Introduction
Pancreatic cancer is a deadly disease and is the
third leading cause of cancer-associated mortality in the United
States of America (1). By 2030, it
will be second only to lung cancer in the USA (1). Pancreatic cancer is often diagnosed at
advanced stages, with local invasion and remote metastasis, making
surgical resection difficult and less effective (2,3).
Therefore, developing chemopreventive measures for pancreatic
cancer is an important avenue of future study.
Cyclooxygenase-2 (COX-2) has been demonstrated to be
an important promoter of tumor growth in various cancer types, and
has been considered as a target for therapy (4,5). Previous
studies revealed that COX-2 was overexpressed in various types of
gastrointestinal and pancreatic cancers and that its expression
level is associated with a poor prognosis (6–8).
Therefore, inhibition of COX-2 may have a potential therapeutic
effect on pancreatic cancer treatment.
Previous data have demonstrated that COX-2 serves an
important role in the development of tumors, and the underlying
mechanisms include the regulation of proliferation, apoptosis,
angiogenesis and metastasis (9,10). It has
been indicated that the supply of nutrition from new blood vessels
assists in increasing the tumor diameter, without which it is
difficult for the tumor to exceed 0.2 cm; a tumor diameter >1.0
cm is an important indicator for increased risk of tumor
metastasis. Therefore, angiogenesis serves an important role in
tumor formation, invasion and metastasis (11). Tsujii et al (12) indicated that COX-2 promoted the
angiogenesis of colon cancer by upregulating angiogenic factors
including vascular endothelial growth factor (VEGF), basic
fibroblast growth factor (bFGF), bFGF binding protein, transforming
growth factor-β, platelet-derived growth factor B, endothelin-1 and
nitric oxide synthase in vitro. In another study, NS398, a
selective COX-2 inhibitor, significantly inhibited angiogenesis in
prostate cancer in vivo (13).
These studies provide evidence that COX-2 participates in the
regulation of angiogenesis, which is important for tumor formation.
Prostaglandin E2 (PGE2), an important
intermediate of COX-2-catalyzed arachidonic acid, is overexpressed
in a wide variety of tumors and is associated with tumor
development (14). A study by Eibl
et al (15) demonstrated that
in a subset of pancreatic cancer cell lines, COX-2 increased
PGE2 which subsequently increased VEGF secretion,
suggesting an important role in the angiogenesis of pancreatic
cancer.
Although COX-2 has been studied in various types of
cancer (4–8), its association with pancreatic cancer
has not been fully elucidated. Therefore, the present study
investigated the effects of COX-2 on the expression of VEGF and
PGE2 in pancreatic cancer in vitro and in
vivo. These data will further assist in revealing the
regulatory mechanisms of COX-2 in pancreatic cancer
angiogenesis.
Materials and methods
Patient samples
A total of 24 paraffin-embedded pancreatic
adenocarcinoma tissues (from 10 males and 14 females; mean age,
55.2 years; range, 42–76 years) collected between January 2010 and
January 2011 and obtained from The Second Affiliated Hospital of
Zhejiang University (Hangzhou, China), were analyzed by
immunohistochemistry. The study protocol was approved by the Ethics
Committees of The Second Affiliated Hospital of Zhejiang University
School of Medicine. Informed consent was obtained from all
patients, agreeing to surgical excision and participation in the
present study.
Cell culture, reagents and
antibodies
The human pancreatic carcinoma PC-3 cell line was a
kind gift from Dr Liu Tonghua (Xiehe Hospital, Beijing, China) and
the AsPC-1 cell line was purchased from Shanghai cell bank
(Shanghai, China). The cell lines were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (GE Healthcare Life Sciences,
Logan, UT, USA) without antibiotics in an incubator with 5%
CO2 at 37°C. Celebrex was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) and was dissolved in 100% dimethyl
sulfoxide (DMSO), then diluted with RPMI-1640 for subsequent
experiments. The final concentration of DMSO for all treatments,
including controls, was maintained at 0.1%. All drug solutions were
prepared on the day the experiments were performed. COX-2 rabbit
polyclonal antibody (cat. no. 160107) was purchased from Cayman
Chemical Company (Ann Arbor, MI, USA). Primary antibodies against
VEGF (cat. no. sc-7269) and collagen IV (cat. no. sc-59814) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
All secondary antibodies (cat. no. BA1080; HRP-conjugated protein A
and cat. no. BA1050, goat anti-mouse secondary antibody) were
obtained from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Immunohistochemistry for COX-2, VEGF
and collagen IV by light microscopy
Immunohistochemical procedures were performed for
the identification of COX-2 and VEGF expression, and microvascular
density (MVD) in the paraffin-embedded tissue samples. Briefly,
following dewaxing with xylene and rehydrated in a series of
decreasing alcohol concentrations (100, 90, 70 and 50% ethanol; 5
min each), 5-µm thick sections were soaked at room temperature in
1% hydrogen peroxide liquid for 15 min, and then blocked at room
temperature with 2% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA) in phosphate-buffered saline (PBS) for 30 min. The sections
were incubated with the primary antibodies, as previously mentioned
(1:100 dilution for COX-2; 1:200 dilution for VEGF and collagen IV,
respectively) in a humidified chamber for 15–18 h at 4°C and
incubated with the corresponding horseradish peroxidase
(HRP)-conjugated secondary antibodies, as previously mentioned
(1:200 dilution) for 1 h at room temperature. This was followed by
incubation with 3,3′-diaminobenzidine solution at room temperature
for 2 min (cat. no. D3939; Sigma-Aldrich; Merck KGaA). The
intensity of COX-2 and VEGF positivity was classified into 4 grades
semi-quantitatively: 0, no staining of cancer cells; 1, weak
staining with a light brown color; 2, moderate staining with brown
color; and 3, strong staining with dark brown. Collagen IV staining
was performed, and the areas with the highest MVD were selected at
magnification, ×100. The number of capillaries was counted in 4
randomly selected fields at magnification, ×200 and the mean value
was calculated. The immunohistochemical intensity and pathological
characteristics of all tumor specimens used in the present study
were examined by an independent pathologist.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from cultured cells by
TRIzol® (Life Technologies; Thermo Fisher Scientific,
Inc.) and was reverse transcribed by M-MLV Reverse Transcriptase
kit (cat no. A1250; Promega Corporation, Madison, WI, USA) using
oligo (dT) primers at 42°C for 60 min. cDNA products were subjected
to 35 cycles of PCR amplification as follows: 94°C for 5 min
followed by 30 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C
for 30 min followed by a final extension step of 72°C for 5 min.
The primers used were as follows: COX-2 forward,
5′-TGAAACCCACTCCAAACACAG-3′ and reverse, 5′-TCACAGGCACAGGAGGAAG-3′
(232 bp); and VEGF forward, 5′-ATGAACTTTCTGCTGTCTTG-3′ and reverse,
5′-TGCATGGTGATGTTGGAC-3′ (382 bp). The primers used for β-actin
forward, 5′-GGGACCTGACTGACTACCTC-3′ and reverse,
5′-TCATACTCCTGCTTGCTGAT-3′ were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China).
VEGF ELISA
The human pancreatic cancer PC-3 cells were plated
in a 96-well plates (5×103 cells/well) and cultured for
24 h in RPMI-1640 supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). In the dose-effect group, the cells were treated
with 0, 20, 60, 100 and 140 µM Celebrex for 3 days. In the
time-effect group, the cells were treated with 100 µM Celebrex for
0, 12, 24, 48 and 72 h. In the PGE2 intervention
experiment, PC-3 cells were treated with 100 µM Celebrex and
different concentrations of PGE2 simultaneously as
indicated in the following four groups at 37°C for 3 days: Celebrex
(C), Celebrex + 0.1 µM PGE2 (C+P1), Celebrex
+ 1 µM PGE2 (C+P2) and Celebrex + 10 µM
PGE2 (C+P3). The supernatant was collected by
centrifugation at 2,500 × g for 10 min at 4°C. VEGF proteins were
assayed using human VEGF ELISA kits (cat no. EK0539; Boster
Biological Technology, Pleasanton, CA, USA).
