Introduction
Ovarian cancer is one of the most common causes of
cancer-associated mortality in women (1). The majority of patients with ovarian
cancer are diagnosed at an advanced stage, as there are no reliable
symptoms for early diagnosis. Even at advanced stages of disease,
signs and symptoms remain nonspecific (2). Upon diagnosis, ovarian cancer has often
already metastasized to the uterus, peritoneum or other organs in
the pelvic cavity (3,4). Radical surgery and adjuvant chemotherapy
are common modes of treatment for ovarian cancer (5,6).
Vitamin D has been reported to inhibit the
recurrence and distant metastasis of 19 types of cancer, of which
ovarian cancer is one (7,8). Furthermore, a previous study
demonstrated that mortality rates of ovarian cancer are lower in
areas with higher levels of ultraviolet-B (UVB) radiation (9). An association between vitamin D
deficiency and the occurrence of ovarian cancer has been suggested
(10).
The germ cell-specific marker DEAD
(Asp-Glu-Ala-Asp)-box helicase 4 (DDX4), which is the human
ortholog of the Drosophila vasa gene, encodes a member of
the DEAD-box family of ATP-dependent RNA helicases (11). DDX4 is expressed solely in germ cells,
including oocytes and spermatocytes, and has been reported to serve
a central role in several aspects of germ cell development
(11). A previous study demonstrated
that DDX4 is overexpressed in ovarian cancer (12).
The present study demonstrated that the
proliferative and invasive capacities of ovarian cancer cells were
suppressed by active vitamin D. Vitamin D treatment downregulated
the expression level of DDX4, and knockdown of DDX4 reduced the
invasive ability of ovarian cancer cells.
Materials and methods
Cell culture
The ovarian epithelial carcinoma SKOV3 and OVCAR3
cell lines were purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). Cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Zhejiang Tianhang Biotechnology Co., Ltd., Sijiqing, China)
and 1% penicillin-streptomycin (Beyotime Institute of
Biotechnology, Haimen, China), and incubated at 37°C with 5%
CO2. When cultured to 70% confluence, SKOV3or OVCAR3
cells were treated with active vitamin D (catalog no. D1530;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Small interfering RNA (siRNA)
treatment
The siRNAs targeting DDX4 were designed by
GenePharma Co., Ltd. (Shanghai, China) and transfected (100 pmol
siRNA for each well containing 2×105 cells) into SKOV3
or OVCAR3 cells using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA was
extracted 3 or 5 days after transfection for the following
experiments. The sequences for DDX4-712 (7656; GenePharma Co.,
Ltd.) were as follows; sense, 5′-GGAAGUGAACGAGGUGGUUTT-3′ and
antisense, 5′-AACCACCUCGUUCACUUCCTT-3′. The sequences for DDX4-121
(7654; GenePharma Co., Ltd.) were as follows; sense,
5′-GCAGAAAUCAACCCUCAUATT-3′ and antisense,
5′-UAUGAGGGUUGAUUUCUGCTT-3′. The sequences for the negative control
(7653; GenePharma Co., Ltd.) were as follows: Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′, and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Cell Counting Kit-8 (CCK-8) assay
The relative cell number was measured using a CCK-8
assay. Briefly, cells were cultured in 96-well plates at a density
of 1×103 cells/well for 24 h, then treated with 0, 25,
50, 100, 250 or 400 µl/ml active vitamin D for 72 h. Subsequently,
10 µl CCK-8 dye (Beyotime Institute of Biotechnology) was added to
each well, according to the manufacturer's protocol. The plates
were read using a microplate reader at a wavelength of 450 nm.
Relative cell number was represented by the absorbance value
relative to that of the untreated control cells.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SKOV3 or OVCAR3 cells
using TRIzol (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. cDNA was synthesized using a ReverTraAce
qPCR kit (Toyobo Life Science, Osaka, Japan, Japan), according to
the manufacturer's protocol. PCR was performed using an ABI PRISM
7500 system (Thermo Fisher Scientific, Inc.), and the thermocycling
conditions were as follows: 95°C for 60 sec, 40 cycles of 95°C of
15 sec and 60°C of 60 sec. The primer sequences for DDX4 are as
follows: Forward, CCAGAGGGCTGGATATTGAA, and reverse,
GCCAGTATTCCCACAACGAC. The primer sequences for GAPDH are as
follows: Forward, AATCCCATCACCATCTTCCA and reverse,
AAATGAGCCCCAGCCTTCT. The
ΔCq=Cqgene-Cqreference calculation was
adopted to scale the relative levels of gene expression, and
2−ΔΔCq method was used to calculate the fold change of
gene expression (13). qPCR was
performed in duplicate for 3 independent groups of treated
cells.
