Introduction
Ovarian cancer is a common type of malignancy, and
also the main cause of gynecological cancer mortality (1). Due to the location of the ovaries, there
are no notable symptoms in the early stage of carcinogenesis
(2). The majority of patients are
diagnosed with advanced-stage ovarian cancer, with the symptoms
including an abdominal mass, ascites and notable weight loss
(3). Surgery combined with
chemotherapy is still the most effective treatment for ovarian
cancer (4). Surgery has the ability
to eliminate the tumor foci, but it is difficult to completely
remove the tumor with surgery alone (5). Furthermore, ovarian cancer cells are
prone to metastasize, and the numerous small tumor metastases are
problematic due to the difficulty in observing them without a
microscope (6). Therefore,
chemotherapy is required following surgery to suppress residual
tumors, though the frequent use of chemotherapeutic agents can
cause adverse reactions and drug resistance (7).
Chinese herbal medicines are extensively reported to
kill tumor cells, as well as to not be susceptible to drug
resistance and to have few adverse effects (8). Naringin is a bioflavonoid and
polyphenolic compound that is located in grapefruit (9). Previous studies have indicated that
naringin has a number of pharmacological activities, including
anti-inflammation, anti-oxidative stress, myocardial protection,
and the regulation of glucose and lipid metabolism (10). Naringin can also induce cancer cell
apoptosis via eliminating free radicals using its antioxidant
activity, and by inhibiting oncogene expression and cell
proliferation (11–13). Naringin can inhibit carcinogenic
substance-induced DNA chain destruction, which maintains the
stability of genetic information and prevents the occurrence of DNA
mutations (10). Additionally,
naringin effectively inhibits β-carotene, which in turn reduces
carcinogenic substance-induced DNA chain destruction (14,15);
however, the antitumor effect of naringin in ovarian cancer and its
underlying mechanism remains unknown.
Xenotransplanted cells are vital for investigating
the mechanisms underlying tumorigenesis and treatments (16). In the present study, an in vivo
tumor model was established via implanting SKOV3 cells in nude
mice, and the intervention of naringin was evaluated by calculating
the size and weight of tumors. Furthermore, apoptosis was detected
in order to investigate the role of naringin in the treatment of
ovarian cancer. The current study aimed to provide preclinical
evidence for the treatment of ovarian cancer with naringin.
Materials and methods
Cell culture
The SKOV3 cell line was purchased from The Shanghai
Institutes for Biological Sciences, Chinese Academy of Science
(Shanghai, China). The cells were cultured at 37°C, in an
atmosphere containing 5% CO2, in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal calf serum (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin
and 100 mg/ml streptomycin.
Establishment of tumor models
A total of 30 female Balb/c nude mice (15–18 g, 4
weeks old) were purchased from The Shanghai Laboratory Animal
Center, Chinese Academy of Science, and housed in specific
pathogen-free conditions that were automatically maintained at a
temperature of 23±2°C, a relative humidity of 45–65%, and a
controlled 12/12 h light/dark cycle. Animals had ad libitum
access to food and water. All protocols were approved and
supervised by the ethics committee of the First Affiliated Hospital
of Nanchang University (Nanchang, China). The mice implanted with
the tumor cell line were randomly divided into six groups (n=5 in
each group): Control, low-dose naringin [0.5 mg/kg, intraperitoneal
(i.p.)], middle-dose naringin (1 mg/kg, i.p.), high-dose naringin
(2 mg/kg, i.p.), positive control (cisplatin, 2 mg/kg, i.p.), and
combination of cisplatin and naringin (cisplatin, 2 mg/kg;
naringin, 2 mg/kg). SKOV3 cells in the logarithmic growth phase
(1×107) were diluted in 0.2 ml PBS and injected (i.p)
into the Balb/c nude mice. Naringin (purity, >90%;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and/or cisplatin
(Sigma-Aldrich; Merck KGaA) were administered for 10 consecutive
days, following attainment of a tumor size of 50 mm3.