Radioimmunoassay (RIA) for
PGE2
According to the aforementioned description, the
cells were divided into two groups: The dose-effect and the
time-effect groups. After 3 days, the supernatant was collected by
centrifugation at 2,500 × g for 10 min at 4°C and the levels of
PGE2 were measured by RIA using the Prostaglandin
E2 125I RIA kit (Amersham; GE Healthcare, Chicago, IL,
USA). Following the establishment of the xenograft mouse model,
described subsequently, nude mouse tumor tissues (100 mg) were
obtained and centrifuged at 7,500 × g for 10 min at 4°C with 1 ml
normal saline to collect the homogenate. The levels of
PGE2 in the supernatant were then measured using
RIA.
Western blot analysis
Tumor tissues were lysed in radioimmunoprecipitation
assay lysis buffer [150 mM NaCl, 20 Mm Tris-HCl (pH 7.4), 5 mM
EDTA, 1% Na-deoxycholate, 1% NP-40, 0.1% SDS, 1 mM PMSF, 20 mg/ml
Aprotini, 20 mg/ml leupeptin and 3 mg/ml Pepstatin A] and the
protein concentration was determined using a BCA kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China). 20 µg of total proteins
per lane were separated using 10% SDS-PAGE and transferred onto a
nitrocellulose membrane. The membranes were blocked at 4°C for 1 h
in 5% BSA in TBST buffer containing 0.1% Tween-20, and then were
incubated with the indicated primary antibodies (previously stated)
overnight at 4°C. Subsequently, HRP-conjugated secondary antibodies
as aforementioned were incubated with the membranes at room
temperature for 1 h at a 1:1,000 dilution, and detected using an
enhanced chemiluminescence detection system (Amersham; GE
Healthcare). Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to quantify the
western blots.
Human pancreatic cancer nude mouse
model
A total of 20 specific pathogen-free, 6-week-old,
female, BALB/C-nu/nu mice were purchased from the Cancer Research
Center of Shanghai (Shanghai, China). The mice were raised under
controlled 12 h light-dark cycles, with constant temperature
(22–24°C) and humidity (55–60%) in a pathogen-free animal research
center in the Zhejiang Chinese Medical University (Hangzhou China),
and had continuous free access to sterilized food (γ-ray-irradiated
food) and autoclaved water. Following 1 week of acclimation,
experiments were initiated. To form the xenograft tumors, human
pancreatic cancer PC-3 cells were cultured and trypsinized, and
then washed and re-suspended in PBS. Next, 1×107 cells
were inoculated subcutaneously into the left lower limbs of the
mice. A total of 2 weeks later, when the tumor nodules were visible
(~5 mm3), the mice were separated randomly into two
groups: The control and the Celebrex treatment groups. Mice in the
Celebrex treatment group were fed with food containing 1,500 ppm
Celebrex, and those in the control group were fed with normal food
alone. The sizes of the tumors were measured weekly using calipers.
Tumors were harvested 3 months after the drug treatment. The length
(L), width (W) and height (H) of the tumors were measured using