Western blotting
SKOV3 and OVCAR3 cells extracts were lysed with
mammalian protein extraction reagent (CWBIO, Beijing, China)
supplemented with 1% protease inhibitors (CWBIO) at 4°C for 30 min.
The suspension was then centrifuged at 10,000 × g for 10 min at
4°C. The supernatant was collected and the protein concentration
was determined using a BCA protein quantitation kit (Pierce; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Proteins (10 µg) were separated by 12% SDS-PAGE and transferred
into nitrocellulose membranes (Merck KGaA, Darmstadt, Germany). The
membranes were blocked with 5% non-fat milk in Tris buffered saline
with 0.5% Tween-20 for 1 h at room temperature, then incubated with
the following primary antibodies overnight at 4°C: DDX4 (dilution
1:1,000, cat. no. ab13840; Abcam, Cambridge, UK) and GAPDH
(dilution 1:1,000, cat. no. AG019-1; Beyotime Institute of
Biotechnology, Haimen, China). The membranes were then incubated
with a horseradish peroxidase-conjugated anti-rabbit secondary
antibody (dilution, 1:1,000, cat. no. SA00001-2; ProteinTech Group,
Inc., Chicago, IL, USA) for DDX4 and anti-mouse for GAPDH (dilution
1:1,000, cat. no. AF0006, Beyotime Institute of Biotechnology) for
2 h at room temperature and visualized by chemiluminescence using
an eECL western blot kit (cat. no. CW0049; CWBIO) and western
enhanced chemiluminescence substrates (cat. nos. 102030838 and
102030839; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
western blotting results were quantified using ImageJ software
(version 1.48; National Institutes of Health, Bethesda, MD,
USA).
Transwell invasion assay
The transwell apparatus was assembled using 8-µm
pore Transwell inserts (Corning Incorporated, Corning, NY, USA) in
24-well plates. Each insert were coated with 100 µl Matrigel
(diluted in PBS, 1:1). A total of 1×105 SKOV3 or OVCAR3
cells were seeded onto the insert and cultured with 250 µl
RPMI-1640 medium supplemented with 1% FBS, while the lower chambers
contained 500 µl RPMI-1640 supplemented with 10% FBS. Subsequent to
incubation for 3 or 5 days, the cells in the upper chambers were
removed carefully using cotton swabs, and cells that traversed the
Matrigel to the lower surface of the insert were fixed using 4%
paraformaldehyde at room temperature for 20 min and stained with
eosin (0.5%, R20593, Shanghai Yuan Ye Biological Technology Co.,
Ltd.) (SKOV3 cells) or hematoxylin (D005, Nanjing Jiancheng
Bioengineering Institute, Nanjing China, 0.5%) (OVCAR3 cells)
alone, both at room temperature for 20 min. Cells were observed and
calculated in five random fields using alight microscope
(magnification ×100).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA). The data are presented as the mean ± standard error of the
mean. Statistically significant differences between mean values of
two groups were identified using unpaired Student's t-test.