General conditions of the mice were monitored daily and the tumor
size was measured every two days. Following 10 days of drug
administration, the mice were anesthetized using isoflurane and
decapitated thereafter, and the whole tumor was removed. The tumor
specimens were fixed in 4% paraformaldehyde in PBS (pH 7.4) at 4°C
overnight and then embedded in paraffin for tissue sectioning.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the tumors using a
TRIzol® kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's instructions. The purity of mRNA was
determined using the optical density (OD) at 280/260 nm. Reverse
transcription was performed using Random Primer kit
(Hexadeoxyribonucleotide mixture; cat. no. 3801; Takara
Biotechnology, Co., Ltd., Dalian, China) and Moloney murine
leukemia virus reverse transcriptase (Promega Corporation, Madison,
WI, USA). The primers were added into a 25-µl PCR reaction system,
following a protocol of denaturation at 95°C for 46 sec, annealing
at 60°C for 45 sec, and then elongation at 72°C for 60 sec for 36
cycles. The primers were as follows: Cyclin D1, forward
AAACTGAAATGGGACTTGG and reverse AGGGTGGATTGGAGATAAA; survivin,
forward TTCATCCACTGCCCTACC and reverse GTGCTTTCTATGCTCCTCTAT;
c-Myc, forward GGGAGTGGTTCAGGATTGG and reverse GCATCGTCGTGGCTGTCT;
caspase-7, forward GTTGACGCCAAGCCAGAC and reverse
AATCCATGCGGTACAGATAAG; caspase-3, forward CTAATCTGACGGTCCTCC and
reverse TCGCCAAATCTTGCTAAT; Bcl-xL, forward GGGCTGTCTGTCGAATCTG and
reverse CTGGAGGCGTAAGGTCAA; Bcl-2, forward ACGTGTAACTTGTAGCGGATAT
and reverse GCTGAAAGGTGAAGAGGC; and GAPDH, forward
GCAAGTTCAACGGCACAG and reverse CGCCAGTAGACTCCACGAC.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL staining was performed on 30 µm slices using
the DeadEnd™ Colorimetric TUNEL System (Promega
Corporation), following the manufacturer's instructions. Following
