Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, with the majority of cases
non-small cell lung cancer (NSCLC) (1). Platinum-based chemotherapy in
combination with radiation therapy is the mainstream in patients
with NSCLC treatment; however, chemoresistance limits the survival
of patients with advanced disease that are receiving chemotherapy
(2). Cisplatin (DDP) is one of the
most commonly used chemotherapeutic agents for patients with lung
cancer owing to its high efficiency, relatively mild side effects
and ease of administration. However, DDP resistance often occurs in
clinical practice (3). Therefore,
investigation of the mechanisms of drug resistance may accelerate
the identification of novel therapeutic targets and strategies for
the treatment of NSCLC (4).
Multidrug resistance (MDR) is a major obstacle of
successful chemotherapy treatment. Thus, an improved understanding
of the different mechanisms underlying MDR or searching for
effective MDR modulators represents a promising strategy to
overcome drug resistance. Several major mechanisms of drug
resistance in cancer cells have been identified, including
alterations to drug metabolism and apoptosis pathways, deficiencies
in DNA repair pathways that induce resistance to chemotherapy and
overexpression of ATP-binding cassette (ABC) transporters that are
involved in the efflux of chemotherapeutic drugs (5–7). The
phosphoinositide 3-kinase (PI3K)/RAC serine/threonine-protein
kinase (Akt) signaling pathway has a substantial role in drug
resistance by enabling tumor cells to escape apoptosis (8). The PI3K/Akt signaling pathway has been
reported to control the expression and function of the numerous
proteins essential for tumor cell drug resistance (9,10).
Inhibitor of DNA binding 3 (ID3) is a member of the
ID family of genes, which are key regulators of cellular
proliferation, differentiation, apoptosis, tumorigenesis and
carcinogenesis (11–13). A previous study demonstrated that ID3
induced apoptosis in immortalized human keratinocytes upon exposure
to ultraviolet B radiation, consistent with its role as a tumor
suppressor (14). ID3 synergizes with
5-FU and DDP therapies for non-melanoma skin cancer cells, and
mediates apoptosis in A431 cells via the ETS domain-containing
protein Elk-1/caspase-8-dependent pathway (15). Overexpression of ID3 has been
documented to trigger apoptosis in A549 human lung adenocarcinoma
cells (16,17), meaning that it could serve as a novel
strategy for inhibiting DDP-sensitive lung cancer (18). Thus, there may be a link between the
ability of ID3 to induce apoptosis and function as a tumor
suppressor gene.
ID3, as a regulator of apoptosis whose expression is
induced by antineoplastic agents, has the potential to enhance the
sensitivity of chemotherapy. However, whether and how ID3 is
involved in regulating DDP resistance in A549/DDP cells remains
unclear. The present study revealed that overexpression of ID3
induced apoptosis in A549/DDP cells and enhanced the sensitivity of
A549/DDP cells to DDP treatment. In addition, the results of the
present study also indicated that ID3 exerted its tumor-suppressing
activity by directly suppressing the PI3K/Akt signaling pathway.
These results may provide novel insights into the molecular
mechanisms of DDP resistance induced by overexpression of ID3
levels in lung adenocarcinoma cells and indicate that ID3 may
represent a novel therapeutic target for the reversal of DDP
resistance in lung adenocarcinoma cells.
Materials and methods
Cell culture
The DDP-resistant A549/DDP cell line and native A549
cells were purchased from the Cancer Institute of the Chinese
Academy of Medical Sciences (Beijing, China). Cells were cultured
as previously described (18). In all
experiments, cells were grown in culture medium to 70% confluence
in 100-mm tissue culture dishes. Cells were serum-starved for 24
h.
Transient transfection
Briefly, A549 and A549/DDP cells (2.5×105
cells/ml) were seeded in 6-well plates and incubated overnight.
Cells were then transfected with pEGFP/ID3 (4 µg/µl, overexpression
ID3 vectors) and pEGFP/control (4 µg/µl, control vectors) plasmid,
which were purchased from Nanjing Realgene Co., Ltd. (Nanjing,
China) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. After 24 h, cells were collected, followed
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), western blot analysis, or cell viability determined by
CCK-8.
CCK-8 assay for proliferation
activity
A549/DDP cells (1×104 cells/ml) that were
untransfected, transfected with pEGFP/ID3 or transfected with
pEGFP/control were seeded into 96-well plates and incubated with
various concentrations of DDP (0, 0.5, 1.0, 2.0, 5.0, 10, 15, 20,
30, 40 and 80 µg/ml) for 24 h in quadruplicate. Next, 10 µl CCK-8
(Dojindo Molecular Technologies, Inc., Shanghai, China) solution
was added to every well and cells were incubated for a further 4 h.
The optical density (OD) was then measured at 570 nm using a
microplate reader. The inhibition rate was calculated as follows:
Inhibition rate (%)=(1-OD570nm value of experimental
group/OD570nm value of control group) ×100. From this,
the half-maximal inhibitory concentration (IC50) was
calculated. The reversal fold (RF) values were calculated using the
following formula: RF=IC50 of pEGFP-ID3
group/IC50 of the pEGFP group. The experiments were
repeated three times.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis assay
Transfected and untransfected A549/DDP cells
(3.5×105 cells/ml) were seeded into 12-well plates and
incubated with 0, 1.0 or 2.0 µg/ml DDP for 24 h. The A549/DDP cells
were then washed with 1X PBS and resuspended in 100 µl binding
buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Next,
Annexin V-FITC and PI (Sigma-Aldrich; Merck KGaA) were added for 30
min at 37°C in the dark. Following dilution with 400 µl binding
buffer, staining was analyzed within 1 h by flow cytometry. The
fluorescence intensity (green, FL1-H; red, FL2-H) was measured
using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA,
USA). CellQuest Pro software 5.1 (BD Biosciences) was used for
acquisition and analysis of data.