Statistically significant differences between mean values of ≥3
groups were identified using analysis of variance and
Student-Newman-Keuls post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Active vitamin D suppresses the
proliferation of ovarian cancer cells
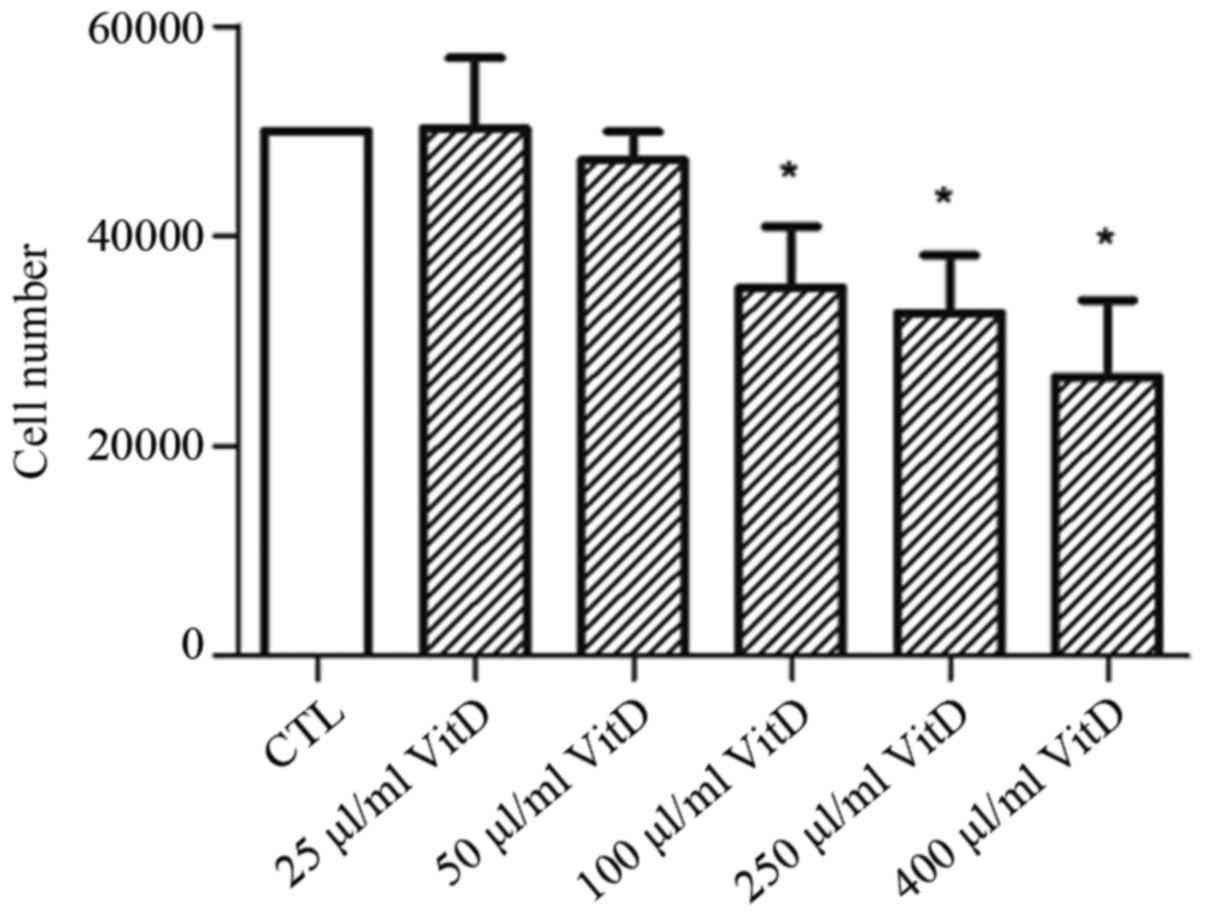
To evaluate the effect of active vitamin D on
ovarian cancer, SKOV3 or OVCAR3 cells were treated with varying
concentrations of biologically active vitamin D for 72 h. The
relative cell number was quantified using CCK-8. It was
demonstrated that 100 µl/ml active vitamin D was able to
significantly inhibit the proliferation of SKOV3 and OVCAR3 cells,
and a further increase in concentration caused a greater inhibitory
effect (Fig. 1).
