Introduction
At present, the death rate of gastric cancer is only
second to lung cancer and its incidence ranks fourth in the world
(1,2).
Clinical treatment for early gastric cancer is mainly focused on
surgery. However, most patients are diagnosed at advanced stage
when the treatment is more complicated. The main treatment method
consists of chemotherapy comprehensive treatment. There is no
standard chemotherapy strategy for advanced gastric cancer, a stage
when chemotherapy cannot significantly improve survival. In
addition, patients suffer adverse reactions caused by chemotherapy
(3–5).
As early stages of gastric cancer have no characteristic features,
it is often neglected. Some reports suggest that 50–60% of patients
are diagnosed with gastric cancer in middle or advanced stages in
China. For confirmed patients, the resection rate is only 40%.
Given the high recurrence and metastasis after tumor resection, the
prognosis is relatively poor. The 5-year survival rate after
surgery is only 20–30%. Therefore, for patients in middle or
advanced stages, especially those with local aggressiveness or
metastasis, surgery alone is not the ideal treatment (6). Currently, the treatment for gastric
cancer has shifted from simple surgery to combination with
radiotherapy and chemotherapy, including target-specific
treatments, like herceptin and other medicines (7).
Current clinical therapies often have serious
adverse reactions, leading to severe pain and serious impact on the
quality of life. Traditional Chinese medicine has many advantages,
including high efficiency and low toxicity. These significant
advantages have attracted recent interest from Chinese and Western
researchers. A report suggested that the traditional Chinese
extracts have relevant ability to inhibit cell proliferation,
inducing apoptosis, reducing the adverse reaction to radiotherapy
and chemotherapy (8). Resveratrol is
a non-flavonoid polyphenol with wide pharmacological actions,
including anti-mutation, antioxidative, and various biological
activities and pharmacological actions. In previous years, the
strong antitumor activity of resveratrol has attracted wide
attention (9,10).
Here, we report the inhibitory effect of resveratrol
on the growth of MGC-803 gastric cancer cells and the involvement
of the Wnt/β-catenin pathway. These results lay the foundation for
clinical treatment of gastric cancer with resveratrol.
Materials and methods
Materials and reagents
Resveratrol, methylthiazolyl tetrazolium (MTT) assay
(both from Sigma; Merck KGaA, Darmstadt, Germany); MGC-803 gastric
cancer cell line (Chinese Academy of Sciences Cell Bank, Shanghai,
China); rabbit anti-human glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), β-catenin, c-myc, cyclin D1 polyclonal antibodies, and
goat anti-rabbit HRP-conjugated secondary antibody (cat. nos.
10494-1-AP, 51067-2-AP, 10828-1-AP, 60186-1-Ig, SA00001-2; Wuhan
Sanying Biotechnology, Wuhan, China); Dulbecco's modified Eagle's
medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); RNA extraction kits, reverse transcription kits, reverse
transcription-polymerase chain reaction (RT-PCR) kits (all from
Invitrogen; Thermo Fisher Scientific, Inc.); primer synthesis
(Takara Biotechnology Co., Ltd., Dalian, China); bicinchoninic acid
(BCA) protein excretion kits, cell lysis buffer (both from Beyotime
Institute of Biotechnology, Nantong, China). The study was approved
by the Ethics Committee of Changchun University of Traditional
Chinese Medicine Affiliated Hospital (Changchun, China).
Cell culture
MGC-803 gastric cancer cells were cultured at 37°C
and 5% CO2. When cells reached 85% confluence, we
trypsinized them, and added DMEM culture medium with 10% fetal
bovine serum to dilute cell suspension. The cell concentration was
adjusted to 2×108 cells/l. After counting, the cells
were inoculated to the corresponding culture plates for the use of
following experiments.
Inhibition of cell proliferation
A total of 100 µl of cell suspension were inoculated
into 96 well plates at 1×105 cells/ml. After 24 h,
resveratrol was added at final concentrations of 0, 50, 75 and 100
µM. Each concentration was repeated in 5 wells and independently
replicated 6 times. The control group receive no treatment. The
cells were cultured at 37°C 5% CO2 for 24, 48, and 72 h,
and then the solution was changed. A total of 10 µl MTT was added
into each well at 5 mg/ml. After 4 h, the optic density (OD) value
at 570 nm was detected in each well. The proliferation inhibitory
rate was calculated according to following formula: Inhibition rate
(%) = (OD in control group - OD in experimental group)/OD in
control group × 100.