staining, the sections were observed using a FV1000 Olympus
Confocal Laser Scanning Microscope (Olympus Corporation, Tokyo,
Japan).
Immunohistochemical staining
(IHC)
Tumor tissues were fixed in 10% formaldehyde at room
temperature for 24 h and embedded in paraffin. The 3-µm sections
were continuously sliced. Following dewaxing by xylene, the tissues
were dehydrated in 70, 75, 80, 85 and 95% alcohol. To retrieve the
antigen, 3% hydrogen peroxide was applied at 100°C. The slices were
blocked in 5% BSA (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) at 37°C for 30 min. Immunostaining of histological sections
was performed using monoclonal antibodies against cyclin D1
(dilution, 1:200; cat no. ab134175), c-Myc (dilution, 1:200; cat
no. ab32072), survivin (dilution, 1:200; cat no. ab469), caspase-3
(dilution, 1:200; cat no. ab13585), caspase-7 (dilution, 1:200; cat
no. ab69540), Bcl-2 (dilution, 1:200; cat no. ab32124) and Bcl-xL
(dilution, 1:200; cat no. ab32370; all from Abcam, Cambridge, MA,
USA) overnight at 4°C, followed by a 30 min incubation with
horseradish peroxidase (HRP)-coupled secondary antibodies goat
anti-mouse immunoglobulin (Ig)G HRP (dilution, 1:500; cat no.
P0447; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) or
goat anti-rabbit IgG HRP (dilution, 1:500, cat no. P0448; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) and then sections
were visualized with diaminobenzidine for 3 min under a light
microscope with ×200 magnification. PBS was used instead of the
primary antibody in the negative control group. The positive
staining was analyzed using ImageJ software version 1.48 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean and analyzed by SPSS 19.0 (IBM Corp., Armonk, NY, USA).
One-way analysis of variance with Bonferroni correction post-hoc