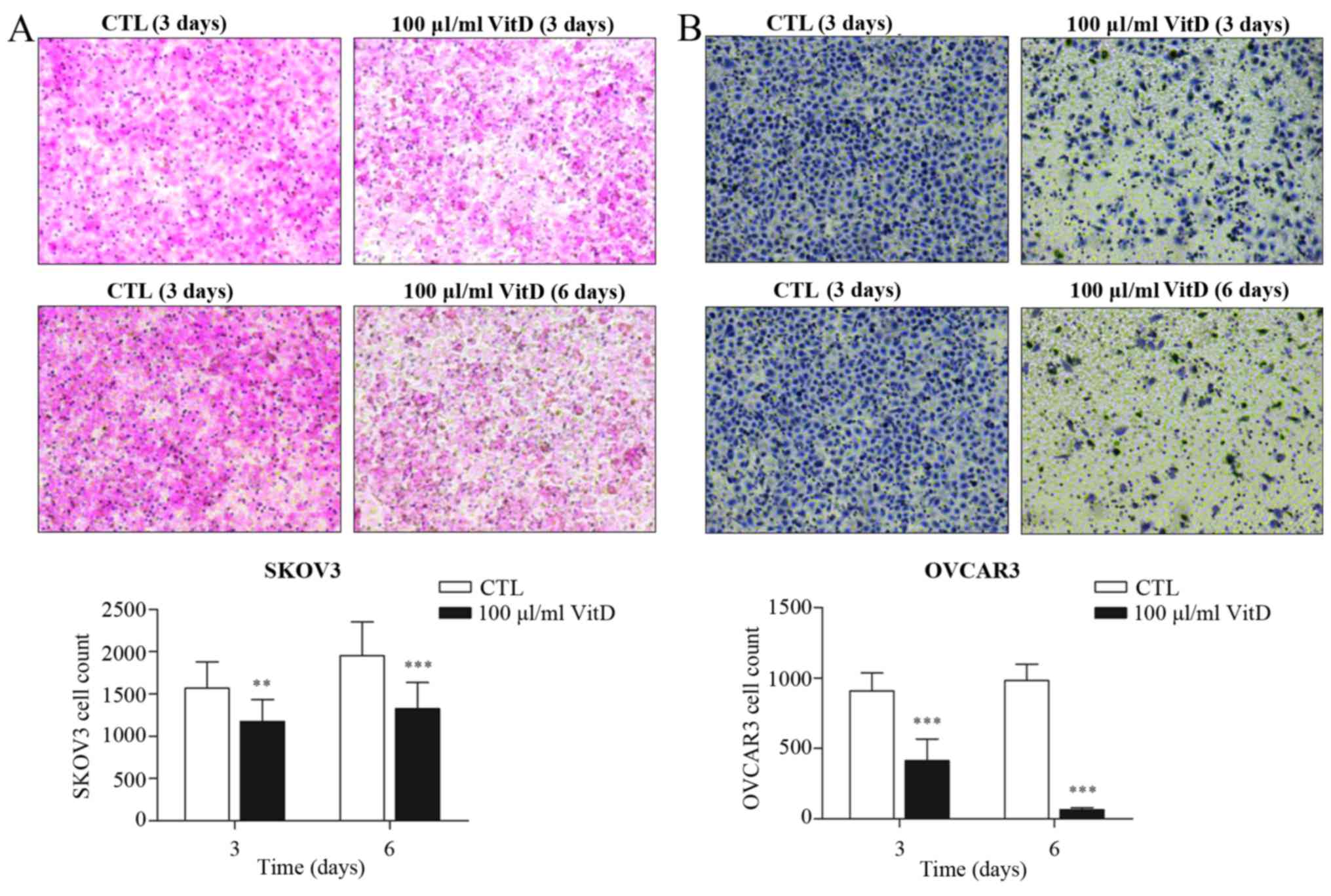
Active vitamin D inhibits the invasion
of ovarian cancer cells
Active vitamin D treatment reduced the number of
SKOV3 or OVCAR3 cells able to migrate to the lower surface of
transwell inserts (Fig. 2A and B),
suggesting that vitamin D could partially block the invasion
ability of ovarian cancer cells.