Cytomorphology observation
When the culture of MGC-803 cells was ready, the
cells were cultured with 50, 75, and 100 µM resveratrol. The
morphologic changes of each group were observed and filmed in an
inverted microscope (Nikon Corp., Tokyo, Japan).
RT-PCR
MGC-803 cells were inoculated in a 6-well plate,
each well containing 104 cells. After 24 h, the
supernatant was discarded. The cells were then cultured with 0, 50,
75, and 100 µM resveratrol for 48 h. After collecting the cells in
each group, the total RNA was extracted according to the
instructions in the RNA extraction kit. Concentration and purity of
total RNA were detected by UV-Vis spectrophotometer (Hitachi, Ltd.,
Tokyo, Japan) (A260/A280 >1.8 is considered to be qualified).
Then cDNA was produced by reverse transcription according to the
instructions of the reverse transcription kits. Then the cDNA was
used as template to determine the expression of β-catenin, c-myc
and cyclin D1 mRNA according to the instructions on the RT-PCR kit.
The primer sequences are shown in Table
I. The reaction conditions were: 95°C 10 min, 95°C 15 sec, 60°C
1 min, and 40 amplification circles. Cq value was obtained from
instrument software. The relative expression was calculated by
2−ΔΔCq method according to following formula: ΔCq
(target gene) = Cq (target gene) - Cq (target gene).
 | Table I.RT-PCR primer sequence. |
Table I.
RT-PCR primer sequence.
| Gene | Primer sequence |
|---|
| β-catenin | F:
5′-GCTTGGAATGAGACTGCTGA-3′ |
|
| R:
5′-CTGGCCATATCCACCAGAGT-3′ |
| c-myc | F:
5′-AGCGACTCTGAGGAGGAACA-3′ |
|
| R:
5′-TCCAGCAGAAGGTGATCCA-3′ |
| cyclin D1 | F:
5′-TGCCACAGATGTGAAGTTCATT-3′ |
|
| R:
5′-CAGTCCGGGTCACACTTGAT-3′ |
| GAPDH | F:
5′-CAAGGTCATCCATGACAACTTTG-3′ |
|
| R:
5′-GTCCACCACCCTGTTGCTGTAG-3′ |
Western blot analysis
MGC-803 cells were inoculated in 6-well plates, each
well containing 104 cells. After 24 h, the supernatant
was discarded. The cells were then cultured with 0, 50, 75, and 100
µM resveratrol for 48 h. After collecting the cells in each group,
they were treated with lysis buffer. Then the cells were
centrifuged for 15 min at 4°C at low temperature and high speed,
and the supernatant was collected. BCA kits were used to detect
protein levels. A total of 50 µg proteins were used for sodium
dodecyl sulphate-polyacrylamide gel electrophoresis electrophoretic
separation. The separated protein was electro-transferred into
polyvinylidene fluoride film. The membranes were sealed for 1 h at
room temperature with blocking buffer. Primary antibody was added
for incubation (1:1,000) overnight at 4°C. After fully washing the
membrane with TTBS, the secondary antibody (1:2,000) was added for
incubation for 1 h at room temperature. The signal was developed
with ECL in a dark room and scanned by gel imaging (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). GADPH was used as internal
reference for band quantitation.
Statistical analysis
Data were expressed as mean ± standard deviation and
processed by SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Data were
analyzed by one-way ANOVA and the post hoc used was Least
Significant Difference test. A P<0.05 was considered to indicate
a statistically significant difference.
Results
Resveratrol inhibits the proliferation
of MGC-803 cells
We first treated MGC-803 cells with 0, 50, 75, and
100 µM resveratrol and measured proliferations at 24, 48, and 72 h.
Resveratrol significantly inhibited MGC-803 cell proliferation in
each group in a time- and dose-dependent manner (P<0.01)
(Table II). Given the dose-dependent
effects of resveratrol, we used 50, 75, and 100 µM resveratrol for
the remaining experiments with an incubation time of 48 h.
 | Table II.Effect of resveratrol on the
proliferation of MGC-803 cells. |
Table II.