calipers, and the volumes of the tumors were calculated using the
following formula: V=л(L × W × H)/6. The mice were anesthetized
prior to cervical dislocation using 45 mg/kg pentobarbital sodium
at the culmination of the experiment.
Statistical analysis
Continuous variables were expressed as the mean ±
standard error and a non-paired Student's t-test was used for
statistical evaluation. Comparisons of means of the VEGF or PGE2
expression rate among groups of different drug concentrations or
different treatment times were performed by one-way analysis of
variance, followed by Tukey's multiple means comparison test.
P<0.05 was considered to indicate a statistically significant
difference. In addition, scatter plotting was applied and an
R2 value was calculated via Pearson's correlation test
to estimate the correlation between VEGF and PGE2 levels. When the
ratio was close to 1, the predominant correlation was indicated.
Statistical analysis was performed with SPSS v.13.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
COX-2 and VEGF expression in
paraffin-embedded tissue samples
The expression levels of COX-2 and VEGF proteins in
the paraffin-embedded tissue samples from 24 patients with
pancreatic adenocarcinomas were investigated by
immunohistochemistry (Fig. 1). COX-2
immunoreactivity was detected in 21 (87.5%) patients with
pancreatic adenocarcinoma (3 negative; 10 weak; and 11 moderate or
strong staining). COX-2 immunoreactivity was localized almost
exclusively in the neoplastic cells, whereas the stroma of the
tumors appeared negative (Fig. 1B).
In the normal pancreatic tissues, pancreatic ductal epithelial and
acinar cells were negative for COX-2, although the normal islet
cells indicated weakly positive staining (Fig. 1A). VEGF immunoreactivity was detected
in 14 patients (58.3%) with pancreatic adenocarcinoma (10 negative;
5 weak; and 9 moderate or strong staining). Positive staining of
VEGF was characterized by uniform intracytoplasmic, tan, fine
granular staining (Fig. 1C).
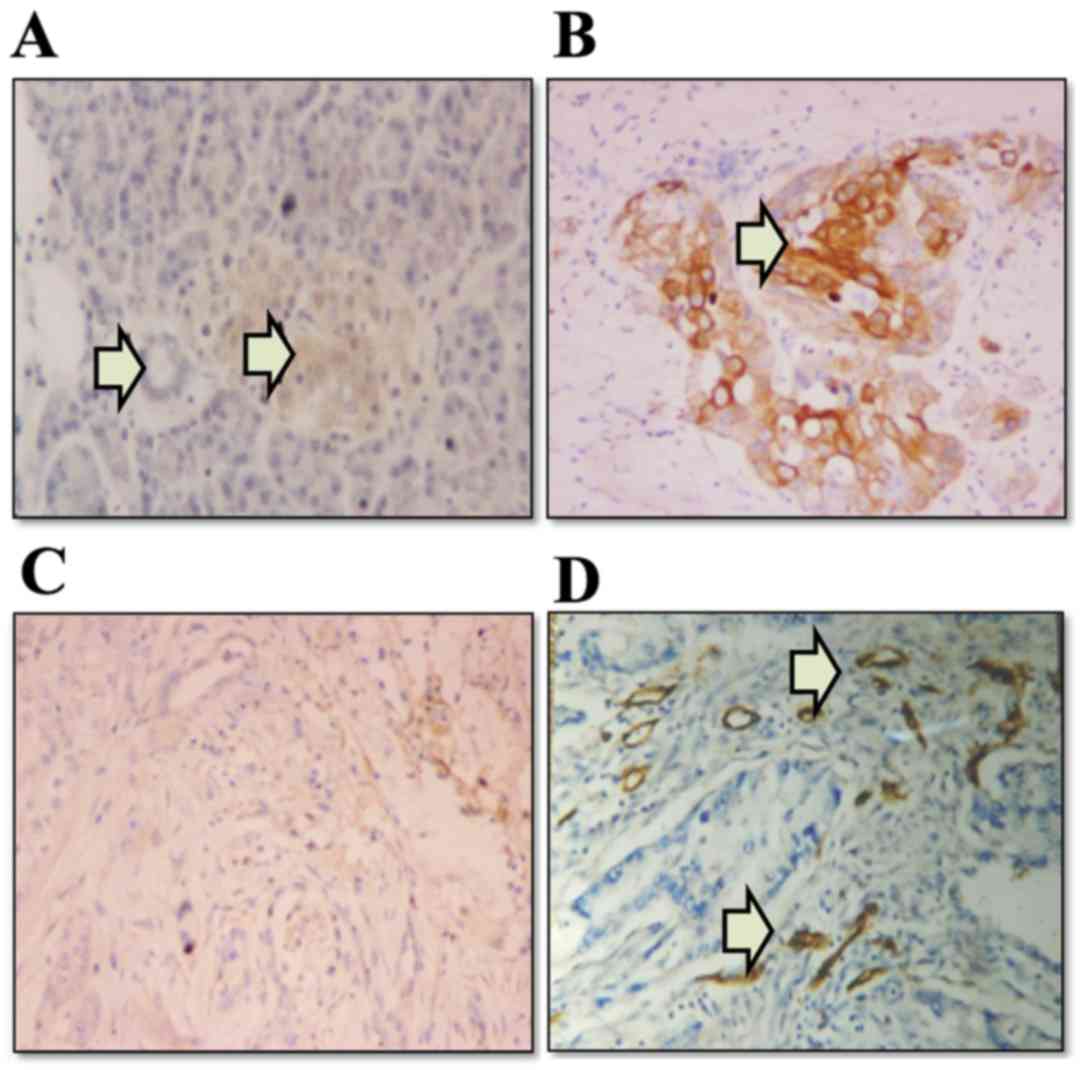 | Figure 1.Expression of COX-2, VEGF and
collagen IV in paraffin-embedded human pancreatic cancer tissues,
as determined by immunohistochemical staining. (A) In normal
pancreatic tissues, islet cells (the right arrow) exhibited weak
staining, whereas epithelial cells of the pancreatic duct and
acinar cells (the left arrow) were negative for COX-2
(magnification, ×200). (B) COX-2 was expressed in the cytoplasm of
pancreatic adenocarcinoma cells (indicated by arrow)
(magnification, ×200). (C) Uniform intracytoplasmic fine granular
VEGF staining was present in pancreatic adenocarcinoma cells
(magnification, ×200). (D) Angiogenesis in pancreatic cancer
tissues was indicated by collagen IV staining (indicated by two
arrows) (magnification, ×200). COX-2, cyclooxygenase-2; VEGF,
vascular endothelial growth factor. |
MVD in paraffin-embedded tissue
samples
Angiogenesis in paraffin-embedded human pancreatic