RT-qPCR analysis
Total RNA was isolated from A549 and A549/DDP cells,
using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.)
in accordance with the manufacturer's protocol. The concentration
and purity of the RNA samples were determined using Eppendorf
Biophotometer (Eppendorf, Hamburg, Germany). In total, 1 µg of
total RNA was used to synthesize cDNA. cDNA was obtained by reverse
transcription with the PrimeScript RT Master Mix (Takara Bio, Inc.,
Otsu, Japan). qPCR was performed using the FastStart Universal SYBR
Green Master (Rox) (Roche Applied Science, Rotkreuz, Switzerland)
with ABI 7500 Real Time PCR system (Applied Biosystems, CA, USA).
The primers were designed with software Premier 5.0 (Premier
Biosoft International, Palo Alto, CA, USA) and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). The sequences of the
primers are depicted in Table I. The
thermocycling conditions were as follows: 95°C for 4 min, followed
by 40 cycles of 95°C for 40 sec and 60°C for 30 sec, and final
elongation at 72°C for 5 min. GAPDH was used as an internal
control, and the fold change was calculated using the
2−ΔΔCq method (19). PCR
was performed in triplicate.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence | Product length,
bp |
|---|
| ID3 | Forward |
5′-ATGAAGGCGCTGAGCCCGGTGC-3′ | 360 |
|
| Reverse |
5′-CGGCCGAGTCAGTGGCAAAAGC-3′ |
|
| Bcl-2 | Forward |
5′-GGGTGGGAGGGAGGAAGAATTT-3′ | 123 |
|
| Reverse |
5′-GCATCACATCGACCCCAATACAG-3′ |
|
| GAPDH | Forward |
5′-CAACTAAGCGGCACAGAATG-3′ | 180 |
|
| Reverse |
5′-GCCAGTGGACTCCACGAC-3′ |
|
Flow cytometry analysis
A549/DDP cells (3.5×105 cells/ml)
transfected with pEGFP/ID3 or pEGFP/control, or untransfected cells
were washed with 1X PBS buffer and resuspended in 200 µl cell
staining buffer (Beyotime Institute of Biotechnology, Shanghai,
China). After blocking with 10% fetal bovine serum (FBS; HyClone
Laboratories, Inc.) at 4°C for 1 h to minimize non-specific
background signals, 5 µl allophycocyanin (APC)-conjugated
anti-human multi-drug resistant protein-1 (MDR-1) antibody (ready
to use dilution; cat. no. 348607; BioLegend, San Diego, CA, USA)
was added for 10–15 min at 37°C in the dark. The expression of
MDR-1 protein on the cell membrane was detected using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA). CellQuest Pro
software 5.1 (BD Biosciences) was used for acquisition and analysis
of data.
Western blotting assay
Cells from each group were cultured in the 6-well
plates were washed in PBS and lysed in Radioimmunoprecipitation
Assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). Protein concentrations was quantified by BCA Protein Assay
kit (Beyotime Institute of Biotechnology), Equal amounts of protein
(20 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). After membranes were blocked with 5% skim milk in TBS
containing 0.2% Tween-20 at room temperature for 2 h, the membranes
were incubated overnight at 4°C with primary antibodies comprising
anti-MDR-1antibody (cat. no. ab-170904; 1:400; Abcam, Cambridge,
UK), anti-RhoE antibody (cat. no. sc-53874; 1:400; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-PI3K antibody (cat. no.