Effect of resveratrol on the
proliferation of MGC-803 cells.
|
| Proliferation
inhibition rate (%) |
|---|
|
|
|
|---|
| Concentration
(µM) | 24 h | 48 h | 72 h |
|---|
| 0 | 0 | 0 | 0 |
| 50 | 7.9±0.12a |
19.32±4.13a |
27.92±4.33a |
| 75 |
13.4±0.31a |
33.32±2.31a |
39.25±5.12a |
| 100 |
18.7±1.32a |
37.51±3.28a |
53.47±6.21a |
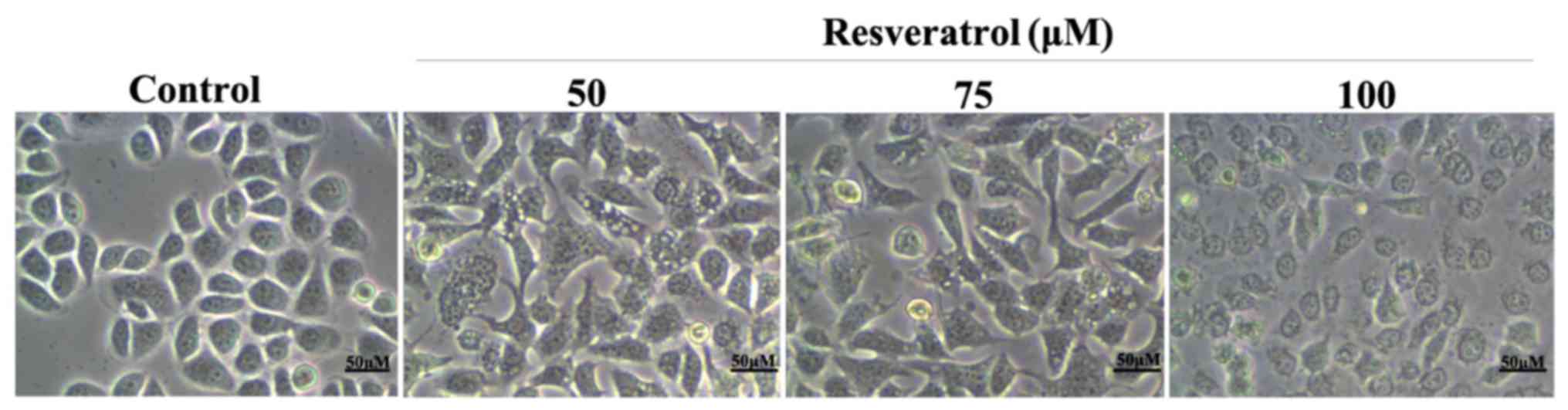
Effect of resveratrol on MGC-803 cell
morphology
We next examined the morphology of MGC-803 cells
after treatment with resveratrol. After 48 h culture with 0, 50, 75
and 100 µM resveratrol, the cell morphology significantly changed
compared with the control group (Fig.
1). Cell shrinkage was observed and the adherence was not firm.
The number of proliferating cells was lower with strong
dose-dependence.
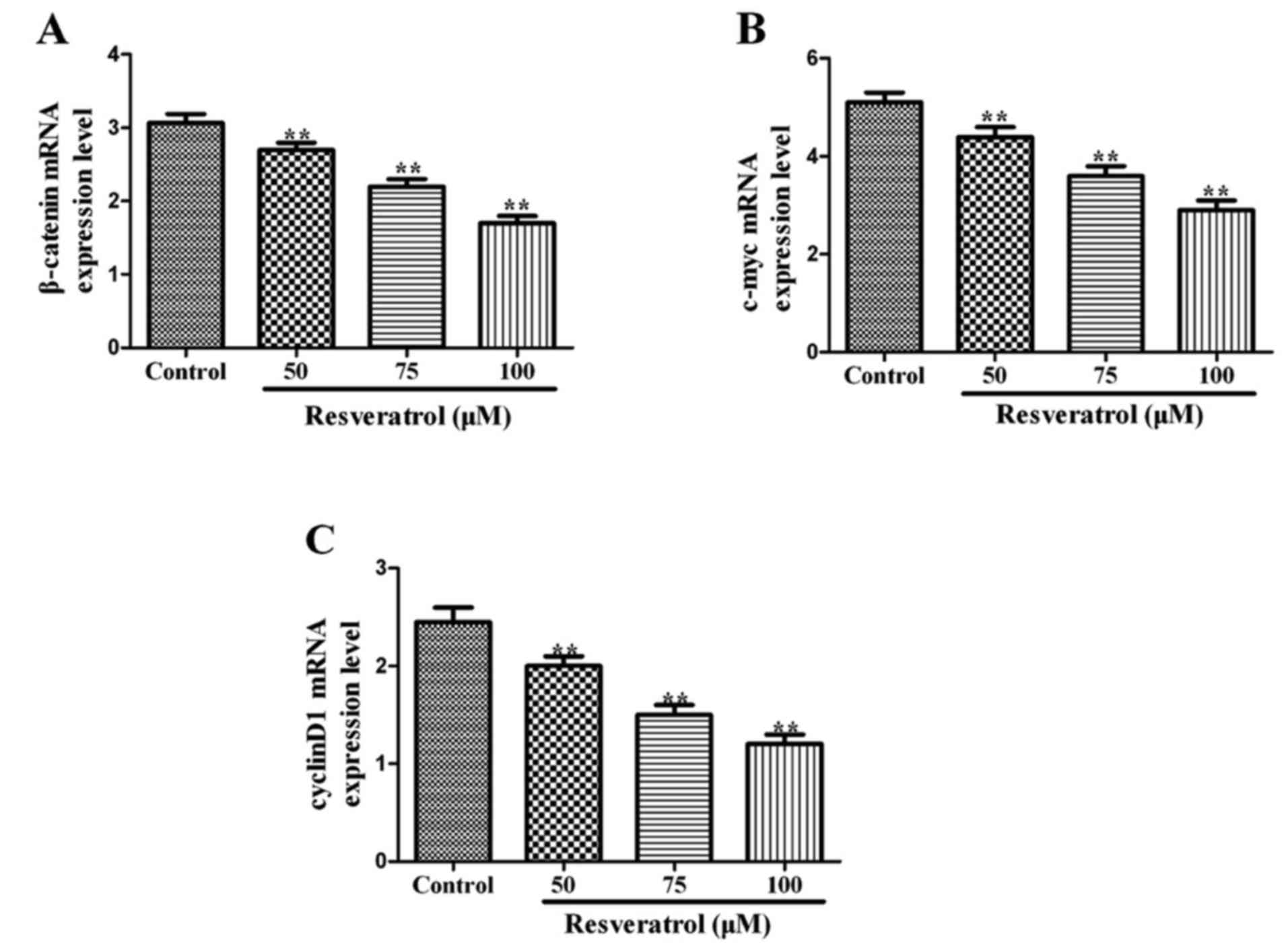
Resveratrol inhibits the expression of
β-catenin, c-myc, and cyclin D1 mRNA
To try to understand mechanistically the effects of
resveratrol, we examined the expression of proliferation markers:
β-catenin, c-myc, and cyclin D1. After culturing MGC-803 cells for
48 h with 0, 50, 75, and 100 µM resveratrol, the levels of
β-catenin, c-myc, and cyclin D1 mRNA were all significantly
downregulated compared with the control group (P<0.01) (Fig. 2). In the three cases, we observed a
dose-dependent response to resveratrol, suggesting its ability to
downregulate the expression of these key cell proliferation
targets.
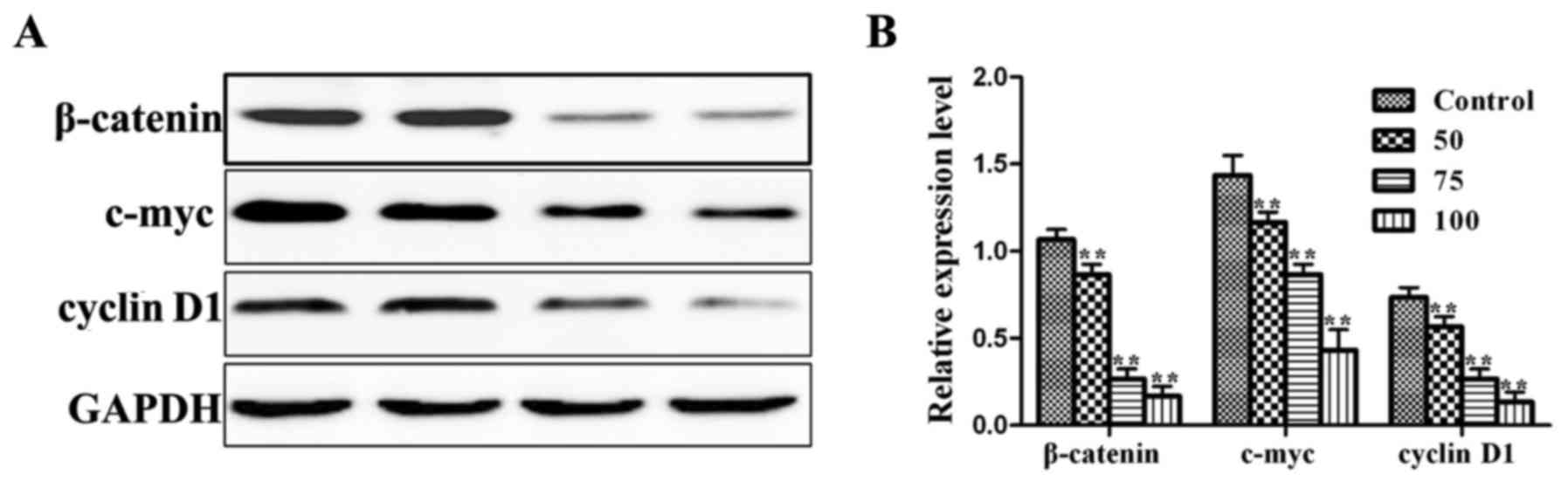
Resveratrol inhibits the expression of
β-catenin, c-myc and cyclin D1 proteins
To confirm the above results on mRNA levels of key
proliferation genes, we next examined the proteins levels for the
same three targets. Culturing MGC-803 cells for 48 h with 0, 50,