cancer tissue samples was also studied by immunostaining for
collagen IV (Fig. 1D). In 11 out of
24 cases, moderate or strong staining for COX-2 expression was
observed, with a mean MVD of 71.6±24.9. In the other 13 cases with
negative or weak COX-2 expression, the average MVD was 38.4±20.9.
There was a significant difference between these two groups
(P<0.05; Table I).
 | Table I.Association between COX-2 or VEGF
expression and MVD. |
Table I.
Association between COX-2 or VEGF
expression and MVD.
| Staining
intensity | MVD, mean ± SE | P-value |
|---|
| COX-2 |
| <0.05 |
|
Moderate/strong (n=11) | 71.6±24.9 |
|
|
Negative/weak (n=13) | 38.4±20.9 |
|
| VEGF |
| >0.05 |
|
Moderate/strong (n=9) | 61.8±31.3 |
|
|
Negative/weak (n=15) | 40.8±21.8 |
|
In the VEGF-negative and weak group, the mean MVD
was 40.8±21.8, while in the VEGF-positive group, the mean MVD was
61.8±31.3. Although the mean MVD in the VEGF-positive group was
increased compared with that in the VEGF-negative group, no
statistical difference between these two groups was observed
(P>0.05; Table I).
VEGF and PGE2 expression in
human pancreatic cancer PC-3 cell line
In order to investigate the inhibitory effects of
Celebrex on the expression of VEGF and PGE2, these two
components were detected in the PC-3 cell supernatants by ELISA and
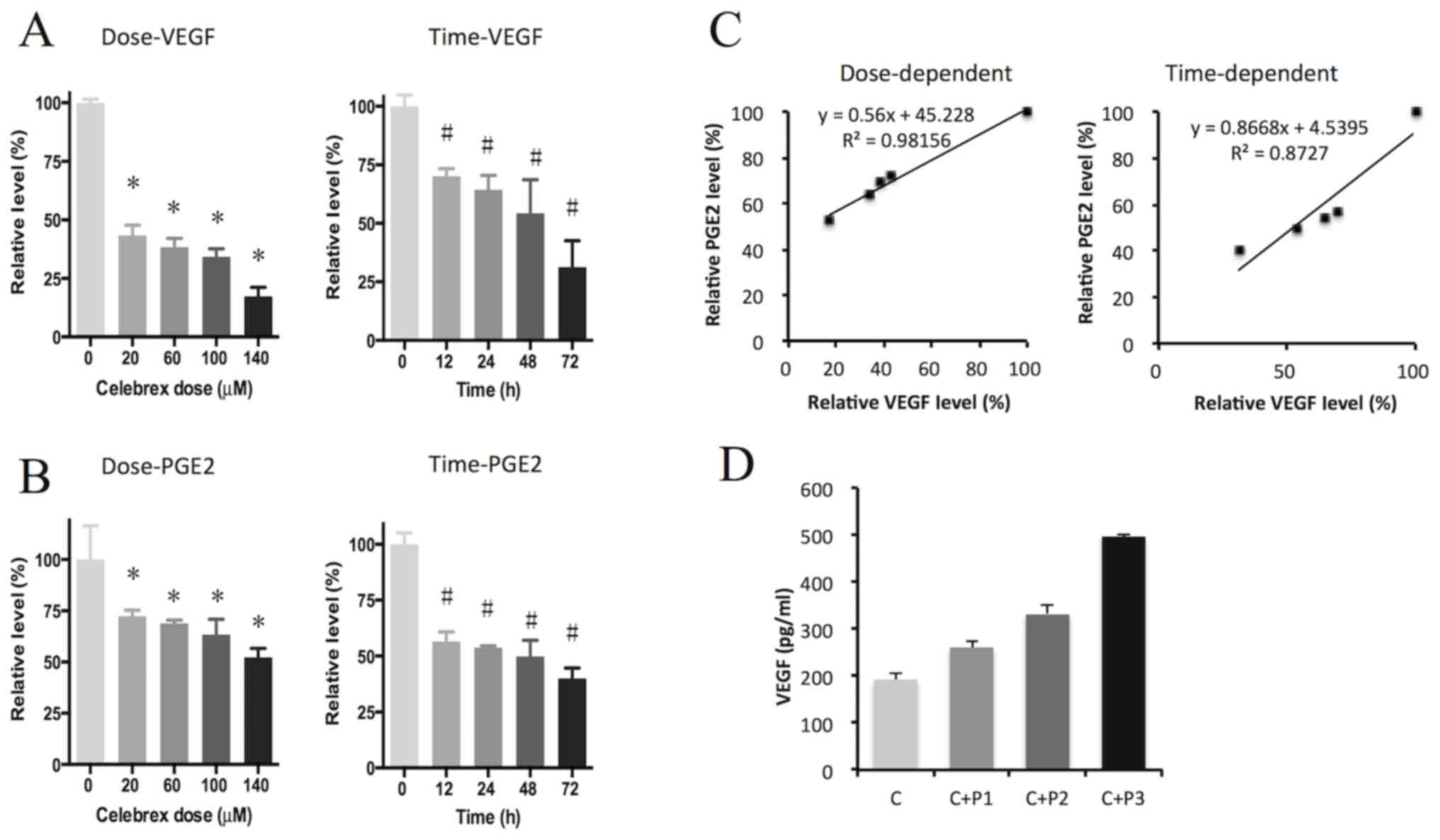
RIA, respectively. The results indicated that VEGF and
PGE2 were significantly suppressed by Celebrex treatment
in a dose- and time-dependent manner (P<0.05; Fig. 2A and B). Scatter plots also showed a
prominent correlation between VEGF and PGE2 levels in a dose- and
time-dependent manner, with an R2 value close to 1
(Fig. 2C). The effect of exogenous
PGE2 on the downregulation of VEGF by Celebrex was also
assessed, and the results demonstrated that exogenous
PGE2 rescued the suppression of VEGF induced by Celebrex
treatment in a dose-dependent manner (Fig. 2D). This experiment was repeated with
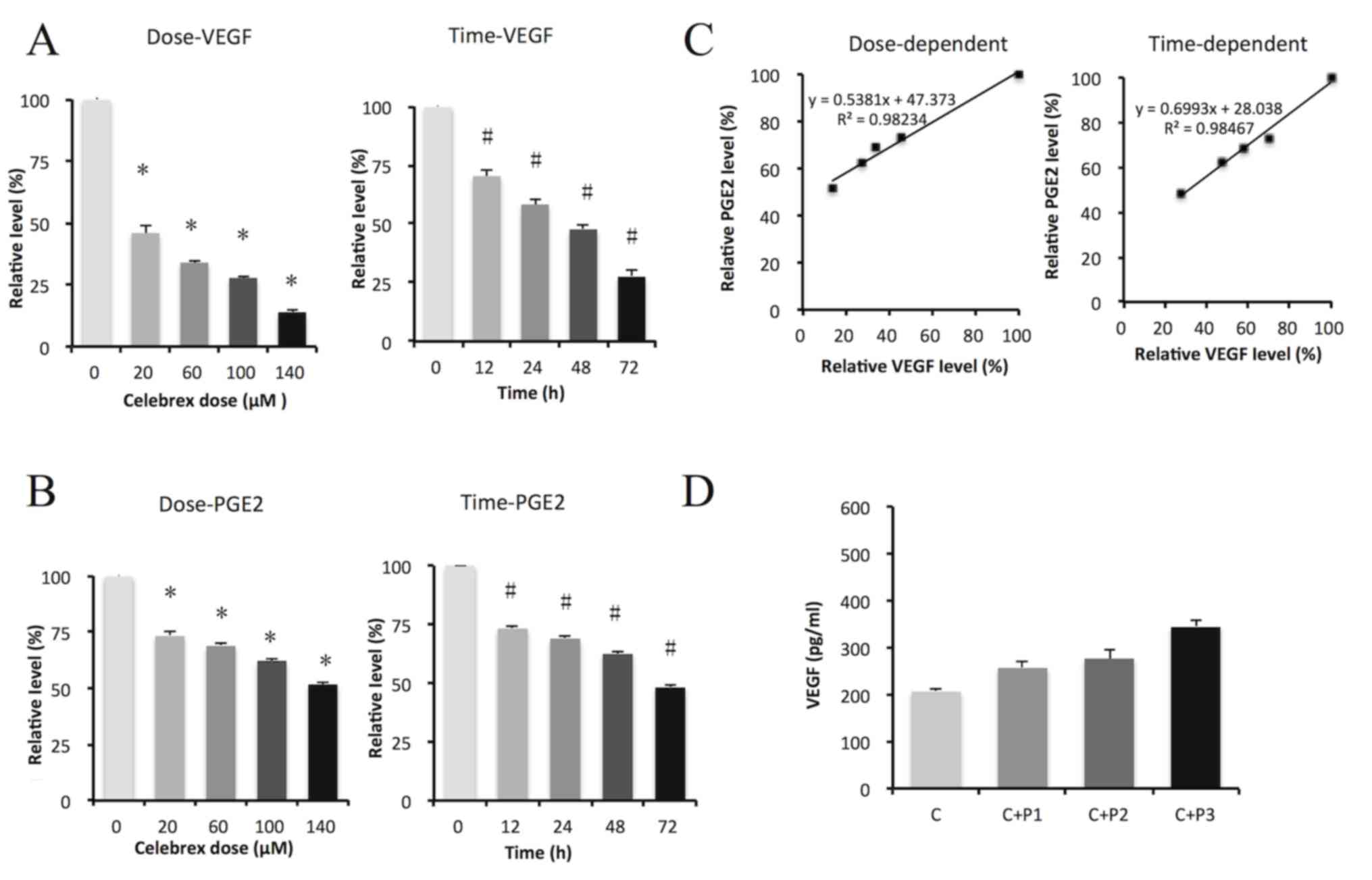
AsPC-1 cells, and similar results were obtained, as demonstrated in
Fig. 3. VEGF and PGE2 were
significantly suppressed by Celebrex treatment in a dose- and
time-dependent manner (P<0.05; Fig. 3A
and B). Scatter plots showed a prominent correlation between
VEGF and PGE2 levels in a dose- and time-dependent manner, with an
R2 value close to 1 (Fig.
3C). Exogenous PGE2 rescued the suppression of VEGF
induced by Celebrex treatment in a dose-dependent manner (Fig. 3D).
VEGF, PGE2 and MVD in PC-3
cell line xenograft nude mice
By the end of the experiment, 9 nude mice in the
control group had survived, and 8 nude mice in the Celebrex
treatment group had survived; all others (n=3) succumbed to lung
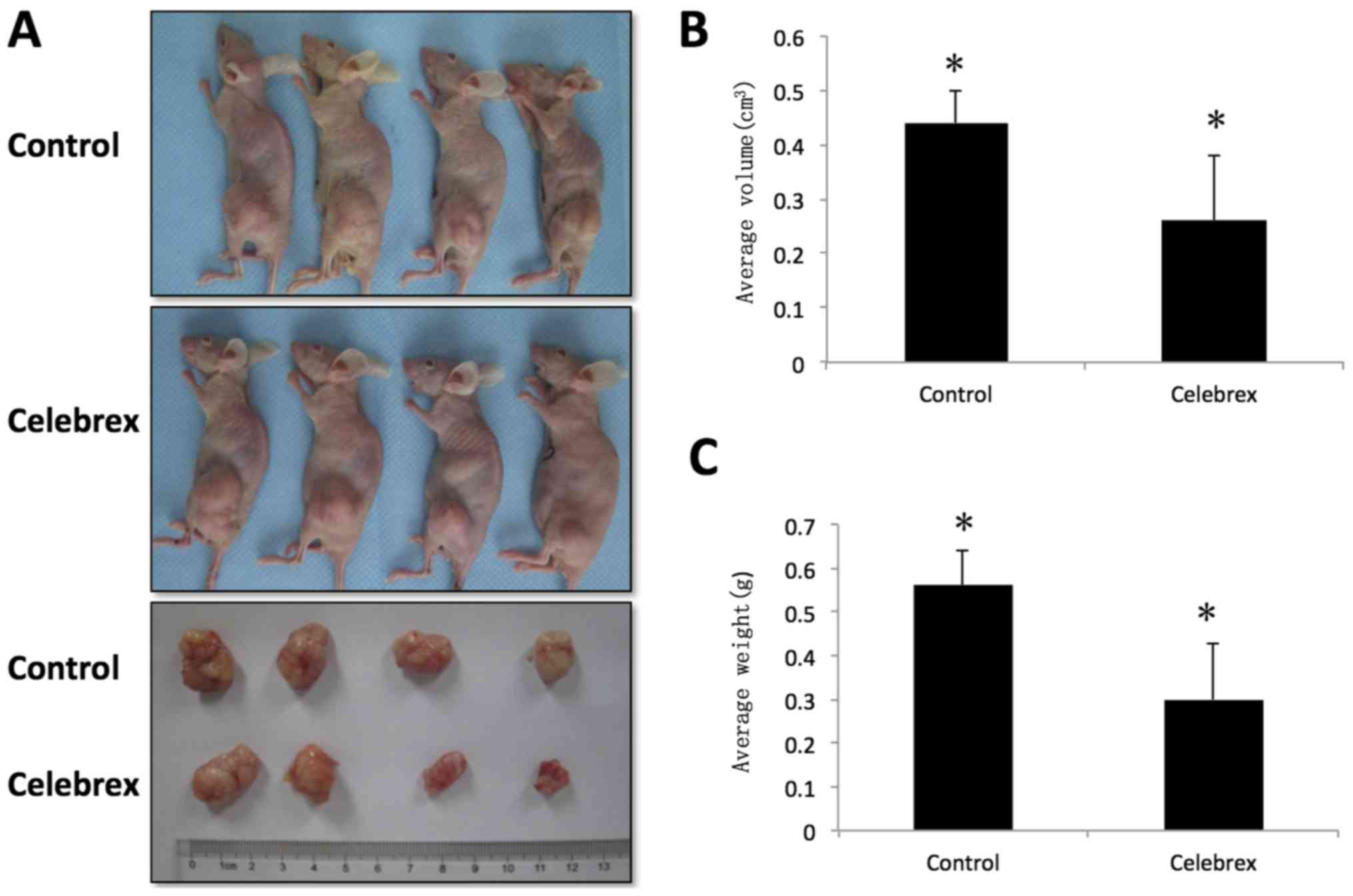
infections. The results of the efficacy trials of Celebrex in PC-3
cell xenografts grown in nude mice are presented in Fig. 4A, and the tumor weight and volume at
the termination of treatment are indicated in Table II and Fig.