ab-40776; 1:400; Abcam). Subsequently, membranes were washed three
times, and then incubated with horseradish peroxidase-conjugated
anti-rabbit (cat. no. sc-51687; 1:1,000; Santa Cruz Biotechnology,
Inc.) or anti-mouse secondary antibodies (cat. no. sc-2954;
1:1,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Anti-β-actin antibody (cat. no. sc-47778; 1:500; Santa
Cruz Biotechnology, Inc.) was used as a control. Immunoreactive
bands were visualized by Enhanced Chemiluminescence Western
Blotting Detection Reagents (GE Healthcare Life Sciences, Little
Chalfont, UK) according to the manufacturer's protocol. The
densitometric analysis of the bands was performed using ImageJ
software (v.2.0; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). The statistical difference of data
between groups was analyzed by one-way analysis of variance (ANOVA)
or Student's t-test. Student-Newman-Keuls (SNK) was used as a post
hoc test following ANOVA. Data are expressed as the means ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
The expression of ID3 mRNA in A549 and
A549/DDP cells was detected by fluorescent quantitative PCR
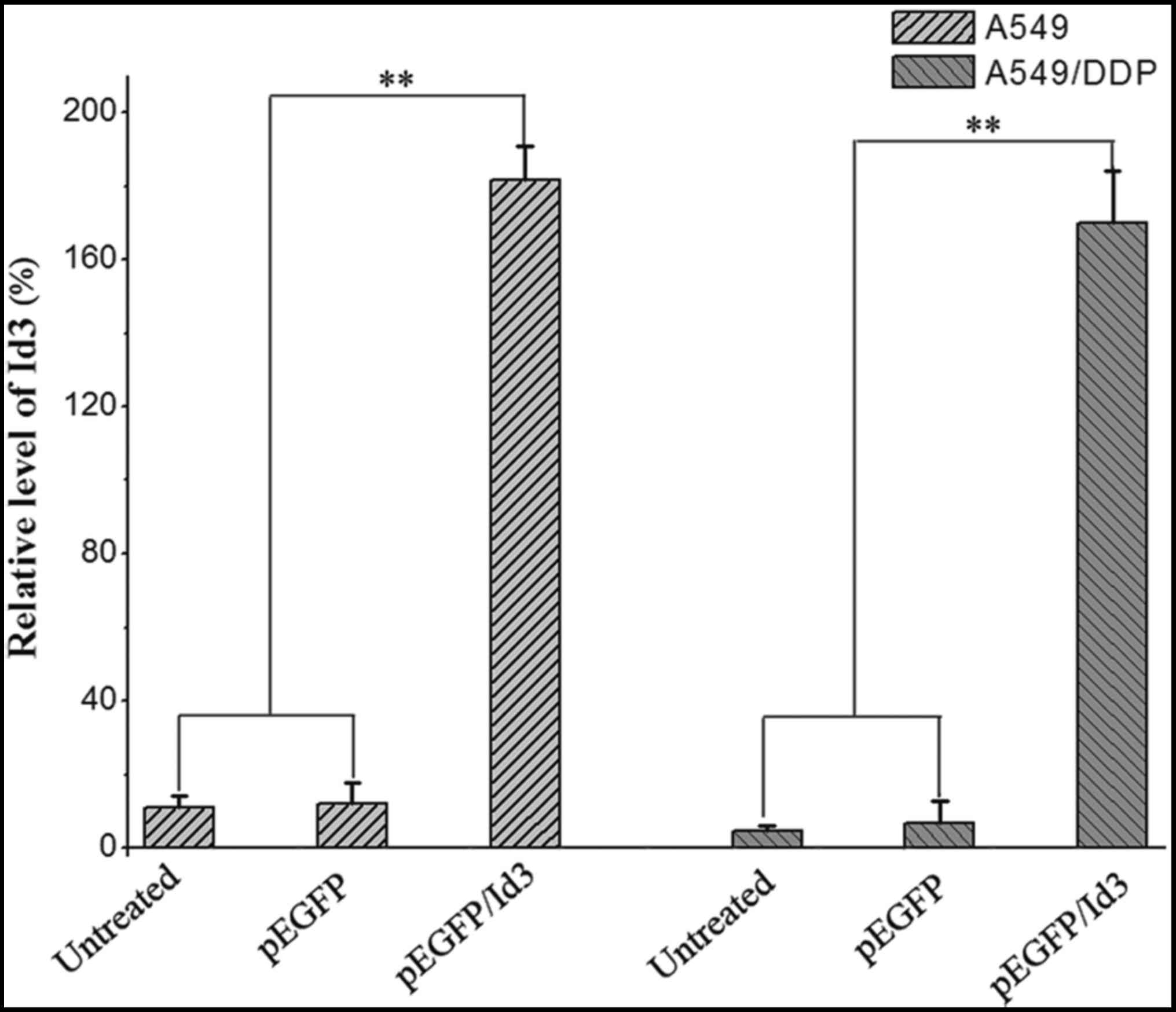
The expression of ID3 was detected by RT-qPCR. Owing
to the low level of expression of ID3 in A549 and A549/DDP cells,
ID3 was overexpressed to elucidate the direct implication of ID3 in
the chemosensitivity of A549/DDP cells. As shown in Fig. 1, the expression of ID3 was
significantly increased in A549/DDP cells transfected with
pEGFP/ID3 compared with those transfected with the empty vector or
untransfected cells at the mRNA level.
Raised sensitivity of chemotherapy in
A549/DDP cells owing to ID3 overexpression
Following pEGFP/ID3 transfection, the growth of
A549/DDP cells was evaluated using a CCK-8 assay. As shown in
Fig. 2A, the IC50 of DDP
in A549/DDP cell decreased from 19.38 to 11.71 µg/ml, representing
a 1.66-fold increase in DDP sensitization after ID3 transfection.
The results of the CCK-8 assay demonstrated that ID3 expression and
DDP treatment had synergistic effect on the inhibition of cell
growth, and the synergistic effect was caused by the change of
biological characteristics of A549/DDP cells following ID3
induction.