for multiple comparisons was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
Naringin inhibits ovarian cancer
growth
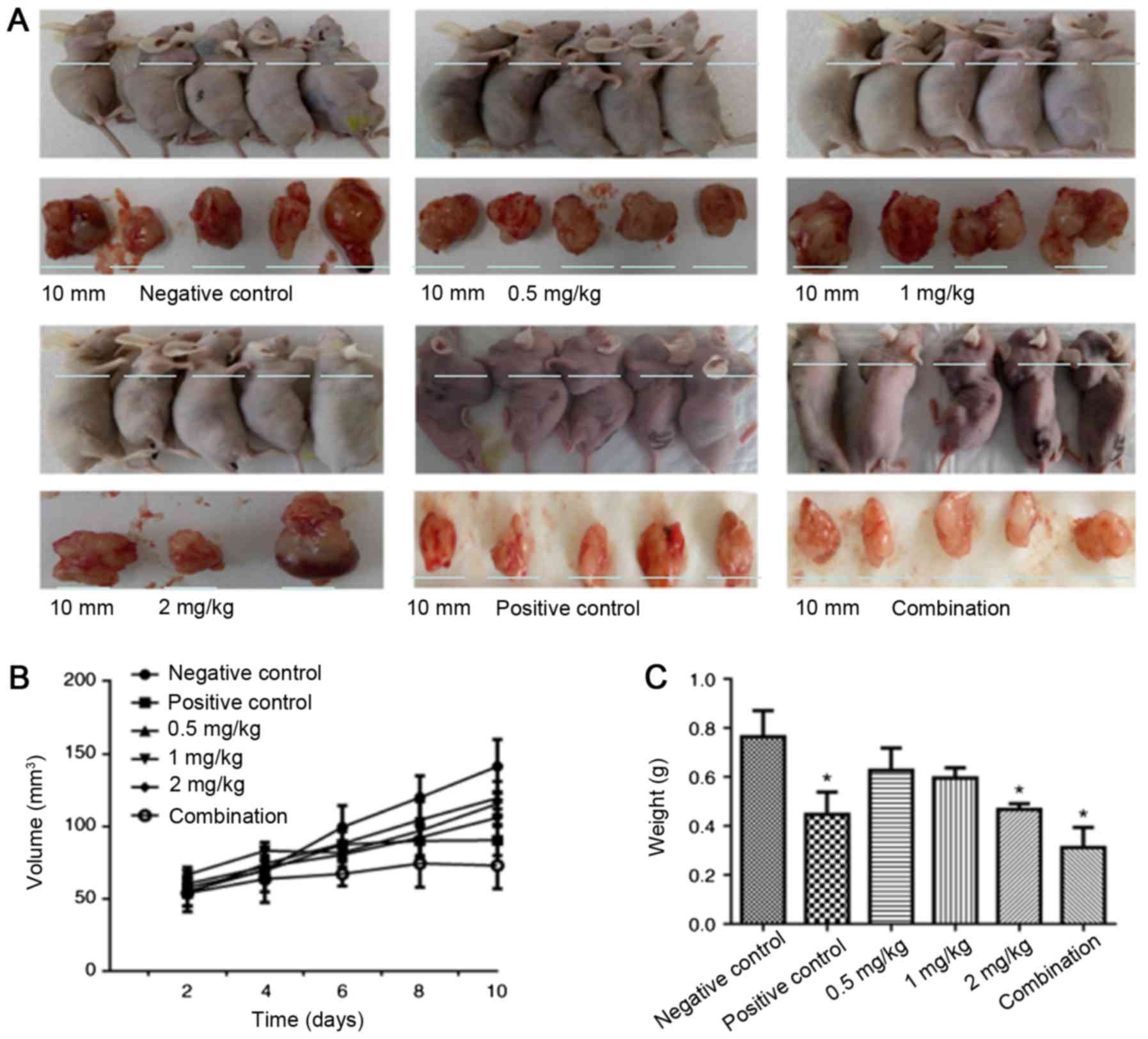
As depicted in Fig. 1A and
B, in the first four days of treatment, the mean values of
tumor sizes in each group were comparable. By contrast, following
treatment for six days, the tumor inhibition by naringin was
observable based upon the tumor sizes, particularly in the naringin
and cisplatin combination group. Following 10 days of treatment,
the tumor size (mean value) in each group was in the following
order (largest to smallest): Control, 0.5 mg/kg naringin, 1 mg/kg
naringin, 2 mg/kg naringin, positive control and combination
(Fig. 1B). The data indicated that
naringin inhibits tumor growth in a dose-dependent manner. The
tumor weight was also measured. As depicted in Fig. 1C, cisplatin, 2 mg/kg naringin, and the
cisplatin and naringin combination significantly reduced the tumor
weight, compared with the control (P<0.05).
Naringin promotes apoptosis in ovarian
tumors
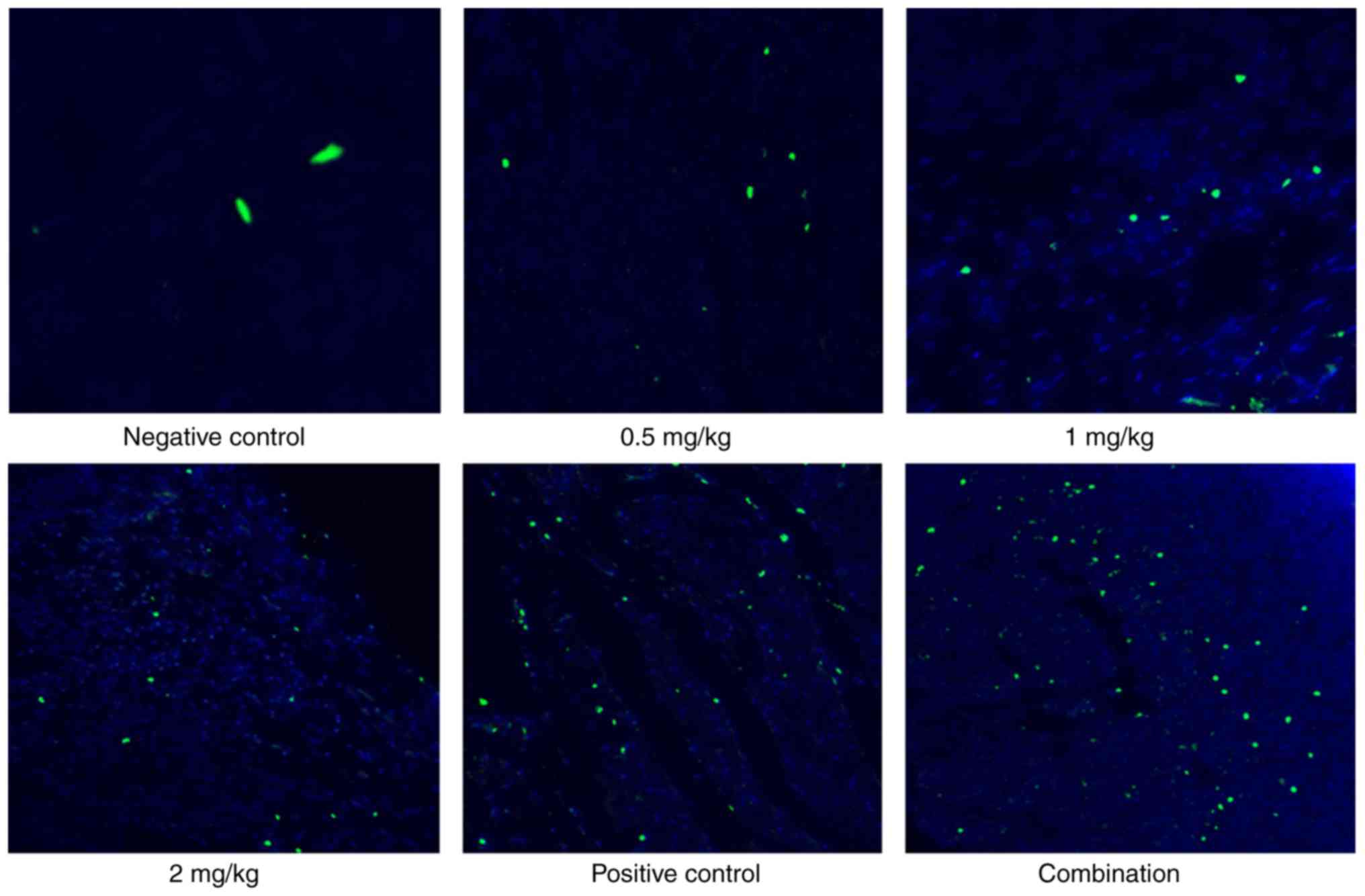
Apoptosis was detected in the tumor cells. As
depicted in Fig. 2, naringin
triggered apoptosis in ovarian tumors in a dose-dependent manner in
the range 0.5–2 mg/kg. The cisplatin, 2 mg/kg naringin, and
cisplatin and naringin combination groups notably promoted
apoptosis.
Naringin regulates
apoptosis-associated gene expression
Apoptosis-associated gene expression was also
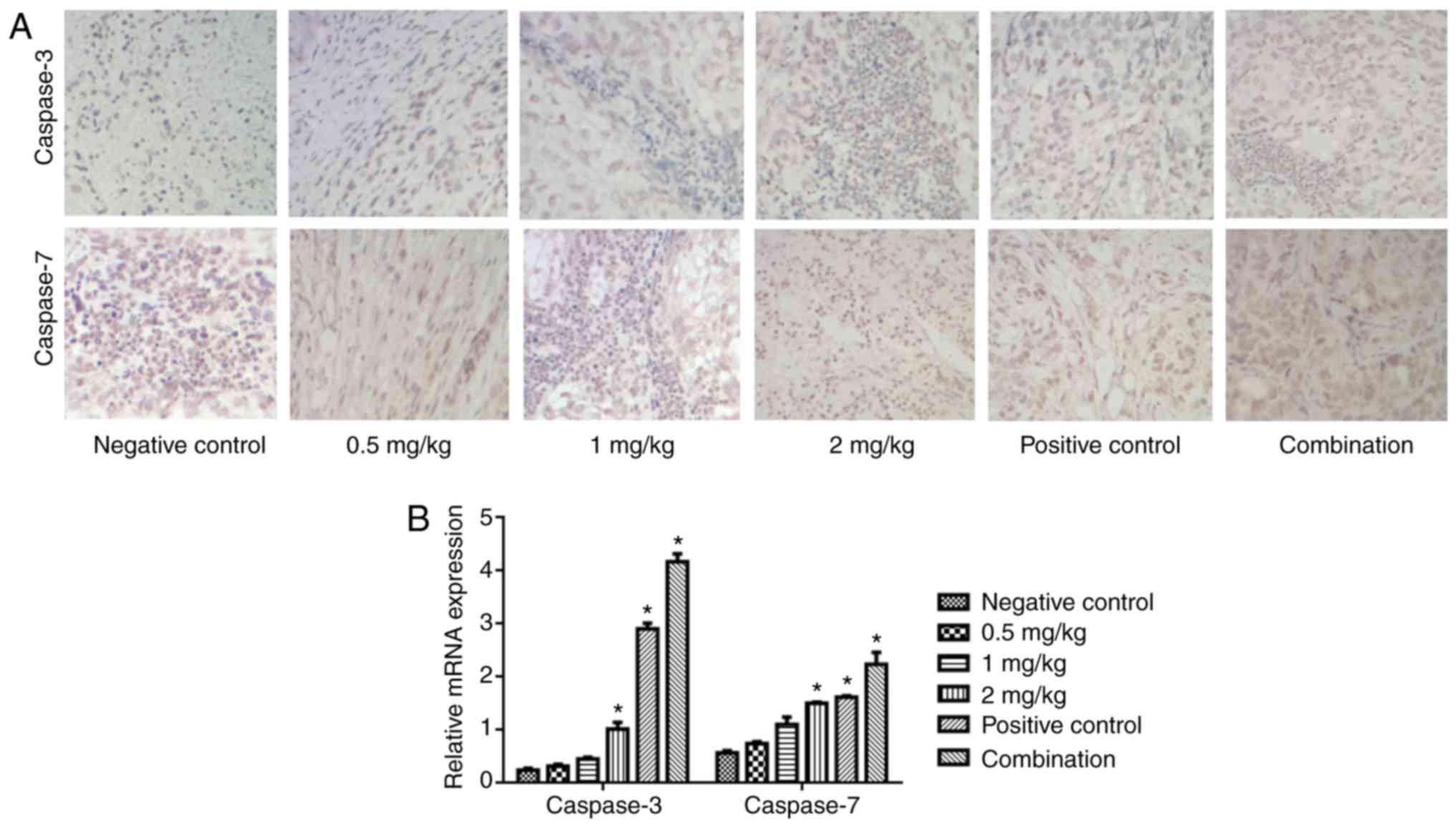
detected. As depicted in Fig. 3,
naringin increased the expression of caspase-7 and caspase-3
protein (Fig. 3A) and mRNA (Fig. 3B) in a dose-dependent manner. In
particular, the cisplatin, 2 mg/kg naringin, and cisplatin and
naringin combination groups notably promoted the expression of
caspase-7 and caspase-3, compared with the control (P<0.05).