4B and C. No multiple tumors were observed in any individual
animal. The volume of the tumors in the group treated with Celebrex
was significantly decreased compared with that in the control group
treated with normal food. A 50% suppression of the tumor volume was
identified in the experimental group, which was statistically
different compared with that of the control group (P<0.01).
 | Table II.Tumor volume and weight in individual
animals. |
Table II.
Tumor volume and weight in individual
animals.
|
| Individual
mice |
|---|
|
|
|
|---|
| Tumor values | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| Volume,
cm3 |
|
|
|
|
|
|
|
|
|
|
Control | 0.98 | 0.65 | 0.54 | 0.45 | 0.32 | 0.38 | 0.33 | 0.37 | 0.49 |
|
Celebrex | 0.92 | 0.36 | 0.25 | 0.23 | 0.21 | 0.17 | 0.15 | 0.19 |
|
| Weight, g |
|
|
|
|
|
|
|
|
|
|
Control | 1.18 | 0.78 | 0.64 | 0.56 | 0.42 | 0.47 | 0.41 | 0.45 | 0.68 |
|
Celebrex | 1.06 | 0.43 | 0.32 | 0.27 | 0.23 | 0.19 | 0.18 | 0.22 |
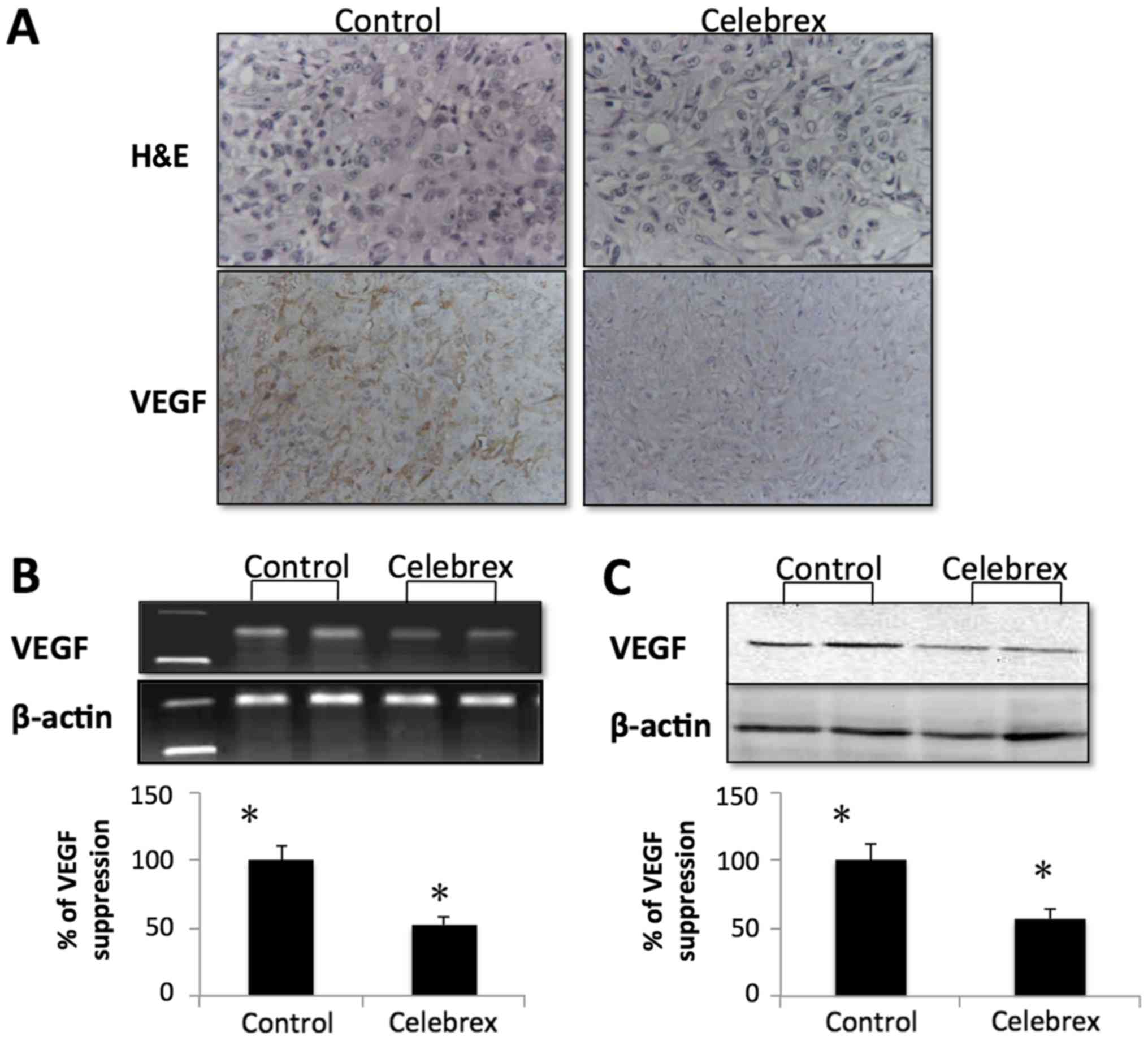
VEGF expression in tumor tissues were examined by
immunohistochemistry, RT-PCR and western blot analysis. Compared
with the control group, as demonstrated in Fig. 5A by immunohistochemistry, the
expression of VEGF was suppressed. The expression of VEGF mRNA was
also markedly downregulated in the Celebrex treatment group
(Fig. 5B). Concurrently, VEGF protein
expression in the tumor tissues was suppressed in the Celebrex
treatment group compared with the control group by western blot
analysis (Fig. 5C).