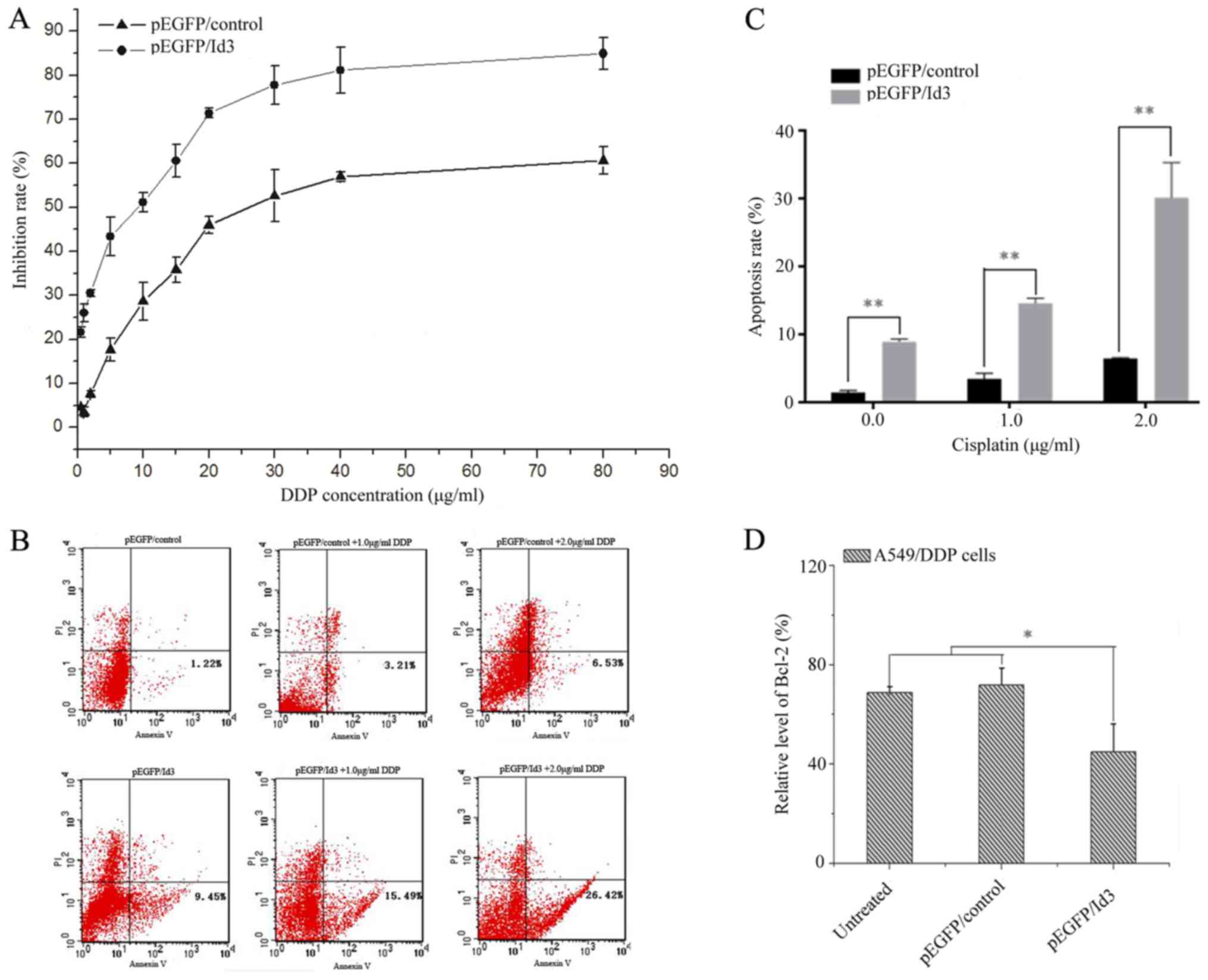 | Figure 2.ID3 enhances DDP sensitivity and
increases apoptosis in A549/DDP cells. (A) The growth inhibitory
effect of ID3/pEGFP on A549 and A549/DDP cells at different DDP
concentrations (0, 0.5, 1.0, 2.0, 5.0, 10, 15, 20, 30, 40 and 80
µg/ml). (B) Flow cytometry analysis of apoptosis in pEGFP/control
or pEGFP/ID3-transfected A549/DDP cells combined with various
concentrations of DDP (0.0, 1.0 or 2.0 µg/ml). (C) The percentage
of apoptotic cells in cells transfected with ID3 and treated with
DDP was significantly higher than that in the pEGFP/control group.
**P<0.01 vs. pEGFP/control group. (D) After A549/DDP cells were
transfected with ID3/pEGFP or pEGFP/control for 24 h, the levels of
Bcl-2 expression were assessed by reverse
transcription-quantitative polymerase chain reaction analysis.
*P<0.05 vs. pEGFP/control group and blank cells. GAPDH served as
an internal reference. Experiment was performed in triplicate.
*P<0.05. ID3, inhibitor of DNA binding 3; DDP, cisplatin; EGFP,
enhanced green fluorescent protein; Bcl-2, B-cell lymphoma-2. |
After transfection with pEGFP/ID3, A549/DDP cells
were treated with DDP at different concentrations (0.0, 1.0 or 2.0
µg/ml) and the apoptotic rate were detected by Annexin V-FITC/PI
double staining. As depicted in Fig. 2B
and C, the apoptotic rate of A549/DDP cells transfected with
pEGFP/ID3 group was significantly higher than that of pEGFP/control
group (P<0.05), and the apoptotic rate increased with the
increase of DDP concentration, indicating that ID3 and DDP have a
synergistic effect on cell apoptosis. This evidence further
demonstrated that ID3 serves a key role in the mechanism of
reversal of DDP resistance in human lung adenocarcinoma cells,
potentially due to the overexpression of ID3, which results in a
series of biological effects leading to enhancement of the
sensitivity of DDP in A549/DDP cells, resulting in apoptosis, and
reversal of drug resistance.
The results of the western blotting assay
demonstrated that the expression of anti-apoptotic gene Bcl-2 was
significantly downregulated in pEGFP/ID3 transfected cells
(P<0.05; Fig. 2D), indicating that
ID3 may be involved in apoptosis as part of the upstream
regulatory/apoptotic-associated genes, thus reversing drug
resistance.