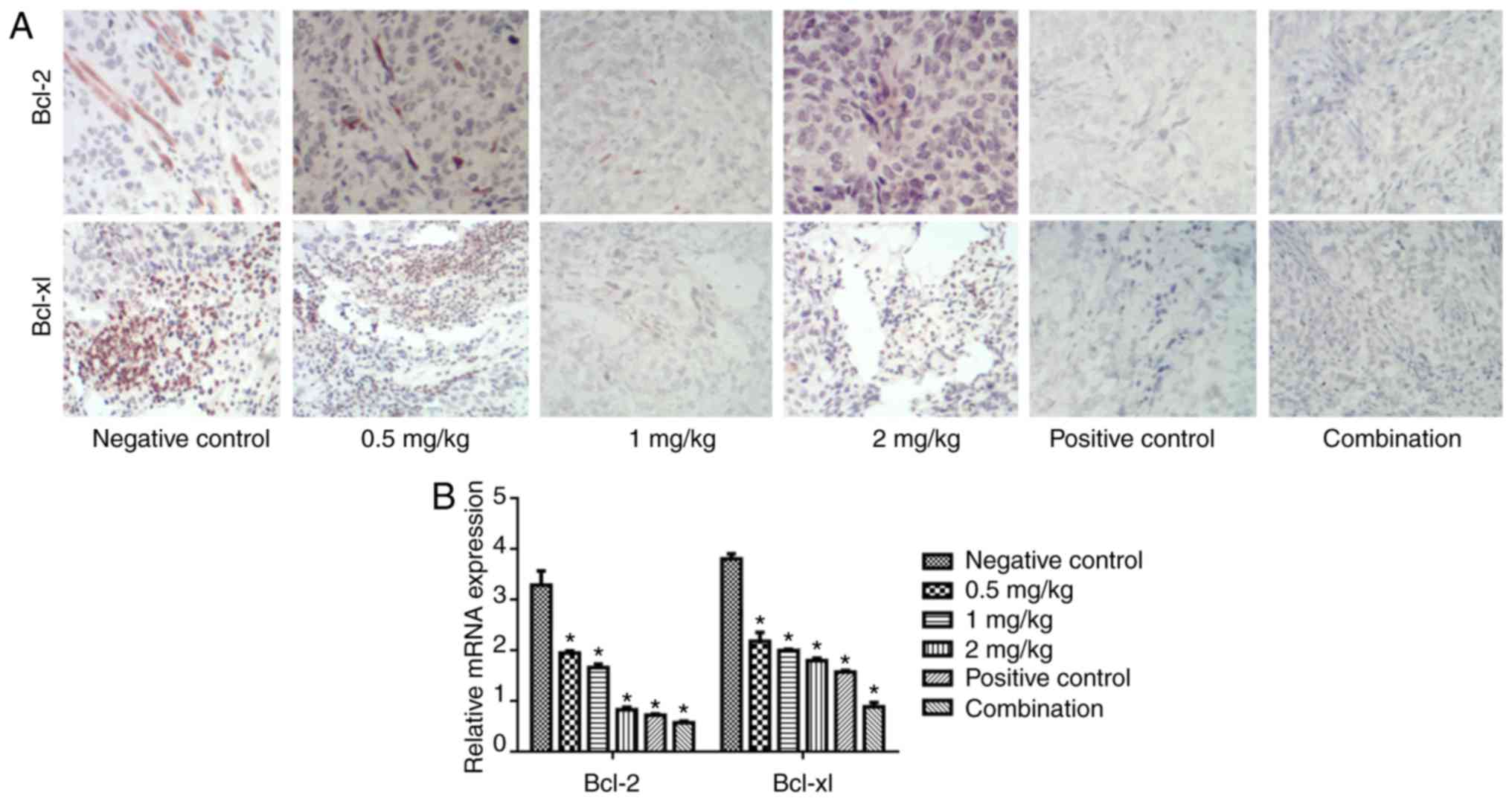
The expression of Bcl-2 and Bcl-xL was also
evaluated via IHC (Fig. 4A) and
RT-PCR (Fig. 4B). Naringin reduced
the expression of Bcl-2 and Bcl-xL protein and mRNA in a
dose-dependent manner. In particular, the cisplatin, 2 mg/kg
naringin, and cisplatin and naringin combination groups
significantly reduced the expression of Bcl-2 and Bcl-xL, compared
with the control (P<0.05).