75, and 100 µM resveratrol, the levels of β-catenin, c-myc, and
cyclin D1 proteins were significantly inhibited compared with the
control group (P<0.01) (Fig. 3).
These results also demonstrated dose-dependency.
Discussion
The incidence of gastric cancer in Asia and South
Africa has been historically higher than that in the USA and West
Europe. Though the incidence of gastric cancer has shown downward
tendency globally since the 1950s, the current incidence keeps
increasing (11). Chemotherapy is the
main treatment methods, but has serious adverse actions and its
cost often results in low treatment adherence. Therefore, it is
essential to explore treatments featuring high effectiveness, low
toxicity, and reasonable price. In previous years, China has
dedicated increasing attention on the production and development of
traditional Chinese medicine. In the field of tumor inhibition,
molecular biology methods have been applied to improve the research
level of traditional Chinese medicine and provided wider prospect
for its application in clinical practice.
β-catenin is a membrane protein that promotes cell
adhesion. When it translocates to the nucleus or is degraded, the
adherent activity will disappear (12). Research suggest that β-catenin has
both cell adhesion and signal transduction functions. The abnormal
activation of the Wnt/β-catenin signal pathway is one of the
critical mechanisms of human tumorigenesis. β-catenin
overexpression is the main manifestation of the activation of this
signal pathway (13). The
proto-oncogenes cyclin D1 and c-myc play important roles in cell
proliferation, differentiation, and apoptosis, and are correlated
with the incidence of many tumors. Interestingly, cyclin D1 and