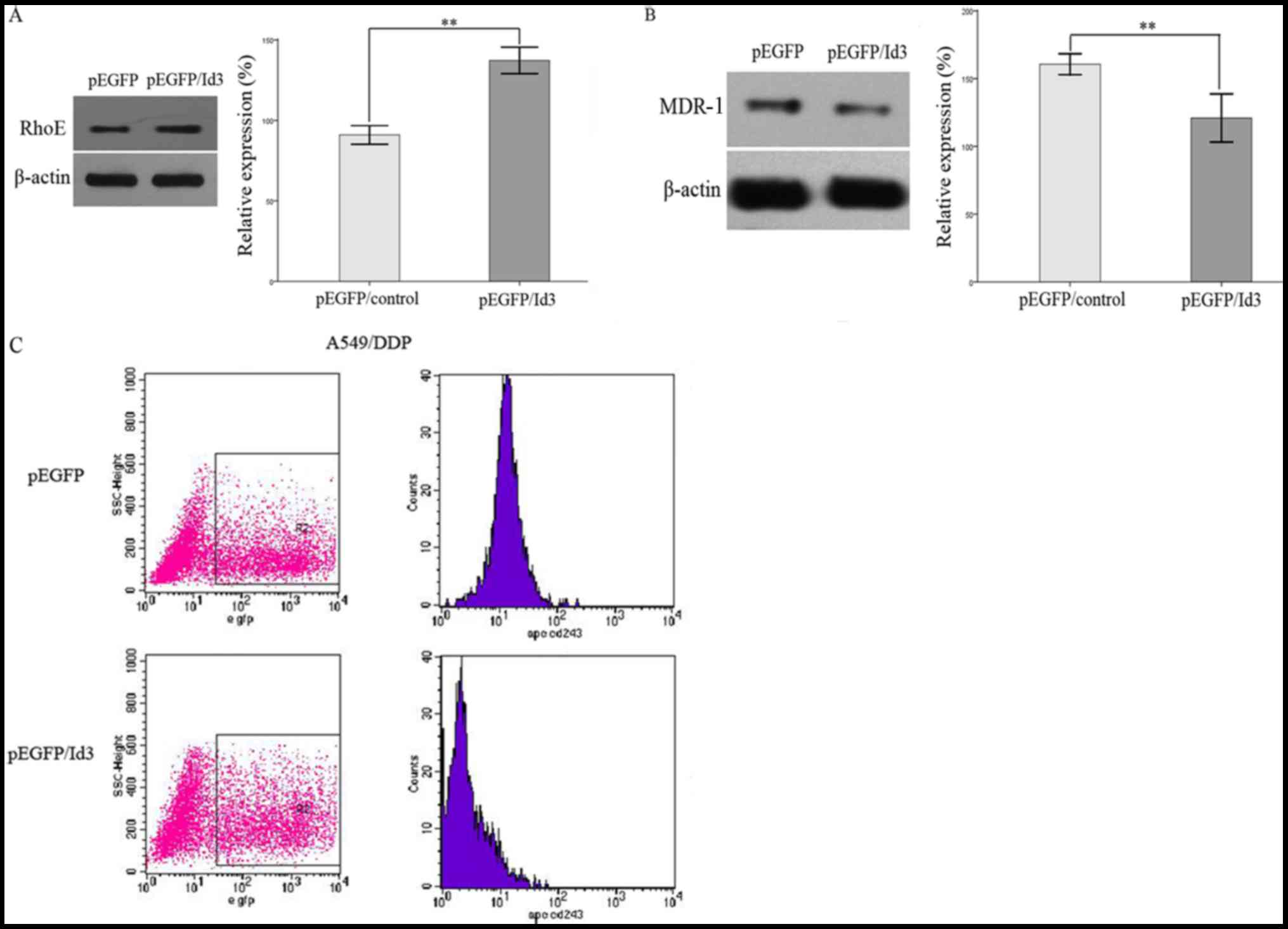
Inhibition of MDR-mediated transport
due to ID3 overexpression
To investigate the possible molecular mechanism of
the ID3-dependent reversal of DDP resistance and promotion of
apoptosis in tumor cells, the changes in the expression of
multidrug resistance-associated genes was assessed. The results of
this analysis revealed that the expression of RhoE protein was
increased in pEGFP/ID3 transfected cells compared with the empty
vector group (Fig. 3A). Furthermore,
western blotting and flow cytometry analysis (Fig. 3B and C) revealed that the expression
of MDR-1 was significantly downregulated in pEGFP/ID3 transfected
cells. This result indicated that the efflux functions mediated by
MDR-1 were significantly inhibited following ID3 overexpression.
The data indicated that ID3 may be implicated in key steps of the
development of multi-drug resistance in human lung adenocarcinoma
cells.