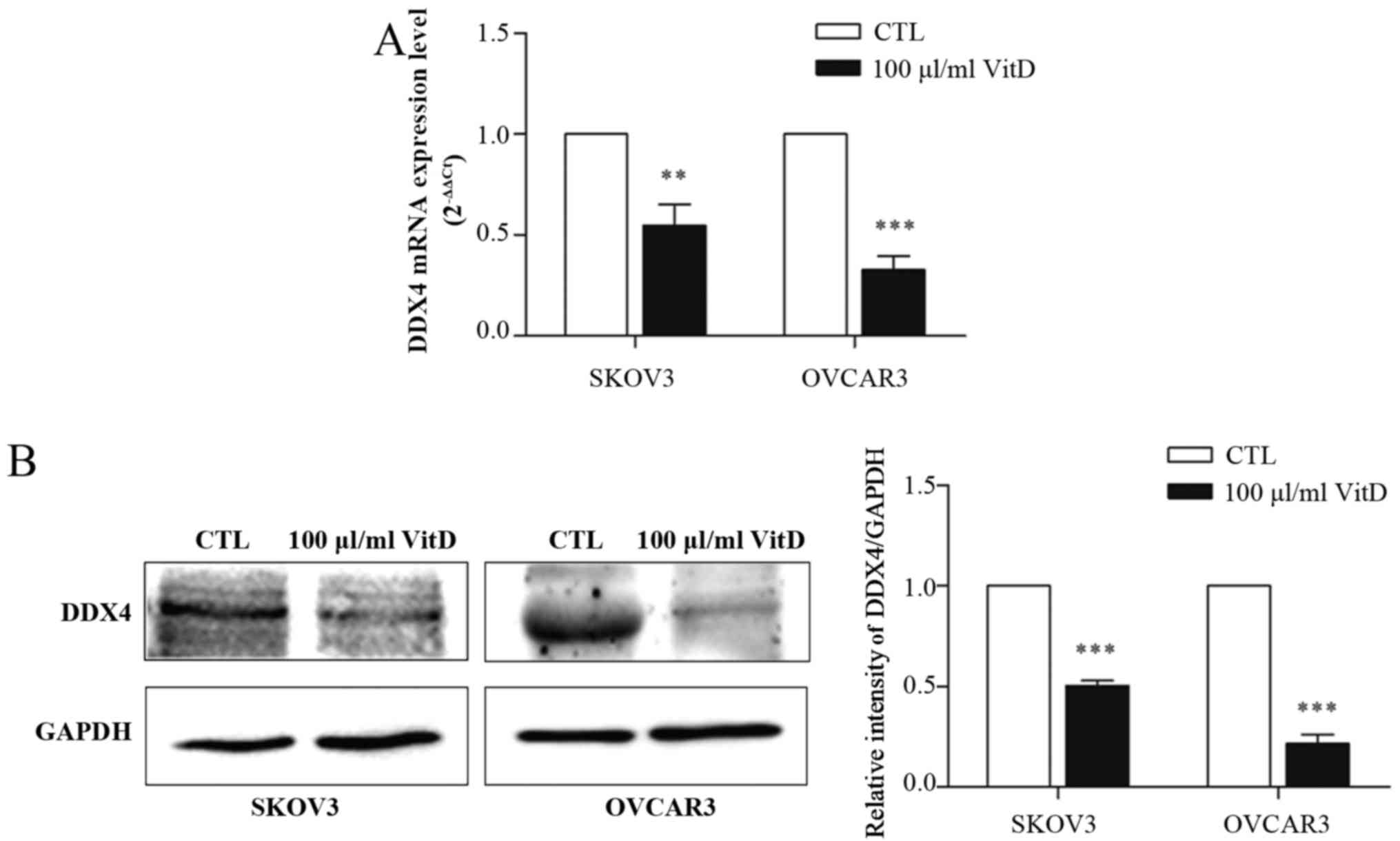
Active vitamin D downregulated the
expression of DDX4 in ovarian cancer cells
It has been established that DDX4 is overexpressed
in epithelial ovarian cancer and can be used as an ovarian cancer
stem cell marker (14). To assess
whether vitamin D affects the expression of DDX4, SKOV3 or OVCAR3
cells were treated with 100 µl/ml active vitamin D for 72 h prior
to RT-qPCR and western blot analyses. It was demonstrated that
vitamin D treatment downregulated the expression of DDX4 at the
mRNA (Fig. 3A) and protein (Fig. 3B) levels.