Naringin reduces cell
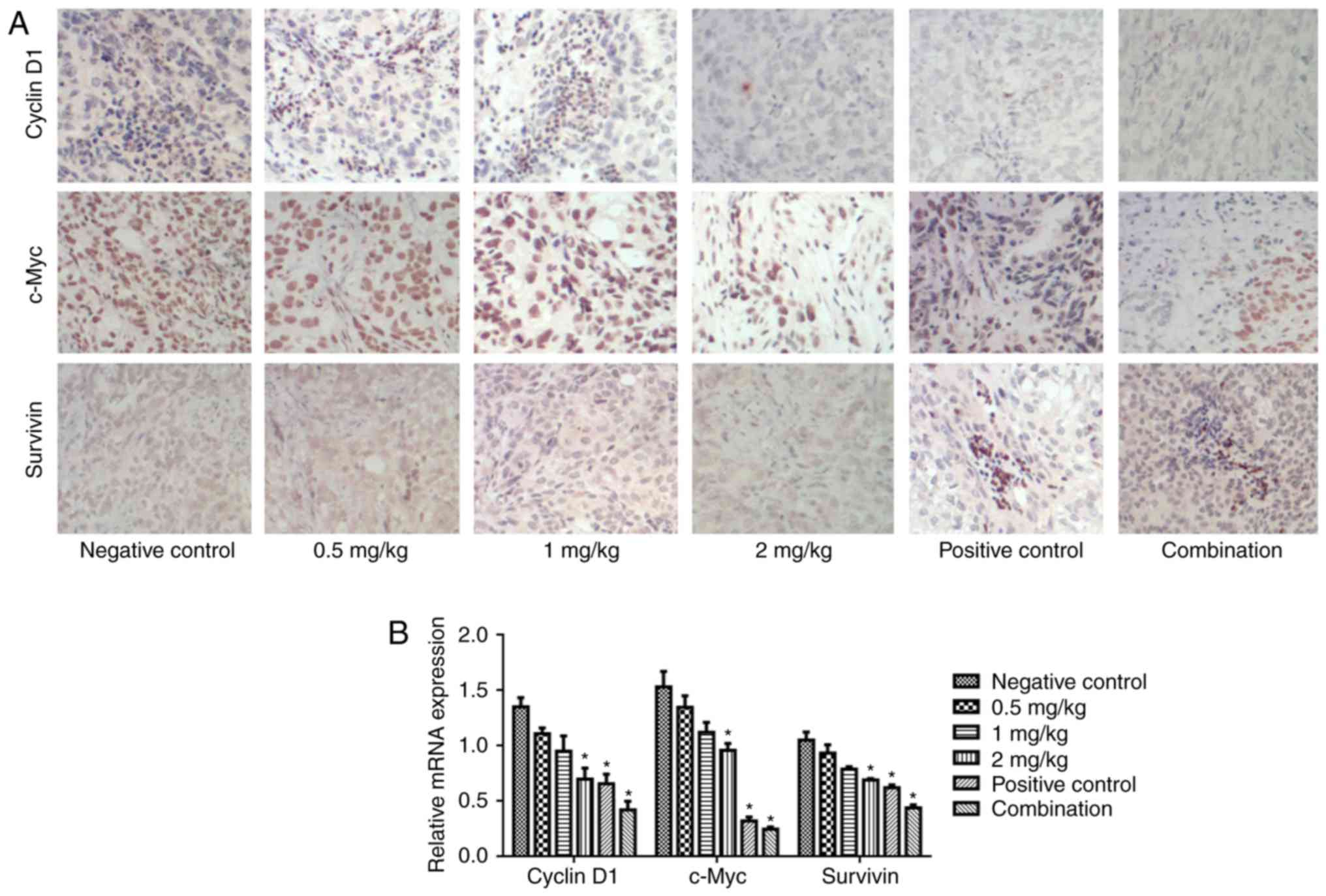
growth-associated gene expression
Cyclin D1, c-Myc and survivin were detected in each
group. As depicted in Fig. 5,
naringin reduced the expression of cyclin D1, c-Myc and survivin
protein (Fig. 5A) and mRNA (Fig. 5B) in a dose-dependent manner. In
particular, the cisplatin, 2 mg/kg naringin and cisplatin and
naringin combination groups significantly reduced cyclin D1, c-Myc
and survivin, compared with the control (P<0.05).
Discussion
In the present study, nude mouse tumor xenograft
models were produced and administered naringin. The data
demonstrated that the tumor volume and weight were reduced
gradually with the increased dose of naringin, and that naringin
has an antitumor effect in vivo.