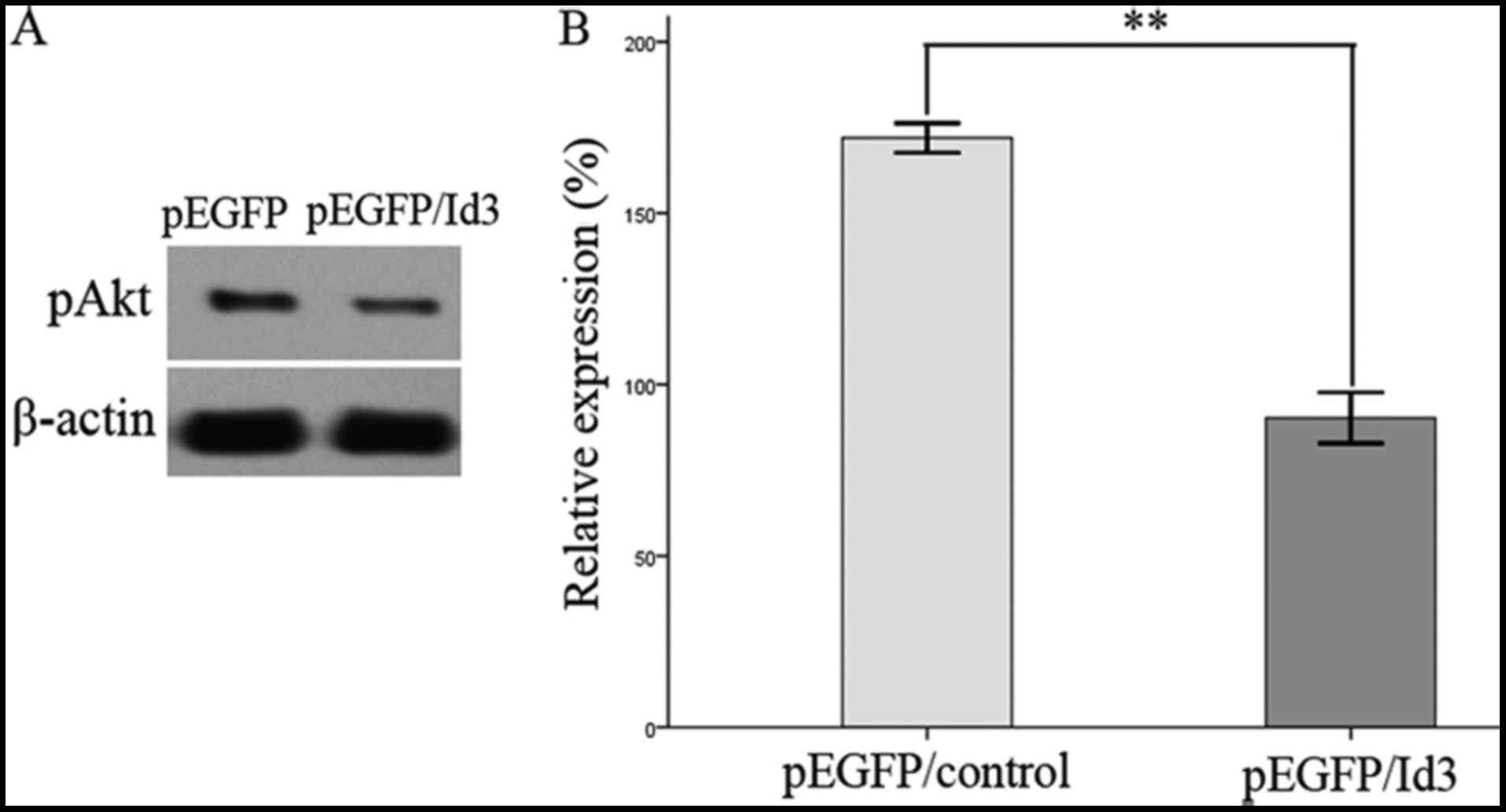
ID3 overexpression results in the
inhibition of PI3K/Akt signaling
The PI3K/Akt pathway is one of the most notable
signaling pathways in cell apoptosis. The activation of the
PI3K/Akt signaling pathway is involved in the regulation of cell
survival and glycogen metabolism. It is closely associated with the
inhibition of tumor cell apoptosis and the occurrence and
development of tumors. Akt activity is positively associated with
drug resistance in lung, ovarian and breast cancer (20). The present study assessed the activity
of PI3K/Akt signaling by pEGFP/ID3 transfection in A549/DDP cells.
Western blotting (Fig. 4) revealed
that the phosphorylation of Akt was significantly decreased
following pEGFP/ID3 transfection, compared with the empty vector
group (P<0.05). Conversely, overexpression of ID3 inhibited
PI3K/Akt signaling pathway activity, thereby inhibiting tumor cell
proliferation and reversing of drug resistance.
Discussion
ID3 is considered to have multiple roles in the
regulation of cell growth; it was identified as a tumor suppressor
gene for Burkitt's lymphoma (21).
Kee et al (22) proposed that
the induction of apoptosis by the retroviral-mediated expression of
ID3 in B lymphocyte progenitors (BLPs) by transforming growth
factor-β1-mediated signaling. In addition, Genetic aberrations to
ID3 indicate that it may function as a tumor suppressor (23). It has been recognized that ID3 serves
as an inducer of apoptosis in response to X-ray irradiation via the
regulation of endogenous β-catenin level (24). Evidence has also demonstrated that ID3
induced apoptosis in immortalized human keratinocytes (14). Recently, it was reported that
ID3-induced apoptosis is mediated through its HLH and C-terminal
domains. ID3 also sensitized SCC cells to chemotherapeutic agents,
including DDP and 5-fluorouracil (5-FU), via
Elk-1/caspase-8-dependent apoptotic pathway (15). Previous studies found that exogenous
ID3 expression induced inhibition of proliferation and apoptosis in
A549 cells and A549/DDP cells (16–18). The
present study aimed to determine whether ID3 overexpression could
enhance the sensitivity of lung cancer cells to DDP.