c-myc are important target genes in Wnt signal pathway (14). Immunohistochemical research has
confirmed that the abnormal expression of cyclin D1, c-myc,
β-catenin correlates with the activation of Wnt signal pathway in
many tumors. When the Wnt signal pathway is activated, β-catenin
enters into the nucleus, further activating the expression of
cyclin D1 and c-myc genes promoting cell proliferation (15). Abnormal nuclear accumulation and
activation of β-catenin becomes a tumorigenic gene. Research has
proved that β-catenin correlates with the incidence of pathologies
of the digestive system, hematological malignant tumors, and the
reproductive system (16).
In this report, we show that resveratrol inhibits
the proliferation of MGC-803 cells, induces apoptotic changes in
cell morphology, and inhibits the expression of the proliferation
factors β-catenin, c-myc, and cyclin D1 at the mRNA and protein
levels. Utsuki et al (17)
found that the expression levels of cyclin D1 and β-catenin
gradually increased along with the progression of tumors. In
addition, other research has found that the positive expression
rate of β-catenin, c-myc, and cyclin D1 in nephroblastoma cells is
significantly increased compared with normal renal tissues
(18), indicating that the
Wnt/β-catenin signaling pathway plays an important role in the
formation of ephroblastoma. Hu et al and Liu et al
(19,20) proposes that resveratrol reduces the
β-catenin abnormally accumulated in HCT116 cells and inhibits the
expression of cyclin D1 and c-myc, and therefore inhibits the tumor
growth in colon cancer cells. This result has provided theoretical
basis for the therapeutic effect of resveratrol for gastric
cancer.