Caspases belong to the aspartic acid specific
cysteine protease family (17).
Caspase-3 and caspase-7 serve important roles in the process of
apoptosis (18). Caspase-3 is a
regulator of cell death (18). When
caspase-3 is inhibited, apoptosis is prohibited, which contributes
to tumorigenesis (19). A previous
study demonstrated that caspase-3 and caspase-7 have synergistic
effects in promoting apoptosis (20).
The results also demonstrated that the expression of caspase-3 and
caspase-7 was upregulated significantly with an increased dose of
naringin. The results indicated that naringin has the ability to
induce apoptosis via promoting the expression of caspase-3 and
caspase-7. Bcl-2 can inhibit the apoptosis of tumor cells (21) and Bcl-xL prohibits apoptosis in the
absence of a cell growth factor (21). Takehara et al (22) revealed that the expression level of
Bcl-xL in hepatocellular carcinoma tissues was significantly
higher, compared with paracancerous tissue, which could inhibit
apoptosis during serum starvation and p53 activation. The results
demonstrated that naringin reduced the expression of Bcl-2 and
Bcl-xL, which promoted apoptosis. The data further indicated that
naringin has an antitumor effect via the promotion of
apoptosis.
Cyclin D1 belongs to the group of cell cycle
regulating proteins, can be expressed continuously under mitogen
stimulation, which can then promote cell proliferation, and
participate in tumorigenesis (23).
Cyclin D1 is highly expressed in tumor cells; it is not only
associated with the occurrence and development of tumors, but also
with tumor prognosis, metastasis and tumor recurrence following
radiotherapy (24). c-Myc affects the
processes of cell growth, differentiation and apoptosis, and the
cell cycle, and the abnormal expression of these molecules
frequently occurs in the early phase of carcinogenesis; in
addition, it is closely associated with tumor proliferation and the
initiation of cancer (25). c-Myc has
dual effects; not only does it promote cell apoptosis, but it also
stimulates cell proliferation (26).
As a nuclear protein-regulating gene, c-Myc serves an important
role in regulating tumor-associated gene expression and modulating
cell invasion, growth and metastasis (27); however, overexpression of c-Myc alone
cannot cause the malignant change in tissues (28,29). The
survivin gene is a novel inhibitor of apoptosis (30), which participates in regulating cell
growth and proliferation (31). The
results demonstrated that with an increase in the naringin
concentration, the protein expression of cyclin D1, c-Myc and
survivin was reduced significantly. These results indicate that
naringin can induce apoptosis in tumor cells.
In conclusion, the data demonstrated that naringin
inhibited ovarian tumor growth in vivo. Its mechanisms may
be associated with caspase-7, caspase-3, Bcl-2 and Bcl-xL-mediated
apoptosis. Furthermore, naringin also reduced the expression of
cyclin D1, c-Myc and survivin. Nevertheless, the clinical
application of naringin in the treatment of ovarian cancer warrants
further study.
Acknowledgements
The present study was supported by The Science and
Technology Support Plan from Jiangxi, China (grant no.
20152ACG70022).
References
|
1
|
Viau M, Renaud MC, Gregoire J,
Sebastianelli A and Plante M: Paraneoplastic syndromes associated
with gynecological cancers: A systematic review. Gynecol Oncol.
146:661–671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banach P, Suchy W, Dereziński P, Matysiak
J, Kokot ZJ and Nowak-Markwitz E: Mass spectrometry as a tool for
biomarkers searching in gynecological oncology. Biomed
Pharmacother. 92:836–842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Gynecologic Oncology Group: Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou YF: High intensity focused ultrasound
in clinical tumor ablation. World J Clin Oncol. 2:8–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
To SKY, Mak ASC, Fung Eva YM, Che CM, Li
SS, Deng W, Ru B, Zhang J and Wong AST: β-catenin downregulates
Dicer to promote ovarian cancer metastasis. Oncogene. 36:5927–5938.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mihanfar A, Fattahi A and Nejabati HR:
MicroRNA-mediated drug resistance in ovarian cancer. J Cell
Physiol. Jun 19–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo YT, Chang TT, Muo CH, Wu MY, Sun MF,
Yeh CC and Yen HR: Use of complementary traditional chinese
medicines by adult cancer patients in Taiwan: A nationwide
population-based study. Integr Cancer Ther. June 1–2017.(Epub ahead
of print).
|
|
9
|
Singh N, Bansal Y, Bhandari R, Marwaha L,
Singh R, Chopra K and Kuhad A: Naringin reverses neurobehavioral
and biochemical alterations in intracerebroventricular
collagenase-induced intracerebral hemorrhage in rats. Pharmacology.
100:172–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: An overview. Pharm Biol.
54:3203–3210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeon SM, Bok SH, Jang MK, Kim YH, Nam KT,
Jeong TS, Park YB and Choi MS: Comparison of antioxidant effects of
naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta.