Inhibitor of differentiation/DNA binding (Id)
proteins, which are negative regulators of basic helix-loop-helix
(bHLH) transcription factors, function as dominant-negative
inhibitors of E-proteins by inhibiting their ability to bind DNA
(12). ID3 belongs to the ID family
and acts as a negative regulator that inhibits apoptosis by
anticancer drugs (25), which is
expected to become a novel therapeutic target for enhancing
sensitivity to chemotherapy. ID3 has been shown to sensitize
sarcoma cells and A431 cells to DDP and 5-FU, respectively
(26). A previous study revealed that
ID1 is a molecular marker of lung cancer prognosis, and
downregulation of the expression of ID1 could increase the
sensitivity of lung cancer chemotherapy; however, its mechanism
remains unclear (27). Additional
evidence revealed that downregulation of ID1 can enhance the
sensitivity of gastric cancer MGC803 and AGS cells to DDP (28). ID1 and ID3 co-expression was
associated with a poor clinical outcome in patients with locally
advanced NSCLC treated with chemoradiotherapy (29). The results of the present study
indicated that ID3 serves an important role in cisplatin resistance
in lung adenocarcinoma, and demonstrated that ID3 overexpression
may enhance cisplatin chemosensitivity and resulted in markedly
attenuated growth inhibition of tumor cells. However, to the best
of our knowledge, no study exists concerning the specific mechanism
of apoptosis driven by ID3 in human lung adenocarcinoma cells and
the mechanism of resistance reversal in A549/DDP.
Bcl-2 can suppress apoptosis, leading to the
generation of drug resistance in several cell types (30). Bcl-2-transfected cancer cells became
more resistant to DDP (30,31). Therefore, the expression of Bcl-2 is
closely associated with drug resistance in tumor cells. The results
of RT-qPCR in the present study revealed that the expression of e
anti-apoptotic gene Bcl-2 was significantly downregulated in
pEGFP/ID3-transfected cells, indicating that ID3 may be involved in
apoptosis as part of the upstream/anti-apoptosis-associated genes
to reverse cell resistance.
Drug resistance is the primary reason for the
failure of cancer treatments (1). MDR
is the main cause of chemotherapy failure, leading to the
recurrence of cancer (32). Thus it
is important to find effective methods to reverse MDR. The present
study demonstrated that the expression of MDR-1 in A549/DDP cells
transfected with pEGFP/ID3 was significantly downregulated, as
analyzed using flow cytometry and western blot analysis
(P<0.05), indicating that ID3 overexpression reverses DDP
resistance in A549/DDP cells. Notably, ID3 transgene expression
increased the expression of RhoE, which may result in inhibition of
tumor growth. These results are consistent with those of recent
studies, which revealed that the downregulation of RhoE expression
in lung cancer cell lines and other cancerous tissues may
contribute to the invasion and metastasis of tumor cells (33,34).
Taken together, the results of the present study
demonstrated that ID3 overexpression in A549/DDP cells inhibited
DDP resistance by suppressing activation of the PI3K/Akt signaling
pathway. Therefore, overexpression of ID3 may be a potential
approach to reverse DDP resistance in DDP-resistant human lung
adenocarcinoma cells. However, the exact molecular mechanisms of
tumor MDR require further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
NSFC-81171652), the Jiangsu Province Science and Technology Program
(grant no. BL2014072) and the National Clinical Key Program (grant
no. 2014ZDZK003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FC and QZ conceptualized the experiments. XLv
analyzed the data. YX performed the statistical analysis. SS
performed cell experiments and flow cytometry. WY contributed to
study design. XLi contributed to study design and manuscript
writing.
Ethics approval and consent to publish
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kievit FM, Wang FY, Fang C, Mok H, Wang K,
Silber JR, Ellenbogen RG and Zhang M: Doxorubicin loaded iron oxide
nanoparticles overcome multidrug resistance in cancer in vitro. J
Control Release. 152:76–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan XL, Moyer AM, Fridley BL, Schaid DJ,
Niu N, Batzler AJ, Jenkins GD, Abo RP, Li L, Cunningham JM, et al:
Genetic variation predicting cisplatin cytotoxicity associated with
overall survival in lung cancer patients receiving platinum-based
chemotherapy. Clin Cancer Res. 17:5801–5811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and de Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancercells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/Akt/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Wei XH, Pan YP, Li HC, Yang H, He
QH, Pang Y, Shan Y, Xiong FX, Shao GZ and Zhou RL: LAPTM4B: A novel
cancer-associated gene motivates multidrug resistance through
efflux and activating PI3K/Akt signaling. Oncogene. 29:5785–5795.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin X, Zhang X, Wang Q, Li J, Zhang P,
Zhao M and Li X: Perifosine downregulates MDR1 gene expression and
reverses multidrug-resistant phenotype by inhibiting
PI3K/Akt/NF-kappaB signaling pathway in a human breast cancer cell
line. Neoplasma. 59:248–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lasorella A, Uo T and Iavarone A: Id
proteins at the cross-road of development and cancer. Oncogene.