In conclusion, we proved that resveratrol can
inhibit the proliferation of gastric cancer MGC-803 cell line, and
its action mechanism may be achieved by inhibiting Wnt signal
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD wrote the manuscript. HBD and HD were responsible
for cell culture. YHW performed PCR. JJG contributed to western
blot analysis. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Changchun University of Traditional Chinese Medicine Affiliated
Hospital (Changchun, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lv Y, Song L, Chang L, Liu Y, Zhang X,
Wang Y, Wang L and Liu W: Bevacizumab followed by chemotherapy is
potential therapy for gastric cancer. J BUON. 21:1466–1470.
2016.PubMed/NCBI
|
|
2
|
Shu B, Lei S, Li F, Hua S, Chen Y and Huo
Z: Laparoscopic total gastrectomy compared with open resection for
gastric carcinoma: A case-matched study with long-term follow-up. J
BUON. 21:101–107. 2016.PubMed/NCBI
|
|
3
|
Shen YH, Xie ZB, Yue AM, Wei QD, Zhao HF,
Yin HD, Mai W, Zhong XG and Huang SR: Expression level of
microRNA-195 in the serum of patients with gastric cancer and its
relationship with the clinicopathological staging of the cancer.
Eur Rev Med Pharmacol Sci. 20:1283–1287. 2016.PubMed/NCBI
|
|
4
|
Zhou SF, Yin JB, Yang H, Zhong J and An P:
Application value of stomach filling ultrasonography and
intravenous contrast agents in diagnosis of advanced gastric
cancer. Eur Rev Med Pharmacol Sci. 20:3206–3210. 2016.PubMed/NCBI
|
|
5
|
Zhang N, Wang AY, Wang XK, Sun XM and Xue
HZ: GAS5 is downregulated in gastric cancer cells by promoter
hypermethylation and regulates adriamycin sensitivity. Eur Rev Med
Pharmacol Sci. 20:3199–3205. 2016.PubMed/NCBI
|
|
6
|
Mello BS, Lucena AF, Echer IC and Luzia
MF: Patients with gastric cancer submitted to gastrectomy: An
integrative review. Rev Gaúcha Enferm. 31:803–811. 2010.(In
Portuguese). View Article : Google Scholar
|
|
7
|
Bang YJ, van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olaku O and White JD: Herbal therapy use
by cancer patients: A literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
10
|
Gescher A, Steward WP and Brown K:
Resveratrol in the management of human cancer: How strong is the
clinical evidence? Ann N Y Acad Sci. 1290:12–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crew KD and Neugut AI: Epidemiology of
upper gastrointestinal malignancies. Semin Oncol. 31:450–464. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyoshi K and Hennighausen L:
Beta-catenin: A transforming actor on many stages. Breast Cancer
Res. 5:63–68. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maruyama K, Ochiai A, Akimoto S, Nakamura
S, Baba S, Moriya Y and Hirohashi S: Cytoplasmic beta-catenin
accumulation as a predictor of hematogenous metastasis in human
colorectal cancer. Oncology. 59:302–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
15
|
Lim SC and Lee MS: Significance of
E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol
Rep. 9:915–928. 2002.PubMed/NCBI
|
|
16
|
Roh MS, Hong SH, Jeong JS, Kwon HC, Kim
MC, Cho SH, Yoon JH and Hwang TH: Gene expression profiling of
breast cancers with emphasis of beta-catenin regulation. J Korean
Med Sci. 19:275–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Utsuki S, Sato Y, Oka H, Tsuchiya B,
Suzuki S and Fujii K: Relationship between the expression of E-,
N-cadherins and beta-catenin and tumor grade in astrocytomas. J
Neurooncol. 57:187–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ehrlich D, Bruder E, Thome MA, Gutt CN,
von Knebel Doeberitz M, Niggli F, Perantoni AO and Koesters R:
Nuclear accumulation of beta-catenin protein indicates activation
of wnt signaling in chemically induced rat nephroblastomas. Pediatr
Dev Pathol. 13:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Y, Wang S, Wu X, Zhang J, Chen R, Chen
M and Wang Y: Chinese herbal medicine-derived compounds for cancer
therapy: A focus on hepatocellular carcinoma. J Ethnopharmacol.
149:601–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|