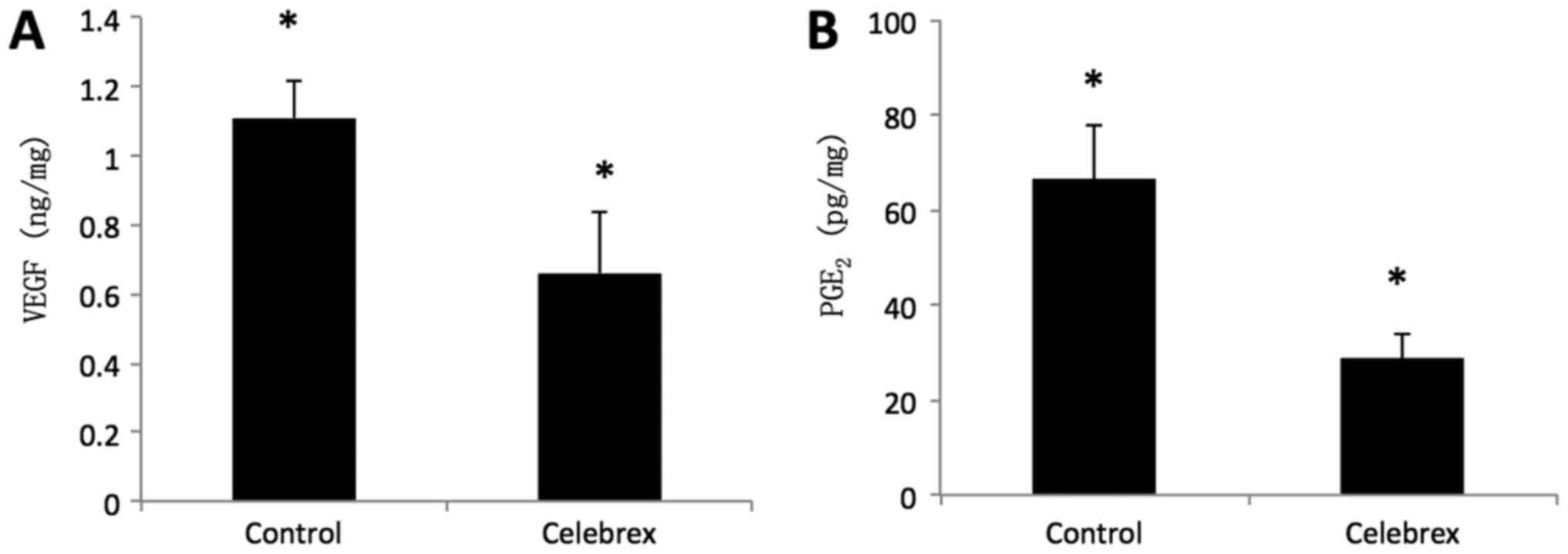
The concentration of VEGF and PGE2 in the
tumor tissues of the nude mice was examined by ELISA and RIA,
respectively. The results revealed that the concentration of VEGF
in the Celebrex group (0.65±0.18 ng/mg) was significantly decreased
compared with that in the control group (1.11±0.12 ng/mg)
(P<0.01; Fig. 6A). Similarly, the
concentration of PGE2 (28.72±4.91 pg/mg) in the Celebrex
treatment group was significantly decreased compared with that in
the control group (66.36±11.60 pg/mg) (P<0.01; Fig. 6B).
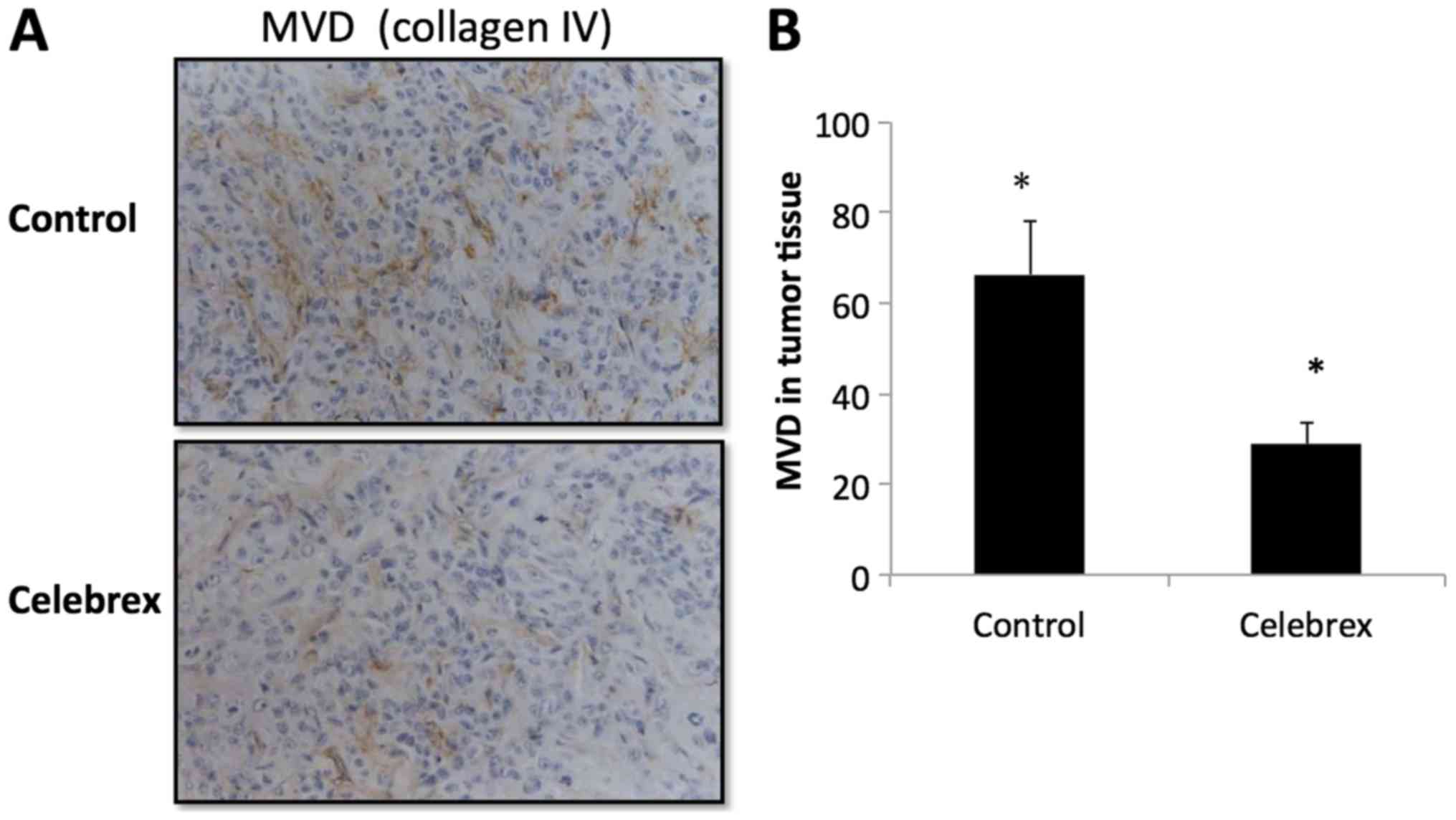
The MVD in the tumor tissues was indicated by
collagen IV staining (Fig. 7). The
data revealed that the mean MVD was 63.89±13.67 in the control
group and 32.25±12.99 in the Celebrex treatment group, indicating
that Celebrex significantly reduced the MVD of tumor tissue in the
nude mice (P<0.01).