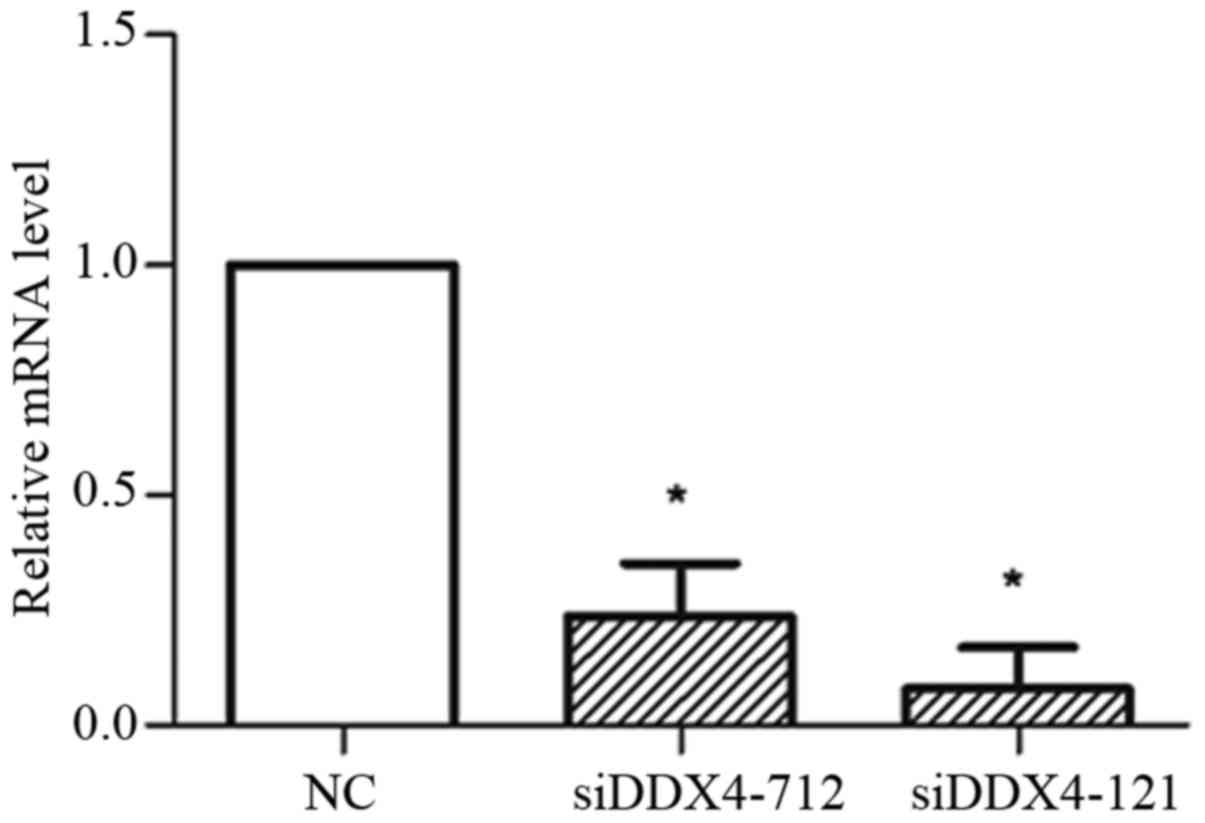
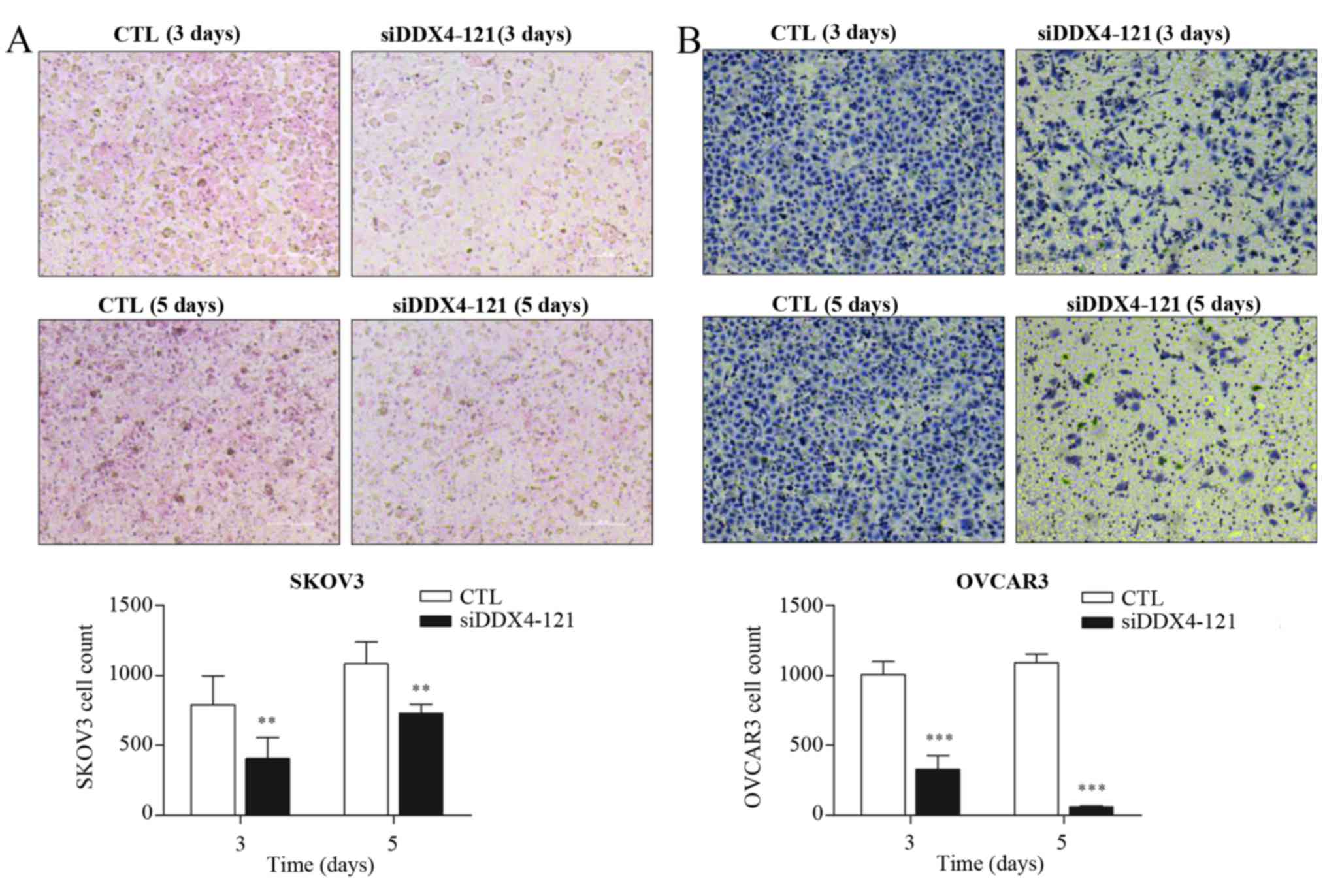
DDX4 knockdown inhibits invasion of
ovarian cancer cells
To investigate whether DDX4 could influence the
invasion of ovarian cancer cells, several independent siRNAs
targeting DDX4 were transfected into SKOV3and OVCAR3 cells
individually. The knockdown efficiency was evaluated by RT-qPCR,
which demonstrated that transfection with siDDX4-121 or siDDX4-712
could significantly reduce DDX4 expression (Fig. 4). siDDX4-121-transfected cells were
selected for the transwell assay, due to the greater inhibition of
DDX4 expression achieved using this siRNA. Invasive cells were
stained 3 or 5 days subsequent to siRNA treatment (Fig. 5A and B). DDX4 knockdown partially
inhibited the invasive ability of SKOV3 and OVCAR3 cells (Fig. 5A and B).
Discussion
Vitamin D was first recognized for its regulatory
function in calcium-phosphorus balance (15). Recently, it has been demonstrated that
active vitamin D affects various cellular processes, including
proliferation, invasion, differentiation and malignant
transformation (8,16–18) in
multiple types of cancer (18,19),
including male reproductive system carcinomas and prostate cancer
(12). It has also been suggested
that vitamin D could prevent ovarian cancer progression (8). The present study demonstrated that the
proliferative and invasive abilities of SKOV3 and OVCAR3 ovarian
cancer cells could be inhibited by active vitamin D. However, the
molecular mechanisms of how vitamin D inhibits the proliferation
and invasion of ovarian cancer cells remain to be further
investigated.
DDX4 is expressed exclusively in the ovaries and
testes. The expression level of DDX4 in SKOV3 and OVCAR3 cells was
downregulated by active vitamin D at the mRNA and protein level.
Knockdown of DDX4 by siRNA partially inhibited the invasion ability
of SKOV3 and OVCAR3 cells. It is speculated that vitamin D may
inhibit the invasion of ovarian cancer cells through downregulating
the expression of DDX4.
In conclusion, vitamin D treatment reduced the
proliferation and invasion of ovarian cancer cells. DDX4, which was
previously found to be overexpressed in ovarian cancer, was
downregulated by vitamin D on the mRNA and protein levels. DDX4
knockdown also inhibited the invasion of ovarian cancer cells.