20:8326–8333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruzinova MB and Benezra R: Id proteins in
development, cell cycle and cancer. Trends Cell Biol. 13:410–418.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rotzer D, Krampert M, Sulyok S, Braun S,
Stark HJ, Boukamp P and Werner S: Id proteins: Novel targets of
activin action, which regulate epidermal homeostasis. Oncogene.
25:2070–2081. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simbulan-Rosenthal CM, Daher A, Trabosh V,
Chen WC, Gerstel D, Soeda E and Rosenthal DS: ID3 induces a
caspase-3- and −9-dependent apoptosis and mediates UVB
sensitization of HPV16 E6/7 immortalized human keratinocytes.
Oncogene. 25:3649–3660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YS, Aubee J, DiVito KA, Zhou H, Zhang
W, Chou FP, Simbulan-Rosenthal CM and Rosenthal DS: ID3 induces an
Elk-1-caspase-8-dependent apoptotic pathway in squamous carcinoma
cells. Cancer Med. 4:914–924. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XJ, Zhu CD, Yu W, Wang P, Chen FF, Xia
XY and Luo B: Overexpression of ID3 induces apoptosis of A549 human
lung adenocarcinoma cells. Cell Prolif. 45:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen FF, Liu Y, Wang F, Pang XJ, Zhu CD,
Xu M, Yu W and Li XJ: Effects of up-regulation of ID3 in human lung
adenocarcinoma cells on proliferation, apoptosis, mobility and
tumorigenicity. Cancer Gene Ther. 22:431–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen F, Zhao Q, Wang S, Wang H and Li X:
Upregulation of ID3 inhibits cell proliferation and induces
apoptosis in A549/DDP human lung cancer cells in vitro. Mol Med
Rep. 14:313–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krystal GW, Sulanke G and Litz J:
Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks
growth, promotes apoptosis, and enhances sensitivity of small cell
lung cancer cells to chemotherapy. Mol Cancer Ther. 1:913–922.
2002.PubMed/NCBI
|
|
21
|
Schmitz R, Young RM, Ceribelli M, Jhavar
S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, et
al: Burkitt lymphoma pathogenesis and therapeutic targets from
structural and functional genomics. Nature. 490:116–120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kee BL, Rivera RR and Murre C: ID3
inhibits B lymphocyte progenitor growth and survival in response to
TGF-β. Nat Immunol. 2:242–247. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellmeier W, Aguzzi A, Kleiner E, Kurzbauer
R and Weith A: Mutually exclusive expression of a helix loop-helix
gene and N-myc in human neuroblastom as and in normal development.
EMBO J. 11:2563–2571. 1992.PubMed/NCBI
|
|
24
|
Lee YS, Mollah ML, Sohn KC, Shi G, Kim DH,
Kim KH, Cho MJ, Kim S, Lee YH, Kim CD and Lee JH: ID3 mediates
X-ray-induced apoptosis of keratinocytes through the regulation of
β-catenin. J Dermatol Sci. 60:138–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sachindra Larribère L, Novak D, Wu H,
Hüser L, Granados K, Orouji E and Utikal J: New role of ID3 in
melanoma adaptive drug-resistance. Oncotarget. 8:110166–110175.
2017.PubMed/NCBI
|
|
26
|
Koyama T, Suzuki H, Imakiire A, Yanase N,
Hata K and Mizuguchi J: ID3-mediated enhancement of
cisplatin-induced apoptosis in a sarcoma cell line MG-63.
Anticancer Res. 24:1519–1524. 2004.PubMed/NCBI
|
|
27
|
Ponz-Sarvisé M, Nguewa PA, Pajares MJ,
Agorreta J, Lozano MD, Redrado M, Pio R, Behrens C, Wistuba II,
García-Franco CE, et al: Inhibitor of differentiation-1 as a novel
prognostic factor in NSCLC patients with adenocarcinoma histology
and its potential contribution to therapy resistance. Clin Cancer
Res. 17:4155–4166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Zhang CH, Hong YL, Li J, Hu YM and
Zhao CF: Inhibitor of DNA-binding-1/inhibitor of differentiation-1
(ID-1) is implicated in various aspects of gastric cancer cell
biology. Mol Biol Rep. 39:3009–3015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castañon E, Bosch-Barrera J, López I,
Collado V, Moreno M, López-Picazo JM, Arbea L, Lozano MD, Calvo A
and Gil-Bazo I: Id1 and ID3 co-expression correlates with clinical
outcome in stage III-N2 non-small cell lung cancer patients treated
with definitive chemoradiotherapy. J Transl Med. 11:132013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon SS, Ahn KS, Kim SH, Shim YM and Kim
J: In vitro establishment of cis-diammine-dichloroplatinum (II)
resistant lung cancer cell line and modulation of apoptotic gene
expression as a mechanism of resistant phenotype. Lung Cancer.
33:221–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grise F, Sena S, Bidaud-Meynard A, Baud J,
Hiriart JB, Makki K, Dugot-Senant N, Staedel C, Bioulac-Sage P,
Zucman-Rossi J, et al: Rnd3/RhoE is down-regulated in
hepatocellular carcinoma and controls cellular invasion.
Hepatology. 55:1766–1775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Wang Y, Liang B, He F, Li Y, Che
J, Li X, Zhao H and Shi G: The Rho GTPase RhoE exerts
tumor-suppressing effects in human esophageal squamous cell
carcinoma via negatively regulating epidermal growth factor
receptor. J Cancer Res Ther. 12:(Suppl):. S60–S63. 2016. View Article : Google Scholar
|