Discussion
The tumor microenvironment is complicated and
maintains the stable survival of pancreatic cancer cells despite
various perturbations, such as hypoxia (16,17). When
the environment is not optimal, pancreatic cancer cells adopt
adaptive changes, such as stimulation of tumor angiogenesis. Tumors
require a blood supply for nutrition, growth and distant
metastasis, and angiogenesis serves a critical role in these
processes and is considered one of the major hallmarks of cancer
(18,19). Previous studies have indicated that
TNP-470, an angiogenesis inhibitor, may significantly inhibit liver
metastasis in pancreatic cancer in mice, alone or in combination
with cisplatin (20). It has also
been suggested that the inhibition of angiogenesis exhibits certain
interventional effects on the growth and metastasis of pancreatic
cancer (21,22).
The incidence of cancer of the digestive system
organs has been demonstrated to be decreased by the use of COX
inhibitors, including aspirin, compared with that in untreated
control subjects (4). Although
non-selective and selective COX-2 inhibitors have been identified
to inhibit the growth of several types of cancer cells,
accumulating evidence indicates that COX-2 serves a more important
role in carcinogenesis compared with COX-1 (5). It has been considered that this
mechanism was associated with cell apoptosis induced by
non-steroidal anti-inflammatories (9,10). Tsujii
et al (12) co-cultured colon
cancer Ca-co-2 cells, which overexpressed COX-2, with endothelial
cells. It was identified that the colon cancer cells secreted a
high concentration of angiogenic factors, including VEGF, basic
fibroblast growth factor, transforming growth factor-β1 and
platelet-derived growth factor, which stimulated the formation of
endothelial tubes, while COX-2 inhibitors significantly inhibited
the expression of angiogenesis factors and the formation of
endothelial tubes. These findings reveal that COX-2 promotes the
growth of colon cancer by promoting tumor angiogenesis.
In the present study, the expression of COX-2 and
VEGF, and MVD in human pancreatic cancer tissues was first
analyzed. It was identified that COX-2 was commonly expressed in
human pancreatic cancer tissues, with staining in the cancer cells
but not in the surrounding stroma cells. This is consistent with a
previous study indicating that COX-2 was identified in the cultured
pancreatic stellate cells, but not in the pancreatic cancer tissue
stroma (23). It was also
demonstrated that mean MVD was significantly increased in the
strongly positive COX-2 expression cases compared with that in the
weak expression and negative cases (P<0.01). Additionally, it
was revealed that Celebrex, a selective COX-2 inhibitor, reduced
the mean MVD in PC-3 xenograft tissues in the nude mice in
vivo, confirming the important role of COX-2 in
angiogenesis.
Concurrently, the present study revealed positive
VEGF expression in 58.3% of human pancreatic cancer tissues, and
the mean MVD in the VEGF-positive cases was increased compared with
that in the VEGF-negative cases, but no statistical differences
were observed. These results were inconsistent with a previous
study (24), potentially due to the
small number of samples in the present study.
A previous study demonstrated that the selective
COX-2 inhibitor reduced the secretion of VEGF in prostate cancer
PG-3ML and LNCaP cell lines, in a dose- and time-dependent manner,
under hypoxic conditions induced by CoCl2 (25). An additional study indicated that the
expression of VEGF secreted by fibroblast cells and macrophages was
in accordance with the dosage of the selective COX-2 inhibitors
(9). In the present study, it was
identified that Celebrex inhibited the secretion of VEGF in PC-3
cells. With the increased drug concentrations and time intervals,
the inhibitory effect became more marked in a dose- and
time-dependent manner, additionally confirming that COX-2
participated in the angiogenesis of pancreatic cancer by regulating
the expression of VEGF.
In our previous study, the effect of Celebrex on
cell viability was confirmed and it was demonstrated that Celebrex
inhibited cell survival through the induction of apoptosis
(26). The study also identified that
although the VEGF secretion was decreased in the supernatant of the
PC-3 cells following Celebrex treatment, the mRNA expression of
VEGF did not exhibit the corresponding change. This suggested that
the VEGF suppression was due to the apoptosis of the cultured
cells. Considering the number of surviving cells, the VEGF
secretion rate per cell was also calculated, and it was revealed
that VEGF concentration was decreased at the lower concentration of
Celebrex treatment (20 µM), but increased marginally at the higher
concentrations (60, 100 and 140 µM). Therefore, we hypothesized
that Celebrex inhibited VEGF expression through the induction of
apoptosis, and that a lower concentration is optimal for the
inhibition of angiogenesis (27).
Notably, in the in vivo nude mouse xenograft model of the
present study, it was identified that Celebrex downregulated VEGF
mRNA and protein expression levels. The aforementioned results
suggest that the mechanism of COX-2 to promote tumor angiogenesis
may not only by regulating VEGF, but also other unknown angiogenic
factors in pancreatic cancer.
COX-2 is an important rate-limiting enzyme involved
in prostaglandin synthesis in the process of arachidonic acid
metabolism (4). Previous studies have
indicated that treatment with PGE2 only may stimulate
angiogenesis (28,29). The expression of PGE2 in
the pancreatic cancer PC-3 cell line was investigated by RIA in the
present study, and it was demonstrated that Celebrex significantly
reduced the expression of PGE2 in PC-3 cells and
transplanted tumor tissues. The effect of exogenous PGE2
on the downregulation of VEGF by Celebrex was also assessed, and
the results indicated that exogenous PGE2 may partly
reverse the decreased expression of VEGF initiated by Celebrex in
PC-3 cells in a dose-dependent manner. The data indicate that
PGE2 may be an important mediator between COX-2 and
VEGF.
A total of 3 mice had succumbed by the endpoint of
the experiment. The mice were dissected, and redness and edema of
the lung tissues were observed. As this occurred in the control and
Celebrex-treated groups, this event was not considered to be
associated with Celebrex treatment. We hypothesized that it may be
due to the tumor burden and individual differences between
mice.
COX-2 expression was upregulated by
mitogen-activated protein kinase and protein kinase C pathways,
which are activated by various factors, and subsequently promoted
prostaglandin synthesis, including PGE1, PGE2
and 15-deoxy-∆-12,14-prostaglandin J2 (30,31). These
prostaglandins interacted with their corresponding receptors and
activated various intracellular kinases by the E-prostanoid
receptor/cyclic adenosine 5′-phosphate pathway (30). They may directly enter into the
nucleus via nuclear receptors including peroxisome
proliferator-activated receptor γ, to induce the production of
various angiogenic factors, including VEGF (31). The findings of the present study also
suggested an important role of PGE2 in regulating the expression of
VEGF.
In conclusion, COX-2 expression was markedly
upregulated in human pancreatic adenocarcinoma cells and serves an
important role in promoting the growth of pancreatic cancer.
PGE2 may act as an important mediator between VEGF
secretion and COX-2 activation. The results of the present study
provide an important theoretical basis for the clinical application
of COX-2 inhibitors in the chemoprevention of pancreatic cancer. In
future studies, we will aim to elucidate the role of Celebrex in
chemoprevention.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Provincial
Natural Science Foundation of China (grant no. LY14C070003) and the
Major Social Development Project of Zhejiang Province Major Science
and Technology Projects (grant no. 2014C03041-1).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CX performed the Celebrex inhibition assay and was a
major contributor in writing the manuscript. XF performed the
statistical analysis. XW and JTC were main contributors for
experiment design and guidance. SW performed the
immunohistochemistry study and pathological analysis. LS and JMC
performed the nude mice xenograft experiments. LJ performed the
RT-PCR and western blot analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committees of the Second Affiliated Hospital of Zhejiang University
School of Medicine. Informed consent was obtained from all
patients, agreeing to surgical excision and participation.