Therefore, the use of vitamin D should be considered as a potential
novel therapy for ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present was supported by the National Natural
Science Foundation of China (grant nos. 81672560 and 31501137), and
Jiangsu Basic Research Funds for Young Scientists (grant no.
BK20150351).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon request.
Authors' contributions
All authors have read and approved the manuscript.
ZS performed the experiment and analyzed the data. JX, PW, JT, XS
and JL contributed to data collection and analysis. YC, FR, and LX
designed the study and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DEAD
|
Asp-Glu-Ala-Asp
|
|
DDX4
|
DEAD-box helicase 4
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Lim HJ and Ledger W: Targeted therapy in
ovarian cancer. Womens Health (Lond). 12:363–378. 2016.PubMed/NCBI
|
|
2
|
Clarke-Pearson DL: Clinical practice.
Screening for ovarian cancer. N Engl J Med. 361:170–177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guenther J, Stiles A and Champion JD: The
lived experience of ovarian cancer: A phenomenological approach. J
Am Acad Nurse Pract. 24:595–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yahara K, Ohguri T, Imada H, Yamaguchi S,
Kawagoe T, Matsuura Y, Hachisuga T and Korogi Y: Epithelial ovarian
cancer: Definitive radiotherapy for limited recurrence after
complete remission had been achieved with aggressive front-line
therapy. J Radiat Res. 54:322–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baruah U, Barmon D, Kataki AC, Deka P,
Hazarika M and Saikia BJ: Neoadjuvant chemotherapy in advanced
epithelial ovarian cancer: A survival study. Indian J Med Paediatr
Oncol. 36:38–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wahler J, So JY, Cheng LC, Maehr H,
Uskokovic M and Suh N: Vitamin D compounds reduce mammosphere
formation and decrease expression of putative stem cell markers in
breast cancer. J Steroid Biochem Mol Biol. 148:148–155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
So JY and Suh N: Targeting cancer stem
cells in solid tumors by vitamin D. J Steroid Biochem Mol Biol.
148:79–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garland CF, Mohr SB, Gorham ED, Grant WB
and Garland FC: Role of ultraviolet B irradiance and vitamin D in
prevention of ovarian cancer. Am J Prev Med. 31:512–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Granato T, Manganaro L, Petri L, Porpora
MG, Viggiani V, Angeloni A and Anastasi E: Low 25-OH vitamin D
levels at time of diagnosis and recurrence of ovarian cancer.
Tumour Biol. 37:2177–2181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castrillon DH, Quade BJ, Wang TY, Quigley
C and Crum CP: The human VASA gene is specifically expressed in the
germ cell lineage. Proc Natl Acad Sci USA. 97:9585–9590. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto H, Sudo T, Mikami Y, Otani M,
Takano M, Tsuda H, Itamochi H, Katabuchi H, Ito M and Nishimura R:
Germ cell specific protein VASA is over-expressed in epithelial
ovarian cancer and disrupts DNA damage-induced G2 checkpoint.
Gynecol Oncol. 111:312–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KH, Kang YJ, Jo JO, Ock MS, Moon SH,
Suh DS, Yoon MS, Park ES, Jeong N, Eo WK, et al: DDX4 (DEAD box
polypeptide 4) colocalizes with cancer stem cell marker CD133 in
ovarian cancers. Biochem Biophys Res Commun. 447:315–322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tavani A, Bertuccio P, Bosetti C, Talamini
R, Negri E, Franceschi S, Montella M and La Vecchia C: Dietary
intake of calcium, vitamin D, phosphorus and the risk of prostate
cancer. Eur Urol. 48:27–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang WL, Welsh J and Tenniswood M:
1,25-Dihydroxyvitamin D3 modulates lipid metabolism in prostate
cancer cells through miRNA mediated regulation of PPARA. J Steroid
Biochem Mol Biol. 136:247–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang WL, Chatterjee N, Chittur SV, Welsh J
and Tenniswood MP: Effects of 1α,25dihydroxyvitamin D3 and
testosterone on miRNA and mRNA expression in LNCaP cells. Mol
Cancer. 10:582011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thorne J and Campbell MJ: The vitamin D
receptor in cancer. Proc Nutr Soc. 67:115–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giovannucci E: Expanding roles of vitamin
D. J Clin Endocrinol Metab. 94:418–420. 2009. View Article : Google Scholar : PubMed/NCBI
|