
one should be cautious when examining a choroidal mass. The patient
should be informed about possible metastases, living expectancy,
treatment options and expected vision outcome. Early detection is
important, thus fundus examination should be considered in patients
over 40 when they are routinely examined for presbyopia.
Acknowledgements
We would like to thank all the clinicians for
providing the data used in this study.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CT was the primary surgeon, who designed and
performed the surgical procedure. GD was the assistant surgeon. AT
designed the study and wrote the manuscript. GNG, GD, DA and DAS
collected the data and critically revised the manuscript.
Ethics approval and consent to
participate
The Ethics Committee for Human Research of the Human
Research of the Konstantopouleio-Patission General Hospital
approved the study.
Consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
Demetrios A. Spandidos is the Editor-in-Chief for
the journal, but had no personal involvement in the reviewing
process, or any influence in terms of adjudicating on the final
decision, for this article.
References
|
1
|
Rodríguez A, Dueñas-Gonzalez A and
Delgado-Pelayo S: Clinical presentation and management of uveal
melanoma. Mol Clin Oncol. 5:675–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Char DH, Stone RD, Irvine AR, Crawford JB,
Hilton GF, Lonn LI and Schwartz A: Diagnostic modalities in
choroidal melanoma. Am J Ophthalmol. 89:223–230. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanski J and Bowling B: Clinical
Ophthalmology: A Systematic Approach (7th ed). Elsevier/Saunders;
New York: pp. 501–504. 2011
|
|
4
|
Kujala E, Makitie T and Kivela T: Very
long-term prognosis of patients with malignant uveal melanoma.
Invest Ophthalmol Visual Sci. 44:4651–4659. 2003. View Article : Google Scholar
|
|
5
|
Shields CL, Kaliki S, Furuta M, Mashayekhi
A and Shields JA: Clinical spectrum and prognosis of uveal melanoma
based on age at presentation in 8,033 cases. Retina. 32:1363–1372.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sisley K, Rennie IG, Parsons MA, Jacques
R, Hammond DW, Bell SM, Potter AM and Rees RC: Abnormalities of
chromosomes 3 and 8 in posterior uveal melanoma correlate with
prognosis. Genes Chromosomes Cancer. 19:22–28. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Raamsdonk CD, Griewank KG, Crosby MB,
Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green
G, Bouvier N, et al: Mutations in GNA11 in uveal melanoma. N
Engl J Med. 363:2191–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Raamsdonk CD, Bezrookove V, Green G,
Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS and Bastian
BC: Frequent somatic mutations of GNAQ in uveal melanoma and blue
naevi. Nature. 457:599–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buecher B, Gauthier-Villars M, Desjardins
L, Lumbroso-Le Rouic L, Levy C, De Pauw A, Bombled J, Tirapo C,
Houdayer C, Bressac-de Paillerets B, et al: Contribution of
CDKN2A/P16 (INK4A), P14 (ARF), CDK4 and
BRCA1/2 germline mutations in individuals with suspected
genetic predisposition to uveal melanoma. Fam Cancer. 9:663–667.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdel-Rahman MH, Pilarski R, Cebulla CM,
Massengill JB, Christopher BN, Boru G, Hovland P and Davidorf FH:
Germline BAP1 mutation predisposes to uveal melanoma, lung
adenocarcinoma, meningioma, and other cancers. J Med Genet.
48:856–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferguson R, Vogelsang M, Ucisik-Akkaya E,
Rai K, Pilarski R, Martinez CN, Rendleman J, Kazlow E, Nagdimov K,
Osman I, et al: Genetic markers of pigmentation are novel risk loci
for uveal melanoma. Sci Rep. 6:311912016.doi:10.1038/srep31191.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harbour JW, Roberson ED, Anbunathan H,
Onken MD, Worley LA and Bowcock AM: Recurrent mutations at codon
625 of the splicing factor SF3B1 in uveal melanoma. Nat
Genet. 45:133–135. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin M, Maßhöfer L, Temming P, Rahmann
S, Metz C, Bornfeld N, van de Nes J, Klein-Hitpass L, Hinnebusch
AG, Horsthemke B, et al: Exome sequencing identifies recurrent
somatic mutations in EIF1AX and SF3B1 in uveal
melanoma with disomy 3. Nat Genet. 45:933–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onken MD, Worley LA, Char DH, Augsburger
JJ, Correa ZM, Nudleman E, Aaberg TM Jr, Altaweel MM, Bardenstein
DS, Finger PT, et al: Collaborative Ocular Oncology Group report
number 1: Prospective validation of a multi-gene prognostic assay
in uveal melanoma. Ophthalmology. 119:1596–1603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Speicher MR, Prescher G, du Manoir S,
Jauch A, Horsthemke B, Bornfeld N, Becher R and Cremer T:
Chromosomal gains and losses in uveal melanomas detected by
comparative genomic hybridization. Cancer Res. 54:3817–3823.
1994.PubMed/NCBI
|
|
16
|
Weis E, Shah CP, Lajous M, Shields JA and
Shields CL: The association between host susceptibility factors and
uveal melanoma: A meta-analysis. Arch Ophthalmol. 124:54–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seddon JM, Gragoudas ES, Glynn RJ, Egan
KM, Albert DM and Blitzer PH: Host Factors, UV radiation, andrisk
of uveal melanoma. Arch Ophthalmol. 108:1274–1280. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith JH, Padnick-Silver L, Newlin A,
Rhodes K and Rubinstein WS: Genetic study of familial uveal
melanoma: Association of uveal and cutaneous melanoma with
cutaneous and ocular nevi. Ophthalmology. 114:774–779. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kath R, Hayungs J, Bornfeld N, Sauerwein
W, Hoffken K and Seeber S: Prognosis and treatment of disseminated
uveal melanoma. Cancer. 72:2219–2223. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shields C, Materin M, Shields J,
Gershenbaum E, Singh A and Smith A: Factors associated with
elevated intraocular pressure in eyes with iris melanoma. Br J
Ophthalmol. 85:666–669. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanski J: Signs in Ophthalmology: Causes
and differential diagnosis. 66824th edition. Elsevier; New York,
NY: pp. 310–318. 2010
|
|
22
|
Singh P and Singh A: Choroidal melanoma.
Oman J Ophthalmol. 5:3–9. 2012. View Article : Google Scholar : PubMed/NCBI
|