317:181–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jagetia GC and Reddy TK: Modulation of
radiation-induced alteration in the antioxidant status of mice by
naringin. Life Sci. 77:780–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajadurai M and Stanely Mainzen Prince P:
Preventive effect of naringin on lipid peroxides and antioxidants
in isoproterenol-induced cardiotoxicity in Wistar rats: Biochemical
and histopathological evidences. Toxicology. 228:259–268. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Wang C, Peng J, Liang J, Jin Y, Liu
Q, Meng Q, Liu K and Sun H: Naringin inhibits TNF-α induced
oxidative stress and inflammatory response in HUVECs via Nox4/NF-κB
and PI3K/Akt pathways. Curr Pharm Biotechnol. 15:1173–1182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramalingayya GV, Nampoothiri M, Nayak PG,
Kishore A, Shenoy RR, Rao Mallikarjuna C and Nandakumar K: Naringin
and rutin alleviates episodic memory deficits in two differentially
challenged object recognition tasks. Pharmacogn Mag. 12 Suppl
1:S63–S70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao X, Lin W, Liang C, Zhang D, Yang F,
Zhang Y, Zhang X, Feng J and Chen C: Naringin rescued the
TNF-α-induced inhibition of osteogenesis of bone marrow-derived
mesenchymal stem cells by depressing the activation of NF-κB
signaling pathway. Immunol Res. 62:357–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang HY and Yang X: Proteases for cell
suicide: Functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu G, Wang X, Wu S and Li Q: Involvement
of activation of PI3K/Akt pathway in the protective effects of
puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell
death. Neurochem Int. 60:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linder M and Tschernig T: Vasculogenic
mimicry: Possible role of effector caspase-3, caspase-6 and
caspase-7. Ann Anat. 204:114–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Médoc M, Dhilly M, Matesic L, Toutain J,
Krause-Heuer AM, Delamare J, Fraser BH, Touzani O, Barré L,
Greguric I and Sobrio F: In vivo evaluation of radiofluorinated
caspase-3/7 inhibitors as radiotracers for apoptosis imaging and
comparison with [18F]ML-10 in a stroke model in the rat. Mol
Imaging Biol. 18:117–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zolota V, Gerokosta A, Melachrinou M,
Kominea A, Aletra C and Scopa CD: Microvessel density,
proliferating activity, p53 and bcl-2 expression in in situ
ductal carcinoma of the breast. Anticancer Res. 19:3269–3274.
1999.PubMed/NCBI
|
|
22
|
Takehara T, Liu X, Fujimoto J, Friedman SL
and Takahashi H: Expression and role of Bcl-xL in human
hepatocellular carcinomas. Hepatology. 34:55–61. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Cui J, Yu Q, Wu X, Pan A and Li L:
Evaluation of CCND1 amplification and CyclinD1 expression: Diffuse
and strong staining of CyclinD1 could have same predictive roles as
CCND1 amplification in ER positive breast cancers. Am J Transl Res.
8:142–153. 2016.PubMed/NCBI
|
|
24
|
Yamamoto K, Lee BJ, Li C, Dubois RL,
Hobeika E, Bhagat G and Zha S: Early B-cell-specific inactivation
of ATM synergizes with ectopic CyclinD1 expression to promote
pre-germinal center B-cell lymphomas in mice. Leukemia.
29:1414–1424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kandela I, Jin HY and Owen K:
Reproducibility Project: Cancer biology: Registered report: BET
bromodomain inhibition as a therapeutic strategy to target c-Myc.
Elife. 4:e070722015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CY, Lovén J, Rahl PB, Paranal RM,
Burge CB, Bradner JE, Lee TI and Young RA: Transcriptional
amplification in tumor cells with elevated c-Myc. Cell. 151:56–67.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang E, Li W, Yin D, De W, Zhu L, Sun S
and Han L: c-Myc-regulated long non-coding RNA H19 indicates a poor
prognosis and affects cell proliferation in non-small-cell lung
cancer. Tumour Biol. 37:4007–4015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu DW, Hsu NY, Wang YC, Lee MC, Cheng YW,
Chen CY and Lee H: c-Myc suppresses microRNA-29b to promote tumor
aggressiveness and poor outcomes in non-small cell lung cancer by
targeting FHIT. Oncogene. 34:2072–2082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia B, Tian C, Guo S, Zhang L, Zhao D, Qu
F, Zhao W, Wang Y, Wu X, Da W, et al: c-Myc plays part in drug
resistance mediated by bone marrow stromal cells in acute myeloid
leukemia. Leuk Res. 39:92–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato J, Kuwabara Y, Mitani M, Shinoda N,
Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J and
Fujii Y: Expression of survivin in esophageal cancer: Correlation
with the prognosis and response to chemotherapy. Int J Cancer.
95:92–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitrović Z, Ilić I, Aurer I, Kinda SB,
Radman I, Dotlić S, Ajduković R and Labar B: Prognostic
significance of survivin and caspase-3 immunohistochemical
expression in patients with diffuse large B-cell lymphoma treated
with rituximab and CHOP. Pathol Oncol Res. 17:243–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|