Consent for publication
All studies participants provided their consent for
the publication of this data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COX-2
|
cyclooxygenase 2
|
|
VEGF
|
vascular endothelial growth factor
|
|
PGE2
|
prostaglandin E2
|
|
MVD
|
microvascular density
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Wientjes MG and Au JL: Pancreatic
cancer: Pathobiology, treatment options, and drug delivery. AAPS J.
12:223–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ranger GS: Current concepts in colorectal
cancer prevention with cyclooxygenase inhibitors. Anticancer Res.
34:6277–6282. 2014.PubMed/NCBI
|
|
5
|
Fajardo AM and Piazza GA: Chemoprevention
in gastrointestinal physiology and disease. Anti-inflammatory
approaches for colorectal cancer chemoprevention. Am J Physiol
Gastrointest Liver Physiol. 309:G59–G70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ristimäki A, Sivula A, Lundin J, Lundin M,
Salminen T, Haglund C, Joensuu H and Isola J: Prognostic
significance of elevated cyclooxygenase-2 expression in breast
cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
7
|
Kondo M, Yamamoto H, Nagano H, Okami J,
Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K, et al:
Increased expression of COX-2 in nontumor liver tissue is
associated with shorter disease-free survival in patients with
hepatocellular carcinoma. Clin Cancer Res. 5:4005–4012.
1999.PubMed/NCBI
|
|
8
|
Masunaga R, Kohno H, Dhar DK, Ohno S,
Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H and
Nagasue N: Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.PubMed/NCBI
|
|
9
|
Xu B, Wang Y, Yang J, Zhang Z, Zhang Y and
Du H: Celecoxib induces apoptosis but up-regulates VEGF via
endoplasmic reticulum stress in human colorectal cancer in vitro
and in vivo. Cancer Chemother Pharmacol. 77:797–806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao Y, Teng Y, Zhang R and Luo L:
Antitumor effect of the selective COX-2 inhibitor celecoxib on
endometrial adenocarcinoma in vitro and in vivo. Oncol Lett.
4:1219–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Netten JP, Cann SA and van der
Westhuizen NG: Angiogenesis and tumor growth. N Engl J Med.
334:920–921. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsujii M, Kawano S, Tsuji S, Sawaoka H,
Hori M and DuBois RN: Cyclooxygenase regulates angiogenesis induced
by colon cancer cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XH, Kirschenbaum A, Yao S, Lee R,
Holland JF and Levine AC: Inhibition of cyclooxygenase-2 suppresses
angiogenesis and the growth of prostate cancer in vivo. J Urol.
164:820–825. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma X, Aoki T, Tsuruyama T and Narumiya S:
Definition of prostaglandin E2-EP2 signals in the colon tumor
microenvironment that amplify inflammation and tumor growth. Cancer
Res. 75:2822–2832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eibl G, Bruemmer D, Okada Y, Duffy JP, Law
RE, Reber HA and Hines OJ: PGE(2) is generated by specific COX-2
activity and increases VEGF production in COX-2-expressing human
pancreatic cancer cells. Biochem Biophys Res Commun. 306:887–897.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson JS, Pirola RC and Apte MV: Stars
and stripes in pancreatic cancer: Role of stellate cells and stroma
in cancer progression. Front Physiol. 5:522014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coffman LG, Choi YJ, McLean K, Allen BL,
di Magliano MP and Buckanovich RJ: Human carcinoma-associated
mesenchymal stem cells promote ovarian cancer chemotherapy
resistance via a BMP4/HH signaling loop. Oncotarget. 7:6916–6932.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang D, Wang LL, Dong TT, Shen YH, Guo XS,
Liu CY, Liu J, Zhang P, Li J and Sun YP: Progranulin promotes
colorectal cancer proliferation and angiogenesis through TNFR2/Akt
and ERK signaling pathways. Am J Cancer Res. 5:3085–3097.
2015.PubMed/NCBI
|
|
19
|
Wang W, Sun QK, He YF, Ma DC, Xie MR, Ji
CS and Hu B: Overexpression of periostin is significantly
correlated to the tumor angiogenesis and poor prognosis in patients
with esophageal squamous cell carcinoma. Int J Clin Exp Pathol.
7:593–601. 2014.PubMed/NCBI
|
|
20
|
Kawarada Y, Ishikura H, Kishimoto T, Saito
K, Takahashi T, Kato H and Yoshiki T: Inhibitory effects of the
antiangiogenic agent TNP-470 on establishment and growth of
hematogenous metastasis of human pancreatic carcinoma in SCID beige
mice in vivo. Pancreas. 15:251–257. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan MA, Srivastava SK, Bhardwaj A, Singh
S, Arora S, Zubair H, Carter JE and Singh AP: Gemcitabine triggers
angiogenesis-promoting molecular signals in pancreatic cancer
cells: Therapeutic implications. Oncotarget. 6:39140–39150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gore J, Craven KE, Wilson JL, Cote GA,
Cheng M, Nguyen HV, Cramer HM, Sherman S and Korc M: TCGA data and
patient-derived orthotopic xenografts highlight pancreatic
cancer-associated angiogenesis. Oncotarget. 6:7504–7521. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pomianowska E, Sandnes D, Grzyb K,
Schjølberg AR, Aasrum M, Tveteraas IH, Tjomsland V, Christoffersen
T and Gladhaug IP: Inhibitory effects of prostaglandin E2 on
collagen synthesis and cell proliferation in human stellate cells
from pancreatic head adenocarcinoma. BMC Cancer. 14:4132014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Georgiadou D, Sergentanis TN, Sakellariou
S, Filippakis GM, Zagouri F, Vlachodimitropoulos D, Psaltopoulou T,
Lazaris AC, Patsouris E and Zografos GC: VEGF and Id-1 in
pancreatic adenocarcinoma: Prognostic significance and impact on
angiogenesis. Eur J Surg Oncol. 40:1331–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XH, Kirschenbaum A, Yao S, Stearns ME,
Holland JF, Claffey K and Levine AC: Upregulation of vascular
endothelial growth factor by cobalt chloride-simulated hypoxia is
mediated by persistent induction of cyclooxygenase-2 in a
metastatic human prostate cancer cell line. Clin Exp Metastasis.
17:687–694. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu XF, Xie CG, Wang XP, Liu J, Yu YC, Hu
HL and Guo CY: Selective inhibition of cyclooxygenase-2 suppresses
the growth of pancreatic cancer cells in vitro and in vivo. Tohoku
J Exp Med. 215:149–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie C, Wang X, Dong Y, Du Q, Cai J and
Qian K: Effects of selective cyclooxygenase-2 inhibitor celebrex on
the expression of VEGF in pancreatic carcinoma PC-3 cell line.
China Oncol. 13:459–461. 2003.
|
|
28
|
Cheng T, Cao W, Wen R, Steinberg RH and
LaVail MM: Prostaglandin E2 induces vascular endothelial growth
factor and basic fibroblast growth factor mRNA expression in
cultured rat Müller cells. Invest Ophthalmol Vis Sci. 39:581–591.
1998.PubMed/NCBI
|
|
29
|
Jain S, Chakraborty G, Raja R, Kale S and
Kundu GC: Prostaglandin E2 regulates tumor angiogenesis in prostate
cancer. Cancer Res. 68:7750–7759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mestre JR, Mackrell PJ, Rivadeneira DE,
Stapleton PP, Tanabe T and Daly JM: Redundancy in the signaling
pathways and promoter elements regulating cyclooxygenase-2 gene
expression in endotoxin-treated macrophage/monocytic cells. J Biol
Chem. 276:3977–3982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lo CJ, Cryer HG, Fu M and Lo FR:
Regulation of macrophage eicosanoid generation is dependent on
nuclear factor kappaB. J Trauma. 45:19–24. 1998. View Article : Google Scholar : PubMed/NCBI
|